Abstract
Background
Warfarin reduces ischemic stroke risk in atrial fibrillation (AF) but increases bleeding risk. Novel anticoagulants challenge warfarin as stroke‐preventive therapy for AF. They are available at fixed doses but are more costly. Warfarin anticoagulation at a time in therapeutic range (TTR) ≥70% is similarly as effective and safe as novel anticoagulants. It is unclear whether AF patients with TTR ≥70% will remain stably anticoagulated and avoid the need to switch to a novel anticoagulant. We assessed stability of warfarin anticoagulation in AF patients with an initial TTR ≥70%.
Methods and Results
Within the community‐based Anticoagulation and Risk Factors in AF (ATRIA) cohort followed from 1996 to 2003, we identified 2841 new warfarin users who continued warfarin over 9 months. We excluded months 1 to 3 to achieve a stable dose. For the 987 patients with TTR ≥70% in an initial 6‐month period (TTR 1; months 4–9), we described the distribution of TTR 2 (months 10–15) and assessed multivariable correlates of persistent TTR ≥70%. Of patients with TTR 1 ≥70%, 57% persisted with TTR 2 ≥70% and 16% deteriorated to TTR 2 <50%. Only initial TTR 1 ≥90% (adjusted odds ratio 1.47, 95% CI 1.07–2.01) independently predicted TTR 2 ≥70%. Heart failure was moderately associated with marked deterioration (TTR 2 <50%); adjusted odds ratio 1.45, 95% CI 1.00–2.10.
Conclusions
Nearly 60% of AF patients with high‐quality TTR1 on warfarin maintained TTR ≥70% over the next 6 months. A minority deteriorated to very poor TTR. Patient features did not strongly predict TTR in the second 6‐month period. Our analyses support watchful waiting for AF patients with initial high‐quality warfarin anticoagulation before considering alternative anticoagulants.
Keywords: anticoagulants, arrhythmia, embolism, prevention, risk factors
Subject Categories: Atrial Fibrillation, Anticoagulants, Primary Prevention, Epidemiology
Introduction
Atrial fibrillation (AF) is the most frequent significant cardiac arrhythmia and the strongest common risk factor for ischemic stroke.1 It increases stroke risk 5‐fold and accounts for ≈15% of all strokes in the United States.2, 3 Moreover, strokes caused by AF are more likely to prove fatal or severely disabling.4, 5 High‐quality anticoagulant therapy can largely prevent AF‐associated thromboembolic events while minimizing bleeding risk.6 AF patients taking warfarin are at the lowest risk for both thromboembolism and intracranial hemorrhage at International Normalized Ratio (INR) levels of 1.8 to 3.5, a range that includes the guideline‐recommended range of 2.0 to 3.0.7, 8 Frequent INR monitoring with dose adjustment is generally needed to maintain INR levels in this target range of 2.0 to 3.0.9 Recently developed novel anticoagulants (ie, dabigatran, rivaroxaban, apixaban, and edoxaban) now compete with warfarin as the primary stroke‐preventive therapy for AF patients. These agents offer fixed oral doses without the need for frequent INR monitoring and with a reduced risk of intracranial hemorrhage compared with average to poor warfarin anticoagulation control.10 These novel agents appear to be particularly attractive for AF patients starting anticoagulant therapy. What remains unclear is whether AF patients who are well anticoagulated on warfarin should switch to a novel agent.
Time in the therapeutic range (TTR) of INR 2.0 to 3.0 is the standard means of assessing quality of warfarin therapy.11 Secondary analyses of randomized trials indicate that warfarin at TTR ≥70% has efficacy and safety comparable to novel anticoagulants, suggesting that patients with TTR ≥70% would gain little clinical benefit from switching to a novel anticoagulant.12, 13, 14 This assumes, however, that such a high TTR is stable over time. Limited analyses have examined the stability of TTR in AF patients taking warfarin over a prolonged period.15, 16, 17 Our study aimed to address this knowledge gap by assessing the stability of high‐quality anticoagulation over 12 months among patients initiating warfarin. Specifically, we used a large, real‐world cohort of AF patients newly taking warfarin to determine the percentage of patients with an initial 6‐month TTR (TTR1) ≥70% whose TTR persisted at ≥70% in the subsequent 6‐month period (TTR2). We then assessed patient features potentially predicting continued high‐quality TTR. Addressing these aims can provide guidance to patients and their providers when making the decision to continue warfarin or to switch to a novel agent.
Methods
Cohort Assembly
The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) cohort consists of 13 559 adults diagnosed with nonvalvular AF who received care at Kaiser Permanente Northern California, a large integrated health care delivery system. Details of cohort identification have been described previously.18 Briefly, patients were identified between July 1, 1996, and December 31, 1997, by searching outpatient databases in which an International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis of AF (427.31) was assigned and by searching electrocardiographic databases for diagnoses of AF. Those with at least 1 outpatient diagnosis of AF and an ECG diagnosis of AF were included in the cohort. Index date was defined as the date of first diagnosis of AF during cohort assembly. Patients were followed until September 30, 2003 (median 6.0 years; interquartile range 3.1–6.7 years), and censored at death or disenrollment from the health plan. Exclusion criteria included patients with diagnoses of mitral stenosis, valvular repair or replacement, transient postoperative AF, or concurrent hyperthyroidism to limit the cohort to those with nontransient, nonvalvular AF.
The study was approved by the institutional review boards of the collaborating institutions. Waiver of informed consent was obtained due to the nature of the study.
Study Variables
Our analysis focused exclusively on AF patients who were new and continuous users of warfarin. Warfarin status was assessed based on dispensed warfarin prescriptions and outpatient INR values in automated pharmacy and laboratory databases and was validated against warfarin status documented in medical records of potential outcome events.19 New users were defined as patients with a new prescription for warfarin during the cohort assembly period who had no prior identified warfarin prescription and <2 outpatient INR measurements in the previous 12 months.19 Continuous warfarin exposure was defined as periods of <60 days between the end of the days supplied and the beginning of the subsequent prescription for any 2 consecutive filled prescriptions. For periods >60 days, continuous warfarin use was assumed if there were intervening INR measurements at least every 42 days. If neither requirement was met, the patient was considered to have discontinued warfarin from day 31 after the end date of the first prescription until the start date of the next prescription. The 30‐day grace period was provided to account for reductions in dose or skipped doses.
TTR is the percentage of time an AF patient maintains an INR between 2.0 and 3.0. Using each patient's INR data, we calculated individual patient TTRs via the standard Rosendaal interpolation method for both an initial 6‐month period and the subsequent 6‐month period.20 This method defines TTR as the number of person‐days with an INR between 2.0 and 3.0 divided by total number of person‐days for which INR could be interpolated. It assumes that changes between consecutive INR measurements are linear over time. We also provided a TTR for the INR range 1.8 to 3.5; there is empirical evidence that this is the full optimal INR range.7 Our 12‐month study period excluded the first 3 months of warfarin use; this is a “break‐in” period during which optimal warfarin dosing is sought. The initial period was defined as months 4 to 9 inclusive, providing our measures of TTR1, and the subsequent period was defined as months 10 to 15 inclusive, providing TTR2. We excluded the 8.1% of time on warfarin during which the inter‐INR interval was >8 weeks.21 Characteristics of patients with and without 9 months of continuous warfarin use following initial prescription are shown for reference in Table S1.
Covariates included demographic features, risk factors for stroke in patients with AF, the CHA2DS2‐VASc and ATRIA stroke risk scores, a current or past diagnosis of cancer (excluding nonmelanoma skin cancer), and common rate and rhythm control drugs used in AF. Covariates were identified using clinical inpatient and ambulatory visit, administrative, and pharmacy databases for the 5 years before each patient's cohort index date. Diabetes mellitus was identified using a validated diabetes registry.18 Ascertainment of individual stroke risk factors was validated against a review of samples of outpatient medical records; crude agreement was high (78–96%), and corresponding κ statistics ranged from 0.51 to 0.89.21
Statistical Analysis
TTR was first assessed as a continuous variable, summarized using mean with standard deviation and median with interquartile range (quartiles 1–3). We dichotomized TTR at 70%. We assessed patient features correlated with TTR1. Among those patients with an initial TTR ≥70%, we recorded the distribution of TTR values in the second 6‐month period (TTR2). We assessed the univariate and multivariable associations of clinical features with persistence of TTR ≥70% and with marked deterioration of TTR (ie, TTR <50%). In sensitivity analyses, we included patients with TTR1 ≥70% who discontinued warfarin in the second 6‐month period as part of a composite outcome of deterioration (ie, TTR <50% or discontinued warfarin). We did not include the following categories of patients in the analysis of TTR2: (1) patients who died in the second period (n=16); (2) patients who discontinued warfarin in period 2 but who restarted warfarin >1 year later (n=42); (3) patients for whom the study follow‐up ended before their second period was complete (n=33); (4) patients who had no interpolatable INR values in period 2 (n=17); and (5) patients who ended their health plan membership during period 2 (n=3). Statistical significance of univariate associations was assessed via chi‐square tests. Features with a univariate association of P≤0.20 were entered into logistic regression models. In these models, P≤0.05 was considered statistically significant.
All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc).
Results
Of the 13 559 patients in the ATRIA cohort, 4460 met the criteria for new starts on warfarin during the study period. Of these, 2928 had ≥9 months of continuous warfarin use, including our initial 3‐month dose‐finding period and our first period assessing TTR (months 4–9). There were 2841 patients who had interpolatable INR values (ie, calculable TTR values) in period 1 (Figure 1). Of those, 43% were aged ≥75 years at baseline, 56% were men, 87% were white, 16% had diabetes mellitus, 55% had hypertension, 28% had coronary artery disease, 27% had heart failure, and 12% had diminished renal function (estimated glomerular filtration rate <45 mL/min per 1.73 m2), among other features. Moreover, 79% had a CHA2DS2‐VASc score22 ≥2, and 49% had an ATRIA stroke risk score23 ≥6 (Table 1).
Figure 1.
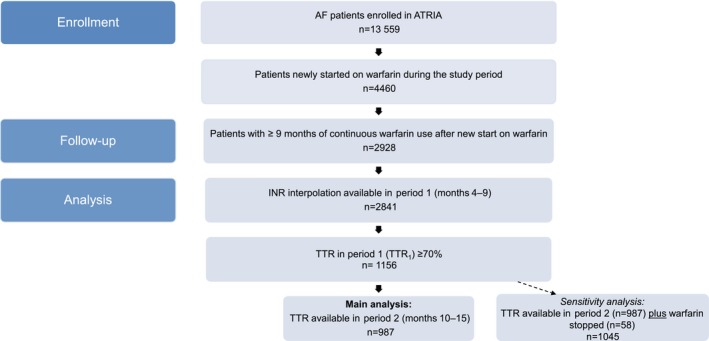
Selection of study cohort. Of the 13 559 patients in the ATRIA AF cohort, 4460 met the criteria for new starts on warfarin during the study period. Of these, 2928 had ≥9 months of continuous warfarin use, including our initial 3‐month dose‐finding period and our first period assessing TTR (months 4–9). There were 2841 patients who had interpolatable INR values (ie, calculable TTR values) in period 1. From these, we identified the 1156 patients newly started on warfarin and who had a TTR ≥70% in period 1 (TTR 1, months 4–9). The primary analysis focused on the 987 patients who had TTR 1 ≥70% and had a calculable TTR in period 2 (TTR 2, months 10–15). In a sensitivity analysis, we also included the 58 patients with TTR 1 ≥70% who discontinued warfarin in period 2. We did not include the following categories of patients in the analysis of TTR 2: (1) died in period 2 (n=16), (2) discontinued warfarin in period 2 but restarted warfarin within 1 year (n=42), (3) period 2 follow‐up was incomplete because the study ended (n=33), (4) no interpolatable INR values in period 2 (n=17), and (5) disenrolled in the health plan during period 2 (n=3). AF indicates atrial fibrillation; ATRIA, anticoagulation and risk factors in atrial fibrillation; INR, International Normalized Ratio; TTR, time in the therapeutic range.
Table 1.
Features of ATRIA Cohort Patients Initiating Warfarin Therapy and Continuing to Take Warfarin Therapy for at Least 9 Months
| Variablea | All Patients, n (%) |
|---|---|
| All | 2841 (100) |
| Age | |
| <75 y | 1614 (56.8) |
| ≥75 y | 1227 (43.2) |
| Sex | |
| Men | 1597 (56.2) |
| Women | 1244 (43.8) |
| Race | |
| White | 2478 (87.2) |
| Other | 363 (12.8) |
| Diabetes mellitus | 466 (16.4) |
| Hypertension | 1575 (55.4) |
| Coronary heart disease | 797 (28.1) |
| Heart failure | 752 (26.5) |
| Peripheral artery disease | 71 (2.5) |
| Renal impairmentb | 351 (12.4) |
| Prior stroke | 262 (9.2) |
| Cancer | 337 (11.9) |
| Prior bleed | 154 (5.4) |
| Beta blockers | 945 (33.3) |
| Antiarrhythmics | 469 (16.5) |
| Calcium channel blockers | 744 (26.2) |
| ATRIA stroke risk score at admissionc | |
| 0–5 | 1455 (51.2) |
| 6 | 404 (14.2) |
| ≥7 | 982 (34.6) |
| CHA2DS2‐VASc score at admissiond | |
| 0 | 161 (5.7) |
| 1 | 439 (15.5) |
| ≥2 | 2241 (78.9) |
ATRIA indicates anticoagulation and risk factors in atrial fibrillation; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease.
Variables assessed at the beginning of the 15‐month follow‐up period.
eGFR <45 mL/min/1.73 m2 or ESRD.
ATRIA risk score includes prior stroke, age, sex, diabetes mellitus, congestive heart failure, hypertension, proteinuria, and eGFR <45 mL/min/1.73 m2 or ESRD.23
CHA2DS2‐VASc score includes congestive heart failure; hypertension; age; diabetes mellitus; stroke, transient ischemic attack, or thromboembolism; vascular disease; and sex.22
For these 2841 new and continuous warfarin users, the mean TTR1 was 61.7% (SD 24.2%) and the median was 64.1% (interquartile range 45.7–80.1%). In total, 1156 (40.7%) achieved TTR1 ≥70% in the initial period, whereas 29.5% demonstrated poor control (TTR1 <50%) (Table 2). Most patient features were unrelated to initial TTR ≥70%, although patients with renal impairment had statistically significantly lower TTR1, and patients taking beta blockers had borderline significantly higher TTR1 (Table 3). In a multivariable logistic model, impaired renal function was the sole significant correlate of TTR1, with an adjusted odds ratio of 0.77 (95% CI 0.61–0.98) for TTR1 ≥70%.
Table 2.
Distribution of TTR1 (Months 4–9) for 2841 New Warfarin Usersa
| TTR1 Category | n (%) |
|---|---|
| ≥70% | 1156 (40.7) |
| 65–69% | 225 (7.9) |
| 60–64% | 213 (7.5) |
| 50–59% | 410 (14.4) |
| <50% | 837 (29.5) |
TTR1 indicates time in the therapeutic range during the first 6‐month period.
Months 4–9 are the first 6‐month period after the initial 3‐month period to establish a stable warfarin dose.
Table 3.
Association of Patient Baseline Features With TTR ≥70% in Period 1
| Variablea | n | Patients With TTR ≥70% in Months 4–9, n (%) | P Valueb |
|---|---|---|---|
| All | 2841 | 1156 (40.7) | |
| Age | 0.45 | ||
| <75 y | 1614 | 647 (40.1) | |
| ≥75 y | 1227 | 509 (41.5) | |
| Sex | 0.99 | ||
| Men | 1597 | 650 (40.7) | |
| Women | 1244 | 506 (40.7) | |
| Race | 0.44 | ||
| White | 2478 | 1015 (41.0) | |
| Other | 363 | 141 (38.8) | |
| Diabetes mellitus | 0.27 | ||
| No | 2375 | 977 (41.1) | |
| Yes | 466 | 179 (38.4) | |
| Hypertension | 0.65 | ||
| No | 1266 | 521 (41.2) | |
| Yes | 1575 | 635 (40.3) | |
| Coronary artery disease | 0.43 | ||
| No | 2044 | 841 (41.1) | |
| Yes | 797 | 315 (39.5) | |
| Heart failure | 0.14 | ||
| No | 2089 | 867 (41.5) | |
| Yes | 752 | 289 (38.4) | |
| Peripheral artery disease | 0.45 | ||
| No | 2770 | 1124 (40.6) | |
| Yes | 71 | 32 (45.1) | |
| Renal impairmentc | 0.016 | ||
| No | 2490 | 1034 (41.5) | |
| Yes | 351 | 122 (34.8) | |
| Prior stroke | 0.96 | ||
| No | 2579 | 1049 (40.7) | |
| Yes | 262 | 107 (40.8) | |
| Cancer | 0.35 | ||
| No | 2504 | 1011 (40.4) | |
| Yes | 337 | 145 (43.0) | |
| Prior bleed | 0.34 | ||
| No | 2687 | 1099 (40.9) | |
| Yes | 154 | 57 (37.0) | |
| Beta blockers | 0.057 | ||
| No | 1896 | 748 (39.5) | |
| Yes | 945 | 408 (43.2) | |
| Antiarrhythmics | 0.82 | ||
| No | 2372 | 963 (40.6) | |
| Yes | 469 | 193 (41.2) | |
| Calcium channel blockers | 0.15 | ||
| No | 2097 | 870 (41.5) | |
| Yes | 744 | 286 (38.4) | |
| ATRIA score at admissiond | 0.76 | ||
| 0 to 5 | 1455 | 601 (41.3) | |
| 6 | 404 | 164 (40.6) | |
| ≥7 | 982 | 391 (39.8) | |
| CHA2DS2‐VASc score at admissione | 0.79 | ||
| 0 | 161 | 66 (41.0) | |
| 1 | 439 | 185 (42.1) | |
| ≥2 | 2241 | 905 (40.4) |
ATRIA indicates anticoagulation and risk factors in atrial fibrillation; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; TTR, time in the therapeutic range.
Variables assessed at the beginning of the 15‐month follow‐up period.
P values from chi‐square tests.
eGFR <45 mL/min/1.73 m2 or ESRD.
ATRIA risk score includes prior stroke, age, sex, diabetes mellitus, congestive heart failure, hypertension, proteinuria, and eGFR <45 mL/min/1.73 m2 or ESRD.23
CHA2DS2‐VASc score includes congestive heart failure; hypertension; age; diabetes mellitus; stroke, transient ischemic attack, or thromboembolism; vascular disease; and sex.22
Among the 987 patients who achieved TTR1 ≥70% and had interpolatable INR values through period 2 (months 10–15), 562 (56.9%) persisted as well‐controlled warfarin users (TTR2 ≥70%). Most INR time that was out of range was below INR 2.0, and this pattern worsened in lower TTR categories (Figure 2). Expanding the definition of good‐quality TTR2 to ≥65%, 650 (65.9%) persisted as good‐quality warfarin users (TTR2 ≥65%). If the target INR range was expanded to include the full optimal INR range of 1.8 to 3.5 in the subsequent period, 891 (90.3%) achieved a TTR2 ≥70%.
Figure 2.
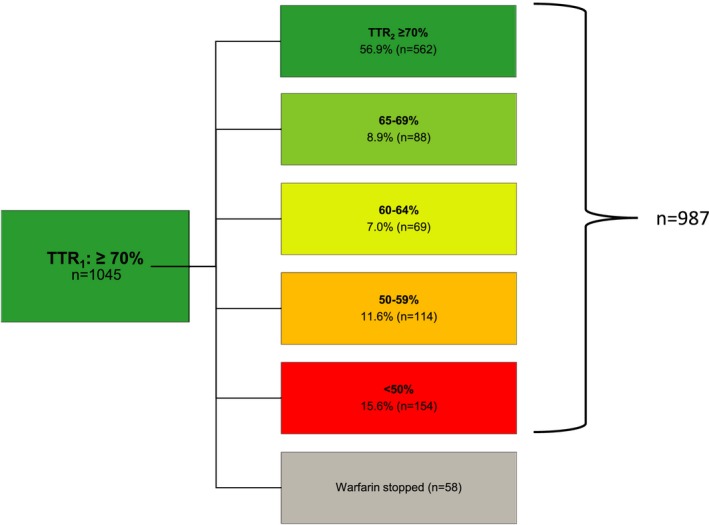
The distribution of TTR values in the second 6‐month period (TTR 2, months 10–15) among the 987 warfarin‐treated ATRIA atrial fibrillation cohort patients whose TTR in the first 6‐month period (TTR 1: months 4–9) was ≥70% and who continued warfarin with a calculable TTR in period 2. An additional 58 patients had TTR 1 ≥70% but discontinued warfarin in period 2. These last patients are included in a sensitivity analysis. Note that the initial 3 months on warfarin (months 1–3) were excluded because time is needed to establish warfarin dosing. The mean percentage of time below INR 2.0 and above INR 3.0, respectively, for the 5 ordered categories of TTR 2 were (1) TTR 2 ≥70%: 8.6% and 5.4%; (2) TTR 2 65–69%: 20.5% and 12.1%; (3) TTR 2 60–64%: 25.5% and 11.6%; (4) TTR 2 50–59%: 29.3% and 15.6%; and (5) TTR 2 <50%: 48.6% and 16.6%. ATRIA indicates anticoagulation and risk factors in atrial fibrillation; TTR, time in the therapeutic range.
In univariate analyses, TTR (using INR 2.0–3.0, the standard therapeutic range) in the initial period, absence of renal impairment, and absence of heart failure were significantly associated with high‐quality TTR in the subsequent period (Table 4). In multivariable analysis, only TTR1 ≥90% remained a strong independent predictor of persistent high‐quality warfarin therapy (adjusted odds ratio 1.47, 95% CI 1.07–2.01) (Table 4).
Table 4.
Univariate and Multivariable Correlates of TTR ≥70% in Months 10–15 Among Those With TTR ≥70% in Months 4–9 (n=987)a
| Variableb | n | Patients With TTR ≥70% in Months 10–15, n (%) | Univariate P Valuec | Multivariable Odds Ratio (95% CI)d |
|---|---|---|---|---|
| All | 987 | 562 (56.9) | NA | NA |
| TTR in months 4–9 | 0.018 | |||
| 70–79% | 375 | 202 (53.9) | Ref | |
| 80–89% | 319 | 173 (54.2) | 1.03 (0.76–1.39) | |
| ≥90% | 293 | 187 (63.8) | 1.47 (1.07–2.01)e | |
| Age | 0.79 | |||
| <75 y | 511 | 293 (57.3) | ||
| ≥75 y | 476 | 269 (56.5) | ||
| Sex | 0.46 | |||
| Men | 552 | 320 (58.0) | ||
| Women | 435 | 242 (55.6) | ||
| Race | 0.74 | |||
| White | 874 | 496 (56.8) | ||
| Other | 113 | 66 (58.4) | ||
| Diabetes mellitus | 0.073 | 0.80 (0.56–1.13) | ||
| No | 823 | 479 (58.2) | ||
| Yes | 164 | 83 (50.6) | ||
| Hypertension | 0.24 | |||
| No | 418 | 229 (54.8) | ||
| Yes | 569 | 333 (58.5) | ||
| Coronary heart disease | 0.69 | |||
| No | 717 | 411 (57.3) | ||
| Yes | 270 | 151 (55.9) | ||
| Heart failure | 0.012 | 0.79 (0.59–1.06) | ||
| No | 705 | 419 (59.4) | ||
| Yes | 282 | 143 (50.7) | ||
| Peripheral artery disease | 0.34 | |||
| No | 958 | 548 (57.2) | ||
| Yes | 29 | 14 (48.3) | ||
| Renal impairmente | 0.027 | 0.74 (0.50–1.11) | ||
| No | 871 | 507 (58.2) | ||
| Yes | 116 | 55 (47.4) | ||
| Prior stroke | 0.89 | |||
| No | 895 | 509 (56.9) | ||
| Yes | 92 | 53 (57.6) | ||
| Cancer | 0.63 | |||
| No | 865 | 495 (57.2) | ||
| Yes | 122 | 67 (54.9) | ||
| Prior bleed | 0.98 | |||
| No | 938 | 534 (56.9) | ||
| Yes | 49 | 28 (57.1) | ||
| Beta blockers | 0.65 | |||
| No | 658 | 378 (57.4) | ||
| Yes | 329 | 184 (55.9) | ||
| Antiarrhythmics | 0.45 | |||
| No | 881 | 498 (56.5) | ||
| Yes | 106 | 64 (60.4) | ||
| Calcium channel blockers | 0.31 | |||
| No | 753 | 422 (56.0) | ||
| Yes | 234 | 140 (59.8) | ||
| ATRIA scoref | 0.91 | |||
| 0–5 | 472 | 272 (57.6) | ||
| 6 | 136 | 77 (56.6) | ||
| ≥7 | 379 | 213 (56.2) | ||
| CHA2DS2‐VASc scoreg | 0.069 | |||
| 0 | 44 | 25 (56.8) | Ref | |
| 1 | 138 | 91 (65.9) | 1.53 (0.76–3.07) | |
| ≥2 | 805 | 446 (55.4) | 1.14 (0.61–2.14) |
ATRIA indicates anticoagulation and risk factors in atrial fibrillation; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; NA, not available; Ref, reference; TTR, time in the therapeutic range.
Included patients had to have a calculable TTR in period 2.
Variables assessed at the start of month 10 of follow‐up.
Univariate P values from chi‐square tests.
Variables entered into the multivariable logistic model were TTR in months 4–9, diabetes mellitus, heart failure, and renal impairment.
eGFR <45 mL/min/1.73 m2 or ESRD.
ATRIA risk score includes prior stroke, age, sex, diabetes mellitus, congestive heart failure, hypertension, proteinuria, and eGFR <45 mL/min/1.73 m2 or ESRD.23
CHA2DS2‐VASc score includes heart failure; hypertension; age; diabetes mellitus; stroke, transient ischemic attack, or thromboembolism; vascular disease; and sex.22
Among patients with TTR1 ≥70%, 154 (15.6%) had the poor outcome of a TTR2 <50% (Figure 2). Only heart failure was significantly associated with deterioration to TTR <50% in the subsequent period (adjusted odds ratio 1.45, 95% CI 1.00–2.10) (Table 5). Of 1045 patients achieving a TTR ≥70% in months 4 to 9, 58 discontinued warfarin in months 10 to 15. If we broadened our definition of poor outcome to the composite of TTR2 <50% or discontinued warfarin, no patient features were significantly related to poor outcome in multivariable models. With this expanded definition of poor outcome, the adjusted odds ratio for heart failure was 1.24 (95% CI 0.89–1.73).
Table 5.
Univariate and Multivariable Correlates of Markedly Deteriorated TTR (<50%) Among Those With TTR ≥70% in Months 4–9 (n=987)
| Variablea | n | Patients With TTR <50% in Months 10–15, n (%) | Univariate P Valueb | Multivariable Odds Ratio (95% CI)c |
|---|---|---|---|---|
| All | 987 | 154 (15.6) | NA | NA |
| TTR in months 4–9 | 0.66 | |||
| 70–79% | 375 | 54 (14.4) | ||
| 80–89% | 319 | 54 (16.9) | ||
| ≥90% | 293 | 46 (15.7) | ||
| Age | 0.63 | |||
| <75 y | 511 | 77 (15.1) | ||
| ≥75 y | 476 | 77 (16.2) | ||
| Sex | 0.71 | |||
| Men | 552 | 84 (15.2) | ||
| Women | 435 | 70 (16.1) | ||
| Race | 0.47 | |||
| White | 874 | 139 (15.9) | ||
| Other | 113 | 15 (13.3) | ||
| Diabetes mellitus | 0.92 | |||
| No | 823 | 128 (15.6) | ||
| Yes | 164 | 26 (15.9) | ||
| Hypertension | 0.83 | |||
| No | 418 | 64 (15.3) | ||
| Yes | 569 | 90 (15.8) | ||
| Coronary heart disease | 0.34 | |||
| No | 717 | 107 (14.9) | ||
| Yes | 270 | 47 (17.4) | ||
| Heart failure | 0.02 | 1.45 (1.00–2.10) | ||
| No | 705 | 98 (13.9) | ||
| Yes | 282 | 56 (19.9) | ||
| Peripheral artery disease | 0.43 | |||
| No | 958 | 151 (15.8) | ||
| Yes | 29 | 3 (10.3) | ||
| Renal impairmentd | 0.18 | 1.25 (0.75–2.07) | ||
| No | 871 | 131 (15.0) | ||
| Yes | 116 | 23 (19.8) | ||
| Prior stroke | 0.68 | |||
| No | 895 | 141 (15.8) | ||
| Yes | 92 | 13 (14.1) | ||
| Cancer | 0.43 | |||
| No | 865 | 132 (15.3) | ||
| Yes | 122 | 22 (18.0) | ||
| Prior bleed | 0.29 | |||
| No | 938 | 149 (15.9) | ||
| Yes | 49 | 5 (10.2) | ||
| Beta blockers | 0.62 | |||
| No | 658 | 100 (15.2) | ||
| Yes | 329 | 54 (16.4) | ||
| Antiarrhythmics | 0.064 | 0.52 (0.27–1.03) | ||
| No | 881 | 144 (16.3) | ||
| Yes | 106 | 10 (9.4) | ||
| Calcium channel blockers | 0.12 | 0.72 (0.46–1.11) | ||
| No | 753 | 125 (16.6) | ||
| Yes | 234 | 29 (12.4) | ||
| ATRIA score at admissione | 0.33 | |||
| 0–5 | 472 | 73 (15.5) | ||
| 6 | 136 | 16 (11.8) | ||
| ≥7 | 379 | 65 (17.2) | ||
| CHA2DS2‐VASc score at admissionf | 0.57 | |||
| 0 | 44 | 5 (11.4) | ||
| 1 | 138 | 19 (13.8) | ||
| ≥2 | 805 | 130 (16.1) |
ATRIA indicates anticoagulation and risk factors in atrial fibrillation; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; NA, not available; TTR, time in the therapeutic range.
Variables assessed at the start of month 10 of follow‐up.
Univariate P values from chi‐square tests.
Variables entered into the multivariable logistic model were heart failure, renal impairment, antiarrhythmics, and calcium channel blockers at the start of month 10.
eGFR <45 mL/min/1.73 m2 or ESRD.
ATRIA risk score includes prior stroke, age, sex, diabetes mellitus, congestive heart failure, hypertension, proteinuria, and eGFR <45 mL/min/1.73 m2 or ESRD.23
CHA2DS2‐VASc score includes heart failure; hypertension; age; diabetes mellitus; stroke, transient ischemic attack, or thromboembolism; vascular disease; and sex.22
Discussion
Using a large real‐world community cohort of AF patients newly and continuously taking warfarin, we assessed how well good control of anticoagulation persisted. We allowed an initial dose‐finding period of months 1 to 3 and then calculated the TTR in study period 1 (months 4–9). We then focused on patients with high‐quality anticoagulation (ie, TTR ≥70%) in period 1 and followed them over a subsequent 6‐month period (months 10–15). We found that 41% of patients achieved TTR ≥70% in period 1. Of these, 57% persisted at TTR ≥70% in period 2. Another 8.9% maintained a TTR2 between 65% and 69%, a range still considered good quality and better than levels seen in recent randomized trials.24 With the target INR range in period 2 defined as 1.8 to 3.5, fully 90% maintained TTR ≥70%. Empirical evidence shows that the optimal INR range for AF is 1.8 to 3.5, not just 2.0 to 3.0.7 Only 16% deteriorated to a TTR <50% (using the standard target of INR 2.0–3.0). Although excellent TTR (ie, ≥90%) predicted continued high TTR levels, no other clinical feature was significantly associated with TTR ≥70% in the second period of warfarin anticoagulation. The presence of heart failure was the only significant predictor of marked deterioration (ie, TTR <50%) in the second follow‐up period. When we expanded our definition of poor outcome in period 2 to include both TTR <50% or discontinuing warfarin, no clinical feature was significantly and independently associated with poor outcome. Our results indicate that most patients with well‐controlled warfarin therapy continue to do well.
Because INR levels between 2.0 and 3.0 are associated with low rates of both ischemic stroke and major bleeding, linearly interpolated TTR has become a standard measure of quality of warfarin management.11 Several clinical trials have provided evidence of warfarin's improved efficacy at high TTR levels, usually defined as approximately ≥70%.12, 14, 25 Multiple studies have aimed to identify predictors of TTR, including language, race, sex, age, and medical history, but such patient features account for only a small fraction of the variance of TTR.26, 27, 28, 29 We found that only impaired renal function was significantly and inversely associated with TTR ≥70% in our first period of follow‐up (months 4–9). Patients with impaired renal function were largely excluded from the trials of novel anticoagulants. These recently developed anticoagulants are not optimal replacements for warfarin in the face of renal insufficiency.24, 30, 31, 32
AF patients receiving warfarin now have the opportunity to switch to novel oral anticoagulants. These agents have comparable efficacy for reducing risk of stroke and do not require frequent INR monitoring. They have a clear advantage in reducing rates of intracranial hemorrhage compared with warfarin by approximately four per thousand per year.33 Although there is some inconsistency across trials,34 warfarin at TTR ≥70% appears to be equivalent to novel anticoagulants in terms of preventing stroke and systemic embolism, with increased risk of hemorrhagic stroke countered by reduced risk of ischemic stroke.12, 14 There may still be a small benefit favoring novel agents in terms of nonstroke intracranial hemorrhage, partially balanced by an increased risk of gastrointestinal hemorrhage.33 Patients are likely to incur greater out‐of‐pocket costs with novel agents than with warfarin.35 Moreover, patients may prefer to continue taking warfarin.36 Our results are directly relevant to patients deciding whether to continue warfarin or to seek a substitute and for whom the convenience of novel agents is not an important factor. Our results support a watchful waiting approach for AF patients who have achieved a TTR ≥70% when taking warfarin because most of these patients will continue to do well. Providers should be particularly attentive to patients with heart failure because such patients are at modestly increased risk of substantial deterioration in TTR.37
Our study represents the experience of AF patients in a community‐based cohort within a large, well‐resourced, integrated health care delivery system. Management of warfarin was coordinated predominantly through dedicated anticoagulation management services. Our results should apply broadly to patients whose anticoagulation was managed in a similar fashion. It is not clear whether our findings generalize to other, less formal systems of managing anticoagulation. High‐quality warfarin anticoagulation depends on patients obtaining frequent INR tests. Patient‐ or system‐level barriers to such a testing regimen will pose a challenge to maintaining TTR levels ≥70%. Our results strictly apply to AF patients at the 9‐month mark after initiating warfarin therapy. Although we assume these results apply more broadly to all AF patients after a prolonged period (ie, 6 months) of high‐quality warfarin treatment, validation of this assumption awaits further study.
Conclusion
Overall, 57% of AF patients with high‐quality 6‐month TTR after initiating warfarin (excluding an initial 3‐month dose‐finding period) maintained TTR ≥70% over the subsequent 6 months. A minority deteriorated to very poor TTR. Patient features do not strongly predict deterioration, although heart failure moderately increases this risk. Our analyses support watchful waiting for AF patients with initial high‐quality warfarin anticoagulation before considering switching to a novel anticoagulant.
Sources of Funding
This study was supported by the National Institute on Aging (R01 AG15478), the National Heart, Lung, and Blood Institute (RC2HL101589 and U19 HL091179) and the Eliot B. and Edith C. Shoolman Fund of the Massachusetts General Hospital (Boston, MA). The funding sources had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or in preparation, review or approval of the manuscript.
Disclosures
Singer has received research support from Bristol‐Myers Squibb, Boehringer Ingelheim, and Medtronic. He has served as a consultant or advisory board member for Boehringer Ingelheim, Bristol‐Myers Squibb, Medtronic, Merck, and CVS Health. Go has received research support from iRhythm. Dallalzadeh, Chang, Borowsky and Fang have no disclosures to report.
Supporting information
Table S1. Characteristics of Patients With and Without 9 Months of Continuous Warfarin Use Following Initial Prescription
(J Am Heart Assoc. 2016;5:e003482 doi: 10.1161/JAHA.116.003482)
References
- 1. Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham study. Stroke. 1991;22:312–318. [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly: the Framingham Heart Study. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 3. Go AS, Hylek EM, Phillips JA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 4. Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, Silver FL, Kapral MK. Potentially preventable strokes in high‐risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–240. [DOI] [PubMed] [Google Scholar]
- 5. Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG; Investigators of the Registry of the Canadian Stroke N, the Stroke Outcomes Research Canada Working G . Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44:99–104. [DOI] [PubMed] [Google Scholar]
- 6. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 7. Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. Should patient characteristics influence target anticoagulation intensity for stroke prevention in nonvalvular atrial fibrillation? The ATRIA study. Circ Cardiovasc Qual Outcomes. 2009;2:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice G . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 9. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest P . Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e44S–e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmad Y, Lip GY. Stroke prevention in atrial fibrillation: where are we now? Clin Med Insights Cardiol. 2012;6:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rose AJ. Improving the management of warfarin may be easier than we think. Circulation. 2012;126:2277–2279. [DOI] [PubMed] [Google Scholar]
- 12. Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, Pais P, Dans A, Eikelboom J, Oldgren J, Pogue J, Reilly PA, Yang S, Connolly SJ; Investigators R‐L . Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983. [DOI] [PubMed] [Google Scholar]
- 13. Giugliano RP, Ruff CT, Rost NS, Silverman S, Wiviott SD, Lowe C, Deenadayalu N, Murphy SA, Grip LT, Betcher JM, Duggal A, Dave J, Shi M, Mercuri M, Antman EM, Braunwald E; Investigators EA‐T . Cerebrovascular events in 21 105 patients with atrial fibrillation randomized to edoxaban versus warfarin: effective anticoagulation with factor Xa next generation in atrial fibrillation‐thrombolysis in myocardial infarction 48. Stroke. 2014;45:2372–2378. [DOI] [PubMed] [Google Scholar]
- 14. O'Donoghue ML, Ruff CT, Giugliano RP, Murphy SA, Grip LT, Mercuri MF, Rutman H, Shi M, Kania G, Cermak O, Braunwald E, Antman EM. Edoxaban vs. warfarin in vitamin K antagonist experienced and naive patients with atrial fibrillation. Eur Heart J. 2015;36:1470–1477. [DOI] [PubMed] [Google Scholar]
- 15. Witt DM, Delate T, Clark NP, Martell C, Tran T, Crowther MA, Garcia DA, Ageno W, Hylek EM, Warped C. Twelve‐month outcomes and predictors of very stable INR control in prevalent warfarin users. J Thromb Haemost. 2010;8:744–749. [DOI] [PubMed] [Google Scholar]
- 16. Witt DM, Delate T, Clark NP, Martell C, Tran T, Crowther MA, Garcia DA, Ageno W, Hylek EM; Warfarin Associated Research P, other EnDeavors C . Outcomes and predictors of very stable INR control during chronic anticoagulation therapy. Blood. 2009;114:952–956. [DOI] [PubMed] [Google Scholar]
- 17. Shalev V, Rogowski O, Shimron O, Sheinberg B, Shapira I, Seligsohn U, Berliner S, Misgav M. The interval between prothrombin time tests and the quality of oral anticoagulants treatment in patients with chronic atrial fibrillation. Thromb Res. 2007;120:201–206. [DOI] [PubMed] [Google Scholar]
- 18. Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–934. [DOI] [PubMed] [Google Scholar]
- 19. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 21. Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. [DOI] [PubMed] [Google Scholar]
- 22. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 23. Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, Reynolds K, Go AS. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e000250 doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; Investigators EA‐T . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 25. Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–2037. [DOI] [PubMed] [Google Scholar]
- 26. Gallego P, Roldan V, Marin F, Galvez J, Valdes M, Vicente V, Lip GY. SAMe‐TT2R2 score, time in therapeutic range, and outcomes in anticoagulated patients with atrial fibrillation. Am J Med. 2014;127:1083–1088. [DOI] [PubMed] [Google Scholar]
- 27. Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Patient characteristics associated with oral anticoagulation control: results of the Veterans Affairs Study to Improve Anticoagulation (VARIA). J Thromb Haemost. 2010;8:2182–2191. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez F, Hong C, Chang Y, Oertel LB, Singer DE, Green AR, Lopez L. Limited English proficient patients and time spent in therapeutic range in a warfarin anticoagulation clinic. J Am Heart Assoc. 2013;2:e000170 doi: 10.1161/JAHA.113.000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe‐TT(2)R(2) score. Chest. 2013;144:1555–1563. [DOI] [PubMed] [Google Scholar]
- 30. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; Committee R‐LS, Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 31. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 32. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; Committees A, Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 33. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 34. Wallentin L, Lopes RD, Hanna M, Thomas L, Hellkamp A, Nepal S, Hylek EM, Al‐Khatib SM, Alexander JH, Alings M, Amerena J, Ansell J, Aylward P, Bartunek J, Commerford P, De Caterina R, Erol C, Harjola VP, Held C, Horowitz JD, Huber K, Husted S, Keltai M, Lanas F, Lisheng L, McMurray JJ, Oh BH, Rosenqvist M, Ruzyllo W, Steg PG, Vinereanu D, Xavier D, Granger CB; Apixaban for Reduction in S, Other Thromboembolic Events in Atrial Fibrillation I . Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127:2166–2176. [DOI] [PubMed] [Google Scholar]
- 35. Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, Spettell CM, Brennan TA, Matlin OS, Avorn J, Choudhry NK. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation‐ quality and cost implications. Am J Med. 2014;127:1075–1082, e1071. [DOI] [PubMed] [Google Scholar]
- 36. Elewa HF, DeRemer CE, Keller K, Gujral J, Joshua TV. Patients satisfaction with warfarin and willingness to switch to dabigatran: a patient survey. J Thromb Thrombolysis. 2014;38:115–120. [DOI] [PubMed] [Google Scholar]
- 37. Kim EJ, Ozonoff A, Hylek EM, Berlowitz DR, Ash AS, Miller DR, Zhao S, Reisman JI, Jasuja GK, Rose AJ. Predicting outcomes among patients with atrial fibrillation and heart failure receiving anticoagulation with warfarin. Thromb Haemost. 2015;114:70–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Patients With and Without 9 Months of Continuous Warfarin Use Following Initial Prescription


