Abstract
Background
The optimal antithrombotic therapy in patients with ST‐segment‐elevation myocardial infarction undergoing primary percutaneous coronary intervention (PCI) remains a matter of debate. This updated meta‐analysis investigated the impact of (1) bivalirudin (with and without prolonged infusion) and (2) prolonged PCI‐dose (1.75 mg/hg per hour) bivalirudin infusion compared with conventional antithrombotic therapy on clinical outcomes in patients undergoing primary PCI.
Methods and Results
Eligible randomized trials were searched through MEDLINE, EMBASE, Cochrane database, and proceedings of major congresses. Prespecified outcomes were major bleeding (thrombolysis in myocardial infarction major and Bleeding Academic Research Consortium 3–5), acute stent thrombosis, as well as all‐cause and cardiac mortality at 30 days. Six randomized trials (n=17 294) were included. Bivalirudin compared with heparin (+/− glycoprotein‐IIb/IIIa inhibitor) was associated with reduction in major bleeding (odds ratio [OR]: 0.65, 95% CI: 0.48–0.88, P=0.006, derived from all 6 trials), increase in acute stent thrombosis (OR: 2.75, 95% CI: 1.46–5.18, P=0.002, 5 trials), and lower rate of all‐cause mortality (OR: 0.81, 95% CI: 0.67–0.98, P=0.03, 6 trials) as well as cardiac mortality (OR: 0.69, 95% CI: 0.55–0.87, P=0.001, 5 trials). The incidence of acute stent thrombosis did not differ between the prolonged PCI‐dose bivalirudin and comparator group (OR: 0.81, 95% CI: 0.27–2.46, P=0.71, 3 trials), whereas the risk of bleeding was reduced despite treatment with high‐dose bivalirudin infusion (OR: 0.28, 95% CI: 0.13–0.60, P=0.001, 3 trials).
Conclusions
Bivalirudin (with and without prolonged infusion) compared with conventional antithrombotic therapy in ST‐segment‐elevation myocardial infarction patients undergoing primary PCI reduces major bleeding and death, but increases the rate of acute stent thrombosis. However, prolonging the bivalirudin infusion at PCI‐dose (1.75 mg/kg per hour) for 3 hours eliminates the excess risk of acute stent thrombosis, while maintaining the bleeding benefits.
Keywords: bivalirudin, meta‐analysis, myocardial infarction, percutaneous coronary intervention
Subject Categories: Myocardial Infarction, Percutaneous Coronary Intervention
Introduction
Primary percutaneous coronary intervention (PCI) is now standard treatment for patients with an ST‐segment‐elevation myocardial infarct (STEMI),1, 2 significantly reducing major adverse cardiovascular events.3, 4
Coronary artery plaque rupture initiates acute coronary syndromes (ACS) by activating the coagulation cascade, and adjunctive antiplatelet as well as antithrombotic therapy is necessary to minimize peri‐ and postprocedural thrombotic events.5, 6, 7, 8 However, the use of these agents is frequently associated with an increase of bleeding, which is itself associated with a higher mortality.9, 10, 11
Unfractionated heparin (UFH) is the standard antithrombotic agent during PCI and in the setting of ACS.12 However, UFH has a number of limitations including the need for anti‐thrombin III as cofactor, and a highly variable dose–response relation, resulting in a narrow therapeutic window.13, 14 These considerations have encouraged investigations of alternative antithrombotic strategies. The role of bivalirudin, a direct thrombin inhibitor, has been investigated in 6 randomized trials in STEMI patients undergoing primary PCI,15, 16, 17, 18, 19, 20 but has resulted in conflicting evidence in terms of major bleeding, acute stent thrombosis, and death.
A recently published meta‐analysis by Shah et al, comparing bivalirudin with heparin in patients undergoing primary PCI, demonstrated a significant reduction in “protocol‐defined” major bleeding and death in the bivalirudin group, but revealed an increase in acute stent thrombosis.21 However, the impact of a prolonged infusion of the short‐acting bivalirudin on clinical outcomes and the optimal dose of the prolonged bivalirudin infusion (low dose at 0.25 mg/kg per hour versus PCI‐dose at 1.75 mg/kg per hour) remains a matter of debate.
The aim of our updated meta‐analysis was to investigate the impact of bivalirudin compared with conventional antithrombotic therapy on major bleeding (defined as thrombolysis in myocardial infarction [TIMI] major and Bleeding Academic Research Consortium [BARC] 3–5 bleeding), acute stent thrombosis, and mortality in STEMI patients undergoing primary PCI, and to assess whether prolonged bivalirudin infusion at PCI‐dose (1.75 mg/kg per hour) influences the rate of acute stent thrombosis and major bleeding.
Methods
This review was guided by methods recommended by the Cochrane Handbook for Systematic Reviews of Intervention22 and accomplished in compliance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) statement in healthcare interventions.23
Eligibility and Search Strategy
We considered data from all randomized controlled trials comparing bivalirudin with heparin among STEMI patients undergoing primary PCI.
Relevant studies were searched through MEDLINE, the Cochrane database, the EMBASE database, as well as www.clinicaltrials.gov, www.clinicaltrialresults.org, www.tctmd.com, www.europcr.com, and www.acc.org websites until February 21, 2016 without language restrictions. In addition, prior meta‐analysis on the same topic as well as the reference lists of included studies were reviewed to identify further citations. The following key words were used: myocardial infarction and bivalirudin.
Inclusion criteria for the meta‐analysis comparing bivalirudin with conventional antithrombotic therapy were the following: randomized treatment allocation, inclusion of STEMI patients, and the intention to undergo PCI. Studies were excluded if thrombolysis rather than PCI was used for reperfusion treatment. For the subgroup analysis comparing prolonged PCI‐dose bivalirudin with heparin in STEMI patients, we included only studies where administration of a prolonged infusion at the higher dose of bivalirudin (1.75 mg/kg per hour) was part of the protocol. The original authors obtained institutional review committee approval and patient informed consent for each included study.
Data Extraction and Validity Assessment
Two independent investigators (G.F. and M.W.) selected the studies for inclusion, and any disagreements were resolved by consensus with a third reviewer (R.K.K.). The data were analyzed according to the intention‐to‐treat principle. The original investigators were contacted to request relevant missing data.
End‐Point Selection
Selected end points were all‐cause mortality, cardiac mortality, and major bleeding at 30 days as well as acute stent thrombosis for the analysis comparing bivalirudin versus conventional antithrombotic therapy. Major bleeding at 30 days and acute stent thrombosis were the prespecified outcome measurements for the subgroup analysis comparing prolonged PCI‐dose bivalirudin with conventional antithrombotic therapy. The prognostically more relevant TIMI major bleeding24 (rather than the protocol‐defined non–coronary artery bypass graft [CABG]–major bleeding) was considered in the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS‐AMI), Bavarian Reperfusion Alternatives Evaluation 4 (BRAVE 4), and European Ambulance Acute Coronary Syndrome Angiography (EUROMAX) trial; and BARC 3 to 5 bleeding was used in the How Effective are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention (HEAT‐PPCI), Bivalirudin in Acute Myocardial Infarction versus Heparin and GPI Plus Heparin (BRIGHT), and Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox (MATRIX) trial (Table). Acute stent thrombosis was defined according to the Academic Research Consortium criteria.25
Table 1.
Study Characteristics
| Study Name (Ref) | ||||||
|---|---|---|---|---|---|---|
| HORIZONS‐AMI15 | EUROMAX16 | BRAVE 417 | HEAT‐PPCI18 | BRIGHT19 | MATRIX20 | |
| Study design | Multicenter, open label | Multicenter, open label | Multicenter, open label | Single‐center, open label | Multicenter, open label | Multicenter, open label |
| Year of publication | 2008 | 2013 | 2014 | 2014 | 2015 | 2015 |
| Patients (n) ITT | 3602 | 2198 | 544a | 1812 | 1925b | 7213c |
| Age, y | 60 | 62 | 61 | 63 | 57 | 65 |
| Radial access (%) | na | 47 | 0 | 81 | 78 | 50 |
| Clopidogrel (%) | 96 | 51 | 97 with Heparin | 11 | 100 | 38 |
| Thienopyridine (%) | 0 | 49 | 99 with Bivalirudin | 89 | 0 | 55 |
| Initial heparin bolus, IU/kg | 60 | 100 without GPI, 60 with GPI | 70 to 100 | 70 | 100 without GPI, 60 with GPI | 70 to 100 without GPI, 50 to 70 with GPI |
| Prolonged bivalirudin infusion after PCI (dose, n%, mean duration) | Stopped immediately after PCI | 1.75 mg/kg per hour (22.5%), 0.25 mg/kg per hour (77.5%), 4.5 hours | Stopped immediately after PCI | Stopped immediately after PCI | 1.75 mg/kg per hour (100%), 3 hours | 1.75 mg/kg per hour (34.4%), 0.25 mg/kg per hour (59.0%), 6.2 hours |
| GPI use in bivalirudin arm (%) | 8 | 12 | 3 | 13 | 5 | 5 |
| GPI use in heparin arm (%) | 98 | 69 | 6 | 15 | 6 and 100 | 26 |
| Definition major bleeding | TIMI major bleedingd at 30 days | TIMI major bleedingd at 30 days | TIMI major bleedingd at 30 days | BARC type 3 to 5e at 28 days | BARC type 3 to 5e at 30 days | BARC type 3 and 5e at 30 days |
BARC indicates Bleeding Academic Research Consortium; BRAVE 4, Bavarian Reperfusion Alternatives Evaluation 4; BRIGHT, Bivalirudin in Acute Myocardial Infarction versus Heparin and GPI Plus Heparin; CABG, coronary artery bypass graft; EUROMAX, European Ambulance Acute Coronary Syndrome Angiography; GPI, glycoprotein IIb/IIIa inhibitors; HEAT‐PPCI, How Effective are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention; HORIZONS‐AMI, Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction; ITT, intention‐to‐treat; MATRIX, Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox; na, not available; NSTEMI, non‐ST‐segment‐elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment‐elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Randomized to bivalirudin plus prasugrel and heparin plus clopidogrel but stopped early due to slow recruitment.
Randomized to bivalirudin, heparin alone, and heparin plus GPI.
STEMI 56% and NSTEMI 44%.
TIMI major bleeding: intracranial bleeding, overt bleeding with hemoglobin drop of >5 g/dL, fatal bleeding.
BARC bleeding: Type 3: overt bleeding with hemoglobin drop of >3 g/dL or requiring transfusion, cardiac tamponade, bleeding requiring intervention or vasoactive agents, intracranial bleeding; Type 4: CABG‐related bleeding within 48 hours; Type 5: fatal bleeding.
Statistical Analysis
Statistical analysis was performed by Review Manager, version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). Odds ratios (OR) and 95% CI were used as summary statistics. The statistical heterogeneity for each end point of interest was assessed by using the Higgins I2 test.26 I2 values between 0% and 30% and P>0.1 were considered not to be relevant, according to the Cochrane guidelines.22 The fixed‐effect model with the Mantel‐Haenszel method was used to calculate the summarized ORs. In case of relevant heterogeneity with an I2>30% or P<0.1, we used the random effects model by DerSimonian and Laird instead to calculate the pooled ORs.22 Visual estimation of the funnel plot was used to assess potential publication bias for each clinical outcome. P values were 2‐tailed, reaching statistical level of significance at 0.05.
Results
Included Studies and Patient Population
The PRISMA statement flowchart describes the process of the literature screening, study selection, and reasons for exclusion (Figure 1). Six hundred fourteen potentially relevant citations were initially identified, of which 50 were retrieved to assess in full‐text. Eventually, results from 6 randomized trials were eligible with a total of 17 294 patients included. Study characteristics are highlighted in (Table). The funnel plots suggest no relevant publication bias.
Figure 1.
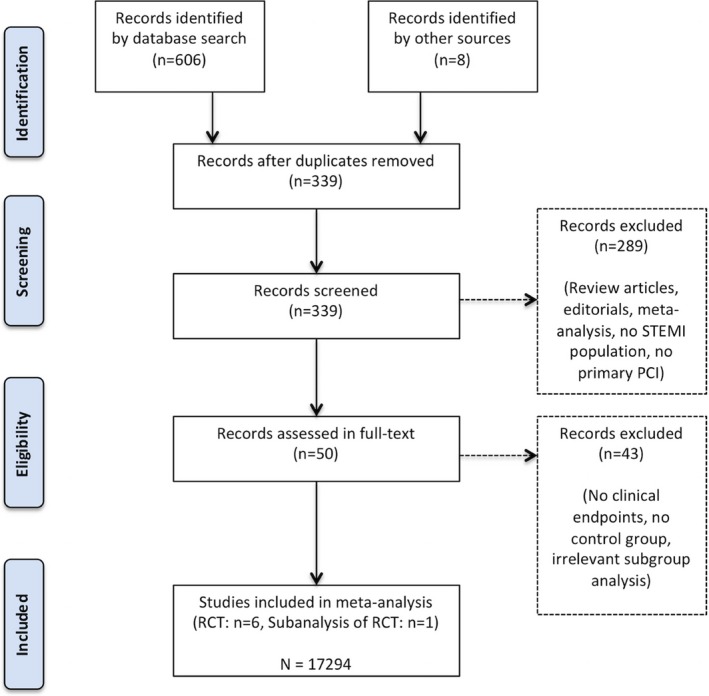
Flow chart of the selection process as per PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analysis) criteria. PCI indicates percutaneous coronary intervention; RCT, randomized clinical trial; STEMI, ST‐segment‐elevation myocardial infarction.
The BRIGHT trial enrolled patients presenting with a non‐STEMI; thus, since the outcome data were available separately, we considered only results from the STEMI group.19 In all studies, bivalirudin was given as initial bolus of 0.75 mg/kg per hour followed by an infusion of 1.75 mg/kg per hour during the procedure. The infusion at PCI‐dose was continued in all patients in the BRIGHT19 trial, as well as partly in the EUROMAX16 and MATRIX20 trial, but was stopped immediately after the intervention in the HORIZONS‐AMI,15 HEAT‐PPCI,18, and BRAVE 4.17 Therefore, 3 studies were considered for the subgroup analysis comparing prolonged PCI‐dose bivalirudin with heparin.
The mean age of the included patients was 62 years. Seventy‐seven percent were male and 18% had diabetes mellitus. In this meta‐analysis, more than 90% of participants underwent PCI.
Clinical Outcome Comparing Bivalirudin Versus Conventional Antithrombotic Therapy in STEMI Patients
Major bleeding at 30 days
All 6 randomized trials contributed to the analysis of major bleeding events, with 17 294 patients included (Figure 2A). The rate of major bleeding was significantly reduced in the bivalirudin (1.92% or 160 of 8328) compared with the control (2.93% or 263 of 8966) arm (OR: 0.65, 95% CI: 0.48–0.88, P=0.006, heterogeneity P=0.10, I2=45%, random effects model).
Figure 2.
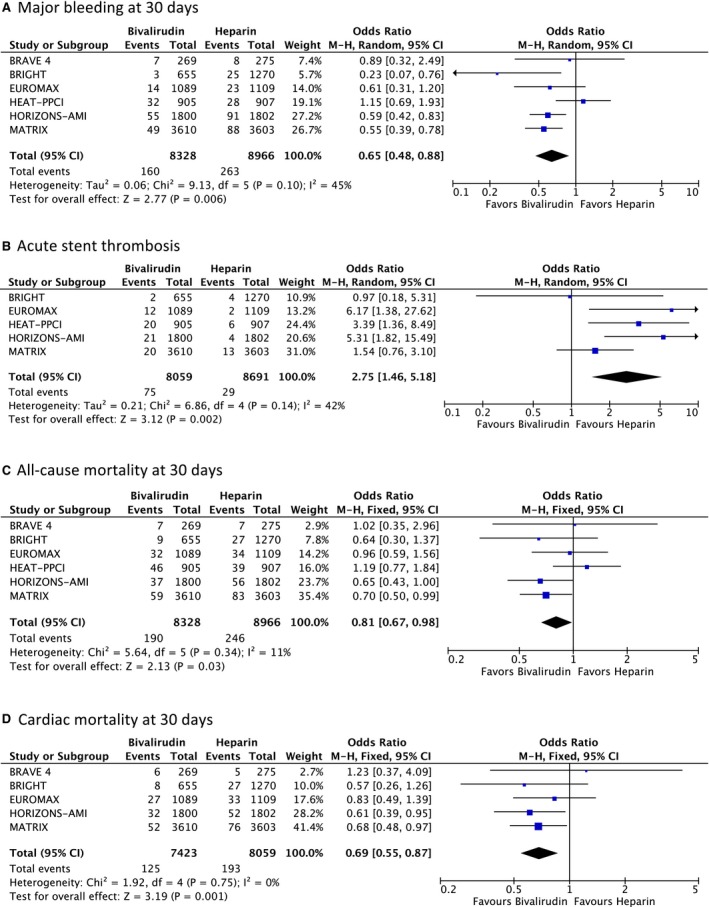
Forest plot of individual and summarized odds ratios for the comparison of bivalirudin vs heparin in STEMI patients for (A) major bleeding at 30 days, (B) acute stent thrombosis, (C) all‐cause mortality at 30 days, and (D) cardiac mortality at 30 days. BRAVE 4, Bavarian Reperfusion Alternatives Evaluation 4; BRIGHT, Bivalirudin in Acute Myocardial Infarction versus Heparin and GPI Plus Heparin; EUROMAX, European Ambulance Acute Coronary Syndrome Angiography; HEAT‐PPCI, How Effective are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention; HORIZONS‐AMI, Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction; MATRIX, Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox; M‐H, Mantel‐Haenszel; STEMI, ST‐segment‐elevation myocardial infarction.
Acute stent thrombosis
Rate of stent thrombosis within 24 hours was reported in 5 studies involving a total of 16 750 patients (Figure 2B). Significant difference emerged between the 2 treatment strategies: 75 of 8059 patients (0.93%) receiving bivalirudin compared with 29 of 8691 (0.33%) receiving conventional treatment had an acute thrombosis (OR: 2.75, 95% CI: 1.46–5.18, P=0.002, heterogeneity P=0.14, I2=42%, random effects model).
All‐cause mortality at 30 days
All 6 randomized clinical trials, involving 17 294 patients, provided data on overall death (Figure 2C).
The rate of death due to any cause was significantly lower in the bivalirudin (2.28% or 190 of 8328) compared with the standard treatment group (2.74% or 246 of 8966) (OR: 0.81, 95% CI: 0.67–0.98, P=0.03, heterogeneity P=0.34, I2=11%, fixed effects model).
Cardiac mortality at 30 days
Cardiac death was assessed by 5 randomized trials involving a total of 15 482 patients (Figure 2D). There were significantly fewer cardiac deaths with bivalirudin: 1.68% (125 of 7423) compared with conventional treatment: 2.39% (193 of 8059), resulting in a 31% OR reduction (OR: 0.69, 95% CI: 0.55–0.87, P=0.001, heterogeneity P=0.75, I2=0%, fixed effects model).
Clinical Outcome Comparing Prolonged PCI‐Dose Bivalirudin Versus Conventional Antithrombotic Therapy in STEMI Patients
Outcome data on acute stent thrombosis and major bleeding in patients treated with extended high‐dose bivalirudin (1.75 mg/kg per hour) are available in 3 of the 6 randomized clinical trials, involving 7337 patients.
Acute stent thrombosis and major bleeding at 30 days
The incidence of acute stent thrombosis did not differ in the prolonged PCI‐dose bivalirudin (0.26% or 4 of 1517) compared with the standard (0.33% or 19 of 5820) treated arm (OR: 0.81, 95% CI: 0.27–2.46, P=0.71, heterogeneity P=0.64, I2=0%, fixed effects model) (Figure 3A).
Figure 3.
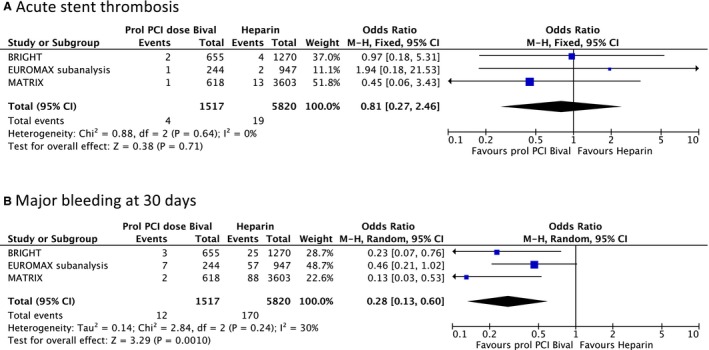
Forest plot of individual and summarized odds ratios for the comparison of prolonged PCI dose bivalirudin vs heparin in STEMI patients for (A) acute stent thrombosis and (B) major bleeding at 30 days. BRIGHT, Bivalirudin in Acute Myocardial Infarction versus Heparin and GPI Plus Heparin; EUROMAX‐ST, European Ambulance Acute Coronary Syndrome Angiography Stent thrombosis; MATRIX, Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox; M‐H, Mantel‐Haenszel; PCI, percutaneous coronary intervention; STEMI, ST‐segment‐elevation myocardial infarction.
The rate of major bleeding events was significantly lower in patient treated with prolonged PCI‐dose bivalirudin (0.79% or 12 of 1517) compared with the controlled group (2.92% or 170 of 5820) (OR: 0.28, 95% CI: 0.13–0.60, P=0.001, heterogeneity P=0.24, I2=30%, random effects model) (Figure 3B).
Discussion
The main findings from our meta‐analysis are that bivalirudin (with and without prolonged infusion) compared with conventional antithrombotic therapy in patients undergoing primary PCI reduces “prognostically relevant” major bleeding (TIMI major and BARC 3–5) and death, but increases the rate of acute stent thrombosis. However, prolonging the bivalirudin infusion at the PCI‐dose (1.75 mg/kg per hour) for 3 hours after the coronary intervention eliminates the excess of acute stent thrombosis, while maintaining the desired bleeding benefits.
Debate on Bleeding
The search for the ideal antithrombotic treatment to reduce the rate of thrombotic complications, and minimize bleeding in STEMI patients, was initiated by the HORIZONS‐AMI trial.15 This multicenter trial involved 3602 patients with STEMI undergoing primary PCI who were randomized to bivalirudin alone or UFH plus routine use of glycoprotein IIb/IIIa inhibitor (GPI). In the bivalirudin group, the rates of net adverse clinical events (NACE) were significantly reduced because of a lower rate of major bleeding.
At this time, routine use of GPI in addition to heparin was standard of care, and GPI was administered to more than 90% of patients presenting for primary PCI in Europe and the United States.27, 28 Previous randomized studies in patients with stable angina, unstable angina, and non‐STEMI showed a reduction in bleeding complications with similar rates of ischemia after PCI when bivalirudin was used instead of heparin plus GPI.29, 30 This formed the basis for the HORIZONS‐AMI study. However, with routine use of dual‐antiplatelet therapy, the recommendation to administer GPI in combination with heparin by default was no longer considered as standard of care and the relevance of HORIZONS‐AMI on contemporary practice was uncertain.31
The EUROMAX trial compared bivalirudin versus heparin in 2218 STEMI patients.16 This time, the use of GPI was left to the operator's discretion. In addition, bivalirudin was continued for 4 hours post PCI (either in a low or a PCI‐dose), and radial access for PCI was used more often. There remained a significant reduction in protocol‐defined non‐CABG major bleeding with bivalirudin compared with heparin.
However, in the HORIZONS‐AMI, BRAVE‐4, and EUROMAX trials, any blood product transfusion, even without overt bleeding or significant drop in hemoglobin, was defined as “non‐CABG” major bleeding. In these trials, blood transfusions accounted for a large proportion of primary end‐point events, though the relationship between transfusion and clinical outcome remains unclear.32, 33 The “prognostically more relevant” TIMI major bleeding (fatal bleeding, intracranial bleeding, or overt bleeding with drop of hemoglobin ≥5 g/dL) was also reported in HORIZONS‐AMI, BRAVE‐4, and EUROMAX, but failed to reach statistical significance in the BRAVE‐4 and EUROMAX trials.
The use of a nonstandardized “protocol‐defined” bleeding definition in earlier ACS trials makes it difficult to interpret relative safety comparisons of different antithrombotic regimens across studies, because results vary according to the definitions used for bleeding. The Bleeding Academic Research Consortium developed the BARC definitions for bleeding end points, providing a consensus for bleeding end points.24
This limitation was addressed in the HEAT‐PPCI study and the BRIGHT studies, which enrolled patients during 2012 and 2013.18, 19 HEAT‐PPCI is a single‐center trial that randomly assigned STEMI patients to bivalirudin or UFH, with GPI reserved for selective bailout use (defined as evidence of massive thrombus, slow‐ or no‐reflow, or a thrombotic complication). The multicenter BRIGHT trial randomized patient into 3 study arms including bivalirudin alone, UFH alone, and UFH plus GPI. As in HEAT‐PPCI, GPI was reserved only for bailout use in the first 2 study arms. The frequency of GPI use was similar within the study groups. HEAT‐PPCI did not demonstrate any difference in bleeding outcomes (BARC 2 and/or 3–5), whereas BRIGHT revealed only a superiority of bivalirudin compared with heparin alone in terms of bleeding requiring medical intervention (BARC 2–5), but not reaching statistical significance for major bleeding (BARC 3–5). PCI in these later trials were largely performed by radial access, which is associated with a lower rate of major bleeding.34 Therefore, it is possible that HEAT‐PPCI and BRIGHT were underpowered to detect a relevant difference in major bleeding outcome.
The recently published MATRIX trial was designed to address the impact of access route and prolonged bivalirudin infusion.20 This multicenter trial randomized 7213 patients with an ACS undergoing PCI to bivalirudin or UFH. Thereafter, patients in the bivalirudin group were randomized to stop or continue the infusion at the end of the procedure. The use of GPI was left to the operator's discretion. The study failed to show a difference in the primary composite end point major adverse cardiac event (MACE) (death, myocardial infarction, or stroke) or NACE (major bleeding or major adverse cardiac event) for the comparison between bivalirudin and UFH. However, major bleeding (BARC 3–5) was significantly lower in the bivalirudin compared with the UFH group. This was irrespective of the duration of the bivalirudin infusion.
Our meta‐analysis that included all of these trials, involving 17 294 patients, shows a significant reduction of major bleeding (TIMI major and BARC 3–5, rather than non‐CABG major bleeding) in patients treated with bivalirudin (1.92%) compared with heparin (2.93%) (OR: 0.65, 95% CI: 0.48–0.88, P=0.006).
There is the concern that the imbalanced use of GPI in the 2 study groups may have an influence on bleeding outcome. Two recent meta‐analysis of over 25 000 patients undergoing PCI for a range of indications (stable and unstable CAD) with balanced use of GPI confirmed a significant reduction in major bleeding with bivalirudin, suggesting that the beneficial effects on bleeding may be independent of GPI use.35, 36
Debate on Stent Thrombosis
Reduction in bleeding is balanced by increased thrombotic complications. The antithrombotic therapy in STEMI patients undergoing primary PCI is especially important since this population has an increased risk of stent thrombosis.37
The positive results of the HORIZONS‐AMI trial in terms of bleeding and mortality benefit with bivalirudin were compromised by an increase in stent thrombosis.15 Assignment to bivalirudin, compared with UFH plus GPI, resulted in a 1.0% absolute excess (1.3% versus 0.3%, P<0.001) of acute stent thrombosis. This difference in stent thrombosis was present within the first 24 hours and no longer significant at 30 days (2.5% versus 1.9%, P=0.30) and 1 year (3.5% versus 3.1%, P=0.53).38
This increase in early stent thrombosis may be caused by residual thrombin activity after the discontinuation of the short‐acting bivalirudin at the end of the procedure and by having low platelet inhibition with oral agents at this time of transition.39
Whether the rate of acute stent thrombosis could be diminished by prehospital initiation of novel P2Y12 blockers and prolonged infusion of bivalirudin at the end of the procedure was assessed in the EUROMAX trial.16 However, there remained an increased rate of acute stent thrombosis in patients receiving bivalirudin compared with heparin (1.1% versus 0.2%, P=0.007).
Dual antiplatelet therapy reduces the risk of stent thrombosis, and the potent third‐generation P2Y12 inhibitors prasugrel and ticagrelor have been shown to reduce stent thrombosis in comparison with clopidogrel in STEMI patients even more.40, 41 However, recent data highlighted that even for novel P2Y12 inhibitors, the time interval to achieve maximal platelet inhibition is significantly delayed in STEMI patients, not reaching peak pharmacodynamic efficacy for 4 to 6 hours,42, 43 and nor does prasugrel and ticagrelor reduce stent thrombosis in comparison to clopidogrel within the first 24 hours.44, 45 Therefore, unsurprisingly, given that oral antiplatelets in EUROMAX were administered 50 minutes (interquartile range 37–66) before the procedure, the patients did not have P2Y12 blockade to prevent acute stent thrombosis, which occurred on average 2.3 hours (interquartile range 1.8–2.8) after stent placement.46 Consequently, stopping the short‐lived and rapidly cleared bivalirudin immediately after stent placement compared to longer‐acting heparin should result in a substantial increase in acute stent thrombosis, as seen in HEAT‐PPCI.18
Extending the anticoagulation with bivalirudin during this vulnerable window for stent thrombosis until the pharmacodynamic effects of P2Y12 blockers become active seems logical, but the exact dosage and duration still need to be defined.
In EUROMAX, all patients received prolonged bivalirudin infusion after the procedure.16 However, only one fifth were treated at a PCI‐dose of 1.75 mg/kg per hour bivalirudin, whereas ≈80% receive a five times lower dose of 0.25 mg/kg per hour. To the best of our knowledge, there are no data showing that anticoagulation with bivalirudin at a dose of 0.25 mg/kg per hour is sufficient to prevent thrombotic complications, especially in a highly thrombotic milieu such as during a myocardial infarction. A post‐hoc analysis of the EUROMAX trial revealed that a prolonged bivalirudin infusion at a PCI‐dose of 1.75 mg/kg per hour was associated with a significantly lower rate of acute stent thrombosis compared to low‐dose bivalirudin and carried the same risk for acute stent thrombosis compared to heparin.46
The recently published MATRIX trial showed a borderline increase in stent thrombosis in the bivalirudin group at 30 days (1.0% versus 0.6%, P=0.048).20 Moreover, there was no difference in the rate of stent thrombosis when comparing bivalirudin with a prolonged infusion to bivalirudin without a prolonged infusion. However, this must be interpreted with some reservation. Prolonged bivalirudin infusion at a PCI‐dose of 1.75 mg/kg per hour was given only to one third of patients, whereas the rest received a low dose of 0.25 mg/kg per hour.
The only randomized trial comparing bivalirudin with a prolonged high‐dose infusion (1.75 mg/kg per hour for 3 hours) to UFH was the BRIGHT trial.19 As the post‐hoc analysis of the EUROMAX trial had indicated,46 the BRIGHT trial confirmed that acute stent thrombosis was no more common with bivalirudin followed by prolonged high‐dose infusion than heparin.
The results of our meta‐analysis suggest that bivalirudin in the overall study population is associated with an increase in acute stent thrombosis compared to heparin (0.93% versus 0.33%, OR: 2.75, 95% CI: 1.46–5.18, P=0.002). However, prolonging the bivalirudin infusion at the PCI‐dose (1.75 mg/kg per hour) for 3 hours eliminates the excess of acute stent thrombosis (0.26% versus 0.33%, OR: 0.81, 95% CI: 0.27–2.46, P=0.71), while maintaining the desired bleeding benefits (0.79% versus 2.92%, OR: 0.28, 95% CI: 0.13–0.60, P=0.001).
Debate on Death
In the HORIZONS‐AMI trial, treatment with bivalirudin rather than UFH plus GPI resulted in a significantly lower rate of death after primary PCI at 30 days.15 The robustness of this finding has been questioned since it was a secondary finding with a broad confidence interval (95% CI=0.44–1.00, P=0.047) and moreover, EUROMAX, BRAVE‐4, HEAT‐PPCI, and BRIGHT failed to reach level of statistical significance.16, 17, 18, 19 However, MATRIX provided further evidence of a reduction in mortality when bivalirudin is compared to UFH.20
The consequences of acute stent thrombosis do not appear to impact on the mortality advantage in STEMI patients treated with bivalirudin. The effect on clinical outcome of stent thrombosis that occurs after hospital discharge in patients following elective coronary intervention with well‐preserved ventricular function might differ from acute stent thrombosis that affects a recently infarcted territory in patients closely monitored and with the possibility of prompt coronary reintervention.
The relationship between major bleeding after PCI and subsequent death has been confirmed in several trials.9, 10, 47, 48 The precise mechanism remains unknown, but the mortality benefit seen with bivalirudin may reflect the early prevention of hemorrhagic complications. Nevertheless, HORIZONS‐AMI showed a reduction in cardiac mortality not only in bivalirudin‐treated patients with major bleeding (5.8% versus 14.6%, P=0.025), but also in those without major bleeding (2.6% versus 3.8%, P=0.048).49 Therefore, prevention of bleeding may only partly explain the lower death rate in the bivalirudin group.
Despite the initial higher rate of acute stent thrombosis, treatment with bivalirudin rather than UFH was associated with reduced rates of reinfarction (6.2% versus 8.2%, P=0.04).50 Discontinuation of antiplatelet therapy to control hemorrhage may increase the risk of reinfarction, including subacute and late stent thrombosis,51, 52, 53 which is known to be a major cause of subsequent death after primary PCI,54, 55 and may partially explain this observation.
Our meta‐analysis shows a significant reduction in all‐cause mortality within 30 days in STEMI patients treated with bivalirudin rather than with heparin (2.28% versus 2.74%, OR: 0.81, 95% CI: 0.67–0.98, P=0.03), which is even more pronounced in cardiac death (1.68% versus 2.39%, OR: 0.69, 95% CI: 0.55–0.87, P=0.001).
Limitations
This meta‐analysis has several limitations. Firstly, the imbalanced use of GPI in the bivalirudin and heparin group may have influenced the outcome. The Cochrane guidelines do not recommend a meta‐regression analysis on confounders if there are fewer than 10 trials in an analysis.22 However, previous meta‐analysis comparing bivalirudin to heparin with balanced GPI use in both groups including more than 25 000 patients undergoing PCI for stable coronary artery disease and ACS showed a significant bleeding benefit in the bivalirudin group.35, 36 Secondly, variation in protocol‐defined major bleedings among the included studies makes a direct comparison difficult. Thus, we used the prognostically more relevant TIMI major bleeding (rather than the protocol‐defined non‐CABG major bleeding) for HORIZONS‐AMI, EUROMAX, and BRAVE‐4 and the recommended BARC 3–5 major bleeding for HEAT‐PPCI, BRIGHT, and MATRIX.25 This ensures that the bleeding outcome in our meta‐analysis is clinically relevant. Thirdly, despite contacting the original investigators, the rates of cardiac death from HEAT‐PPCI were not available.18 However, calculating the summarized OR with a random effect model, assuming that all deaths are caused by cardiac mortality, still resulted in a significant difference in favor of bivalirudin. Fourthly, the MATRIX trial also included non‐STEMI patients and outcome for STEMI patients is not available separately.20 However, this non‐STEMI population contributes only 18.5% of the whole study population in our meta‐analysis, and the rate of acute stent thrombosis in the MATRIX trial treated with prolonged PCI‐dose bivalirudin coincides with the results of the BRIGHT STEMI population, in which all received a prolonged PCI‐dose bivalirudin infusion. Therefore, we believe that this is unlikely to have a relevant impact on our overall findings. Fifthly, the bivalirudin infusion at PCI‐dose (1.75 mg/kg per hour) was continued in all patients in the BRIGHT trial.19 However, prolonging the infusion at the higher dose, rather than a lower dose (0.25 mg/kg per hour), was left to the operators' discretion in the EUROMAX and MATRIX trials.16, 20 Consequently, data for the subgroup analysis are in part not randomized. Nevertheless, these are the best data available to answer this remaining important question.
Conclusions
Bivalirudin (with and without prolonged infusion) compared with conventional therapy in STEMI patients undergoing primary PCI reduces major bleeding (TIMI major and BARC 3–5) and death, but increases the risk of acute stent thrombosis. However, prolonging the bivalirudin infusion at a PCI‐dose (1.75 mg/kg per hour) for 3 hours eliminates the excess of acute stent thrombosis, while maintaining the bleeding benefits.
Sources of Funding
Fahrni was supported by fellowship grants from the Bangerter‐Rhyner‐Stiftung, Freiwillige Akademische Gesellschaft Basel, and NIHR Oxford Biomedical Research Centre.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003515 doi: 10.1161/JAHA.116.003515)
References
- 1. Steg PG, James SK, Atar D, Badano LP, Blömstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ‘t Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 2. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140. [DOI] [PubMed] [Google Scholar]
- 3. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 4. Schömig A, Neumann FJ, Kastrati A, Schühlen H, Blasini R, Hadamitzky M, Walter H, Zitzmann‐Roth EM, Richardt G, Alt E, Schmitt C, Ulm K. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary‐artery stents. N Engl J Med. 1996;334:1084–1089. [DOI] [PubMed] [Google Scholar]
- 5. Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–1040. [DOI] [PubMed] [Google Scholar]
- 6. Sciulli TM, Mauro VF. Pharmacology and clinical use of bivalirudin. Ann Pharmacother. 2002;36:1028–1041. [DOI] [PubMed] [Google Scholar]
- 7. White CM. Thrombin‐directed inhibitors: pharmacology and clinical use. Am Heart J. 2005;149:S54–S60. [DOI] [PubMed] [Google Scholar]
- 8. Morrow DA. Antithrombotic therapy to support primary PCI. N Engl J Med. 2008;358:2280–2282. [DOI] [PubMed] [Google Scholar]
- 9. Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. [DOI] [PubMed] [Google Scholar]
- 10. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. [DOI] [PubMed] [Google Scholar]
- 11. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–2566. [DOI] [PubMed] [Google Scholar]
- 12. Kadakia MB, Desai NR, Alexander KP, Chen AY, Foody JM, Cannon CP, Wiviott SD, Scirica BM. Use of anticoagulant agents and risk of bleeding among patients admitted with myocardial infarction: a report from the NCDR ACTION Registry–GWTG (National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With the Guidelines. JACC Cardiovasc Interv. 2010;3:1166–1177. [DOI] [PubMed] [Google Scholar]
- 13. Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low‐molecular‐weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S–94S. [DOI] [PubMed] [Google Scholar]
- 14. Weitz JI, Hudoba M, Massel D, Maraganore J, Hirsh J. Clot‐bound thrombin is protected from inhibition by heparin‐antithrombin III but is susceptible to inactivation by antithrombin III‐independent inhibitors. J Clin Invest. 1990;86:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230. [DOI] [PubMed] [Google Scholar]
- 16. Steg PG, van ‘t Hof A, Hamm CW, Clemmensen P, Lapostolle F, Coste P, Ten Berg J, Van Grunsven P, Eggink GJ, Nibbe L, Zeymer U, Campo dell’ Orto M, Nef H, Steinmetz J, Soulat L, Huber K, Deliargyris EN, Bernstein D, Schuette D, Prats J, Clayton T, Pocock S, Hamon M, Goldstein P. Bivalirudin started during emergency transport for primary PCI. N Engl J Med. 2013;369:2207–2217. [DOI] [PubMed] [Google Scholar]
- 17. Schulz S, Richardt G, Laugwitz K‐L, Morath T, Neudecker J, Hoppmann P, Mehran R, Gershlick AH, Tölg R, Anette Fiedler K, Abdel‐Wahab M, Kufner S, Schneider S, Schunkert H, Ibrahim T, Mehilli J, Kastrati A. Prasugrel plus bivalirudin vs. clopidogrel plus heparin in patients with ST‐segment elevation myocardial infarction. Eur Heart J. 2014;35:2285–2294. [DOI] [PubMed] [Google Scholar]
- 18. Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, Andron M, Appleby C, Fisher M, Khand A, Kunadian B, Mills JD, Morris JL, Morrison WL, Munir S, Palmer ND, Perry RA, Ramsdale DR, Velavan P, Stables RH. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT‐PPCI): an open‐label, single centre, randomised controlled trial. Lancet. 2014;384:1849–1858. [DOI] [PubMed] [Google Scholar]
- 19. Han Y, Guo J, Zheng Y, Zang H, Su X, Wang Y, Chen S, Jiang T, Yang P, Chen J, Jiang D, Jing Q, Liang Z, Liu H, Zhao X, Li J, Li Y, Xu B, Stone GW. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA. 2015;313:1336–1346. [DOI] [PubMed] [Google Scholar]
- 20. Valgimigli M, Frigoli E, Leonardi S, Rothenbühler M, Gagnor A, Calabrò P, Garducci S, Rubartelli P, Briguori C, Andò G, Repetto A, Limbruno U, Garbo R, Sganzerla P, Russo F, Lupi A, Cortese B, Ausiello A, Ierna S, Esposito G, Presbitero P, Santarelli A, Sardella G, Varbella F, Tresoldi S, de Cesare N, Rigattieri S, Zingarelli A, Tosi P, van ‘t Hof A, Boccuzzi G, Omerovic E, Sabaté M, Heg D, Jüni P, Vranckx P. Bivalirudin or unfractionated heparin in acute coronary syndromes. N Engl J Med. 2015;373:997–1009. [DOI] [PubMed] [Google Scholar]
- 21. Shah R, Rogers KC, Matin K, Askari R, Rao SV. An updated comprehensive meta‐analysis of bivalirudin vs heparin use in primary percutaneous coronary intervention. Am Heart J. 2016;171:14–24. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collab.; 2011. Available at: www.handbook.cochrane.org. Accessed January 20, 2016. [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 25. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es G‐A, Steg PG, Morel M, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dauerman HL, Frederick PD, Miller D, French WJ. Current incidence and clinical outcomes of bivalirudin administration among patients undergoing primary coronary intervention for stent thrombosis elevation acute myocardial infarction. Coron Artery Dis. 2007;18:141–148. [DOI] [PubMed] [Google Scholar]
- 28. Fox KAA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Granger CB, Flather MD, Budaj A, Quill A, Gore JM; GRACE Investigators . Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892. [DOI] [PubMed] [Google Scholar]
- 29. Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE‐2 randomized trial. JAMA. 2003;289:853–863. [DOI] [PubMed] [Google Scholar]
- 30. Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht H‐J, Hoekstra J, Mehran R, Ohman EM. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. [DOI] [PubMed] [Google Scholar]
- 31. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann F‐J, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Intervention (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 32. Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, Srinivas V, Menegus MA, Marroquin OC, Rao SV, Noveck H, Passano E, Hardison RM, Smitherman T, Vagaonescu T, Wimmer NJ, Williams DO. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–971.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, Panza JA. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol. 2011;108:1108–1111. [DOI] [PubMed] [Google Scholar]
- 34. Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Andò G, Repetto A, Limbruno U, Cortese B, Sganzerla P, Lupi A, Galli M, Colangelo S, Ierna S, Ausiello A, Presbitero P, Sardella G, Varbella F, Esposito G, Santarelli A, Tresoldi S, Nazzaro M, Zingarelli A, de Cesare N, Rigattieri S, Tosi P, Palmieri C, Brugaletta S, Rao SV, Heg D, Rothenbühler M, Vranckx P, Jüni P. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–2476. [DOI] [PubMed] [Google Scholar]
- 35. Cassese S, Byrne RA, Laugwitz K‐L, Schunkert H, Berger PB, Kastrati A. Bivalirudin versus heparin in patients treated with percutaneous coronary intervention: a meta‐analysis of randomised trials. EuroIntervention. 2015;11:196–203. [DOI] [PubMed] [Google Scholar]
- 36. Bavry AA, Elgendy IY, Mahmoud A, Jadhav MP, Huo T. Critical appraisal of bivalirudin versus heparin for percutaneous coronary intervention: a meta‐analysis of randomized trials. PLoS One. 2015;10:e0127832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz K‐L, Kastrati A. Risk of stent thrombosis among bare‐metal stents, first‐generation drug‐eluting stents, and second‐generation drug‐eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv. 2013;6:1267–1274. [DOI] [PubMed] [Google Scholar]
- 38. Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Wong SC, Nikolsky E, Gambone L, Vandertie L, Parise H, Dangas GD, Stone GW. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS‐AMI): 1‐year results of a randomised controlled trial. Lancet. 2009;374:1149–1159. [DOI] [PubMed] [Google Scholar]
- 39. Gurbel PA, Bliden KP, Zaman KA, Yoho JA, Hayes KM, Tantry US. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation. 2005;111:1153–1159. [DOI] [PubMed] [Google Scholar]
- 40. Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, Emanuelsson H, Finkelstein A, Husted S, Katus H, Kilhamn J, Olofsson S, Storey RF, Weaver WD, Wallentin L. Ticagrelor versus clopidogrel in patients with ST‐elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–2141. [DOI] [PubMed] [Google Scholar]
- 41. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST‐elevation myocardial infarction (TRITON‐TIMI 38): double‐blind, randomised controlled trial. Lancet. 2009;373:723–731. [DOI] [PubMed] [Google Scholar]
- 42. Valgimigli M, Tebaldi M, Campo G, Gambetti S, Bristot L, Monti M, Parrinello G, Ferrari R. Prasugrel versus tirofiban bolus with or without short post‐bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (Facilitation through Aggrastat By drOpping or shortening Infusion Line in patients with ST‐segment elevation myocardial infarction compared to or on top of PRasugrel given at loading dOse) Trial. JACC Cardiovasc Interv. 2012;5:268–277. [DOI] [PubMed] [Google Scholar]
- 43. Parodi G, Valenti R, Bellandi B, Migliorini A, Marcucci R, Comito V, Carrabba N, Santini A, Gensini GF, Abbate R, Antoniucci D. Comparison of prasugrel and ticagrelor loading doses in ST‐segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61:1601–1606. [DOI] [PubMed] [Google Scholar]
- 44. Wiviott SD, Braunwald E, McCabe CH, Horvath I, Keltai M, Herrman J‐PR, Van de Werf F, Downey WE, Scirica BM, Murphy SA, Antman EM. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON‐TIMI 38 trial: a subanalysis of a randomised trial. Lancet. 2008;371:1353–1363. [DOI] [PubMed] [Google Scholar]
- 45. Steg PG, Harrington RA, Emanuelsson H, Katus HA, Mahaffey KW, Meier B, Storey RF, Wojdyla DM, Lewis BS, Maurer G, Wallentin L, James SK. Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial. Circulation. 2013;128:1055–1065. [DOI] [PubMed] [Google Scholar]
- 46. Clemmensen P, Wiberg S, Van't Hof A, Deliargyris EN, Coste P, Ten Berg J, Cavallini C, Hamon M, Dudek D, Zeymer U, Tabone X, Kristensen SD, Bernstein D, Anthopoulos P, Prats J, Steg PG. Acute stent thrombosis after primary percutaneous coronary intervention: insights from the EUROMAX trial (European Ambulance Acute Coronary Syndrome Angiography). JACC Cardiovasc Interv. 2015;8:214–220. [DOI] [PubMed] [Google Scholar]
- 47. Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. [DOI] [PubMed] [Google Scholar]
- 48. Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB, Ohman EM, Stone GW. Impact of major bleeding on 30‐day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–1368. [DOI] [PubMed] [Google Scholar]
- 49. Stone GW, Clayton T, Deliargyris EN, Prats J, Mehran R, Pocock SJ. Reduction in cardiac mortality with bivalirudin in patients with and without major bleeding: the HORIZONS‐AMI trial (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction). J Am Coll Cardiol. 2014;63:15–20. [DOI] [PubMed] [Google Scholar]
- 50. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Fahy M, Parise H, Mehran R. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel‐eluting stents versus bare‐metal stents in acute myocardial infarction (HORIZONS‐AMI): final 3‐year results from a multicentre, randomised controlled trial. Lancet. 2011;377:2193–2204. [DOI] [PubMed] [Google Scholar]
- 51. Ferrari E, Benhamou M, Cerboni P, Marcel B. Coronary syndromes following aspirin withdrawal. J Am Coll Cardiol. 2005;45:456–459. [DOI] [PubMed] [Google Scholar]
- 52. Biondi‐Zoccai GGL, Lotrionte M, Agostoni P, Abbate A, Fusaro M, Burzotta F, Testa L, Sheiban I, Sangiorgi G. A systematic review and meta‐analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27:2667–2674. [DOI] [PubMed] [Google Scholar]
- 53. Grines CL, Bonow RO, Casey DE, Gardner TJ, Lockhart PB, Moliterno DJ, O'Gara P, Whitlow P. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, With Representation From the American College of Physicians. Circulation. 2007;115:813–818. [DOI] [PubMed] [Google Scholar]
- 54. Kernis SJ, Harjai KJ, Stone GW, Grines LL, Boura JA, Yerkey MW, O'Neill W, Grines CL. The incidence, predictors, and outcomes of early reinfarction after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2003;42:1173–1177. [DOI] [PubMed] [Google Scholar]
- 55. De Luca G, Ernst N, van ‘t Hof AWJ, Ottervanger JP, Hoorntje JCA, Gosselink ATM, Dambrink J‐HE, de Boer M‐J, Suryapranata H. Predictors and clinical implications of early reinfarction after primary angioplasty for ST‐segment elevation myocardial infarction. Am Heart J. 2006;151:1256–1259. [DOI] [PubMed] [Google Scholar]


