Abstract
Background
There is limited evidence of long‐term impact of right ventricular pacing on left ventricular (LV) systolic function in pacemaker recipients with preserved LV ejection fraction (LVEF). The objective of the study was to evaluate the outcome and echocardiographic course of baseline preserved LVEF in a large cohort of pacemaker recipients with respect to pacing indication and degree of right ventricular pacing.
Methods and Results
We enrolled 991 patients (73±10 years, 54% male) with baseline normal (>55%) LVEF (n=791) or mildly reduced (41–55%) LVEF (n=200) who had paired echocardiographic data on LV systolic function recorded at implantation and last follow‐up. According to pacing indication, patients were divided into atrioventricular block group A (n=500) and sinus node disease group B (n=491). Main outcome measures were all‐cause mortality and deterioration of LV function ≥2 LVEF categories at last follow‐up. Patients were followed for an average of 44 months. Death from any cause occurred in 166 (17%), and deterioration of LV function ≥2 LVEF categories in 56 (6%) patients. There was no significant difference in outcome between group A and group B either in patients with normal LVEF or in those with mildly reduced LVEF. Mean percentage of right ventricular pacing was not predictive of outcome.
Conclusions
In a large cohort of pacemaker recipients with predominantly normal LVEF, clinically relevant LV dysfunction develops rather infrequently. No significant difference in all‐cause mortality and development of severe LV dysfunction is observed between patients with atrioventricular block and sinus node disease. Accordingly, de novo biventricular pacing cannot be recommended for patients with preserved LVEF.
Keywords: bradycardia, cardiac resynchronization therapy, heart failure, left ventricular ejection fraction, pacemaker, right ventricular pacing, ventricular dyssynchrony
Subject Categories: Arrhythmias, Electrophysiology
Introduction
Several studies have demonstrated that conventional right ventricular (RV) apical pacing causes changes of the electrical and mechanical activation pattern of the heart. These changes can result in an altered regional perfusion, mechanical dyssynchrony, and adverse left ventricular (LV) remodeling.1, 2, 3, 4 Eventually pacing‐induced mechanical dyssynchrony may promote progressive impairment of LV systolic and diastolic function, resulting in severe clinical heart failure and cardiac death.5 These effects seem to be of particular clinical relevance for pacing‐dependent patients with preexisting LV dysfunction at time of pacemaker insertion.6, 7
Nonetheless, patients with baseline normal cardiac function may also be affected by the detrimental effects of RV pacing.8, 9, 10 However, it does not necessarily mean that every conventionally RV‐paced heart is prone to mechanical dyssynchrony. The development of mechanical dyssynchrony and associated LV dysfunction during long‐term RV pacing rather seems to be an individual and multifactorial process. This would explain why certain individuals with normal LV function at time of pacemaker insertion, who represent the vast majority of conventional pacemaker recipients, are susceptible to the potentially harmful effects of RV pacing while many others are not.
To the best of our knowledge, no investigation thus far has studied the incidence of pacing‐induced LV dysfunction in a large, nonpreselected population of conventional pacemaker recipients with baseline preserved LV systolic function. Therefore, the main objective of the present study was to evaluate the overall outcome and echocardiographic course of LV function in a large cohort of pacemaker recipients with baseline normal or at the most mildly reduced LV systolic function with respect to antibradycardia pacing indication and anticipated cumulative degree of RV pacing.
Methods
Study Population
All consecutive patients implanted with a single‐ or dual‐chamber pacemaker for a standard antibradycardia pacing indication at University Heart Center Bad Krozingen between 2005 and 2009 were screened for analysis. For the purpose of the study, we only enrolled eligible pacemaker recipients who had a normal or at the most mildly reduced LV ejection fraction (LVEF ≥41%) at time of device implantation and who also had a clinical and echocardiographic follow‐up of at least 6 months. Exclusion criteria were a reduced LVEF of 40% or less at baseline, an indication for an implantable cardioverter‐defibrillator or cardiac resynchronization therapy (CRT) device, or the lack of sophisticated paired echocardiographic examination at baseline and follow‐up of at least 6 months after device implantation. All available clinical, echocardiographic, and device‐related data were prospectively gathered and electronically stored at University Heart Center Bad Krozingen. Enrolled subjects were classified into 2 groups according to the initial antibradycardia pacing indication and anticipated degree of ventricular pacing: group A, patients with atrioventricular (AV) conduction block, expected to require a high percentage of RV pacing (“AVB cohort”); and group B, patients with sinus node disease, expected to require a low percentage of RV pacing (“SND cohort”). Pacemaker recipients who could not be clearly assigned to either group were excluded from analysis. The SND cohort was defined as control (minimally or nonpaced) group since they have an intermittent or minimal need for ventricular pacing in contrast to group A patients with advanced AV conduction block. To achieve this goal, all group B patients were programmed to minimize ventricular pacing, including the use of algorithms that favor atrial pacing over AV sequential pacing.
Follow‐up treatment was adjusted to the patient's clinical needs at the discretion of the treating physician. An upgrade to CRT was performed in patients who developed severe clinical heart failure during follow‐up and featured an established class I or IIa indication for CRT according to the 2008 guidelines.11
The study was approved by the institutional review board, and all included subjects gave informed consent to participate in the registry.
Echocardiographic Assessments
Standard transthoracic echocardiography was performed by an experienced examiner to assess LV function at the time of implantation and at least once during pacemaker follow‐up according to the current practice guidelines.12 At any echocardiographic examination, a minimum of 3 cardiac cycles were recorded and analyzed. Preserved LV function was defined as normal global LV systolic function with fractional shortening ≥30% without regional wall motion abnormalities and estimated LVEF >55%. Deterioration of LV systolic function with or without regional wall motion abnormalities was classified as mild, moderate, and severe, depending on the assessed LVEF at last follow‐up. Following the current recommendations,12 we defined 4 categories of LV systolic function to which the patients were finally assigned: LV‐0: LVEF >55% (preserved); LV‐1: LVEF 41% to 55% (mildly reduced); LV‐2: LVEF 31% to 40% (moderately reduced); and LV‐3: LVEF ≤30% (severely reduced). The most recent echocardiographic examination performed at last visit was considered to define the follow‐up category of LV systolic function.
Outcome Measures
The predefined main outcome measures were (1) time to death from any cause, and (2) deterioration of LV systolic function ≥2 LVEF categories at time of last follow‐up as compared with the value at time of implantation. Outcome measures were compared between the pacing indication groups A and B with respect to the baseline category of LV systolic function (LV‐0 versus LV‐1).
Statistical Analysis
All data have been evaluated using standard frequency distributions for categorical variables as numbers and percentage, whereas continuous variables are presented as means±SD. The χ2 test was performed to compare categorical parameters of the pacing indication groups A and B. To compare continuous variables, the unpaired t test or the Mann–Whitney test was used as appropriate. In order to reveal predictors for the main outcomes, effects of explanatory variables (LVEF category, pacing group assignment, percentage RV pacing, age, sex, atrial fibrillation, coronary artery disease, cardiovascular disease, hypertension, and diabetes mellitus) were evaluated using univariate and multivariate Cox proportional hazard regression models, which were also used to test for interactions between pacing indication group and LVEF category. Survival curves were plotted by use of the Kaplan–Meier method and analyzed by the log‐rank test. A 2‐tailed probability value <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 21.0 software package (SPSS Inc, Chicago, IL) for all computations.
Results
Patient Characteristics
Between January 2005 and December 2009, a total of 1552 patients received a conventional single‐ or dual‐chamber pacemaker at University Heart Center Bad Krozingen and were assessed for eligibility. Among all pacemaker recipients, 561 (36%) did not meet the entry criteria and were excluded from analysis. Hence, the study population consisted of 991 patients (530 male, 54%) with a mean age of 73±10 years. Five hundred patients (51%) were assigned to group A (AVB cohort), and 491 patients (49%) to group B (SND cohort) (Figure 1). Important clinical characteristics of the study population and the 2 groups, including detailed information on the pacing indications and implanted cardiac pacing devices, are presented in Table 1. Female sex, atrial fibrillation, and hypertension were significantly more prevalent among group B than group A patients. At time of pacemaker implantation, 791 patients (80%) had a preserved LVEF (LV‐0), and 200 patients (20%) had a mildly reduced LVEF (LV‐1). There was no significant difference in baseline LV systolic function between group A and group B (P=0.26). Patients were followed for a mean of 44.2±21.2 months with no significant difference in time to follow‐up between groups (44.2±21.5 versus 44.1±21.0 months, P=0.22; median 41.2 versus 43.6 months).
Figure 1.

Study enrollment and patient population. #Exclusion criteria were baseline reduced LVEF ≤40%, indication for an ICD or CRT device, and lack of paired echocardiographic data recorded at baseline and follow‐up. According to pacing indication and anticipated degree of RV pacing, patients were classified into 2 groups: group A, patients with AV block (AVB cohort), and group B, patients with sinus node disease (SND cohort). LV‐0, baseline normal LVEF (>55%); LV‐1, baseline mildly reduced LVEF (41% to 55%). AV indicates atrioventricular; AVB, atrioventricular block; CRT, cardiac resyncynchronization therapy; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; RV, right ventricular; SND, sinus node disease.
Table 1.
Clinical Characteristics, Pacing Indication, and Implanted Pacing Device
| All Patients (n=991) | Group A (n=500) | Group B (n=491) | |
|---|---|---|---|
| Age, y | 73±10 | 72±11a | 74±9a |
| Male, n (%) | 530 (54) | 295 (59)b | 235 (48)b |
| Baseline LVEF category, n (%) | |||
| LV‐0 (LVEF >55%) | 791 (80) | 392 (78)a | 399 (81)a |
| LV‐1 (LVEF 41–55%) | 200 (20) | 108 (22)a | 92 (19)a |
| Cardiovascular disease, n (%) | 437 (44) | 243 (49)b | 194 (40)b |
| Coronary artery disease | 277 (28) | 148 (30)a | 129 (26)a |
| Atrial fibrillation, n (%) | 258 (26) | 117 (23)b | 141 (29)b |
| Hypertension | 642 (65) | 298 (60)b | 344 (70)b |
| Diabetes mellitus, n (%) | 67 (7) | 39 (8)a | 28 (6)a |
| Indication for pacing, n (%) | |||
| AV conduction disease | 500 (51) | 500 (100) | — |
| Long PR interval and 1:1 AVC | 1 (0.1) | 1 (0.2) | — |
| AVB type Mobitz 1 | 14 (1.4) | 14 (2.8) | — |
| AVB type Mobitz 2 | 77 (7.8) | 77 (15.4) | — |
| Advanced AVB | 123 (12.4) | 123 (24.6) | — |
| Complete AVB | 189 (19.1) | 189 (37.8) | — |
| AF with slow ventricular rate | 57 (5.8) | 57 (11.4) | — |
| AV junction ablation | 39 (3.9) | 39 (7.8) | — |
| Sick‐sinus syndrome | 452 (45.6) | — | 452 (92.1) |
| Carotid sinus syndrome | 25 (2.5) | — | 25 (5.1) |
| Cardioinhibitory reflex syncope | 14 (1.3) | — | 14 (2.8) |
| Cardiac pacing device, n (%) | |||
| Single‐chamber ventricular PM (VVI(R)) | 138 (14) | 109 (22) | 29 (6) |
| Single‐chamber atrial PM (AAI(R)) | 6 (0.6) | 0 (0) | 6 (1) |
| Dual‐chamber PM (DDD(R)) | 847 (86) | 391 (78) | 356 (93) |
Plus‐minus values are means±SD. AF indicates atrial fibrillation; AV, atrioventricular; AVB, atrioventricular block; AVC, atrioventricular conduction; LVEF, left ventricular ejection fraction; ns, not significant; PM, pacemaker.
P=ns.
P≤0.001.
Echocardiographic Follow‐Up of LV Systolic Function
After a mean follow‐up of 44.2±21.2 months, 74% of the whole study population (732 of 991 patients) revealed a preserved LV systolic function. Among the 791 patients with baseline normal LVEF, 658 (83%) had a preserved (LV‐0) and 93 (12%) had a mildly reduced (LV‐1) LVEF. Forty patients (5%) experienced deterioration of LV systolic function ≥2 LVEF categories (LVEF ≤40%; LV‐2 or LV‐3) (Figure 2). Among the 200 patients with baseline mildly reduced LVEF, 74 (37%) showed an improvement to normal (LV‐0), 71 (35.5%) remained unchanged with a mildly reduced LVEF (LV‐1), and 55 (27.5%) had worsening of LVEF below 41%. Sixteen patients (8%) experienced deterioration of LV systolic function ≥2 LVEF categories (LV‐3) (Figure 2).
Figure 2.

Echocardiographic follow‐up of LV systolic function in patients with baseline normal LVEF (LV‐0) and baseline mildly reduced LVEF (LV‐1). Presented is the percentage (plus 95% CI) of patients in each LVEF category assessed at time of last follow‐up. LV‐0: LVEF >55%; LV‐1: LVEF 41% to 55%; LV‐2: LVEF 31% to 40%; LV‐3: LVEF ≤30%. LV indicates left ventricular; LVEF, left ventricular ejection fraction.
There was no significant difference in the long‐term course of LV systolic function between group A and group B. The development and severity of LV dysfunction was similar in both groups for patients with baseline normal LVEF (P=0.58) and patients with baseline mildly reduced LVEF (P=0.12) (Figure 3). Eighteen patients out of the whole study population (1.8%) developed severe clinical heart failure and were upgraded to a CRT device. Eleven of these patients presented with baseline normal LVEF, and 7 with baseline mildly reduced LVEF (P=ns). There was no significant difference in the need for CRT upgrade between group A and group B (1.8% versus 1.8%; P=0.97).
Figure 3.

Echocardiographic follow‐up of LV systolic function in group A and group B patients with baseline normal LVEF (LV‐0) (upper panel) and baseline mildly reduced LVEF (LV‐1) (lower panel). There was no significant difference in the course of LV systolic function between group A and group B patients with (LV‐1; P=0.12) and without (LV‐0; P=0.58) baseline mildly reduced LVEF. Presented is the percentage (plus 95% CI) of patients in each LVEF category assessed at time of last follow‐up. LV‐0: LVEF >55%; LV‐1: LVEF 41% to 55%; LV‐2: LVEF 31% to 40%; LV‐3: LVEF ≤30%. LV indicates left ventricular; LVEF, left ventricular ejection fraction.
Outcome
Death from any cause
During the observation period, 166 of the 991 study patients (17%) died. Death from any cause occurred in 85 of 500 patients (17%) in group A and 81 of 491 patients (17%) in group B. The average time to death from any cause was not significantly different between group A and group B (47.3±21.6 versus 46.7±20.5 months, P=0.31; median 44.4 versus 45.5 months). The left panel in Figure 4 shows the Kaplan–Meier estimate of cumulative survival according to pacing indication group. The occurrence of death was also similar in both groups when patients were classified according to baseline LVEF category (Table 2). Kaplan–Meier analysis showed no significant difference in time to death from any cause between group A and group B either in patients with baseline normal LVEF (LV‐0: 46.8±21.5 versus 47.1±20.4 months, P=0.25) (Figure 4, middle panel) or in patients with baseline mildly reduced LVEF (LV‐1: 48.8±22.0 versus 44.9±21.0 months, P=0.43) (Figure 4, right panel). Importantly, patients with baseline mildly reduced LVEF (LV‐1) performed significantly worse compared to patients with baseline normal LVEF (LV‐0) irrespective of the pacing indication group (P<0.001).
Figure 4.
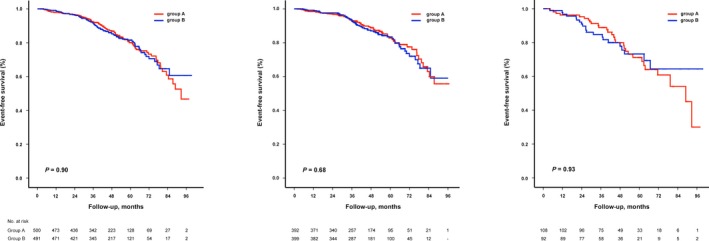
Cumulative survival of all patients (left), patients with baseline normal LVEF (middle), and patients with baseline mildly reduced LVEF (right) according to pacing indication group. Group A, patients with atrioventricular block (AVB cohort); group B, patients with sinus node disease (SND cohort). Log‐rank test, ns for all 3 survival analyses as indicated. LV indicates left ventricular; LVEF, left ventricular ejection fraction; ns, not significant.
Table 2.
Outcome Measures According to Baseline LV Systolic Function
| Outcome | All Patients (n=991) | LV‐0 | LV‐1 | ||
|---|---|---|---|---|---|
| Group A (n=392) | Group B (n=399) | Group A (n=108) | Group B (n=92) | ||
| Death from any cause, n (%)a | 166 (17) | 56 (14.3) | 60 (15.0) | 29 (26.9) | 21 (22.8) |
| Deterioration ≥2 LVEF categories, n (%)b | 56 (6.0) | 24 (6.1) | 16 (4.0) | 6 (5.6) | 10 (10.9) |
| CRT upgrade, n (%)b | 18 (1.8) | 4 (1.0) | 7 (1.8) | 5 (4.6) | 2 (2.2) |
CRT indicates cardiac resynchronization therapy; LV‐0, subgroup with baseline normal LVEF (>55%); LV‐1, subgroup with baseline mildly reduced LVEF (41% to 55%); LVEF, left ventricular ejection fraction.
P<0.001, LV‐0 vs LV‐1.
P=ns, LV‐0 vs LV‐1.
Deterioration of LV systolic function ≥2 LVEF categories
Deterioration of LV systolic function ≥2 LVEF categories was observed in 56 of the 991 study patients (6%). The outcome occurred in 30 group A patients (6%) and 26 group B patients (5%). Kaplan–Meier estimate of event‐free survival according to pacing indication group is depicted in the left panel in Figure 5. With respect to baseline LV systolic function, the event rate was comparable among groups (Table 2). There was no significant difference in time to event between group A and group B patients with (LV‐1: 45.1±22.9 versus 42.5±22.6 months, P=0.17) (Figure 5, middle panel) and without (LV‐0: 44.3±21.8 versus 45.2±21.2 months, P=0.18) baseline mildly reduced LVEF (Figure 5, right panel). Table 2 summarizes the outcomes of group A and group B according to baseline LVEF category.
Figure 5.

Freedom from deterioration of LV systolic function ≥2 LVEF categories during follow‐up in all patients (left), patients with baseline normal LVEF (middle), and patients with baseline mildly reduced LVEF (right) according to pacing indication group. Group A, patients with atrioventricular block (AVB cohort); group B, patients with sinus node disease (SND cohort). Log‐rank test, ns for all 3 survival analyses as indicated. LV indicates left ventricular; LVEF, left ventricular ejection fraction; ns, not significant.
Impact of RV pacing on outcome
In line with our hypothesis, the mean cumulative percentage of RV pacing was significantly higher in group A (AVB cohort) compared with group B (SND cohort) (85.5% versus 22.7%, P<0.001). By univariate analysis, mean percentage of RV pacing was a predictor of death from any cause (hazard ratio [HR]: 1.007; 95% CI: 1.001–1.013; P=0.014) but not deterioration of LV systolic function ≥2 LVEF categories (HR: 1.008; 95% CI 0.999–1.017; P=0.09). However, multivariate analysis failed to identify mean percentage of RV pacing as a predictor of death from any cause (HR: 1.005; 95% CI: 0.999–1.011; P=0.10). With respect to baseline LV systolic function, univariate analysis identified mean percentage of RV pacing as a predictor of death from any cause (HR: 1.008; 95% CI: 1.001–1.014; P=0.02) and deterioration of LV systolic function ≥2 LVEF categories (HR: 1.011; 95% CI: 1.001–1.022; P=0.04) for patients with baseline normal LVEF (LV‐0) but not for patients with baseline mildly reduced LVEF (LV‐1). Multivariate analysis, however, failed to predict death from any cause (HR: 1.006; 95% CI: 0.999–1.013; P=0.10) and deterioration of LV systolic function ≥2 LVEF categories (HR: 1.009; 95% CI: 0.998–1.020; P=0.13) in these patients (Table 3).
Table 3.
Impact of RV Pacing on Main Outcomes
| Outcome | Group A (%) | Group B (%) | Univariate Analysis Hazard Ratio (95% CI) | P Value | Multivariate Analysis Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Death from any cause | 17 | 17 | 1.007 (1.001–1.013) | 0.01 | 1.005 (0.999–1.011) | 0.10 |
| Deterioration ≥2 LVEF categories | 6 | 5 | 1.008 (0.999–1.017) | 0.09 | 1.008 (0.999–1.018) | 0.10 |
| LV‐0 | ||||||
| Death from any cause | 14.3 | 15.0 | 1.008 (1.001–1.014) | 0.02 | 1.006 (0.999–1.013) | 0.10 |
| Deterioration ≥2 LVEF categories | 6.1 | 4.0 | 1.011 (1.001–1.022) | 0.03 | 1.009 (0.9998–1.020) | 0.13 |
| LV‐1 | ||||||
| Death from any cause | 26.9 | 22.8 | 1.004 (0.992–1.016) | 0.51 | — | — |
| Deterioration ≥2 LVEF categories | 5.6 | 10.9 | 0.995 (0.975–1.015) | 0.60 | — | — |
There were no significant interactions between pacing indication group and LVEF category. LV‐0 indicates subgroup with baseline normal LVEF (>55%); LV‐1, subgroup with baseline mildly reduced LVEF (41–55%); LVEF, left ventricular ejection fraction.
Predictors of main outcomes
Table 4 summarizes the results of univariate and multivariate Cox proportional hazard regression analyses. In multivariate analysis, age was found to predict death from any cause, whereas age and diabetes mellitus were the only predictors of deterioration of LV systolic function ≥2 LVEF categories.
Table 4.
Predictors of Main Outcomes
| Outcome | Univariate Analysis Hazard Ratio (95% CI) | P Value | Multivariate Analysis Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Death from any cause | ||||
| LVEF category | 1.683 (1.207–2.346) | <0.01 | 1.408 (0.852–2.327) | 0.18 |
| Pacing indication group | 1.020 (0.752–1.385) | 0.90 | — | — |
| Percentage RV pacing | 1.007 (1.001–1.013) | 0.01 | 1.004 (0.998–1.010) | 0.19 |
| Age | 1.100 (1.076–1.124) | <0.01 | 1.111 (1.074–1.148) | <0.01 |
| Sex | 0.920 (0.676–1.249) | 0.59 | — | — |
| Cardiovascular disease | 2.031 (1.470–2.806) | <0.01 | 1.418 (0.779–2.582) | 0.25 |
| Coronary artery disease | 1.907 (1.402–2.593) | <0.01 | 1.159 (0.643–2.091) | 0.62 |
| Diabetes mellitus | 2.070 (1.282–3.342) | <0.01 | 1.628 (0.774–3.426) | 0.20 |
| Atrial fibrillation | 1.280 (0.901–1.809) | 0.17 | — | — |
| Hypertension | 0.915 (0.671–1.248) | 0.58 | — | — |
| Deterioration ≥2 LVEF categories | ||||
| LVEF category | 1.362 (0.751–2.473) | 0.31 | — | — |
| Pacing indication group | 0.938 (0.554–1.590) | 0.81 | — | — |
| Percentage RV pacing | 1.008 (0.999–1.017) | 0.09 | — | — |
| Age | 1.056 (1.022–1.091) | 0.01 | 1.057 (1.021–1.094) | <0.01 |
| Sex | 0.506 (0.282–0.906) | <0.01 | 0.617 (0.328–1.162) | 0.14 |
| Cardiovascular disease | 1.733 (1.008–2.979) | 0.05 | 1.324 (0.629–2.787) | 0.46 |
| Coronary artery disease | 1.914 (1.126–3.255) | 0.02 | 1.132 (0.533–2.404) | 0.75 |
| Diabetes mellitus | 3.080 (1.447–6.555) | <0.01 | 2.482 (1.148–5.363) | 0.02 |
| Atrial fibrillation | 1.641 (0.902–2.986) | 0.11 | — | — |
| Hypertension | 1.218 (0.703–2.110) | 0.48 | — | — |
There were no significant interactions between pacing indication group and LVEF category. LVEF indicates left ventricular ejection fraction; RV, right ventricular.
Discussion
The main findings of our study show that overall survival of conventional pacemaker recipients with baseline normal or mildly reduced LV systolic function is basically not influenced by the pacing indication and anticipated degree of RV pacing. Patients with AVB who required frequent or permanent RV pacing had a similar survival after pacemaker insertion compared to patients with SND who required only intermittent or minimal RV pacing. Importantly, this held true even for the subgroup of patients with preexisting mildly reduced LVEF (41% to 55%). Moreover, there was no significant difference in the development of clinically relevant LV dysfunction or deterioration of preexisting mild LV dysfunction between the pacing indication groups.
The majority (83%) of our large pacemaker population with baseline normal LV systolic function revealed a preserved LVEF after >3.5 year follow‐up, and only 5% experienced clinically relevant deterioration of LV systolic function, as defined by a significant decrease in LVEF ≥2 categories (ie, LVEF ≤40%). Among these patients, only 1 of 4 was indicated for an upgrade to biventricular pacing, accounting for no more than 1.4% of all pacemaker recipients with baseline normal LVEF. Our findings lent further evidence and support to the practice that patients with normal LV systolic function and need for antibradycardia pacing should be implanted with a conventional (dual‐chamber) pacemaker irrespective of the pacing indication and anticipated degree of RV pacing.
Several studies have shown that upgraded patients derive similar benefit from biventricular pacing compared with de novo implanted patients.13, 14, 15 This further argues in favor of an initially conventional pacing approach, with later upgrade in case of pacing‐induced deterioration of LV systolic function (LVEF↓ to <35%) and worsening of heart failure symptoms (New York Heart Association class III or ambulatory IV) despite adequate medical treatment.16
Notably, recent follow‐up data from the PACE trial suggest that de novo biventricular pacing may prevent the reduction in LVEF and increase in LV end‐systolic volume observed with RV apical pacing in patients with baseline normal LV systolic function.17, 18 However, these functional and structural changes were not associated with any difference in clinical outcome at 2‐year follow‐up.18 In contrast, the multicenter randomized PREVENT‐HF trial could not demonstrate significant differences in adverse LV remodeling, LVEF, and clinical end points after >1 year between RV apical and biventricular pacing in AVB patients without LV dysfunction.19 In addition, preliminary data from the most recent BIOPACE trial20 showed no significant difference in the incidence of death and heart failure hospitalization after >5 years between the 2 pacing modes in a large cohort of patients (n=1239) with conventional pacemaker indication and preserved LV systolic function. Thus, de novo biventricular antibradycardia pacing cannot be recommended at present for patients with baseline normal LVEF.
The situation may look different in patients with moderate‐to‐severe LV dysfunction.6 The potentially detrimental effects of RV apical pacing have been attributed to pacing‐induced development of mechanical LV dyssynchrony.1, 2 However, only a subset of conventional pacemaker recipients exhibits relevant LV dyssynchrony with long‐term RV apical pacing.4 The amount of pacing‐induced LV dyssynchrony seems to be related to the presence and severity of LV dysfunction.7 Hence, specifically patients with preexisting LV dysfunction and underlying conduction disease have been identified to be susceptible to the deleterious effects of conventional RV pacing.6, 21
There is now emerging evidence that patients with preexisting reduced LV systolic function may benefit from de novo biventricular instead of conventional RV apical pacing. Several randomized controlled studies demonstrated that de novo CRT is able to reduce heart failure–related symptoms and hospitalization, and improve quality of life in these patients.22, 23, 24 In particular, the BLOCK HF trial24 provided convincing evidence in favor of de novo biventricular antibradycardia pacing for AVB in patients with LV systolic dysfunction. The 691 study patients (mean LVEF 40±8%) were randomly assigned to biventricular pacing and conventional RV pacing with or without implantable cardioverter‐defibrillator back‐up and were followed for >3 years (mean ventricular pacing rate >97%). Patients assigned to biventricular pacing had a significant lower incidence of primary outcome, a composite of all‐cause mortality, urgent care visit for heart failure, or ≥15% increase in LV end‐systolic volume index, compared to those assigned to conventional RV pacing. In secondary analysis, the rate of heart failure–related hospitalization was 30% lower in the CRT group.24
In line with these data is our finding that conventional pacemaker recipients with baseline mildly reduced LV systolic function (LVEF 41–55%) performed significantly worse compared to those with baseline normal cardiac function. However, we could not observe a significant difference in outcome between the pacing indication groups, which differed substantially with respect to mean percentage of RV pacing during follow‐up (85.5% versus 22.7%, P<0.001). Importantly, the mean percentage of RV pacing did not predict outcome in multivariate analysis. Moreover, patients with baseline mildly reduced LVEF experienced more frequently an improvement to normal (>55%) than worsening below 41%. These observations suggest that diverse underlying (individual) cardiac conditions and beneficial effects of antibradycardia pacing itself may have influenced outcome in this study. In support of our data, it has been reported previously that conventional dual‐chamber pacing can improve cardiac function in selected heart failure patients with dilated cardiomyopathy.25 However, further randomized studies are certainly needed to better characterize those patients who likely benefit from an initial biventricular pacing approach to bradycardia.
In everyday clinical practice, whenever one considers a patient with baseline mildly reduced LVEF for de novo biventricular antibradycardia pacing, it is worth the effort to distinguish to what extent clinical presentation may be secondary to the underlying bradycardia rather than to the LV systolic dysfunction. In any case, the potential benefit of de novo or upgraded biventricular pacing should be weighed against the added complication rate and shorter service life of the more complex CRT system along with the subsequent need for more revision procedures and higher costs.16
Study Limitations
The major limitations of the present study are imposed by its retrospective nature. The analysis of echocardiographic data was restricted to the assessment of LVEF categories according to the practice guidelines for clinical application of echocardiography.12 Therefore, patients with slightly reduced LVEF between 51% and 55%, for instance, who were considered in the BIOPACE trial20 as patients with preserved LVEF, have been put on a par with patients with more significant LV dysfunction and LVEF between 41% and 45%, who represented the average pacemaker cohort in the BLOCK HF trial.24 This may further explain why a substantial number of our LV‐1 patients (baseline LVEF 41% to 55%) improved to normal or remained stable irrespective of the initial antibradycardia pacing indication. Moreover, we did not systematically assess and analyze sophisticated echocardiographic parameters of mechanical LV dyssynchrony and structural LV remodeling. However, despite the provocative echocardiographic data from the PACE trial,18 clinical relevance of changes in functional and structural parameters in pacemaker recipients with preserved LVEF needs to be proven. Another study limitation is imposed by the lack of detailed information on the development (SND cohort) or improvement (AVB cohort) of cardiac conduction disease, which has an impact on the need for atrial‐based RV pacing and potential development of pacing‐induced LV dysfunction. Based on the purpose of our study and the “intention‐to‐treat”–like assignment, such situations were not considered for analysis, which might have skewed the outcome findings to the disadvantage of the SND cohort. On the other hand, the mean percentage of RV pacing was <25% in our SND cohort, which is far below the deleterious 40% cut‐off reported for the DDDR pacing group in the MOST trial.26
Conclusions
In a large cohort of nonpreselected conventional pacemaker recipients with predominantly normal LVEF, development of clinically relevant LV systolic dysfunction is a rather infrequent event irrespective of pacing indication and cumulative percentage of RV pacing. There is no significant difference in death from any cause and development of severe LV dysfunction requiring upgrade to biventricular pacing between patients implanted for AVB and patients implanted for SND. Accordingly, prophylactic de novo biventricular antibradycardia pacing cannot be recommended for patients with conventional pacemaker indication and preserved LV systolic function.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003485 doi: 10.1161/JAHA.116.003485)
References
- 1. Tops LF, Schalij MJ, Holman ER, van Erven L, van der Wall EE, Bax JJ. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J Am Coll Cardiol. 2006;48:1642–1648. [DOI] [PubMed] [Google Scholar]
- 2. Thambo JB, Bordachar P, Garrigue S, Lafitte S, Sanders P, Reuter S, Girardot R, Crepin D, Reant P, Roudaut R, Jais P, Haissaguerre M, Clementy J, Jimenez M. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004;110:3766–3772. [DOI] [PubMed] [Google Scholar]
- 3. Tse HF, Lau CP. Long‐term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol. 1997;29:744–749. [DOI] [PubMed] [Google Scholar]
- 4. Tops LF, Suffoletto MS, Bleeker GB, Boersma E, van der Wall EE, Gorcsan J III, Schalij MJ, Bax JJ. Speckle‐tracking radial strain reveals left ventricular dyssynchrony in patients with permanent right ventricular pacing. J Am Coll Cardiol. 2007;50:1180–1188. [DOI] [PubMed] [Google Scholar]
- 5. Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation. 2006;113:2082–2088. [DOI] [PubMed] [Google Scholar]
- 6. Sweeney MO, Prinzen FW. A new paradigm for physiologic ventricular pacing. J Am Coll Cardiol. 2006;47:282–288. [DOI] [PubMed] [Google Scholar]
- 7. Pastore G, Noventa F, Piovesana P, Cazzin R, Aggio S, Verlato R, Zanon F, Baracca E, Roncon L, Padeletti L, Barold SS. Left ventricular dyssynchrony resulting from right ventricular apical pacing: relevance of baseline assessment. Pacing Clin Electrophysiol. 2008;31:1456–1462. [DOI] [PubMed] [Google Scholar]
- 8. Gillis AM. Redefining physiologic pacing: lessons learned from recent clinical trials. Heart Rhythm. 2006;3:1367–1372. [DOI] [PubMed] [Google Scholar]
- 9. Zhang XH, Chen H, Siu CW, Yiu KH, Chan WS, Lee KL, Chan HW, Lee SW, Fu GS, Lau CP, Tse HF. New‐onset heart failure after permanent right ventricular apical pacing in patients with acquired high‐grade atrioventricular block and normal left ventricular function. J Cardiovasc Electrophysiol. 2008;19:136–141. [DOI] [PubMed] [Google Scholar]
- 10. Delgado V, Tops LF, Trines SA, Zeppenfeld K, Marsan NA, Bertini M, Holman ER, Schalij MJ, Bax JJ. Acute effects of right ventricular apical pacing on left ventricular synchrony and mechanics. Circ Arrhythm Electrophysiol. 2009;2:135–145. [DOI] [PubMed] [Google Scholar]
- 11. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. [DOI] [PubMed] [Google Scholar]
- 12. Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography). Circulation. 2003;108:1146–1162. [DOI] [PubMed] [Google Scholar]
- 13. Foley PW, Muhyaldeen SA, Chalil S, Smith RE, Sanderson JE, Leyva F. Long‐term effects of upgrading from right ventricular pacing to cardiac resynchronization therapy in patients with heart failure. Europace. 2009;11:495–501. [DOI] [PubMed] [Google Scholar]
- 14. Frohlich G, Steffel J, Hurlimann D, Enseleit F, Luscher TF, Ruschitzka F, Abraham WT, Holzmeister J. Upgrading to resynchronization therapy after chronic right ventricular pacing improves left ventricular remodelling. Eur Heart J. 2010;31:1477–1485. [DOI] [PubMed] [Google Scholar]
- 15. Bogale N, Witte K, Priori S, Cleland J, Auricchio A, Gadler F, Gitt A, Limbourg T, Linde C, Dickstein K. The European Cardiac Resynchronization Therapy Survey: comparison of outcomes between de novo cardiac resynchronization therapy implantations and upgrades. Eur J Heart Fail. 2011;13:974–983. [DOI] [PubMed] [Google Scholar]
- 16. Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Blomstrom‐Lundqvist C, Badano LP, Aliyev F, Bansch D, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Van Gelder IC, Wilson CM. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 17. Yu CM, Chan JY, Zhang Q, Omar R, Yip GW, Hussin A, Fang F, Lam KH, Chan HC, Fung JW. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123–2134. [DOI] [PubMed] [Google Scholar]
- 18. Chan JY, Fang F, Zhang Q, Fung JW, Razali O, Azlan H, Lam KH, Chan HC, Yu CM. Biventricular pacing is superior to right ventricular pacing in bradycardia patients with preserved systolic function: 2‐year results of the PACE trial. Eur Heart J. 2011;32:2533–2540. [DOI] [PubMed] [Google Scholar]
- 19. Stockburger M, Gomez‐Doblas JJ, Lamas G, Alzueta J, Fernandez‐Lozano I, Cobo E, Wiegand U, Concha JF, Navarro X, Navarro‐Lopez F, de Teresa E. Preventing ventricular dysfunction in pacemaker patients without advanced heart failure: results from a multicentre international randomized trial (PREVENT‐HF). Eur J Heart Fail. 2011;13:633–641. [DOI] [PubMed] [Google Scholar]
- 20. Funck RC, Blanc JJ, Mueller HH, Schade‐Brittinger C, Bailleul C, Maisch B. Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the ‘biventricular pacing for atrioventricular block to prevent cardiac desynchronization (BioPace)’ study. Europace. 2006;8:629–635. [DOI] [PubMed] [Google Scholar]
- 21. Hayes JJ, Sharma AD, Love JC, Herre JM, Leonen AO, Kudenchuk PJ. Abnormal conduction increases risk of adverse outcomes from right ventricular pacing. J Am Coll Cardiol. 2006;48:1628–1633. [DOI] [PubMed] [Google Scholar]
- 22. Kindermann M, Hennen B, Jung J, Geisel J, Bohm M, Frohlig G. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: the homburg biventricular pacing evaluation (HOBIPACE). J Am Coll Cardiol. 2006;47:1927–1937. [DOI] [PubMed] [Google Scholar]
- 23. Martinelli Filho M, de Siqueira SF, Costa R, Greco OT, Moreira LF, D'Avila A, Heist EK. Conventional versus biventricular pacing in heart failure and bradyarrhythmia: the COMBAT study. J Card Fail. 2010;16:293–300. [DOI] [PubMed] [Google Scholar]
- 24. Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, Shinn T, Sutton MS. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. [DOI] [PubMed] [Google Scholar]
- 25. Brecker SJ, Xiao HB, Sparrow J, Gibson DG. Effects of dual‐chamber pacing with short atrioventricular delay in dilated cardiomyopathy. Lancet. 1992;340:1308–1312. [DOI] [PubMed] [Google Scholar]
- 26. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. [DOI] [PubMed] [Google Scholar]


