Abstract
Background
Cardiac magnetic resonance (CMR) can detect inflammatory myocardial alterations in patients suspected of having acute myocarditis. There is limited information regarding the degree of normalization of CMR parameters during the course of the disease and the time window during which quantitative CMR should be most reasonably implemented for diagnostic work‐up.
Methods and Results
Twenty‐four patients with suspected acute myocarditis and 45 control subjects underwent CMR. Initial CMR was performed 2.6±1.9 days after admission. Myocarditis patients underwent CMR follow‐up after 2.4±0.6, 5.5±1.3, and 16.2±9.9 weeks. The CMR protocol included assessment of standard Lake Louise criteria, T1 relaxation times, extracellular volume fraction, and T2 relaxation times. Group differences between myocarditis patients and control subjects were highest in the acute stage of the disease (P<0.001 for all parameters). There was a significant and consistent decrease in all inflammatory CMR parameters over the course of the disease (P<0.01 for all parameters). Myocardial T1 and T2 relaxation times—indicative of myocardial edema—were the only single parameters showing significant differences between myocarditis patients and control subjects on 5.5±1.3‐week follow‐up (T1: 986.5±44.4 ms versus 965.1±28.1 ms, P=0.022; T2: 55.5±3.2 ms versus 52.6±2.6 ms; P=0.001).
Conclusions
In patients with acute myocarditis, CMR markers of myocardial inflammation demonstrated a rapid and continuous decrease over several follow‐up examinations. CMR diagnosis of myocarditis should therefore be attempted at an early stage of the disease. Myocardial T1 and T2 relaxation times were the only parameters of active inflammation/edema that could discriminate between myocarditis patients and control subjects even at a convalescent stage of the disease.
Keywords: diagnosis, follow‐up study, magnetic resonance imaging, mapping, myocarditis
Subject Categories: Magnetic Resonance Imaging (MRI), Diagnostic Testing
Introduction
Myocarditis has been identified as an important cause of cardiac morbidity and mortality, accounting for up to 20% of sudden unexpected deaths in adults younger than 40 years.1 In addition, dilated cardiomyopathy may result from chronic inflammatory disease in patients with inadequate immune response.2 Endomyocardial biopsy combined with immunohistology is regarded as the current “gold standard” for diagnosis, but for many reasons it is mostly performed in hemodynamically unstable patients with unexplained or therapy‐refractory cardiomyopathy.3 Instead of undergoing early endomyocardial biopsy, cardiac magnetic resonance (CMR) is increasingly used in patients with clinical manifestation of myocarditis.4, 5 In these patients, CMR can reliably characterize acute inflammatory myocardial alterations by the use of a combination of imaging sequences that detect edema, hyperemia, and necrosis. This combined imaging approach is the essential part of the so‐called “Lake Louise” criteria (LLC) and a diagnosis of myocarditis is made if 2 of 3 criteria are met.6 In patients suspected of having myocarditis and negative LLC on initial CMR exam, a repeat scan is currently recommended in order to establish the diagnosis.6 Newer quantitative imaging techniques such as myocardial T1 and T2 mapping can assess diffuse myocardial inflammation and have recently been described to further enhance the diagnostic performance of CMR.4, 5, 7 However, most of these results are only valid in the acute stage of the disease, and there is limited information regarding the time window during which quantitative CMR should be most reasonably implemented for diagnostic work‐up. Furthermore, in follow‐up of myocarditis, quantitative T1 and T2 relaxation times may provide additional information about the healing process and degree of inflammation. Repetitive CMR in acute myocarditis may also help to identify patients with persistent myocardial inflammation who might require subsequent endomyocardial biopsy for a more specific therapeutic treatment.
In this prospective study, we performed repetitive comprehensive follow‐up CMR examinations in an intraindividual cohort of patients suspected of having acute myocarditis. The purpose of our study was to investigate (1) at which time point comprehensive CMR should be best implemented for diagnostic work‐up, (2) the usefulness of repetitive CMR to monitor disease activity in acute myocarditis, and (3) the diagnostic target and value of different CMR parameters including myocardial T1 and T2 mapping for the follow‐up of patients with acute myocarditis.
Methods
The institutional review board approved the study and all subjects gave written informed consent prior to CMR. The study population of this prospective study was recruited during April 2014 and August 2015 and consisted of patients with clinically defined acute myocarditis and control subjects. Patients with clinically suspected myocarditis had the following: acute chest pain, evidence of acute myocardial injury (ECG changes and/or elevated troponin), and a history of viral infection during the last 4 weeks with elevated serum markers indicating infectious disease (eg, C‐reactive protein). Coronary artery disease was ruled out by invasive cardiac catheterization prior to CMR in all patients. Exclusion criteria for the study were contraindications for CMR, previous acute myocardial conditions (myocarditis or myocardial infarction), or other medical history of cardiac disease. In accordance with the pathophysiological course of viral myocarditis,8 3 time frames were defined (2–3, 4–8, and >8 weeks) for follow‐up CMR investigations. Patients with suspected myocarditis were treated in accordance with European guidelines. The control group was only scanned once and consisted of healthy volunteers and outpatients referred for nonspecific thoracic pain and in which a detailed diagnostic workup and clinical follow‐up were unremarkable and without signs of cardiac disease.
Cardiac Magnetic Resonance
CMR scans were performed on a 1.5‐Tesla CMR system (Ingenia 1.5T; Philips Healthcare, Best, the Netherlands). For functional analysis, ECG‐gated steady‐state free precession cine images were obtained including short‐axis, 4‐chamber and 2‐chamber views. Edema‐sensitive black blood T2‐weighted short‐tau inversion‐recovery sequences were acquired in the short‐axis and transversal orientation. To correct for torso coil–related signal inhomogeneities, a signal intensity correction algorithm based on a calibration measurement using the body coil was performed. Early gadolinium enhancement was assessed using transverse free‐breathing fast spin echo T1‐weighted images, which were acquired in 3 identical slices both before and after intravenous injection of a bolus of 0.2 mmol/kg of body weight of gadobutrol (Gadovist; Bayer Healthcare, Leverkusen, Germany) using the body coil for signal reception. For late gadolinium enhancement (LGE) imaging, segmented inversion‐recovery gradient‐echo sequences were performed in short‐axis, 4‐chamber and 2‐chamber views. Optimal inversion time was determined by using the Look‐Locker technique.9 T1 and T2 mapping were performed in end‐diastole in short‐axis orientation (basal, midventricular, and apical sections). For myocardial T1 mapping a 3(3)3(3)5 modified Look‐Locker inversion recovery acquisition scheme was applied.10 T1 maps were acquired before and 10 minutes after contrast administration. For myocardial T2 mapping, an optimized 6‐echo gradient spin echo sequence was used as previously described.11 T1 and T2 maps were reconstructed directly on the imager console after image acquisition. Detailed sequence parameters are given in Table S1.
Image Analysis
Two CMR‐experienced physicians analyzed the data and performed the measurements. Readers were blinded to the patient information. Cardiac functional analysis was performed offline using dedicated software (IntelliSpace Portal 6; Philips Healthcare, the Netherlands). Papillary muscles were included in the left ventricular cavity volume. The presence of focal myocardial edema on T2 short‐tau inversion‐recovery and/or nonischemic lesions on LGE images was visually assessed by consensus agreement of the 2 readers. In addition, enhanced areas were measured quantitatively using dedicated software (IntelliSpace Portal 6). After manually contouring the endocardial and epicardial contours of short‐axis LGE images, enhanced areas were defined as those with signal intensitiy ≥3.0 SDs above the mean signal intensity of normal myocardium.12 Enhanced volume percentage was calculated from enhanced and nonenhanced myocardial volumes.
T2 ratio for the presence of global myocardial edema as well as early gadolinium enhancement ratio (EGEr) for the presence of inflammation‐induced hyperemia were calculated as recommended for the assessment of the LLC.4, 5, 6, 13 Myocardial T1 and T2 relaxation times were extracted from the relaxation maps by using freely available software (Segment, version 1.9, R2783; http://segment.heiberg.se).14 Endocardial and epicardial borders were carefully contoured to exclude epicardial fat, blood pool, and pericardial effusion from analysis. Myocardial T1 and T2 maps were analyzed by using a segmental approach5, 15 and global T1 and T2 relaxation times were calculated from the segmental data. Hematocrit‐corrected extracellular volume fraction values were calculated separately from pre‐ and postcontrast T1 values as previously described.4, 5
Statistical Analysis
Statistical analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC) and SPSS Statistics 22.0 (IBM, Armonk, NY). Patient characteristics are presented as mean±SD or as absolute frequency. Continuous variables were tested for normal distribution. The independent 2‐sample Student t test or the Mann–Whitney U test was used for comparison of continuous variables between 2 different groups. Dichotomous variables were compared using the χ2 test (with a cell count >5) or Fisher exact test (with a cell count ≤5). For intra‐individual group comparisons, a generalized linear model was applied introducing a random effect for patients to account for the repeated measurements over time. Correlation analysis was performed using Pearson correlation coefficient. Cut‐off values for the calculation of sensitivities, specificities, positive predictive values (PPV), and negative predictive values were obtained from receiver operating characteristic analyses. The level of statistical significance was set to P<0.05.
Results
Population Characteristics
A total of 69 subjects were included in this study (24 patients with acute myocarditis and 45 control subjects). Mean time from admission to initial baseline CMR (n=24) was 2.6±1.9 days. After initial CMR, patients with myocarditis were followed up at 2.4±0.6 (2–3 weeks follow‐up, n=11), 5.5±1.3 (4–8 weeks follow‐up, n=20), and 16.2±9.9 (>8 weeks follow‐up, n=24) weeks. No deaths or heart transplantations occurred during the study period. Mean age of myocarditis patients was 41.4±16.7 (range: 18–74) years. Mean age of healthy controls was 40.1±16.7 (range: 18–75) years. Age (P=0.758), sex (P=0.982), and body mass index (P=0.241) did not differ significantly between both groups. Baseline clinical characteristics for myocarditis and control subjects are given in Table 1.
Table 1.
Baseline Clinical Characteristics in Myocarditis and Control Subjects
| Variable | Myocarditis Baseline (n=24) | Control Subjects (n=45) | P Value |
|---|---|---|---|
| Age, y | 41.4±16.7 | 40.1±16.7 | 0.758 |
| Male patients | 15 (62%) | 28 (62%) | 0.982 |
| Heart rate, beats/min | 70.7±14.8 | 66.4±12.6 | 0.212 |
| Body mass index, kg/m2 | 26.4±4.8 | 25.1±4.3 | 0.241 |
| Troponin I, ng/mL | 9.5±13.8 | Below detection limit | — |
| White blood cell count, 103/μL | 8.97±3.90 | 6.7±1.9 | 0.003 |
| C‐reactive protein, mg/L | 75.2±85.8 | 1.3±0.9 | 0.006 |
Data are mean±SD or absolute frequency with percentages in parentheses.
Baseline CMR Results
During the acute phase, all CMR parameters indicating inflammatory alterations of the myocardium were significantly elevated in the myocarditis group compared to the control group: T2 ratio (1.9±0.4 versus 1.6±0.3; P<0.001), EGEr (4.7±3.2 versus 2.3±2.1; P<0.001), native T1 relaxation times (1047.7±44.0 ms versus 965.1±28.1 ms; P<0.001), T2 relaxation times (62.2±6.2 ms versus 52.6±2.6 ms; P<0.001), and extracellular volume fraction (30.0±4.6% versus 26.1±3.2%; P<0.001). Nonischemic LGE was found in 91% (22/24) of all myocarditis patients. Ninety‐one percent (22/24) of all patients had a CMR diagnosis of myocarditis with positive LLC. All CMR parameters evaluated are given in Table 2. There was a significant correlation between baseline native T1 relaxation times and T2 relaxation times (r=0.743; P<0.001). Myocardial T2 relaxation times also showed a significant correlation with baseline left ventricular ejection fraction (EF) (r=−0.460; P=0.024). All other CMR parameters did not show a significant correlation with baseline EF.
Table 2.
CMR Parameters of Myocarditis Patient (Baseline and Follow‐Up) and Control Subjects
| Parameter | Myocarditis Baseline (n=24) | 2 to 3 Weeks Follow‐Up (n=11) | 4 to 8 Weeks Follow‐Up (n=20) | >8 Weeks Follow‐Up (n=24) | Control Subjects (n=45) |
|---|---|---|---|---|---|
| Left ventricular ejection fraction, % |
55.5±9.7 P<0.001 |
59.5±13.1 P=0.313 |
61.4±7.0 P=0.779 |
61.6±7.3 P=0.937 |
61.8±4.3 — |
| Left ventricular end diastolic volume/body surface area, mL/m2 |
70.8±12.8 P=0.878 |
71.1±18.3 P=0.968 |
71.2±13.1 P=0.972 |
71.0±13.0 P=0.9145 |
71.3±11.5 — |
| T2 ratio |
1.9±0.4 P<0.001 |
1.8±0.2 P=0.037 |
1.7±0.2 P=0.154 |
1.7±0.2 P=0.088 |
1.6±0.3 — |
| Visible myocardial edema |
21 (88%) P<0.001 |
8 (72%) P<0.001 |
7 (35%) P<0.001 |
1 (4%) 0.348 |
0 (0%) — |
| Early gadolinium enhancement ratio |
4.7±3.2 P<0.001 |
2.5±0.8 P=0.707 |
2.5±2.0 P=0.719 |
2.1±1.1 P=0.551 |
2.3±2.1 — |
| Visible late gadolinium enhancement |
22 (91%) P<0.001 |
10 (91%) P<0.001 |
18 (90%) P<0.001 |
16 (67%) P<0.001 |
0 (0%) — |
| Late gadolinium enhancement, % |
15.8±12.0 P<0.001 |
13.5±10.9 P<0.001 |
9.2±9.0 P=0.010 |
7.2±5.9 P=0.150 |
4.8±4.4 — |
| Lake Louise criteria |
22 (91%) P<0.001 |
9 (82%) P<0.001 |
8 (40%) P<0.001 |
3 (13%) P=0.039 |
0 (0%) — |
| Native T1, ms |
1047.7±44.0 P<0.001 |
1018.1±44.8 P<0.001 |
986.5±44.4 P=0.022 |
963.0±30.0 P=0.846 |
965.1±28.1 — |
| Native T2, ms |
62.2±6.2 P<0.001 |
61.8±7.7 P<0.001 |
55.5±3.2 P=0.001 |
54.9±3.0 P=0.005 |
52.6±2.6 — |
| Extracellular volume fraction, % |
30.0±4.6 P<0.001 |
26.4±4.8 P=0.780 |
27.1±6.5 P=0.419 |
26.4±4.8 P=0.811 |
26.1±3.2 — |
CMR indicates cardiac magnetic resonance.
Data are mean±SD or absolute frequency with percentages in parentheses. P values given indicate differences against control subjects.
Follow‐Up CMR Results
Over the follow‐up period there was a significant and consistent decrease in all inflammatory CMR parameters in the myocarditis group: T2 ratio (P=0.001), EGEr (P<0.001), native T1 relaxation times (P<0.001), native T2 relaxation times (P<0.001), and extracellular volume fraction (P<0.001) (Table 2, Figures 1 and 2). Quantitative LGE also significantly decreased over time (P<0.001). Qualitative LGE was still visible in 67% (16/24) of myocarditis patients at last follow‐up (>8 weeks). EF significantly improved from baseline to last follow‐up (55.5±9.7% versus 61.6±7.3%; P<0.001).
Figure 1.
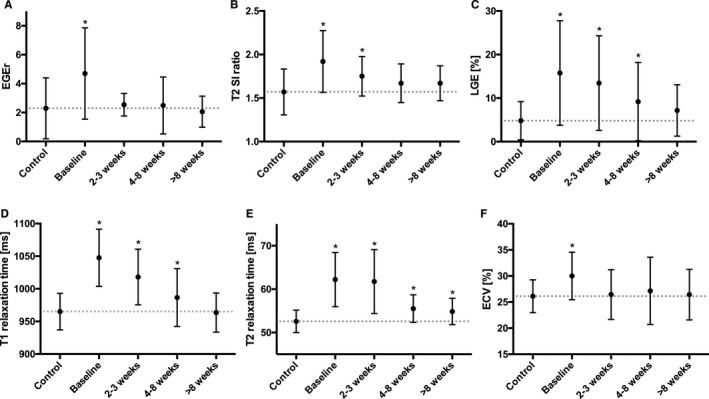
Mean plots of different cardiac magnetic resonance parameters for control subjects and myocarditis patients during the course of the disease. Dots represent the mean of the data and errors bars the SD. Differences are shown for (A) early gadolinium enhancement ratio (EGEr), (B) T2 signal intensity (SI) ratio, (C) quantitative late gadolinium enhancement (LGE), (D) T1 relaxation times, (E) T2 relaxation times, and (F) extracellular volume fraction (ECV). *Statistical significance compared to control subjects.
Figure 2.
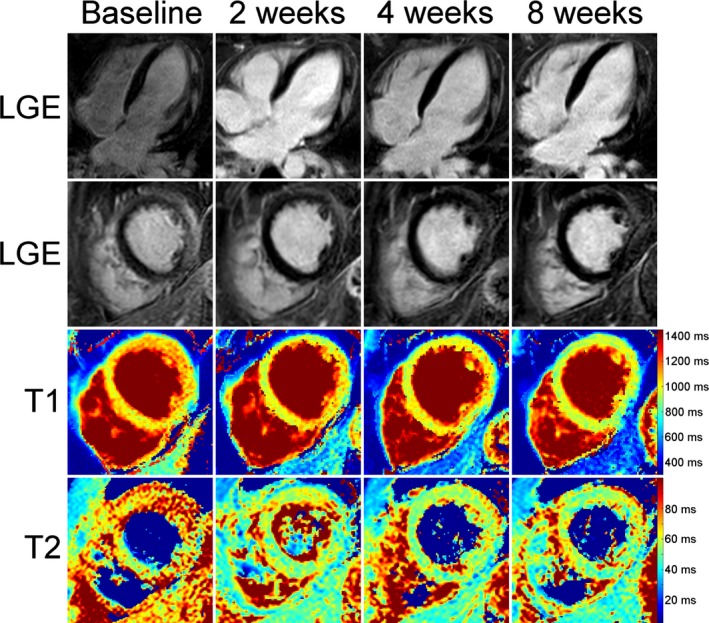
Baseline and follow‐up CMR examinations in a 38‐year‐old male with typical inflammatory lesions at the subepicardium of the lateral wall. The composition of pictures exemplarily illustrates the continuous normalization of T1 and T2 relaxation times during the course of the disease. As a marker of irreversible myocardial damage, late gadolinium enhancement (LGE) was persistently visible even at 8 weeks follow‐up. In this regard, LGE cannot be used for the discrimination between acute and convalescent stages of the disease. CMR indicates cardiac magnetic resonance.
Among all single CMR parameters indicating ongoing inflammation and edema, only T1 and T2 relaxation times could discriminate between residual inflammation in myocarditis patients and healthy controls at second follow‐up (4–8 weeks) (T1 relaxation times: 986.5±44.4 ms versus 965.1±28.1 ms; P=0.022, and T2 relaxation times: 55.5±3.2 ms versus 52.6±2.6 ms; P=0.001). In diagnosing residual inflammation, myocardial T1 mapping (cut‐off value: 967.2 ms) yielded a sensitivity of 55%, a specificity of 58%, a positive predictive value of 37%, and a negative predictive value of 74%. Myocardial T2 mapping (cut‐off value: 53.8 ms) yielded higher values with a sensitivity of 70%, a specificity of 67%, a positive predictive value of 48%, and a negative predictive value of 83%. At the last follow‐up, only T2 relaxation times showed a group difference between myocarditis patients and control subjects (54.9±3.0 ms versus 52.6±2.6 ms; P=0.005). Three of 24 (13%) patients had persistent positive LLC at the last follow‐up (Figure 3). These patients had a lower EF compared with myocarditis patients who showed a full recovery according to the LLC (53.7±10.2% versus 63.1±6.3%; P=0.032). T1 and T2 relaxation times were also elevated in these patients (T1 relaxation times: 983.2±41.1 ms versus 960.8±28.2 ms, T2 relaxation times: 57.5±3.1 ms versus 54.4±2.9 ms; P>0.05, respectively). However, compared to baseline, ∆EF was comparable between both groups (9.7±5.8% versus 7.2±9.3%; P=0.523). There was a significant correlation between ∆EF and baseline T1 and T2 relaxation times (T1: r=0. 519; P=0.009; T2: r=0.440; P=0.031). The change in myocardial T1 and T2 relaxation times between baseline and last follow‐up (∆T1 and ∆T2) also showed a significant correlation (r=0.659; P<0.001).
Figure 3.
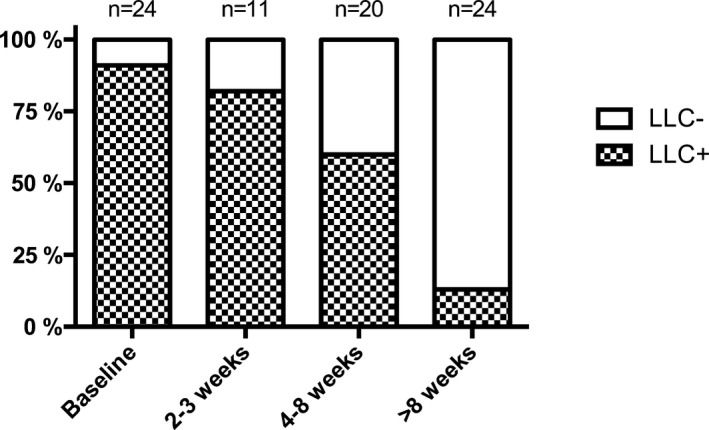
Stacked column chart on the percentage distribution of the Lake Louise criteria (LLC) for patients with suspected acute myocarditis during the course of the disease.
Discussion
In this prospective study, we evaluated whether repetitive CMR in acute myocarditis may reliably assess the decrease of inflammatory myocardial alterations and to what extent it may discriminate between diseased and control subjects even at convalescent stages of disease. The main findings of our follow‐up study on patients with acute myocarditis are that (1) all CMR parameters indicating inflammatory alterations of the myocardium showed a rapid and consistent decrease during the course of the disease, (2) the group differences between diseased and control subject were highest in the acute phase of the disease, and (3) T1 and T2 relaxation times were the only single parameters of ongoing myocardial inflammation/edema to show significant differences between myocarditis and control subjects even at 4‐to‐8‐week follow‐up examinations.
Myocardial Edema
Black‐blood T2‐weighted imaging is regarded as the standard imaging technique for detection of myocardial edema.6, 16 In CMR, myocardial edema represents a sign of inflammation and is one of its main diagnostic targets.13, 16 Myocardial edema in myocarditis can best be visualized during the first days of the disease: In a study performed by Monney et al, visible myocardial edema decreased from 84% to 39% in 20 patients with acute myocarditis after a >3‐week follow‐up.17 In our study cohort, the presence of visible myocardial edema also decreased from 88% to 35% at 4‐to‐8‐week follow‐up, whereas at the last follow‐up myocardial edema was visible in only 4% of all patients. Semiquantitative T2 ratio, another surrogate for the presence myocardial edema, also showed a normalization over time, and after 3 weeks no significant elevation in mean T2 ratio could be observed in our study. This normalization of T2 ratio was also described in several other myocarditis follow‐up studies.18, 19, 20 In this regard the presence of visible myocardial edema or of increased T2 ratio values may be regarded as one of the main imaging findings in patients with acute or subacute myocarditis.4, 5, 17 The observed changes and presence of myocardial edema fit well with the pathophysiological course of viral myocarditis, in which significant cardiac damage and reduction of myocardial function is mainly described during the acute phase 1 (last 1–7 days) and subacute phase 2 (last 1–4 weeks).8
In this study, we evaluated 2 new parameters of myocardial edema (native T1 relaxation time and T2 relaxation times), which have been shown to have high diagnostic accuracies for diagnosing myocarditis during the acute stage.4, 5, 21 Both new parameters have been described to be especially helpful for the detection of diffusive myocardial inflammation, as the assessment of T1 or T2 relaxation times is independent on a direct visualization of inflammatory myocardium or of signal intensities of a reference tissue.4, 5, 21 Interestingly, we found that in contrast to standard T2‐weighted imaging, both parameters were significantly elevated compared to controls even in later stages of the disease. With their unique sensitivity and specificity to detect even diffuse and discreet myocardial edema as a sign of ongoing/residual inflammation,4, 16, 22 myocardial mapping techniques may especially prove to be useful in the discrimination between acute and convalescent myocarditis and healthy controls. This presumption is supported by a study from Hinojar et al in which native T1 relaxation times were an independent discriminator between patients with acute and convalescent myocarditis.18 Interestingly, a recent study by Lurz et al,7 in which the diagnostic performance of myocardial mapping techniques was compared with endomyocardial biopsy, found that especially myocardial T2 mapping is a useful diagnostic tool not only in patients presenting with acute, but also with chronic symptoms (sensitivity: 71%, specificity: 72%). In accordance with these findings, we also found myocardial T2 mapping to be the only reasonable parameter in diagnosing residual inflammation in patients with initial acute symptoms even at 2‐to‐4‐week follow‐up (sensitivity: 70%, specificity: 67%). Beyond this, our study results provided evidence that T2 mapping may also serve as a discriminator between different disease stages and healthy patients, as we could observe a consistent decrease in myocardial T2 relaxation time during intraindividual disease follow‐up. Furthermore, in the context of acute or convalescent myocarditis, T1 relaxation time should mainly be regarded as a marker of myocardial edema and not of myocardial fibrosis, as we observed a significant positive correlation between the change in ∆T1 and ∆T2 and a complete normalization of myocardial T1 values at the last follow‐up. Overall our findings suggest that myocardial mapping techniques are a far more sensitive technique in detecting myocardial edema compared to T2‐weighted imaging,16 and slightly elevated values can be found even late in the course of the disease. Thus, implementation of myocardial T1 and T2 mapping into routine protocols may be warranted, as both techniques might be especially useful for intraindividual disease monitoring.
Myocardial Hyperemia
Wagner et al described a significantly increased EGEr up to 14 days after onset of symptoms in patients with acute myocarditis.23 In our study we did observe a significant increase in EGEr at the baseline exam only, and no further increase was seen at follow‐up exams. Previous studies have reported a complete normalization of EGEr after 1 year.19, 23 According to our data, myocardial hyperemia represents another CMR marker of active inflammation in the acute stage of myocarditis; however, its usefulness beyond the acute stage is questionable. As early gadolinium enhancement imaging is time consuming, susceptible to artifacts, and offers only sparse diagnostic value, it is becoming more infrequently performed in myocarditis CMR.4, 5, 18, 21
Myocardial Necrosis and Fibrosis
Several CMR myocarditis follow‐up studies have described a persistence of nonischemic scar in 43% to 97% of all patients with convalescent myocarditis.19, 20, 24 In our study, the prevalence of LGE at the last follow‐up was 67%. This persistent high prevalence might be based on the healing process during which initial necrotic tissue is replaced by fibrosis.19, 24 In this regard, LGE should be mainly regarded as a marker of irreversible myocardial injury.20, 24 As LGE cannot differentiate between necrosis (active inflammation) and scar (healed inflammation), it cannot be used for the discrimination between acute and convalescent stages of the disease. Rather, the presence of LGE without any signs of active myocardial inflammation (eg, the absence of myocardial edema in T1 and T2 mapping) should be used for the discrimination of convalescent myocarditis and healthy patients.18 Although we observed no deaths during our study period, it is important to know that the presence of positive LGE is associated with a higher long‐term mortality.25 Interestingly, the total extent of LGE consistently decreases during the course of the disease, and at the last follow‐up there was only a difference of 2.4% between myocarditis patients and control subjects. This finding suggests that although nonischemic scars can be observed more frequently in patients with convalescent myocarditis, the total volume of fibrosis is relatively low. This assumption is supported by a nonsignificant difference of follow‐up extracellular volume fraction values between both groups, which are known to represent an indirect measure of diffuse interstitial myocardial fibrosis.26
Outcome and Clinical Implication
Thirteen percent of all patients had a CMR diagnosis of myocarditis according to the LLC at the last follow‐up. In addition, the positive LLC at the last follow‐up were associated with a lower EF. These results are comparable to the results of a study by Vermes et al, who also reported the persistence of LLC to be associated with a lower EF at follow‐up.24 Furthermore, baseline myocardial edema was described to correlate significantly with the increase of EF during the course of the disease.24 In our study we could reproduce these results by showing a significant correlation between ∆EF and baseline T1 and T2 relaxation times. These findings emphasize once more the importance of myocardial edema as an indicator of reversible myocardial injury, which can predict functional recovery.24, 27
Our findings furthermore suggest that CMR should be implemented as early as possible in the diagnostic work‐up of patients suspected of having myocarditis, since most CMR parameters showed significant alterations, especially at the early disease stage. Due to often nonspecific clinical symptoms and the standardized management pathways of acute coronary syndrome,28 CMR is currently often performed late during diagnostic work. In a clinical setting, repetitive CMR may have a strong impact on the follow‐up of patients as it offers various image techniques to detect a persistent and active myocardial inflammation. If intraindividual multiparametric CMR is unable to show a decrease of inflammatory parameters during follow‐up, ongoing myocarditis may be considered. Therefore, repetitive CMR in acute myocarditis may help to identify patients with persistent myocardial inflammation, who might require subsequent endomyocardial biopsy for a more specific therapeutic treatment.
Limitations
This study was performed by using clinical validation for patients suspected of having acute myocarditis, and a systematic endomyocardial biopsy as a reference standard was not performed. Instead, the presence of acute myocarditis was defined by combining typical clinical features, exclusion of coronary artery disease, and elevated biomarkers as reported previously in multiple CMR validation studies.4, 5, 13, 21 As follow‐up myocardial biopsy was not performed, we cannot exclude the development of a persistent inflammation/chronic myocarditis in some patients, which might have led to abnormal measurements for some parameters (eg, T2 relaxation times and LLC).
The statistical evaluation was mainly explorative, and no additional regression analysis was performed because of the limited sample size. Furthermore, we could not recruit each patient for every follow‐up examination, which might have influenced some results for the second and third follow‐up examinations. Most of the patients included in our study were of younger age and were admitted to our hospital because of persistent typical anginal chest pain suggestive of an acute myocardial infarction. Therefore, the results of this study are only valid for the described subgroup of patients with suspected myocarditis and caution must be exercised when transferring the results to patients with other clinical presentations.
Conclusions
In patients suspected of having acute myocarditis, a comprehensive quantitative CMR approach may serve as a useful tool to monitor disease activity. In order to reliably detect inflammatory alterations of the myocardium, CMR should be implemented early in the diagnostic cascade. Repetitive CMR has the potential to differentiate between active and convalescent myocarditis and may help to identify patients with persistent myocardial inflammation, who might require subsequent endomyocardial biopsy.
Disclosures
Stehning and Gieseke are employees of Philips Research (Hamburg, Germany).
Supporting information
Table S1. CMR Sequence Parameters
(J Am Heart Assoc. 2016;5:e003603 doi: 10.1161/JAHA.116.003603)
References
- 1. Drory Y, Turetz Y, Hiss Y, Lev B, Fisman EZ, Pines A, Kramer MR. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol. 1991;68:1388–1392. [DOI] [PubMed] [Google Scholar]
- 2. Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. [DOI] [PubMed] [Google Scholar]
- 3. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R; America EbtHFSo, Cardiology tHFAotESo . The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. [DOI] [PubMed] [Google Scholar]
- 4. Luetkens JA, Homsi R, Sprinkart AM, Doerner J, Dabir D, Kuetting DL, Block W, Andrie R, Stehning C, Fimmers R, Gieseke J, Thomas DK, Schild HH, Naehle CP. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging. 2016;17:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luetkens JA, Doerner J, Thomas DK, Dabir D, Gieseke J, Sprinkart AM, Fimmers R, Stehning C, Homsi R, Schwab JO, Schild H, Naehle CP. Acute myocarditis: multiparametric cardiac MR imaging. Radiology. 2014;273:383–392. [DOI] [PubMed] [Google Scholar]
- 6. Friedrich MG, Sechtem U, Schulz‐Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel‐Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P; International Consensus Group on Cardiovascular Magnetic Resonance in M . Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lurz P, Luecke C, Eitel I, Fohrenbach F, Frank C, Grothoff M, de Waha S, Rommel KP, Lurz JA, Klingel K, Kandolf R, Schuler G, Thiele H, Gutberlet M. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer‐Trial. J Am Coll Cardiol. 2016;67:1800–1811. [DOI] [PubMed] [Google Scholar]
- 8. Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. [DOI] [PubMed] [Google Scholar]
- 9. Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum. 1970;41:250–251. [Google Scholar]
- 10. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look‐Locker inversion recovery (MOLLI) for high‐resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. [DOI] [PubMed] [Google Scholar]
- 11. Sprinkart A, Luetkens J, Traber F, Doerner J, Gieseke J, Schnackenburg B, Schmitz G, Thomas D, Homsi R, Block W, Schild H, Naehle C. Gradient Spin Echo (GraSE) imaging for fast myocardial T2 mapping. J Cardiovasc Magn Reson. 2015;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdel‐Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz‐Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. [DOI] [PubMed] [Google Scholar]
- 14. Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of segment–freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial S, Registration for Cardiac I . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 16. Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S. Non‐contrast T1‐mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2‐weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monney PA, Sekhri N, Burchell T, Knight C, Davies C, Deaner A, Sheaf M, Baithun S, Petersen S, Wragg A, Jain A, Westwood M, Mills P, Mathur A, Mohiddin SA. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart. 2011;97:1312–1318. [DOI] [PubMed] [Google Scholar]
- 18. Hinojar R, Foote L, Arroyo Ucar E, Jackson T, Jabbour A, Yu CY, McCrohon J, Higgins DM, Carr‐White G, Mayr M, Nagel E, Puntmann VO. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging. 2015;8:37–46. [DOI] [PubMed] [Google Scholar]
- 19. Mavrogeni S, Spargias C, Bratis C, Kolovou G, Markussis V, Papadopoulou E, Constadoulakis P, Papadimitropoulos M, Douskou M, Pavlides G, Cokkinos D. Myocarditis as a precipitating factor for heart failure: evaluation and 1‐year follow‐up using cardiovascular magnetic resonance and endomyocardial biopsy. Eur J Heart Fail. 2011;13:830–837. [DOI] [PubMed] [Google Scholar]
- 20. Zagrosek A, Abdel‐Aty H, Boye P, Wassmuth R, Messroghli D, Utz W, Rudolph A, Bohl S, Dietz R, Schulz‐Menger J. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging. 2009;2:131–138. [DOI] [PubMed] [Google Scholar]
- 21. Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2‐weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. 2013;6:1048–1058. [DOI] [PubMed] [Google Scholar]
- 22. Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent‐onset heart failure. Circ Cardiovasc Imaging. 2015;8:e003073. [DOI] [PubMed] [Google Scholar]
- 23. Wagner A, Schulz‐Menger J, Dietz R, Friedrich MG. Long‐term follow‐up of patients with acute myocarditis by magnetic resonance imaging. MAGMA. 2003;16:17–20. [DOI] [PubMed] [Google Scholar]
- 24. Vermes E, Childs H, Faris P, Friedrich MG. Predictive value of CMR criteria for LV functional improvement in patients with acute myocarditis. Eur Heart J Cardiovasc Imaging. 2014;15:1140–1144. [DOI] [PubMed] [Google Scholar]
- 25. Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert E‐M, Hill S, Ong P, Klingel K, Kandolf R, Sechtem U, Mahrholdt H. Long‐term follow‐up of biopsy‐proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. [DOI] [PubMed] [Google Scholar]
- 26. de Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, Pasquet A, Vancraeynest D, Pouleur AC, Vanoverschelde JL, Gerber BL. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look‐Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson. 2015;17:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eitel I, von Knobelsdorff‐Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz‐Menger J, Thiele H, Friedrich MG. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. [DOI] [PubMed] [Google Scholar]
- 28. Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Hasdai D, Astin F, Åström‐Olsson K, Budaj A, Clemmensen P, Collet J‐P, Fox KA, Fuat A, Gustiene O, Hamm CW, Kala P, Lancellotti P, Maggioni AP, Merkely B, Neumann F‐J, Piepoli MF, Van de Werf F, Verheugt F, Wallentin L. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. The Task Force on the management of ST‐segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). European Heart Journal. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CMR Sequence Parameters


