Abstract
Background
Adults <50 years old, with diabetes mellitus, or a history of stroke were not enrolled in the Systolic Blood Pressure Intervention Trial (SPRINT). Estimating the size and characteristics of these excluded groups who meet the other SPRINT eligibility criteria may provide information on the potential impact of providers extending the SPRINT findings to these populations.
Methods and Results
We analyzed the National Health and Nutrition Examination Survey 2003–2012 (n=25 076) to estimate the percentage and characteristics of US adults ≥20 years in 3 populations (age <50 years, diabetes mellitus, or history of stroke) excluded from SPRINT who otherwise meet the trial eligibility criteria: age ≥50 years, systolic blood pressure (SBP) 130–180 mm Hg, high cardiovascular disease risk, and not having trial exclusion criteria. Overall, 1.0% (95% CI 0.8–1.3) of US adults age <50 years, 25.4% (95% CI 23.4–27.6) with diabetes mellitus, and 19.0% (95% CI 16.0–22.4) with history of stroke met the other SPRINT eligibility criteria. Among US adults with SBP ≥130 mm Hg, other SPRINT eligibility criteria were met by 7.5% (95% CI 6.1–9.2) of those age <50 years, 32.9% (95% CI 30.5–35.4) with diabetes mellitus, and 23.0% (95% CI 19.4–27.0) with history of stroke. Among US adults meeting the other SPRINT eligibility criteria, antihypertensive medication was being taken by 31.0% (95% CI 23.9–41.3) of those <50 years, 63.0% (95% CI 58.2–67.6) with diabetes mellitus, and 68.9% (95% CI 59.4–77.1) with a history of stroke.
Conclusions
A substantial percentage of US adults with diabetes mellitus or history of stroke and a small percentage <50 years old meet the other SPRINT eligibility criteria.
Keywords: diabetes mellitus, high blood pressure, hypertension, stroke, systolic blood pressure, systolic blood pressure intervention trial, treatment
Subject Categories: High Blood Pressure, Hypertension, Epidemiology
Introduction
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated reductions in cardiovascular disease (CVD) events and all‐cause mortality among participants randomized to a systolic blood pressure (SBP) target goal of <120 mm Hg versus <140 mm Hg.1 We previously estimated that 7.6% of the US adult population meets the SPRINT eligibility criteria.2
Adults <50 years of age were not enrolled in SPRINT due to the low average CVD risk in this population.3 Also, SPRINT excluded patients with diabetes mellitus or a history of stroke. At the time SPRINT was being designed, other National Institutes of Health–funded trials including the Action to Control Cardiovascular Risk in Diabetes blood pressure trial (ACCORD BP) and the Secondary Prevention of Small Subcortical Strokes trial were evaluating the benefits and harms of lower versus conventional SBP target goals in these populations.4, 5, 6 In a recent meta‐analysis of large‐scale blood pressure–lowering trials, which included ACCORD BP and Secondary Prevention of Small Subcortical Strokes, greater SBP reductions achieved were associated with statistically significant lower risk for CVD events among adults with diabetes mellitus or with a history of stroke.7 There was no effect modification between diabetes mellitus or a history of stroke and lower SBP on CVD events, suggesting that these groups may experience CVD risk reduction from a SBP target goal of <120 mm Hg as demonstrated in SPRINT. US adults age <50 years and with diabetes mellitus or a history of stroke represent substantial segments of the US adult population. There are more than 120 million US adults age <50 years, 20 million US adults with diabetes mellitus, and 6 million US adults with a history of stroke.8, 9
The goal of this study was to estimate the prevalence, number, and characteristics of US adults with 1 of 3 major SPRINT exclusion criteria who might would otherwise be eligible for the trial: <50 years of age, with diabetes mellitus, or with a history of stroke. Estimating the size and characteristics of these excluded groups who meet the other SPRINT eligibility criteria may provide information on the potential impact of providers extending the SPRINT findings to these populations.
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) was designed to track the overall health of the civilian noninstitutionalized US population. Details on the design and conduct of NHANES are available online.10 In brief, NHANES uses a multistage stratified probability sampling approach to identify potential participants for enrollment. Since 1999, NHANES has been conducted in 2‐year cycles. Multiple cycles can be pooled together to provide stable estimates in population subgroups.11 To provide sufficient sample sizes to characterize US adults <50 years of age, with diabetes mellitus, or with a history of stroke meeting the SPRINT criteria, we pooled data from the 2003–2004, 2005–2006, 2007–2008, 2009–2010, and 2011–2012 NHANES cycles. We restricted the analyses to participants who were ≥20 years of age and completed a medical evaluation at the NHANES mobile examination center (n=26 600). Participants were excluded if they did not have 3 SBP and diastolic blood pressure measurements taken during their NHANES medical evaluation (n=1445) or were missing self‐reported information on the use of antihypertensive medication (n=79), leaving 25 076 participants for the analyses. The National Center for Health Statistics institutional review board approved the protocol for each NHANES cycle and all participants provided written informed consent.
Data Collection
NHANES data collected by interview included age, race/ethnicity, sex, cigarette smoking, a previous self‐reported diagnosis of hypertension, diabetes mellitus, heart failure, myocardial infarction, angina, coronary heart disease (CHD) and stroke, receipt of dialysis in the past 12 months, and use of antihypertensive medication, insulin, and oral hypoglycemic medication. We categorized participants who reported having smoked ≥100 cigarettes in their entire life and smoking “some days” or “every day” at the time of the study interview as current smokers.
At the NHANES medical evaluation, height and weight were measured and used to calculate body mass index. Total and high‐density lipoprotein cholesterol, serum creatinine, serum glucose, and hemoglobin A1c were measured from the blood sample. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation, which includes age, sex, race‐ethnicity, and serum creatinine.12 Urine albumin and creatinine were measured using random spot urine samples. Diabetes mellitus was defined by a prior diagnosis, excluding gestational diabetes mellitus, with concurrent use of insulin or oral hypoglycemic medication; or fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or a hemoglobin A1c ≥6.5%.
Blood Pressure Measurement
Blood pressure measurements in NHANES were conducted by a trained physician using a mercury sphygmomanometer with an appropriately sized cuff, determined by midarm circumference measurement, after 5 minutes of seated rest. Blood pressure was measured 3 times at 1‐minute intervals. SBP and diastolic blood pressure were defined as the mean of the 3 measurements. Participants with less than 3 measurements were excluded from the analysis. To ensure the quality of the blood pressure measurements, study physicians underwent quarterly recertification with retraining as necessary. Blood pressure certification consisted of video test recognition of Korotkoff sounds and measurement performance on live volunteer subjects.
Pill Bottle Review
Participants were instructed to bring all prescription medications taken in the previous 2 weeks to their medical evaluation. Study personnel reviewed the pill bottles, and medication names were recorded and coded into drug classes based on their generic equivalents. Medication dosages were not recorded. We identified the following antihypertensive medication classes: angiotensin‐converting enzyme inhibitors, α‐blockers, aldosterone receptor antagonists, angiotensin‐receptor blockers, β‐blockers, calcium channel blockers, central‐acting agents, loop diuretics, potassium‐sparing diuretics, thiazide diuretics, renin inhibitors, and direct vasodilators. Single‐pill combinations were classified into their component classes. Treated hypertension was defined by self‐reported use of antihypertensive medication with 1 or more classes of antihypertensive medication identified through the pill bottle review.
SPRINT Eligibility Criteria
For this analysis, we categorized the SPRINT eligibility criteria into 6 domains: (1) age ≥50 years, (2) elevated SBP (SBP 130–180 mm Hg on 0 or 1 antihypertensive medication classes, 130–170 mm Hg on up to 2 classes, 130–160 mm Hg on up to 3 classes, 130–150 mm Hg on up to 4 classes); (3) high CVD risk; (4) not having diabetes mellitus; (5) not having a history of stroke; and (6) not having other SPRINT exclusion criteria (more than 1 g of proteinuria daily, heart failure, being on dialysis, or an eGFR <20 mL/min per 1.73 m2). High CVD risk conditions included history of CHD, defined in NHANES as self‐report of a prior diagnosis of myocardial infarction, angina, or CHD, eGFR of 20 to 59 mL/min per 1.73 m2, 10‐year risk for CVD ≥15% calculated using the Framingham risk score for general clinical practice,13 and age ≥75 years.
We conducted 3 parallel analyses applying combinations of the SPRINT eligibility criteria to US adults <50 years of age, with diabetes mellitus, or with a history of stroke. For comparison, we also applied criteria to identify US adults meeting all of the SPRINT eligibility criteria.
Statistical Analysis
The percent and number of US adults <50 years, with diabetes mellitus, and with a history of stroke meeting the sequential SPRINT eligibility criteria were calculated (Figure 1). We performed these calculations for the overall population and within subgroups defined by age, sex, race‐ethnicity (non‐Hispanic whites, non‐Hispanic blacks, and Hispanics), SBP (130–139 and ≥140 mm Hg), and by treated hypertension status. In addition, we calculated the percentage meeting SPRINT eligibility criteria among those with (1) SBP ≥130 mm Hg, (2) any high CVD risk condition, and (3) SBP ≥130 mm Hg with any high‐risk condition. For the diabetes mellitus and history of stroke cohorts, we also calculated the percentage and number of US adults meeting SPRINT eligibility criteria among those (4) age ≥50 years, (5) age ≥50 years with SBP ≥130 mm Hg, and (6) age ≥50 years with SBP ≥130 mm Hg and any high‐risk condition. For each cohort, we calculated demographic and clinical characteristics of US adults meeting the other SPRINT eligibility criteria. We also calculated the number of antihypertensive medication classes being taken and the use of angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers, β‐blockers, calcium channel blockers, and thiazide diuretics in each of these populations. Because individuals with diabetes mellitus or a history of stroke are at high risk for CVD,14, 15 in a sensitivity analysis, we recalculated the percent of US adults in these populations meeting the SPRINT eligibility criteria without requiring other high CVD risk criteria to be present.
Figure 1.

Flowchart showing the eligibility criteria for SPRINT applied to adults <50 years (A), with diabetes mellitus (B), and with a history of stroke (C) in the National Health and Nutrition Examination 2003–2012. SPRINT indicates Systolic Blood Pressure Intervention Trial.
All analyses were performed using SUDAAN 10.1 (Research Triangle Institute, Research Triangle Park, NC), accounting for the complex sampling design of NHANES. Sampling weights, recalibrated based on the proportion of participants missing data by age, sex, and race‐ethnicity, were applied to all calculations to obtain US nationally representative prevalence estimates. Recalibration of the sampling weights corrects for differences in missing data across sex and race‐ethnicity strata and assumes that data within strata are missing at random.16
Results
Between 2003 and 2012, 1.0% (95% CI 0.8–1.3) of US adults <50 years of age met the other SPRINT eligibility criteria (Figure 2 and Table S1). The primary reasons for not meeting the SPRINT eligibility criteria were not having SBP in the required range and not having a high CVD risk condition. The percentage meeting the other SPRINT eligibility criteria was higher among adults 40 to 49 versus <40 years of age; males versus females; with SBP ≥140 mm Hg versus 130 to 139 mm Hg; and with, versus without, treated hypertension.
Figure 2.
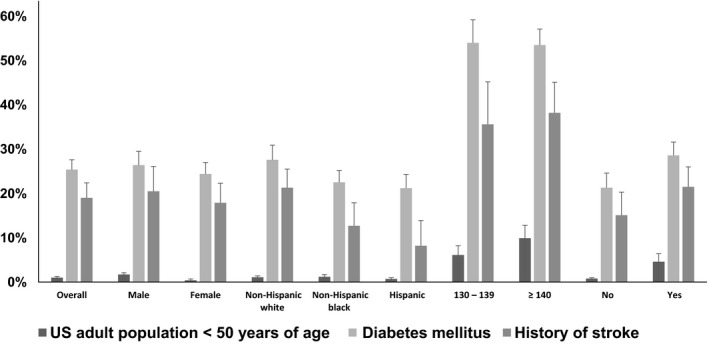
Percentage of US adults <50 years of age, with diabetes mellitus, and history of stroke meeting the other SPRINT eligibility criteria, overall and in subgroups. SPRINT indicates Systolic Blood Pressure Intervention Trial.
Overall, 25.4% (95% CI 23.4–27.6) of US adults with diabetes mellitus met the other SPRINT eligibility criteria (Figure 2 and Table S2, top panel). The primary reason for not meeting the SPRINT eligibility criteria were being younger than 50 years of age, not having SBP in the required range, and having an exclusionary factor. The percentage of US adults with diabetes mellitus who met the other SPRINT eligibility criteria was higher at age 60 to 74 and ≥75 years versus 50 to 59 years, among non‐Hispanic whites compared with non‐Hispanic blacks or Hispanics, and those with versus without treated hypertension. When not requiring an additional high CVD risk condition, 27.5% (95% CI 25.4–29.8) of US adults with diabetes mellitus met the other SPRINT eligibility criteria.
Overall, 19.0% (95% CI 16.0–22.4) of US adults with a history of stroke met the other SPRINT eligibility criteria (Figure 2 and Table S2, bottom panel). Similar to the population with diabetes mellitus, the primary reasons for not meeting the other SPRINT eligibility criteria were being younger than 50 years of age, not having SBP in the required range, and having an exclusionary factor. The percentage of US adults with a history of stroke meeting the other SPRINT eligibility criteria increased with age and was higher among non‐Hispanic whites compared with non‐Hispanic blacks and Hispanics, and those with versus without treated hypertension. When repeating the analysis without requiring an additional high CVD risk condition, 23.1% (95% CI 19.8–26.8) of US adults with a history of stroke met the other SPRINT eligibility criteria.
The percentage of US adults <50 years, with diabetes mellitus or a history of stroke meeting the other SPRINT eligibility criteria was higher when restricted to subgroups with SBP ≥130 mm Hg and with a high CVD risk condition (Table 1). Among the diabetes mellitus and history of stroke populations, the percentage meeting the other SPRINT eligibility criteria was also higher when restricted to US adults ≥50 years of age. Overall, 1.3 million (95% CI 1.0–1.6 million) US adults age <50 years, 5.5 million (95% CI 4.8–6.2 million) with diabetes mellitus, and 1.2 million (95% CI 0.9–1.4 million) with a history of stroke met the other SPRINT eligibility criteria (Figure 3, Tables S3 and S4).
Table 1.
Percentage of US Adults Age <50 Years, With Diabetes Mellitus, and With a History of Stroke Meeting the Other SPRINT Eligibility Criteria in Select Subgroups
| Subgroup | Percentage (95% CI) Meeting Other SPRINT Eligibility Criteria | ||
|---|---|---|---|
| Age <50 Years | Diabetes Mellitus | History of Stroke | |
| SBP ≥130 mm Hg | 7.5 (6.1–9.2) | 53.7 (50.4–57.0) | 37.3 (32.2–42.7) |
| Any high CVD risk condition | 22.1 (18.1–26.6) | 35.4 (32.8–38.0) | 25.1 (21.4–29.3) |
| History of coronary heart disease | 6.7 (3.0–14.2) | 21.3 (17.7–25.3) | 12.3 (7.4–19.6) |
| eGFR 20 to 59 mL/min per 1.73 m2 | 16.1 (8.3–29.0) | 26.2 (22.2–30.6) | 21.8 (17.1–27.4) |
| Framingham risk score ≥15% | 31.7 (26.2–37.8) | 37.5 (34.9–40.2) | 28.6 (24.1–33.5) |
| SBP ≥130 mm Hg with any high CVD risk condition | 56.1 (49.2–62.8) | 62.3 (58.9–65.5) | 43.4 (38.0–48.9) |
| Age ≥50 years | — | 32.9 (30.5–35.4) | 23.0 (19.4–27.0) |
| Age ≥50 years and SBP ≥130 mm Hg | — | 63.5 (60.1–66.7) | 40.4 (35.1–45.9) |
| Age ≥50 years and SBP ≥130 mm Hg with any high CVD risk condition | — | 67.8 (64.4–71.0) | 45.1 (39.5–50.7) |
Criteria for high CVD risk condition include the following: history of CHD (defined in NHANES as self‐report of a prior diagnosis of myocardial infarction, angina, or CHD), eGFR of 20 to 59 mL/min per 1.73 m2, 10‐year risk for CVD ≥15% calculated using the Framingham risk score for general clinical practice.13 CHD indicates coronary heart disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial.
Figure 3.
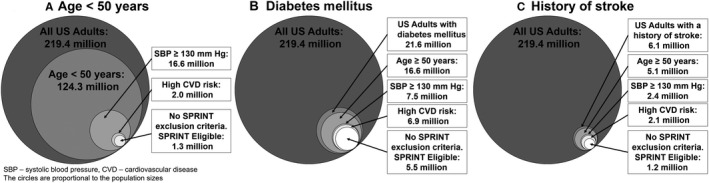
Number of US adults age <50 years (A), or with diabetes mellitus (B), or with a history of stroke (C) otherwise meeting each sequential SPRINT eligibility criterion. SPRINT indicates Systolic Blood Pressure Intervention Trial.
Compared to US adults meeting the full SPRINT eligibility criteria, those age <50 years meeting the other SPRINT eligibility criteria were less likely to be women, non‐Hispanic white, have a history of CHD, eGFR of 20 to 59 mL/min per 1.73 m2 or a 10‐year CVD risk ≥15% and were more likely to be a current smoker, obese, and have SBP between 130 and 139 mm Hg (Table 2). US adults with diabetes mellitus meeting the other SPRINT eligibility criteria were less likely to be ≥80 years of age and non‐Hispanic white and more likely to be obese and have a 10‐year CVD risk ≥15% compared to the population meeting the full SPRINT eligibility criteria. US adults with a history of stroke meeting the other SPRINT eligibility criteria were more likely to be ≥80 years of age, women, underweight or normal weight, have a history of CHD, and have an eGFR of 20 to 59 mL/min per 1.73 m2 compared to the overall population of US adults meeting the full SPRINT eligibility criteria.
Table 2.
Characteristics of US Adults Meeting SPRINT Eligibility in the Overall US Adult Population and Among US Adults <50 Years of Age, With Diabetes Mellitus, and With a History of Stroke
| Group | Full SPRINT Criteria Met | Meeting SPRINT Criteria Except | ||
|---|---|---|---|---|
| Age <50 Years | Diabetes Mellitus | History of Stroke | ||
| Age, y | ||||
| <30 | — | 1.8 (0.3–11.5)a | — | — |
| 30 to 39 | — | 7.9 (4.4–14.1) | — | — |
| 40 to 49 | — | 90.3 (83.1–94.6) | — | — |
| 50 to 59 | 22.1 (19.5–24.9) | — | 24.2 (20.5–28.4) | 11.2 (5.8–20.4) |
| 60 to 69 | 29.6 (27.1–32.3) | — | 36.0 (31.9–40.3) | 26.1 (17.8–36.5) |
| 70 to 79 | 28.8 (26.8–31.0) | — | 26.9 (23.8–30.4) | 21.7 (16.6–28.0) |
| ≥80 | 19.5 (17.8–21.3) | — | 12.8 (10.7–15.3) | 41.0 (32.6–49.9) |
| Female sex | 44.9 (42.4–47.4) | 20.0 (12.6–30.4) | 47.4 (43.6–51.2) | 54.4 (45.5–63.1) |
| Race/ethnicity | ||||
| Non‐Hispanic white | 81.4 (78.2–84.2) | 66.0 (56.6–74.2) | 66.0 (59.5–71.9) | 80.1 (72.0–86.3) |
| Non‐Hispanic black | 7.4 (6.1–8.9) | 14.8 (10.0–21.4) | 14.9 (11.8–18.7) | 10.2 (6.5–15.7) |
| Hispanic | 6.7 (5.1–8.7) | 11.2 (7.4–16.6) | 12.2 (9.0–16.3) | 2.9 (1.5–5.3) |
| Other | 4.5 (3.5–5.9) | 8.0 (3.8–16.2) | 6.9 (4.9–9.6) | 6.8 (3.2–14.1) |
| Current smoker | 17.7 (15.6–19.9) | 59.1 (46.4–70.6) | 13.3 (10.8–16.4) | 16.6 (11.2–24.0) |
| Body mass index | ||||
| Underweight | 2.1 (1.6–2.6) | 0.5 (0.1–3.5)a | 2.4 (1.5–3.8) | 6.7 (4.0–11.1) |
| Normal weight | 26.5 (24.1–29.0) | 18.1 (10.0–30.6) | 13.1 (10.7–16.0) | 31.6 (24.2–40.0) |
| Overweight | 39.1 (36.7–41.5) | 37.0 (26.7–48.6) | 31.0 (27.5–34.7) | 37.4 (30.2–45.4) |
| Obese | 32.4 (30.3–34.6) | 44.4 (33.6–55.7) | 53.5 (49.3–57.6) | 24.3 (17.3–32.9) |
| History of coronary heart disease | 10.9 (9.4–12.5) | 6.8 (3.3–13.5) | 12.7 (10.2–15.7) | 17.7 (11.1–27.0) |
| eGFR 20 to 59 mL/min per 1.73 m2 | 23.5 (21.2–26.0) | 18.7 (10.1–32.0) | 19.7 (16.8–22.9) | 36.2 (28.2–45.1) |
| 10‐year CVD risk ≥15% | 86.0 (84.0–87.8) | 74.4 (61.8–83.9) | 97.1 (95.4–98.2) | 86.1 (77.1–91.9) |
| Mean 10‐year CVD risk | 25.2 (24.7–25.8) | 16.6 (14.8–18.5) | 38.1 (36.6–39.6) | 27.3 (25.3–29.3) |
| Systolic blood pressure, mm Hg | ||||
| 130 to 139 | 38.1 (35.4–40.9) | 50.1 (39.5–60.6) | 41.7 (37.9–45.5) | 33.6 (25.4–42.9) |
| 140 to 149 | 30.2 (27.7–32.8) | 29.6 (21.2–39.6) | 30.0 (26.6–33.7) | 33.8 (25.4–43.4) |
| 150 to 159 | 17.7 (15.8–19.7) | 10.5 (5.1–20.3) | 16.9 (14.5–19.6) | 21.6 (15.4–29.5) |
| ≥160 | 14.0 (12.3–16.0) | 9.9 (6.1–15.6) | 11.4 (8.7–14.9) | 10.9 (7.0–16.7) |
Numbers in table are column percent (95% CI). Body mass index: underweight <18.5 kg/m2, normal weight 18.5 to 24.9 kg/m2, overweight 25.0 to <30.0 kg/m2, and obese ≥30.0 kg/m2. 10‐year CVD risk was calculated by the Framingham risk score for general clinical practice.13 CVD indicates cardiovascular disease; eGFR, estimated glomerular filtration rate; SPRINT, Systolic Blood Pressure Intervention Trial.
Calculations for these percentages are based on small sample sizes and should be interpreted with caution.
US adults age <50 years meeting the other SPRINT eligibility criteria were less likely to be taking antihypertensive medication, while those with diabetes mellitus or a history of stroke were more likely to be taking antihypertensive medication compared with the overall US population meeting the full SPRINT eligibility criteria (Table 3). Angiotensin‐converting enzyme inhibitors use was higher among the diabetes mellitus and history of stroke populations compared with the overall SPRINT‐eligible population. The use of angiotensin‐receptor blockers was higher in the diabetes mellitus group while the use of thiazide diuretics and calcium channel blockers was higher in those with a history of stroke, each compared to the overall SPRINT eligible population. As <10% of US adults were taking other antihypertensive medication classes, these are not reported individually.
Table 3.
Antihypertensive Medication Use Among US Adults Meeting the Full SPRINT Eligibility Criteria and All Criteria Except <50 Years of Age, Diabetes Mellitus, and History of Stroke
| Full SPRINT Criteria Met | Meet All SPRINT Criteria Except | |||
|---|---|---|---|---|
| Age <50 Years | Diabetes Mellitus | History of Stroke | ||
| Taking antihypertensive medication | ||||
| No | 53.8 (51.1–56.4) | 68.1 (58.7–76.1) | 37.0 (32.4–41.8) | 31.1 (22.9–40.6) |
| Yesa | 46.2 (43.6–48.9) | 31.9 (23.9–41.3) | 63.0 (58.2–67.6) | 68.9 (59.4–77.1) |
| Number of antihypertensive medication classes | ||||
| 1 | 19.1 (17.1–21.3) | 19.9 (13.0–29.4) | 22.7 (19.0–26.7) | 26.9 (19.6–35.7) |
| 2 | 17.1 (14.9–19.6) | 9.3 (4.8–17.1) | 21.2 (17.9–24.9) | 21.2 (14.7–29.6) |
| ≥3 | 10.0 (8.4–11.9) | 2.7 (1.0–7.4)b | 19.1 (15.2–23.9) | 20.8 (14.3–29.5) |
| Classes of antihypertensive medication | ||||
| ACE inhibitor | 18.1 (16.4–20.0) | 11.2 (5.7–20.7) | 33.1 (29.5–36.8) | 32.1 (24.1–41.2) |
| Angiotensin receptor blocker | 10.6 (9.2–12.1) | 5.7 (2.9–10.6) | 20.7 (17.2–24.6) | 13.4 (8.8–20.0) |
| β‐Blocker | 21.3 (19.1–23.7) | 10.3 (6.3–16.5) | 26.7 (23.1–30.5) | 26.8 (21.0–33.6) |
| Calcium channel blocker | 15.1 (13.4–17.1) | 7.8 (4.1–14.4) | 21.6 (18.0–25.7) | 31.4 (23.3–40.9) |
| Thiazide diuretic | 17.7 (15.5–20.1) | 11.2 (6.5–18.8) | 19.0 (15.8–22.5) | 26.5 (19.2–35.5) |
Numbers in table are column percent (95% CI). Antihypertensive medication classes included angiotensin‐converting enzyme inhibitors, α‐blockers, aldosterone receptor antagonists, angiotensin‐receptor blockers, β‐blockers, calcium channel blockers, central‐acting agents, loop diuretics, potassium‐sparing diuretics, thiazide diuretics, renin inhibitors, and direct‐acting vasodilators. ACE indicates angiotensin‐converting enzyme; SPRINT, Systolic Blood Pressure Intervention Trial.
Taking antihypertensive medication includes adults who both self‐report taking antihypertensive medication and had 1 or more classes of antihypertensive medication identified during the pill bottle review.
Calculations for these percentages are based on small sample sizes and should be interpreted with caution.
Discussion
In the current study, only 1.0% or 1.3 million US adults age <50 years met the SPRINT SBP criteria, had high CVD risk, and were free of the trial's exclusion criteria. Less than one third of US adults <50 years old meeting the other SPRINT eligibility criteria were taking antihypertensive medication. In contrast, ≈20% to 25% of US adults with diabetes mellitus or a history of stroke met the other SPRINT eligibility criteria. The majority of US adults with diabetes mellitus or a history of stroke meeting the other SPRINT eligibility criteria were taking antihypertensive medication.
Most US adults age <50 years of age have SBP <130 mm Hg and do not have high CVD risk, and these were the primary reasons why younger US adults did not meet the eligibility criteria for SPRINT. Among the subgroup of US adults <50 years of age with SBP ≥130 mm Hg or at high CVD risk, a substantial percentage met the other SPRINT eligibility criteria. US adults <50 years of age meeting the other SPRINT eligibility criteria had a high mean 10‐year CVD risk. Observational studies have demonstrated a strong and graded association between SBP and CVD events beginning as early as age 40 years, and the beneficial effects of antihypertensive medication have been demonstrated in clinical trials of populations with a mean age of 50 years.17, 18, 19, 20 Therefore, antihypertensive medication initiation or titration to an SBP target goal <120 mm Hg may yield a substantial CVD event reduction for high‐risk younger adults.
A large percentage of US adults with diabetes mellitus, 25.4% or 5.5 million people, met the other SPRINT eligibility criteria. In the ACCORD BP trial, an intensive SBP target goal (SBP <120 mm Hg) versus standard SBP target goal (SBP <140 mm Hg) resulted in a non–statistically significant 12% lower risk of CVD events over 5 years of treatment.21 The ACCORD BP trial used a double 2×2 factorial design to simultaneously study SBP, lipid, and glycemic control interventions. In a post‐hoc analysis of ACCORD BP, intensive SBP control reduced CVD events in those assigned to the standard glycemia intervention arm but not in the intensive glycemia arm.21, 22 Also, findings from a recent meta‐analysis of blood pressure–lowering trials demonstrated CVD and all‐cause mortality risk reductions with lower SBP target goals in patients with diabetes mellitus.7 Among participants with diabetes mellitus, each 10 mm Hg more intensive SBP lowering was associated with a hazard ratio for CVD events and all‐cause mortality of 0.88 (95% CI 0.82–0.94) and 0.87 (95% CI 0.79–0.97), respectively.7 No evidence was present to suggest the CVD or all‐cause mortality risk reduction associated with SBP lowering diminished below 130 mm Hg. These results suggest that treatment effects of more intensive SBP control observed in SPRINT may extend to those with diabetes mellitus. The current analysis shows that a substantial population of US adults with diabetes mellitus meet the other SPRINT eligibility criteria.
Among US adults with a history of stroke, 19.0% or 1.2 million met the other SPRINT eligibility criteria. Both observational studies and clinical trials demonstrate a reduced risk of stroke with lower SBP regardless of age.23 The Secondary Prevention of Small Subcortical Strokes trial compared an intensive SBP target goal (<130 mm Hg) to a standard SBP target goal (<150 mm Hg) in patients who had a recent lacunar stroke.6 Participants randomized to an intensive versus a standard SBP target goal experienced a 19% reduced risk for recurrent stroke (hazard ratio 0.81; 95% CI 0.64–1.03) over 3.7 years of treatment.6 These findings were extended by a recent meta‐analysis wherein each 10 mm Hg more intensive SBP lowering was associated with a hazard ratio for CVD events of 0.77 (95% CI 0.69–0.85) among those with a history of stroke at baseline.7 However, there was no association between SBP lowering and all‐cause mortality in those with a prior stroke (hazard ratio 0.96; 95% CI 0.86–1.07).7 Collectively, these results suggest that there may be CVD risk reduction benefits of intensive SBP control for those with a history of stroke.
Extrapolating SPRINT results to populations not enrolled in the trial should be done with caution. Some of the risks associated with a SBP target goal of <120 mm Hg in SPRINT and ACCORD BP include falls, renal dysfunction, bradyarrhythmias, and electrolyte abnormalties.1, 5 These risks may be more pronounced in some subgroups such as those with a history of stroke or older individuals, and potential adverse effects should be considered when deciding SBP target goals for individual patients. Previous meta‐analyses of blood pressure–lowering randomized trials did not report side effects associated with intensive SBP lowering, likely because these events were too disparate and inconsistently reported to allow for a formal analysis.7, 24
The findings from the current study provide insight into the potential numbers of patients affected if providers generalize the more intensive SBP target goal of <120 mm Hg to populations beyond those enrolled in SPRINT. While only 1% of US adults <50 years of age otherwise met the SPRINT eligibility criteria, over 50% of US adults <50 years of age with SBP ≥130 mm Hg and with high CVD risk met the other SPRINT eligibility criteria. Among US adults with diabetes mellitus or a history of stroke and SBP ≥130 mm Hg, ≈40% to 50% met the other SPRINT eligibility criteria. Screening of these populations to assess whether they meet the other SPRINT criteria may be warranted.
Clinicians will need to decide on how to incorporate SPRINT results into clinical practice. For example, a SBP goal of 120 mm Hg may be most appropriate for patients who strictly meet the SPRINT eligibility criteria. However, clinicians often make decisions based on extrapolation of trial results beyond the studied populations because it is not always feasible to repeat the trial in those who were excluded. Based on the evidence available, clinicians may also consider appropriate a SBP target goal of <120 mm Hg for patients with diabetes mellitus or a history of stroke. Our results show that a substantial percentage of US adults with diabetes mellitus or a history of stroke meet the other SPRINT eligibility criteria. Future studies are needed to evaluate the risks, benefits, and cost‐effectiveness of a SBP target goal of <120 mm Hg among populations not enrolled in SPRINT.
A major strength of the current analysis is the sampling design employed by NHANES that facilitates the generation of US nationally representative estimates. Additional strengths include the extensive data collection following protocols with quality control procedures. The current study has some limitations. NHANES did not assess coronary calcium score, ankle brachial index, or left ventricular hypertrophy, which were SPRINT inclusion criteria. There were also some SPRINT exclusion criteria that NHANES does not have information on, including reduced left ventricular ejection fraction or a history of medication nonadherence. An additional limitation is the small sample size within some subgroups, especially for US adults <50 years.
In conclusion, a substantial percentage of US adults with diabetes mellitus or a history of stroke but only a small percentage of US adults <50 years old meet the other SPRINT eligibility criteria. Many of these people were not taking antihypertensive medication. Until trials are conducted in these populations, healthcare providers may consider extrapolating the results of SPRINT to younger adults or those with diabetes mellitus or stroke. The results from the current analysis provide estimates of the numbers and percentages of US adults in 3 subgroups who may have antihypertensive treatment initiated or intensified if SPRINT results are applied.
Sources of Funding
This work was supported by the National Institutes of Health (K24‐HL125704, Shimbo‐PI) from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Disclosures
Dr Muntner receives grant support from Amgen Inc. unrelated to the topic of this manuscript. The remaining authors have no disclosures to report.
Supporting information
Table S1. Percentage of US Adults <50 Years of Age Meeting Each Sequential SPRINT Eligibility Criterion, Overall and in Subgroups
Table S2. Percentage of US Adults With Diabetes (Top Panel) and History of Stroke (Bottom Panel) Meeting Each Sequential SPRINT Eligibility Criterion, Overall and in Subgroups
Table S3. Number of US Adults <50 Years of Age Meeting Each Sequential SPRINT Eligibility Criterion, Overall (Top Panel) and in Subgroups
Table S4. Number of US Adults With Diabetes (Top Panel) and History of Stroke (Bottom Panel) Meeting Each Sequential SPRINT Eligibility Criterion, Overall and in Subgroups
(J Am Heart Assoc. 2016;5:e003547 doi: 10.1161/JAHA.116.003547)
References
- 1. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of SPRINT results to the U.S. adult population. J Am Coll Cardiol. 2016;67:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Systolic blood pressure intervention trial (SPRINT) protocol version 4.0. 2012. Available at: https://www.sprinttrial.org/public/Protocol_Current.pdf. Accessed July 23, 2016.
- 4. Cushman WC, Whelton PK, Fine LJ, Wright JT Jr, Reboussin DM, Johnson KC, Oparil S; Group SSR . SPRINT trial results: latest news in hypertension management. Hypertension. 2016;67:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons‐Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail‐Beigi F. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood‐pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 8. United States Census Bureau American fact finder. 2016. Available at: http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed March 1, 2016.
- 9. Writing Group M , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e60. [DOI] [PubMed] [Google Scholar]
- 10. National Health and Nutrition Examination Survey: questionnaires, datasets, and related documentation. 2016. Available at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed March 1, 2016.
- 11. Mirel LB, Mohadjer LK, Dohrmann SM, Clark J, Burt VL, Johnson CL, Curtin LR. National Health and Nutrition Examination Survey: estimation procedures, 2007–2010. Vital Health Stat 2. 2013;159:1–17. [PubMed] [Google Scholar]
- 12. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 13. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 14. Carson AP, Tanner RM, Yun H, Glasser SP, Woolley JM, Thacker EL, Levitan EB, Farkouh ME, Rosenson RS, Brown TM, Howard G, Safford MM, Muntner P. Declines in coronary heart disease incidence and mortality among middle‐aged adults with and without diabetes. Ann Epidemiol. 2014;24:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dhamoon MS, Elkind MS. Inclusion of stroke as an outcome and risk equivalent in risk scores for primary and secondary prevention of vascular disease. Circulation. 2010;121:2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. [DOI] [PubMed] [Google Scholar]
- 17. Parry CA. Australian therapeutic trial in mild hypertension. Lancet. 1980;2:425. [DOI] [PubMed] [Google Scholar]
- 18. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies C . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 19. Five‐year findings of the hypertension detection and follow‐up program. II. Mortality by race‐sex and age. Hypertension Detection and Follow‐Up Program Cooperative Group. JAMA. 1979;242:2572–2577. [DOI] [PubMed] [Google Scholar]
- 20. MRC trial of treatment of mild hypertension: principal results. Medical Research Council Working Party. Br Med J (Clin Res Ed). 1985;291:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Margolis KL, O'Connor PJ, Morgan TM, Buse JB, Cohen RM, Cushman WC, Cutler JA, Evans GW, Gerstein HC, Grimm RH Jr, Lipkin EW, Narayan KM, Riddle MC Jr, Sood A, Goff DC Jr. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the accord randomized trial. Diabetes Care. 2014;37:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Group AS , Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr, Grimm RH Jr, Margolis KL, Probstfield JL, Simons‐Morton DG, Sullivan MD. Action to control cardiovascular risk in diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. [DOI] [PubMed] [Google Scholar]
- 23. Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A; American Heart Association Stroke C, Council on C, Stroke N, Council on Quality of C, Outcomes R, Council on Functional G, Translational B . Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure‐lowering treatment. 6. Prevention of heart failure and new‐onset heart failure—meta‐analyses of randomized trials. J Hypertens. 2016;34:373–384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Percentage of US Adults <50 Years of Age Meeting Each Sequential SPRINT Eligibility Criterion, Overall and in Subgroups
Table S2. Percentage of US Adults With Diabetes (Top Panel) and History of Stroke (Bottom Panel) Meeting Each Sequential SPRINT Eligibility Criterion, Overall and in Subgroups
Table S3. Number of US Adults <50 Years of Age Meeting Each Sequential SPRINT Eligibility Criterion, Overall (Top Panel) and in Subgroups
Table S4. Number of US Adults With Diabetes (Top Panel) and History of Stroke (Bottom Panel) Meeting Each Sequential SPRINT Eligibility Criterion, Overall and in Subgroups


