Abstract
Background
Although patients with kidney disease have potential to benefit from revascularization, they are also at higher risk of complications, which may affect quality of life.
Methods and Results
We studied a cohort of 8198 adults who underwent coronary angiography in Alberta, between 2004 and 2008, and completed health‐related quality‐of‐life (HR‐QOL) surveys. Changes in HR‐QOL measures were most favorable among patients who received coronary artery bypass graft (CABG), but did not significantly differ by kidney function within groups of patients who received CABG, percutaneous coronary intervention (PCI), or medical therapy (P value for interaction between estimated glomerular filtration rate [eGFR] and revascularization status >0.10 for all outcomes). Among those who received CABG, the adjusted mean EuroQol 5 dimensions (EQ‐5D) utility score for those with eGFR >90 mL/min per 1.73 m2 increased by 0.11 (95% CI, 0.09–0.14) and for those with eGFR <30 mL/min per 1.73m2 by 0.13 (95% CI, 0.05–0.21). The adjusted mean EQ‐5D utility score also increased similarly at all levels of eGFR for those who received PCI and for those who received medical management. Mean changes in Seattle Angina Questionnaire (SAQ) scores were also similar across all levels of eGFR within each treatment group for the quality of life, angina frequency, angina stability, physical limitations, and treatment satisfaction domains of the SAQ. Among those who received CABG, the adjusted mean SAQ quality of life score for those with eGFR >90 mL/min per 1.73m2 increased by 22.1 (95% CI, 18.5–25.7) and for those with eGFR <30 mL/min per 1.73m2 by 14.0 (95% CI, 2.31–25.63).
Conclusions
Changes in HR‐QOL do not vary by kidney function among patients selected for CABG, PCI, or medical management of coronary disease.
Keywords: chronic kidney disease, coronary disease, health‐related quality of life, kidney, revascularization
Subject Categories: Quality and Outcomes, Coronary Artery Disease, Revascularization
Introduction
Chronic kidney disease (CKD), defined by reduced estimated glomerular filtration rate (eGFR), affects more than 1 in 10 adults, and more than 1 in 5 North Americans aged 65 years or older.1 Coronary artery disease increases in prevalence with lower eGFR2 and is the leading cause of mortality in patients with CKD.3, 4 Among patients referred for coronary angiography, those with CKD have a higher prevalence of severe coronary artery disease,5 often have significant coronary artery lesions amenable to angioplasty,6, 7 and more frequently have high‐risk coronary anatomy, including left main or 3‐vessel coronary disease, compared to those without CKD.8, 9, 10 These observations suggest that coronary revascularization has the potential to improve outcomes of patients with CKD, including symptoms of coronary artery disease.
Nonetheless, patients with CKD are less likely to receive invasive management of coronary artery disease.11, 12, 13 The presence of CKD may discourage patients and clinicians from pursuing coronary revascularization because of the higher risk of complications after revascularization procedures, including prolonged mechanical ventilation, bleeding, stroke, and acute kidney injury.14, 15 For example, 10% to 30% of patients with preexisting CKD may experience acute kidney injury after percutaneous or surgical coronary revascularization,16, 17 which may, in turn, further increase the risk of acute dialysis18 or progression to end‐stage renal disease (ESRD) requiring chronic renal replacement therapy.19, 20, 21 Given the dramatic effects that these complications can have on health status,22, 23 the potential associations between kidney function and changes in quality of life after coronary revascularization are clinically relevant to treatment decisions.
We used data from a large registry of patients referred for coronary angiography to examine the associations between coronary artery disease management strategies, kidney function, and changes in coronary artery disease symptoms and quality of life. Given the higher risk of complications associated with invasive coronary procedures in people with CKD, we hypothesized that improvement in health‐related quality‐of‐life (HR‐QOL) after percutaneous or surgical coronary revascularization would be less impressive among people with lower eGFR than in those with normal kidney function.
Methods
Study Population
We derived the study cohort from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH). APPROACH prospectively collects data on demographic and clinical characteristics on all patients undergoing coronary angiography in the province of Alberta, Canada.24 Individuals in the APPROACH registry are followed longitudinally after angiography, thus allowing for assessment of subsequent procedures (ie, percutaneous coronary intervention [PCI] or coronary artery bypass graft surgery [CABG]), as well as quality of life in patients who consent to follow‐up. All‐cause mortality is determined for all patients in APPROACH by linkage to provincial vital statistics.
The cohort consisted of all Alberta residents, ≥18 years of age, who received coronary angiography from January 1, 2004 to December 31, 2008 and who consented in APPROACH to be contacted for follow‐up. Eligible participants required at least 1 outpatient serum creatinine measurement within a 6‐month period before coronary angiography. Patients with a renal transplant or who were receiving dialysis before coronary angiography were excluded based on the Northern and Southern Alberta Renal Program registries.25
Measurement of Kidney Function and Coronary Revascularization
We obtained all serum creatinine measurements made in Alberta from the Alberta Kidney Disease Network repository of laboratory data.26 Baseline kidney function was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation to estimate glomerular filtration rate (eGFR).27 To reduce between‐laboratory variation, we standardized creatinine measurements across provincial laboratories to an isotope dilution mass spectrometry reference standard and applied a laboratory‐specific correction factor where necessary. We categorized the index eGFR as 90 or higher, 60 to 89, 45 to 59, 30 to 44, and <30 mL/min per 1.73 m2 based on the last outpatient creatinine measurement made within 6 months preceding the index coronary angiogram.
We identified subsequent receipt of coronary revascularization procedures (CABG, PCI, and none/medical management) preceding the 1 year follow‐up survey after the index coronary angiogram from the APPROACH database.24
Measurement of Quality of Life
Eligible and consenting patients received baseline and 1‐year follow‐up quality‐of‐life questionnaires within 1 week of the initial angiogram and 1 year later, respectively.28 The questionnaire included the EuroQol (EQ‐5D), a generic 5‐item HR‐QOL instrument,29, 30 and the Seattle Angina Questionnaire (SAQ), a 19‐item disease‐specific HR‐QOL instrument.31, 32
The EQ‐5D is a generic scale for measuring HR‐QOL over the past 4 weeks. It expresses health status using a single index score (range, 0–1) based on societal based utility theory.29 The EQ‐5D covers 5 dimensions of health, including mobility, self‐care, family and leisure activities, pain, and mood.30 A unique health state is defined by combining 1 level from each of the dimensions. The EQ‐5D is a reliable instrument for measuring HR‐QOL (intraclass correlation coefficient of 0.91 from a study of patients with acute coronary syndrome) and a minimal increment of 0.03 is considered a clinically important difference.29, 30, 31 The SAQ is a 19‐item self‐administered questionnaire on 5 dimensions of HR‐QOL assessed over the past 4 weeks. Five dimensions of coronary artery disease are measured, generating 5 independent scales, including quality of life, angina frequency, anginal stability, physical limitation, and treatment satisfaction. The SAQ has been shown to be a valid, responsive, and reliable instrument.32, 33 The SAQ is scored by assigning each response an ordinal value, beginning with 1 for the response that implies the lowest level of functioning and summing across items within each of the 5 dimensional scales. Scale scores are then transformed to a 0 to 100 range by subtracting the lowest possible score, dividing by the range of the scale, and multiplying by 100. With the exception of the angina stability dimension, the reproducibility of the quality of life, angina frequency, physical limitations, and treatment satisfaction dimensions of the SAQ is high (intraclass correlation coefficients, 0.76–0.83), and it is sensitive to changes accompanying coronary revascularization.32, 33
Measurement of Covariates
Age, sex, comorbidities, anatomical distribution of coronary artery disease, and left ventricular ejection fraction were collected in the APPROACH registry at the time of the index coronary angiogram. The 6‐digit residential postal code for each participant was linked to the 2001 or 2006 Canadian Census (whichever was closest to the index date) using the postal code conversion file to determine median neighborhood household income quintile and rural/urban location of residence.
Statistical Analysis
We compared baseline characteristics according to the eGFR categories described earlier and by revascularization status (CABG, PCI, or medical management) using ANOVA and chi‐squared tests for continuous and categorical variables, respectively. We then fit six separate models (one for the EQ‐5D and one for each of the 5 SAQ domains) using ANCOVA to examine the relationships between eGFR, revascularization, and the change in HR‐QOL at 1 year. These models used each of the 1‐year change in HR‐QOL measurements as the outcome and included the respective baseline HR‐QOL measurements as an independent variable. We included variables for eGFR categories and revascularization status and also included an interaction term between these terms to test whether associations between revascularization and HR‐QOL were modified by eGFR. All models were also adjusted for age, sex, comorbidities (diabetes mellitus, hypertension, chronic pulmonary disease, cerebrovascular disease, heart failure, hyperlipidemia, malignancy, and peripheral vascular disease) distribution of coronary artery disease, left ventricular systolic function, smoking status, residence location, and income quintile as independent variables. Individual missing SAQ domain or EQ‐5D measurements at 1‐year follow‐up for surviving patients were assumed missing at random and imputed using multiple partial imputation.34 Sensitivity analyses were performed repeating the modeling using a complete case analysis for each HR‐QOL measurement. The assumptions of normality and homoskedasticity of ANCOVA were tested and satisfied. All statistical analyses were conducted using SAS (version 9.3; SAS Institute Inc., Cary, NC) and R software (version 3.0; The R Project for Statistical Computing; www.r-project.org). The conjoint health research ethics board of the University of Calgary (Calgary, Alberta, Canada) approved the study, and all participants gave informed consent.
Results
Cohort Formation and Baseline Characteristics
We identified 8384 Alberta residents who were 18 years of age or older, received coronary angiography during the cohort entry period, and completed at least 1 HRQOL survey. We excluded 31 without a creatinine measurement within 3 months before coronary angiography, and 45 with ESRD receiving renal replacement therapy before coronary angiography, and 110 who died within 1 year of the initial angiogram.
Of the 8198 participants included in the final cohort, 1574 (19.2%) received CABG, 4642 (56.5%) PCI, and 1982 (24.2%) medical management alone. Table 1 presents patient characteristics in these 3 treatment groups according to baseline eGFR, and reveals that participants who received CABG were more likely to have high risk (3‐vessel disease or 2‐vessel with proximal left anterior descending artery disease) or left main coronary artery disease, whereas medically treated patients were more likely to have cerebrovascular disease, peripheral vascular disease, and heart failure, particularly among those with an eGFR <30 mL/min per 1.73 m2. Within each treatment group, participants with lower eGFR were older, were more likely to have comorbidities (including diabetes mellitus, hypertension, and heart failure), and reduced left ventricular ejection fraction.
Table 1.
Baseline Characteristics of the Cohort
| CABG | PCI | Medical Therapy | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFR | eGFR | eGFR | |||||||||||||
| ≥90 | 60 to 89 | 45 to 60 | 30 to 44 | <30 | ≥90 | 60 to 89 | 45 to 60 | 30 to 44 | <30 | ≥90 | 60 to 89 | 45 to 60 | 30 to 44 | <30 | |
| n=316 | n=916 | n=248 | n=77 | n=17 | n=1134 | n=2718 | n=589 | n=169 | n=32 | n=388 | n=1088 | n=331 | n=131 | n=44 | |
| Age, y, mean (SD) | 57.56 (7.75) | 67.02 (8.81) | 72.38 (8.38) | 74.29 (7.3) | 68.38 (9.81) | 54.37 (8.08) | 63.85 (10.28) | 70.88 (8.86) | 75.27 (8.29) | 73.9 (10.34) | 56.34 (8.06) | 66.78 (9.72) | 73.53 (8.26) | 74.84 (8.07) | 76.1 (9.12) |
| Male, % | 86.08 | 85.26 | 81.45 | 75.32 | 64.71 | 81.22 | 78.37 | 68.42 | 62.13 | 40.63 | 77.06 | 76.75 | 69.79 | 62.6 | 54.55 |
| Diabetes mellitus, % | 32.28 | 24.89 | 28.63 | 42.86 | 64.71 | 20.9 | 17.22 | 21.22 | 24.85 | 37.5 | 24.23 | 26.84 | 33.84 | 35.11 | 56.82 |
| Hypertension, % | 74.05 | 74.24 | 83.06 | 94.81 | 94.12 | 58.99 | 65.78 | 73.01 | 79.88 | 84.38 | 65.72 | 73.44 | 77.04 | 88.55 | 84.09 |
| Left ventricular ejection fraction, % | |||||||||||||||
| >50% | 63.92 | 60.59 | 56.85 | 44.16 | 5.88 | 67.2 | 67.03 | 60.44 | 52.07 | 43.75 | 67.53 | 63.33 | 57.7 | 43.51 | 22.73 |
| 35% to 50% | 22.47 | 20.31 | 16.53 | 19.48 | 5.88 | 18.25 | 17.95 | 18.17 | 11.24 | 25 | 17.78 | 16.45 | 17.52 | 16.03 | 13.64 |
| 20% to 34% | 3.16 | 4.91 | 4.44 | 1.3 | 5.88 | 2.29 | 2.61 | 3.9 | 4.14 | 0 | 4.64 | 4.96 | 8.16 | 6.11 | 4.55 |
| <20% | 0.32 | 0.44 | 0.81 | 2.6 | 5.88 | 0.26 | 0.37 | 0.68 | 1.18 | 0 | 0.52 | 2.11 | 1.21 | 3.82 | 2.27 |
| Unmeasured | 10.13 | 13.76 | 21.37 | 32.47 | 76.47 | 11.99 | 12.03 | 16.81 | 31.36 | 31.25 | 9.54 | 13.14 | 15.41 | 30.53 | 56.82 |
| Chronic pulmonary disease, % | 9.81 | 14.74 | 16.94 | 24.68 | 29.41 | 11.02 | 10.34 | 14.26 | 17.75 | 28.13 | 19.07 | 16.45 | 19.94 | 22.9 | 22.73 |
| Cerebrovascular disease, % | 4.75 | 6.44 | 9.27 | 18.18 | 5.88 | 1.68 | 4.23 | 8.66 | 8.28 | 12.5 | 4.9 | 6.62 | 9.67 | 12.21 | 20.45 |
| Heart failure, % | 6.33 | 8.3 | 12.5 | 23.38 | 17.65 | 4.14 | 4.53 | 8.49 | 18.93 | 21.88 | 8.51 | 12.78 | 18.43 | 27.48 | 47.73 |
| Hyperlipidemia, % | 87.97 | 84.72 | 81.45 | 77.92 | 94.12 | 78.66 | 78.88 | 81.49 | 80.47 | 84.38 | 81.44 | 79.6 | 77.04 | 79.39 | 68.18 |
| Malignancy, % | 4.43 | 4.91 | 4.44 | 5.19 | 5.88 | 1.59 | 3.97 | 4.92 | 7.1 | 3.13 | 3.87 | 4.5 | 6.95 | 7.63 | 9.09 |
| Peripheral vascular disease, % | 6.33 | 8.52 | 13.31 | 16.88 | 5.88 | 3.26 | 4.27 | 6.62 | 10.65 | 3.13 | 4.64 | 8.27 | 8.76 | 16.79 | 29.55 |
| Coronary vascular disease, % | |||||||||||||||
| Normal/minimal/low risk | 9.81 | 12.99 | 13.71 | 11.69 | 11.76 | 71.34 | 67.81 | 63.67 | 60.36 | 50 | 77.84 | 68.38 | 61.03 | 56.49 | 50 |
| High risk | 57.59 | 54.69 | 55.24 | 44.16 | 52.94 | 27.51 | 30.17 | 33.96 | 36.69 | 46.88 | 20.1 | 27.02 | 34.14 | 32.06 | 38.64 |
| Left main | 32.59 | 32.31 | 31.05 | 44.16 | 35.29 | 1.15 | 2.02 | 2.38 | 2.96 | 3.13 | 2.06 | 4.6 | 4.83 | 11.45 | 11.36 |
| CCS grading of angina | |||||||||||||||
| 0 none | 2.85 | 4.69 | 5.65 | 2.6 | 11.76 | 0.97 | 0.85 | 2.04 | 2.37 | 0 | 3.35 | 4.04 | 6.04 | 5.34 | 6.82 |
| I strenuous | 5.7 | 5.13 | 6.85 | 2.6 | 0 | 1.85 | 2.1 | 2.21 | 2.96 | 3.13 | 7.99 | 7.63 | 5.44 | 6.11 | 9.09 |
| II slight limit on norm act | 31.33 | 33.73 | 33.87 | 28.57 | 17.65 | 15.7 | 18.29 | 16.81 | 19.53 | 12.5 | 24.48 | 27.76 | 32.93 | 29.01 | 11.36 |
| III marked limit on normal | 14.56 | 18.56 | 17.74 | 16.88 | 29.41 | 7.32 | 10.74 | 11.38 | 12.43 | 0 | 11.34 | 11.95 | 13.29 | 10.69 | 22.73 |
| IV unstable angina/ACS | 40.19 | 32.65 | 29.44 | 42.86 | 23.53 | 71.34 | 64.97 | 62.31 | 60.36 | 81.25 | 48.45 | 41.27 | 35.35 | 39.69 | 47.73 |
| Atypical | 2.22 | 0.87 | 0.81 | 2.6 | 5.88 | 0.79 | 0.63 | 0.34 | 0.59 | 3.13 | 2.32 | 1.56 | 1.21 | 0.76 | 2.27 |
| Missing | 3.16 | 4.37 | 5.65 | 3.9 | 11.76 | 2.03 | 2.43 | 4.92 | 1.78 | 0 | 2.06 | 5.79 | 5.74 | 8.4 | 0 |
| Current smoker, % | 30.06 | 16.38 | 9.27 | 12.99 | 11.76 | 43.03 | 23.55 | 15.62 | 12.43 | 18.75 | 39.69 | 17.92 | 12.99 | 9.16 | 9.09 |
| Urban residents, % | 77.53 | 77.95 | 80.24 | 79.22 | 82.35 | 77.34 | 78.4 | 74.53 | 77.51 | 78.13 | 78.35 | 81.43 | 79.76 | 79.39 | 81.82 |
| Income quintile, % | |||||||||||||||
| 1 (lowest) | 19.62 | 14.63 | 15.32 | 6.49 | 35.29 | 15.96 | 14.2 | 16.98 | 18.34 | 18.75 | 17.78 | 17.19 | 18.13 | 19.08 | 20.45 |
| 2 | 11.71 | 14.85 | 19.76 | 29.87 | 5.88 | 18.43 | 17.18 | 18.34 | 14.2 | 21.88 | 17.01 | 16.64 | 20.24 | 20.61 | 15.91 |
| 3 | 16.77 | 20.74 | 20.97 | 19.48 | 23.53 | 20.02 | 18.98 | 21.9 | 19.53 | 21.88 | 20.88 | 19.21 | 22.66 | 20.61 | 6.82 |
| 4 | 26.27 | 21.51 | 18.15 | 15.58 | 11.76 | 20.46 | 22.26 | 17.49 | 22.49 | 18.75 | 21.13 | 20.13 | 16.62 | 17.56 | 22.73 |
| 5 (highest) | 19.62 | 23.69 | 24.6 | 24.68 | 23.53 | 19.93 | 22.44 | 20.03 | 18.93 | 18.75 | 17.27 | 21.6 | 19.03 | 19.85 | 29.55 |
| Missing | 6.01 | 4.59 | 1.21 | 3.9 | 0 | 5.2 | 4.93 | 5.26 | 6.51 | 0 | 5.93 | 5.24 | 3.32 | 2.29 | 4.55 |
ACS indicates acute coronary syndrome; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate (mL/min per 1.73 m2); PCI, percutaneous coronary intervention. Coronary artery disease: minimal/low risk=single‐ or 2‐vessel disease; high risk=3‐vessel disease or 2‐vessel with proximal left anterior descending artery disease. CCS (Canadian Cardiovascular Society) grading of angina: 0=none; I=angina with strenuous activity; II=angina with slight limit on normal activity; III=angina with marked limitation on normal activity; IV=angina with any physical activity or at rest; V=atypical angina.
During the 1‐year follow‐up, 110 patients in the cohort died. Sixteen patients progressed to end‐stage kidney disease, the majority of whom (56%) had a baseline eGFR <30 mL/min per 1.73 m2. A total of 4685 members of the full cohort (58.5%) completed 1‐year follow‐up HR‐QOL surveys.
Revascularization Status and Change in HR‐QOL
Compared to participants who received CABG, the adjusted changes in all HR‐QOL measures at 1 year were lower for those who received PCI (P<0.001 for all outcomes) and those who received medical management (P<0.001 for all HR‐QOL outcomes). For example, in models adjusted for all covariates including baseline eGFR, the EQ‐5D utility score improved over 1 year in all treatment groups; however, the mean change in the EQ‐5D score of those who received PCI was 0.076 (95% CI, 0.062–0.090) lower than that of those who received CABG and 0.095 (95% CI, 0.081–0.109) lower for those who received medical therapy alone than for those who received CABG.
Similarly, compared to those who received CABG, the adjusted mean change in the SAQ quality of life domain score was 16.9 (95% CI, 14.4–19.4) lower for those who received PCI and 20.4 (95% CI, 17.9–22.9) lower for those who received medical therapy alone. Compared to those who received CABG, mean changes in SAQ scores were also lower for those who received PCI, and medical management in the domains of angina frequency, and physical limitation, whereas SAQ domain scores for angina stability and treatment satisfaction did not improve for patients who received PCI or medical management. Findings were similar in complete case analyses, excluding individuals with missing follow‐up measurements.
Revascularization and Change in HR‐QOL Stratified by Kidney Function
Changes in HR‐QOL measures did not significantly differ by baseline eGFR within strata defined by receipt of CABG, PCI, or medical therapy (P value for interaction between eGFR and revascularization status >0.10 for all outcomes). For example, among those who received CABG, the adjusted mean EQ‐5D utility score for those with eGFR >90 mL/min per 1.73 m2 increased by 0.11 (95% CI, 0.09–0.14) and for those with eGFR <30 mL/min per 1.73 m2 by 0.13 (95% CI, 0.05–0.21; Figure 1). Changes in the adjusted mean EQ‐5D utility score increased similarly at all levels of eGFR for those who received PCI and for those who received medical management (Figure 1).
Figure 1.
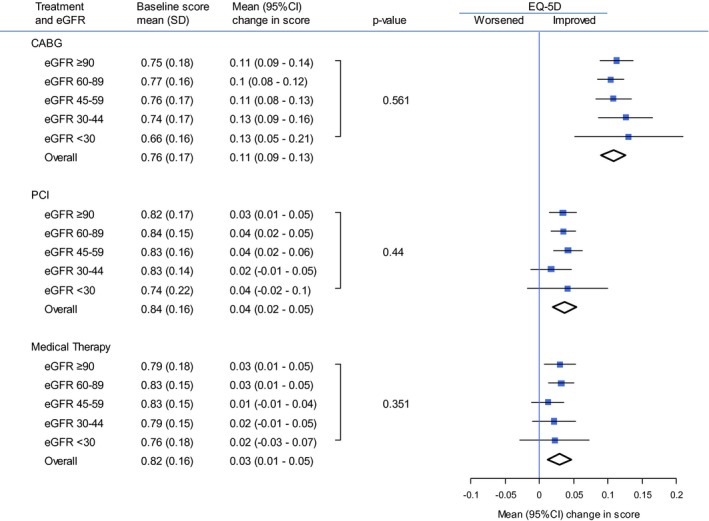
Mean baseline and change in EQ‐5D scores, stratified by treatment and eGFR. CABG indicates coronary artery bypass graft; eGFR, estimated glomerular filtration rate (mL/min per 1.73 m2); EQ‐5D, EuroQol 5 dimensions; PCI, percutaneous coronary intervention.
Similarly, among those who received CABG, the adjusted mean SAQ quality‐of‐life domain score increased for those with eGFR >90 mL/min per 1.73 m2 by 22.1 (95% CI, 18.5–25.7) and for those with eGFR <30 mL/min per 1.73 m2 by 14.0 (2.3–25.6; Figure 2). As for the EQ‐5D, the adjusted mean SAQ quality‐of‐life domain score for those who received PCI increased similarly at all levels of eGFR, and for those who received medical management it changed similarly at all levels of eGFR (Figure 2). Mean changes in the SAQ scores were also similar across all levels of eGFR within each treatment group (CABG, PCI, and medical management) for the angina frequency, angina stability, physical limitations, and treatment satisfaction domains of the SAQ (Figure 2; Table 2). Sensitivity analyses excluding individuals with missing follow‐up measurements produced similar findings, with no evidence of effect modification by eGFR on estimates of changes in EQ‐5D or SAQ domain scores according to treatment group.
Figure 2.
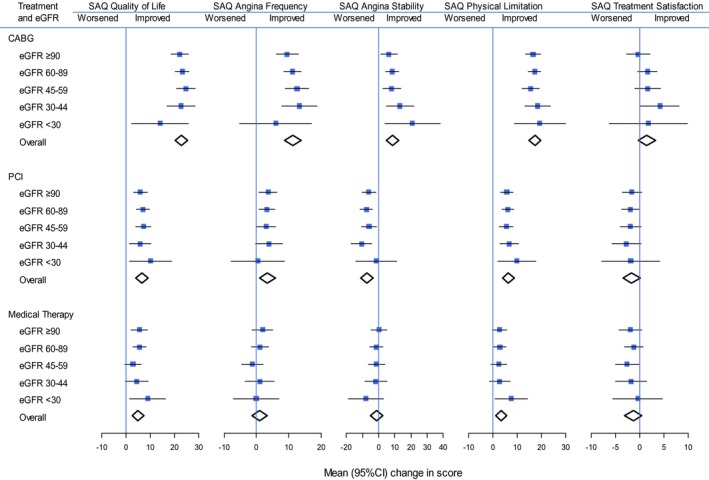
Mean changes in SAQ quality of life, angina frequency, angina stability, physical limitation, and treatment satisfaction scores, stratified by treatment and eGFR. CABG indicates coronary artery bypass graft; eGFR, estimated glomerular filtration rate (mL/min per 1.73 m2); PCI, percutaneous coronary intervention; SAQ, Seattle Angina Questionnaire.
Table 2.
Mean Baseline and Change in SAQ Scores, Stratified by Treatment and eGFR
| Treatment and eGFR | SAQ Domain | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quality of Life | Angina Frequency | Angina Stability | Physical Limitation | Treatment Satisfaction | |||||||||||
| Baseline Score, Mean (SD) | Mean (95% CI) Change in Score | P Value | Baseline Score, Mean (SD) | Mean (95% CI) Change in Score | P Value | Baseline Score, Mean (SD) | Mean (95% CI) Change in Score | P Value | Baseline Score, Mean (SD) | Mean (95% CI) Change in Score | P Value | Baseline Score, Mean (SD) | Mean (95% CI) Change in Score | P Value | |
| CABG | |||||||||||||||
| eGFR ≥90 | 51.2 (26.2) | 22.1 (18.5–25.7) | 0.38 | 78.0 (25.4) | 9.5 (6.0–12.9) | 0.39 | 64.0 (31.5) | 6.4 (1.1–11.6) | 0.32 | 64.4 (24.2) | 16.6 (13.3–19.8) | 0.57 | 88.2 (14.8) | −0.3 (−2.7 to 2.2) | 0.22 |
| eGFR 60 to 89 | 54.2 (26.4) | 23.1 (20.3–25.9) | 77.6 (25.0) | 11.1 (8.4–13.8) | 62.4 (30.7) | 8.5 (4.3–12.6) | 63.1 (23.2) | 17.2 (14.7–19.8) | 87.7 (16.2) | 1.6 (−0.3 to 3.6) | |||||
| eGFR 45 to 59 | 55.1 (27.1) | 24.6 (20.8–28.4) | 76.9 (25.9) | 12.5 (8.9–16.2) | 60.5 (27.7) | 8 (2.4–13.6) | 60.5 (23.8) | 15.6 (12.1–19.0) | 87.4 (15.1) | 1.7 (−1.0 to 4.3) | |||||
| eGFR 30 to 44 | 56.2 (24.8) | 22.6 (16.8–28.4) | 77.9 (26.9) | 13.3 (7.8–18.8) | 61.0 (29.2) | 13.2 (4.7–21.7) | 54.7 (25.2) | 18.5 (13.2–23.7) | 86 (16.1) | 4.3 (0.3–8.3) | |||||
| eGFR <30 | 58.2 (28.1) | 14.0 (2.3–25.6) | 79.1 (26.9) | 5.9 (−5.2 to 17.1) | 50 (34.4) | 20.8 (3.8–27.9) | 41.7 (17.4) | 19.3 (8.8–29.9) | 84.2 (18.6) | 1.8 (−6.2 to 9.9) | |||||
| Overall | 53.9 (26.5) | 22.8 (20.2–25.4) | 77.6 (25.37) | 11.3 (8.8–13.8) | 62.3 (30.4) | 8.4 (4.5–12.2) | 62.3 (23.8) | 17.4 (15.0–19.8) | 87.0 (15.8) | 1.5 (−0.3 to 3.3) | |||||
| PCI | |||||||||||||||
| eGFR ≥90 | 64.4 (24.3) | 5.8 (2.9–8.7) | 0.51 | 82.0 (22.2) | 3.6 (0.8–6.4) | 0.86 | 76.8 (29.5) | −6.2 (−10.4, −1.9) | 0.31 | 76.5 (22.0) | 5.7 (3.1–8.3) | 0.61 | 87.5 (15.5) | −1.6 (−3.6 to 0.5) | 0.83 |
| eGFR 60 to 89 | 67.3 (23.1) | 7.0 (4.3–9.6) | 84.2 (21.5) | 3.2 (0.7–5.8) | 78.7 (27.2) | −7.7 (−11.6, −3.8) | 75.7 (23.1) | 6.1 (3.7–8.5) | 89.0 (14.7) | −1.8 (−3.7 to 0.0) | |||||
| eGFR 45 to 59 | 68.6 (23.2) | 7.2 (4.1–10.3) | 83.9 (23.0) | 2.9 (−0.1 to 5.9) | 75 (28.1) | −6.0 (−10.6, −1.5) | 71.9 (24.1) | 5.5 (2.7–8.3) | 89.5 (14.1) | −1.8 (−4.0 to 0.3) | |||||
| eGFR 30 to 44 | 68.8 (23.2) | 5.8 (1.5–10.2) | 82.8 (24.0) | 3.8 (−0.3 to 8.0) | 79.0 (27.5) | −10.7 (−17.0, −4.3) | 63.3 (24.7) | 6.8 (2.8–10.7) | 88.7 (14.2) | −2.7 (−5.7 to 0.3) | |||||
| eGFR <30 | 63.7 (26.4) | 10.0 (1.4–18.7) | 87.2 (19.9) | 0.4 (−7.9 to 8.7) | 77.3 (26.1) | −1.5 (−14.2, 11.2) | 56.8 (25.0) | 9.8 (2.0–17.7) | 91.2 (13.4) | −1.8 (−7.8 to 4.2) | |||||
| Overall | 66.8 (23.5) | 6.6 (4.0–9.1) | 83.6 (22.0) | 3.4 (1.0–5.8) | 77.8 (27.9) | −7.3 (−11.0 to −3.6) | 74.9 (23.3) | 6.4 (4.1–8.7) | 88.7 (14.8) | −1.6 (−3.7 to 0.1) | |||||
| Medicine | |||||||||||||||
| eGFR ≥90 | 60.5 (24.1) | 5.4 (2.0–8.8) | 0.28 | 79.6 (22.7) | 1.9 (−1.4 to 5.1) | 0.43 | 64.9 (27.0) | 0.2 (−4.8 to 5.2) | 0.59 | 72.4 (23.8) | 2.7 (−0.4 to 5.8) | 0.5 | 83.4 (18.1) | −1.8 (−4.2 to 0.5) | 0.58 |
| eGFR 60 to 89 | 66.2 (23.4) | 5.5 (2.8–8.3) | 83.1 (21.4) | 1.1 (−1.6 to 3.7) | 66.5 (26.8) | −1.4 (−5.4 to 2.7) | 71.7 (23.8) | 2.9 (0.4–5.4) | 85 (16.9) | −1.1 (−3.0 to 0.8) | |||||
| eGFR 45 to 59 | 67.9 (23.1) | 2.8 (−0.7 to 6.3) | 83.9 (20.9) | −1.2 (−4.6 to 2.1) | 63.9 (25.9) | −1.4 (−6.5 to 3.8) | 66.9 (24.6) | 2.4 (−0.8 to 5.6) | 86.3 (15.3) | −2.6 (−5.0, −0.1) | |||||
| eGFR 30 to 44 | 65.7 (23.3) | 4.4 (−0.3 to 9.1) | 79.5 (23.4) | 1 (−3.4 to 5.5) | 60.9 (28.67) | −1.9 (−8.8 to 4.9) | 62.4 (22.5) | 2.8 (−1.4 to 7.1) | 85.8 (14.7) | −1.8 (−5.0, 1.5) | |||||
| eGFR <30 | 62.0 (23.8) | 8.9 (1.4–16.3) | 82.7 (24.0) | −0.1 (−7.2 to 7.0) | 70.0 (25.3) | −7.9 (−18.8 to 2.9) | 54.0 (27..0) | 7.5 (0.8–14.2) | 85.0 (16.3) | −0.3 (−5.4 to 4.8) | |||||
| Overall | 65.3 (23.6) | 4.9 (2.4–7.5) | 82.3 (21.9) | 0.9 (−1.5 to 3.4) | 65.5 (26.8) | −1.3 (−5.0 to 2.5) | 70.0 (24.2) | 3.5 (1.2–5.8) | 85.0 (16.8) | −1.3 (−3.0 to 0.5) | |||||
eGFR indicates estimated glomerular filtration rate (mL/min per 1.73m2); SAQ, Seattle Angina Questionnaire.
Discussion
In this large cohort of patients with coronary artery disease who completed HR‐QOL questionnaires, we found that level of kidney function did not appear to modify the quality‐of‐life improvements associated with coronary revascularization. Specifically, within treatment groups defined by CABG, PCI, or medical therapy alone, changes in both general and coronary artery disease–specific HR‐QOL remained consistent across a broad range of underlying kidney function. These findings provide evidence that reduced kidney function should not dissuade otherwise suitable candidates from coronary revascularization, when other clinical consideration indicate the potential to benefit.
Randomized trials published more than 30 years ago first showed improvements in short‐ and long‐term measures of health status among patients who received CABG.10 More recently, trials comparing PCI with medical management in patients with stable coronary artery disease reported incremental improvements for those treated with PCI, across all domains of the SAQ, although differences became attenuated by 36 months.35, 36 Similarly, trials enrolling patients with acute coronary syndrome have shown higher generic and disease‐specific quality‐of‐life scores at 1 year for patients who received invasive compared with conservative management.37, 38 However, because patients with abnormal kidney function were excluded from most of these trials, it has been unclear whether these benefits of revascularization on HR‐QOL are generalizable to people with CKD.
There is limited information about whether kidney function modifies changes in health status associated with coronary revascularization. In a systematic search of the literature, we identified only 2 other published studies addressing this question.39, 40 In the first study, Parikh et al. followed 1160 patients, all of whom received CABG and completed the 36‐Item Short Form Survey (SF‐36) generic HR‐QOL tool.39 They found that people with the lowest levels of kidney function (eGFR <30 mL/min per 1.73 m2) reported lower average levels of physical functioning 6 months after CABG; however, changes in HR‐QOL with other treatment strategies were not included nor were coronary artery disease–specific HR‐QOL measures assessed in the study. A more recent secondary analysis of results from the COURAGE trial compared health status measures of patients with and without CKD (defined as eGFR <60 mL/min per 1.73 m2) randomized to either PCI plus optimal medical therapy versus optimal medical therapy alone.40 Mean SAQ scores for quality of life, angina frequency, and physical limitation significantly improved in both patients with and without CKD, and early improvement was more common in patients treated with PCI and optimal medical therapy than optimal medical therapy alone in both patients with and without CKD.
Our study adds to the findings from the COURAGE trial and provides additional, real‐world information about changes in quality‐of‐life measures across a broader range of kidney function with both surgical and percutaneous revascularization strategies. Importantly, our results show that improvements in generic and coronary artery disease–specific quality of life after revascularization were consistent across all levels of eGFR. In contrast to the findings of Parikh et al., we did not find evidence that changes in physical functioning or a generic HR‐QOL measure differed with low eGFR for any treatment strategy, although it remains possible that differences in the selection of participants in these cohorts, or the use of different HR‐QOL instruments, could underlie this inconsistency.
These findings are important because people with CKD are less likely to receive invasive coronary procedures, including revascularization, despite their high‐risk status.13, 41 These differences cannot be fully explained by differences in clinical severity of disease or predicted risks,11, 12 suggesting there may be aversion to pursuing revascularization approaches in patients with CKD attributed to concerns over risk of harm or lack of effectiveness. Wong et al. previously found that the most common reasons for avoiding invasive management of patients with CKD were attributed to physicians deeming patients as being not at high enough risk to warrant invasive management, or that the approach was not supported by evidence.42 Our findings show that patients with CKD can experience similar improvements in coronary disease–related symptoms and general HR‐QOL to those of patients without CKD who are selected for PCI or CABG.
There are limitations to this study. First, participant selection was limited to patients who completed baseline and follow‐up HRQOL questionnaires. Patients who received revascularization procedures were more likely to complete HR‐QOL questionnaires, and selection bias could be introduced if the relationships between kidney function, treatment strategy, and HRQOL measures differed among patients who responded to the surveys from those who did not respond. Second, because this was an observational study, there may be residual confounding attributed to treatment‐by‐indication bias, despite our best attempts at adjustment for important covariates. Third, changes in HR‐QOL were measured over 1 year of follow‐up, and these improvements may be attenuated over longer time periods. Finally, the total number of patients with eGFR <30 mL/min per 1.73 m2 included in our study remained small, the variance of estimates within this group was relatively large, and we did not include patients with kidney failure receiving dialysis at cohort entry in our study. Thus, although our study provides more information about most patients with CKD (including advanced forms of CKD) than past studies, there is still uncertainty among estimates of HR‐QOL in patients receiving dialysis. Additional studies with larger sample sizes of patients with very low levels of kidney function are required to better characterize the outcomes of such patients.
In conclusion, we found that changes in both general and disease‐specific HR‐QOL did not significantly differ across a broad range of kidney function among patients selected for CABG, PCI, or medical management. These findings suggest that patients with CKD can experience significant improvements in several domains of HR‐QOL after coronary revascularization. This knowledge should inform clinical decision making about the use of revascularization procedures to improve coronary artery disease–related symptoms and quality of life in people with kidney disease.
Sources of Funding
The study was funded by the MSI Foundation. The study funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. APPROACH was initially funded with a grant from the W. Garfield Weston Foundation and has also received contributions from Alberta Health and Wellness and the following industry sponsors: Merck Frosst Canada Inc., Eli Lily Canada Inc., Roche Canada, Bristol‐Myers Squibb, and Philips Medical Systems Canada to support the basic infrastructure of this cardiac registry initiative; the ongoing operation of the APPROACH project has been made possible by support from Alberta Health Services (Calgary Zone, Edmonton Zone), the Libin Cardiovascular Institute of Alberta and Mazankowski Alberta Heart Institute. Dr James was supported by a Kidney REsearch SCiEntist National Training Program New Investigator Award (cofunded by the Kidney Foundation of Canada, Canadian Society of Nephrology, and the Canadian Institutes for Health Research).
Disclosures
None.
Supporting information
Appendix S1. Members of the AKDN and APPROACH Investigators.
Acknowledgments
This study is based, in part, on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta nor Alberta Health or Alberta Health Services express any opinion in relation to this study.
(J Am Heart Assoc. 2016;5:e003642 doi: 10.1161/JAHA.116.003642)
References
- 1. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. [DOI] [PubMed] [Google Scholar]
- 2. Ohtake T, Kobayashi S, Moriya H, Negishi K, Okamoto K, Saito S. High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: an angiographic examination. J Am Soc Nephrol. 2005;16:1141–1148. [DOI] [PubMed] [Google Scholar]
- 3. Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, Salem DN, Levey AS, Sarnak MJ. Cardiovascular outcomes and all‐cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48:392–401. [DOI] [PubMed] [Google Scholar]
- 4. Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. [DOI] [PubMed] [Google Scholar]
- 5. Nunes JPL, Faria MDS, Garcia JMM, Gonalves FR. Glomerular filtration rate and coronary artery disease burden in patients with acute coronary syndrome. Clin Cardiol. 2007;30:464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abaci A, Sen N, Yazici H, Tulmac M, Tukoglu TY, Yalcin R. Renal dysfunction is the most important predictor of the extent and severity of coronary artery disease in patients with diabetes mellitus. Coron Artery Dis. 2007;18:463–469. [DOI] [PubMed] [Google Scholar]
- 7. Khalique O, Aronow WS, Ahn C, Mazar M, Schair B, Shao J, Channamsetty V. Relation of moderate or severe reduction in glomerular filtration rate to number of coronary arteries narrowed >50% in patients undergoing coronary angiography for suspected coronary artery disease. Am J Cardiol. 2007;100:415–416. [DOI] [PubMed] [Google Scholar]
- 8. Hemmelgarn BR, Southern DA, Humphries KH, Culleton BF, Knudtson ML, Ghali WA. Refined characterization of the association between kidney function and mortality in patients undergoing cardiac catheterization. Eur Heart J. 2006;27:1191–1197. [DOI] [PubMed] [Google Scholar]
- 9. Chonocol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28:354–360. [DOI] [PubMed] [Google Scholar]
- 10. Yusuf S, Zucker D, Peduzzi P, Fischer L, Takaro T, Kennedy J. Effects of coronary artery bypass graft surgery on survival: overview of 10 year results from randomized trials by the Coronary Artery Bypass Graft Surgery Trialist Collaboration. Lancet. 1994;344:563–570. [DOI] [PubMed] [Google Scholar]
- 11. Chertow GM, Normand ST, McNeil BJ. Renalism: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15:2462–2468. [DOI] [PubMed] [Google Scholar]
- 12. Charytan DM, Setoguchi S, Solomon DH, Avorn J, Winkelmayer WC. Clinical presentation of myocardial infarction contributes to lower use of coronary angiography in patients with chronic kidney disease. Kidney Int. 2007;71:938–945. [DOI] [PubMed] [Google Scholar]
- 13. James M, Tonelli M, Ghali W, Knudtson ML, Faris P, Manns BJ, Pannu N, Galbraith PD, Hemmelgarn BR. Renal outcomes associated with invasive versus conservative management of acute coronary syndrome: propensity matched cohort study. BMJ. 2013;347:f4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson RJ, O'Brien M, MaWhinney S. Renal failure predisposes patients to adverse outcome after coronary artery bypass surgery: VA Cooperative Study #5. Kidney Int. 1999;55:1057–1062. [DOI] [PubMed] [Google Scholar]
- 15. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Matthew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 16. Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 17. Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast‐induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–19. [DOI] [PubMed] [Google Scholar]
- 18. Harjai KJ, Raizada A, Shenoy C, Sattur S, Orshaw P, Yeger K, Boura J, Aboufares A, Sporn D, Stapleton D. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol. 2008;101:812–819. [DOI] [PubMed] [Google Scholar]
- 19. Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ. Long‐term risk of mortality and end‐stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–616. [DOI] [PubMed] [Google Scholar]
- 20. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2008;20:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn BR. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–416. [DOI] [PubMed] [Google Scholar]
- 22. Manns B, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C. Quality of life in patients treated with hemodialysis or peritoneal dialysis: what are the important determinants? Clin Nephrol. 2007;60:341–351. [DOI] [PubMed] [Google Scholar]
- 23. Fukuhara S, Lopes AA, Bragg‐Gresham JL, Kurokawa K, Mapes DL, Akizawa T. Health‐related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;64:1903–1910. [DOI] [PubMed] [Google Scholar]
- 24. Ghali WA, Knudtson ML. Overview of the Alberta provincial project for outcome assessment in coronary heart disease. On behalf of the APPROACH investigators. Can J Cardiol. 2000;16:1225–1230. [PubMed] [Google Scholar]
- 25. Manns BJ, Mortis GP, Taub K, McLaughlin K, Donaldson C, Ghali WA. The Southern Alberta Renal Program database: a prototype for patient management and research initiatives. Clin Invest Med. 2001;24:164–170. [PubMed] [Google Scholar]
- 26. Hemmelgarn BR, Clement F, Manns BJ, Klarenbach SW, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott‐Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M. Overview of the Alberta kidney disease network. BMC Nephrol. 2009;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Norris CM, Ghali WA, Saunders LD. Comparison of four different statistical analysis strategies for analyzing Seattle Anginal Questionnaire quality of life data. Qual Life Res. 2001;9:309. [Google Scholar]
- 29. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. [DOI] [PubMed] [Google Scholar]
- 30. EuroQol Group . EuroQol: a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 31. Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart. 2006;92:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spertus JA, Winder JA, Dewhurst TA, Day RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. [DOI] [PubMed] [Google Scholar]
- 33. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonnell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 34. Van Buuren S. Multiple imputation of and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 35. Hueb W, Soares PR, Gersh BJ, Csar LA, Luz PL, Puig LB, Martinez EM, Oliveira SA, Ramires JA. The medicine, angioplasty, or surgery study (MASS‐II): a randomized controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one‐year results. J Am Coll Cardiol. 2004;43:1743. [DOI] [PubMed] [Google Scholar]
- 36. Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang J, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O'Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. [DOI] [PubMed] [Google Scholar]
- 37. Kim J, Henderson RA, Pocock SJ, Clayton T, Sculpher MJ, Fox KA. Health‐related quality of life after interventional or conservative strategy in patients with unstable angina or non‐ST‐segment elevation myocardial infarction: one year results of the third randomized intervention trial of unstable angina (RITA‐3). J Am Coll Cardiol. 2005;45:221–228. [DOI] [PubMed] [Google Scholar]
- 38. Hoenig MR, Aroney CN, Scott IA. Early invasive versus conservative strategies for unstable angina and non‐ST elevation myocardial infarction in the stent era. Cochrane Database Syst Rev. 2010;17:3. [DOI] [PubMed] [Google Scholar]
- 39. Parikh CR, Coca SG, Smith GL, Vaccarino V, Krumholz HM. Impact of chronic kidney disease on health‐related quality‐of‐life improvement after coronary artery bypass surgery. Arch Intern Med. 2006;166:2014–2019. [DOI] [PubMed] [Google Scholar]
- 40. Sedlis S, Jurkovitz CT, Hartigan PM, Kolm P, Golgfarb DS, Lorin JD, Dada M, Maron DJ, Spertus JA, Mancini GB, Teo KK, Boden WE, Weintraub WS. Health status and quality of life in patients with stable coronary artery disease and chronic kidney disease treated with optimal medical therapy or percutaneous coronary intervention (post hoc findings from the COURAGE trial). Am J Cardiol. 2013;112:1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szummer K, Lundman P, Jacobson SH, Schon S, Lindback J, Stenestrand U, Wallenttin L, Jernberg T. Influence of renal function on the effects of early revascularization in non‐ST‐elevation myocardial infarction: data from the Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009;120:851–858. [DOI] [PubMed] [Google Scholar]
- 42. Wong JA, Goodman SC, Yan RT, Wald R, Bagnall AJ, Welsh RC, Wong GC, Kornder J, Eagle KA, Steg PG, Yan AT. Temporal management patterns and outcomes of non‐ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J. 2009;30:549–557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Members of the AKDN and APPROACH Investigators.


