Abstract
Background
Little is known regarding the relationship between hospital performance on adverse event rates and hospital performance on 30‐day mortality and unplanned readmission rates for Medicare fee‐for‐service patients hospitalized for acute myocardial infarction (AMI).
Methods and Results
Using 2009–2013 medical record‐abstracted patient safety data from the Agency for Healthcare Research and Quality's Medicare Patient Safety Monitoring System and hospital mortality and readmission data from the Centers for Medicare & Medicaid Services, we fitted a mixed‐effects model, adjusting for hospital characteristics, to evaluate whether hospital performance on patient safety, as measured by the hospital‐specific risk‐standardized occurrence rate of 21 common adverse event measures for which patients were at risk, is associated with hospital‐specific 30‐day all‐cause risk‐standardized mortality and unplanned readmission rates for Medicare patients with AMI. The unit of analysis was at the hospital level. The final sample included 793 acute care hospitals that treated 30 or more Medicare patients hospitalized for AMI and had 40 or more adverse events for which patients were at risk. The occurrence rate of adverse events for which patients were at risk was 3.8%. A 1% point change in the risk‐standardized occurrence rate of adverse events was associated with average changes in the same direction of 4.86% points (95% CI, 0.79–8.94) and 3.44% points (95% CI, 0.19–6.68) for the risk‐standardized mortality and unplanned readmission rates, respectively.
Conclusions
For Medicare fee‐for‐service patients discharged with AMI, hospitals with poorer patient safety performance were also more likely to have poorer performance on 30‐day all‐cause mortality and on unplanned readmissions.
Keywords: Medicare, mortality, myocardial infarction, patient safety, readmission
Subject Categories: Complications, Quality and Outcomes, Myocardial Infarction
Introduction
For over a decade, improving hospital performance on patient safety and patient outcomes have been national priorities in the United States.1, 2, 3, 4, 5, 6, 7 Hospitals with high in‐hospital adverse event, mortality, or unplanned readmission rates are considered to provide poorer quality of care.7, 8, 9 Studies estimate that the excess annual cost attributed to measurable medical errors is around $17 billion10 and that unplanned readmissions result in an additional $15 billion in annual Medicare expenditures.4 Although extensive nation‐wide efforts have focused on improving patient safety and outcomes for acute myocardial infarction (AMI) in particular,11, 12, 13, 14, 15, 16, 17, 18 with some recent data showing that patient safety and outcomes for acute cardiovascular disease have improved,19, 20, 21 in‐hospital adverse events, short‐term mortality, and unplanned readmission rates for AMI patients remain high, with considerable variation across hospitals.22
Efforts to improve patient safety, reduce hospital mortality, and reduce unplanned readmission rates are largely pursued independently. Their impact on one another is unclear or thought to be small.23, 24, 25, 26 Previous studies have shown that patients with 1 or more adverse events are more likely to have higher mortality or to be readmitted, but these studies were limited to a few measures and local data sources.27, 28, 29, 30, 31, 32 They also focused on patient‐level analyses, rather than connecting hospital‐level performance on patient safety with other important hospital‐level outcomes. An association between adverse events and outcomes of individual patients indicates that adverse events may lead to worse outcomes, but it may not reflect hospital performance. A patient experiencing more adverse events may be sicker, and these events may not reflect the performance of the hospital that treated that patient. Depending on case mix, a hospital with a high raw adverse event rate may also have a high raw mortality or readmission rate, but a high raw adverse event rate does not indicate that the hospital has worse performance in patient safety. How hospital performance on patient safety associates with hospital performance on outcomes is unclear from a patient‐level analysis. Without evidence of a link between patient safety and mortality and readmission at the hospital level, hospitals may not view their investment in improving safety as benefiting readmission rates or even mortality and hence may not recognize harm reduction as a potential strategy for lessening these key patient outcomes.
Accordingly, we sought to investigate the association at the hospital‐level between a wide range of in‐hospital adverse event rates and both mortality and readmission rates for Medicare fee‐for‐service patients with AMI, an acute condition that may be more sensitive to in‐hospital adverse events. To accomplish this analysis on a national scale, we used data from the Agency for Healthcare Research and Quality (AHRQ)'s Medicare Patient Safety Monitoring System (MPSMS), the nation's largest randomly selected hospital medical record‐abstracted patient safety database,33 and data from the Centers for Medicare & Medicaid Services (CMS), which includes hospital performance on mortality and readmissions for over 4000 Medicare‐certified hospitals, to assess the relationship between hospital performance on patient safety and hospital performance on 30‐day all‐cause mortality and unplanned readmissions for Medicare fee‐for‐service patients discharged with AMI.
Methods
Study Sample
The MPSMS data, at the individual patient level, includes 21 in‐hospital adverse event measures19 (Table 1) jointly developed by federal agencies and private health care organizations.33 Starting from 2009, MPSMS medical records were drawn from the CMS “validation sample” for process‐of‐care measures required for the Hospital Inpatient Quality Reporting Program, which includes a multistage random sample of all‐payer patients hospitalized for AMI, heart failure, pneumonia, and major surgical conditions. All hospitals in the MPSMS data were randomly selected. Hospital Inpatient Quality Reporting program validation sample also includes 200 hospitals targeted based on past data quality or performance (selected after the random sample was drawn); these were not included in MPSMS data. Approximately 17 500 records were randomly selected from 4000 hospitals for 2009 and 34 000 records from ≈1400 hospitals for 2010 and 2011. The 2012 data included 27 200 records from 1110 hospitals and 2013 data included 17 900 records from 730 hospitals. Each hospital in 2010–2013 contributed approximately equal numbers of randomly selected records to the MPSMS. Medical record abstraction was conducted at the CMS Clinical Data Abstraction Center. Based on 80 monthly reabstractions, the agreement between abstraction and reabstraction ranged from 94% to 99% for data elements used to identify adverse events.19, 27, 33
Table 1.
List of the 21 Adverse Event Measures
| Adverse Event Measures for Which Patients Were at Risk During Hospitalizations |
|---|
| Adverse events associated with digoxin |
| Adverse events associated with hypoglycemic agents |
| Adverse events associated with heparin |
| Adverse events associated with low‐molecular‐weight heparin and factor Xa inhibitors |
| Adverse events associated with warfarin |
| Hospital‐acquired pressure ulcers |
| Inpatient falls |
| Central line‐associated blood stream infections |
| Postoperative pneumonia |
| Hospital‐acquired antibiotic‐associated Clostridium difficile |
| Catheter‐associated urinary tract infections |
| Hospital‐acquired methicillin‐resistant Staphylococcus aureus |
| Hospital‐acquired vancomycin‐resistant Enterococcus |
| Ventilator‐associated pneumonia |
| Adverse events associated with hip joint replacement |
| Adverse events associated with knee joint replacement |
| Mechanical complications associated with central lines |
| Postoperative venous thromboembolic events |
| Postoperative cardiac events (cardiac and noncardiac surgeries) |
| Adverse events associated with femoral artery puncture for catheter angiographic procedures |
| Contrast nephropathy associated with catheter angiography |
The CMS mortality and readmission data for AMI are available from the Hospital Compare website, at the individual hospital level. The data includes hospital‐specific total discharges, 30‐day all‐cause risk‐standardized mortality, and readmission rates for all acute care hospitals treating Medicare fee‐for‐service patients aged 65 years or older. CMS combined data from a 3‐year period to report mortality and readmission rates for each hospital. The 2 recent reporting periods were July 1, 2009 through June 30, 2012 and July 1, 2010 through June 30, 2013. Not all hospitals were repeated in each reporting period. To include the maximum number of hospitals from both the MPSMS and CMS data, we combined the two 3‐year‐period data sets into a single 4‐year‐period data set from July 1, 2009 to June 30, 2013. If a hospital had data in both periods, we averaged its mortality and readmission rates weighted by its total discharges in each period for each outcome. To align with the CMS data, we restricted MPSMS data to only Medicare fee‐for‐service patients aged 65 years or older discharged for AMI from July 1, 2009 to June 30, 2013. Patients discharged to a skilled nursing facility or other level‐of‐care facilities, except transferred to an acute care hospital, were included in the MPSMS as well as in the CMS data.
Patient and Hospital Characteristics
Patient characteristics for the CMS data have been reported elsewhere.24, 25, 26 Patient characteristics for MPSMS data were obtained from medical records, including demographics (age, sex, and race), common clinical comorbidities (heart failure, obesity, coronary artery disease, renal disease, cerebrovascular disease, chronic obstructive pulmonary disease, cancer, and diabetes), and smoking status. Hospital characteristics were obtained from the American Hospital Association's 2013 Annual Survey Database, including teaching status (teaching vs nonteaching); Joint Commission certification status (yes/no); geographical location (urban vs rural); ownership (private not‐for‐profit vs others); bed size; nurse‐to‐patient ratio; perform cardiac catheterization and/or percutaneous coronary intervention procedures (yes/no); and perform coronary artery bypass graft surgery (yes/no).
Outcomes
Our primary outcomes were the hospital‐specific risk‐standardized 30‐day all‐cause mortality and all‐cause unplanned readmission rates. Mortality was measured from the date of admission; readmission was measured from the date of discharge for patients who were discharged alive and were not transferred to another acute‐care hospital.
In‐Hospital Adverse Event Rates
We measured in‐hospital adverse events as the rate of occurrence of in‐hospital adverse events for which patients were at risk. The numerator for this rate is the number of adverse events that occurred, and the denominator is the number of adverse events for which patients were at risk.19 All patients were at risk for at least 2 adverse events, in‐hospital falls, and hospital‐acquired pressure ulcers, but only a subset of patients were at risk for other events (eg, only patients who received warfarin were at risk for a warfarin‐associated adverse event). We reported the rate of patients with 1 or more adverse events during a hospitalization. The numerator for this rate is the number of patients who experienced 1 or more adverse events, and the denominator is the number of patients in the MPSMS data (Table 2).19 Among the 21 in‐hospital adverse event measures, no patient had a knee joint replacement and 3 had a hip joint replacement during the index AMI hospitalization. We included these 3 patients because they had a principal discharge diagnosis code of AMI.
Table 2.
Illustration of Calculating of Adverse Event Rates
| Numerator and Denominator | Patient ID | Total | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| No. of adverse events that occurred during a hospitalization (n) | 2 | 1 | 0 | 0 | 3 |
| No. of adverse events for which a patient was at risk during a hospitalization (n) | 8 | 7 | 4 | 9 | 28 |
| No. of patients who experienced 1 or more adverse events | 1 | 1 | 0 | 0 | 2 |
| No. of patients (n) | 1 | 1 | 1 | 1 | 4 |
| Calculating of adverse event rates | |||||
| Rate of occurrence of adverse events (%)a | 3÷28×100=10.7% | ||||
| Rate of patients with 1 or more adverse events (%)b | 2÷4×100=50.0% | ||||
The numerator is the number of adverse events that occurred and the denominator is the number of adverse events for which patients were at risk.
The numerator is the number of patients who experienced 1 or more adverse events and the denominator is the number of hospitalizations.
Using the CMS risk‐standardized method for profiling hospitals,34, 35, 36, 37 we fitted a hierarchical generalized linear model with a Poisson link function to model the occurrence of adverse events as a function of patients’ age, sex, and clinical comorbidities. The number of adverse events for which patients were at risk was the offset in the model. Using this model, we obtained the risk‐standardized occurrence rate of adverse events. We linked the hospital‐specific risk‐standardized occurrence rate of adverse events data with the hospital‐specific risk‐standardized mortality and readmission data at the hospital level. To ensure that each hospital had an adequate sample size, we included only hospitals with at least 30 patients in the CMS data and at least 40 adverse events for which patients were at risk over the study period (Table 3). The threshold of 40 was selected because the median number of adverse events for which patients were at risk per hospital was 36 for AMI. With this larger threshold, we reduced the likelihood that a hospital's low adverse event rate is attributed to an insufficient number of adverse events for which patients were at risk.
Table 3.
No. of Hospitals by Outcome
| Data Sources | No. of Hospitals by Outcome (n) | Unique Hospitals (n) | |
|---|---|---|---|
| 30‐Day Mortality | 30‐Day Readmission | Overall | |
| CMS mortality and readmission data | |||
| Hospitals with 30 or more patients | 2523 | 2275 | 2530 |
| MPSMS patient safety data | |||
| With 1 or more adverse events for which patients were at riska | 2087 | 2087 | 2087 |
| With 40 or more adverse events for which patients were at risk | 921 | 921 | 921 |
| Linked mortality readmission data and patient safety data | 791 | 723 | 793 |
We identified 2530 unique hospitals that each had 30 or more patients from the CMS mortality and readmission data and 921 unique hospitals that each had 40 or more adverse events for which patients were at risk from the MPSMS adverse events data. The threshold of 40 was selected based on the fact that the median numbers of adverse events for which patients were at risk per hospital was 36 for AMI. With this larger threshold, we reduced the likelihood that a hospital's low adverse event rate is attributed to an insufficient number of adverse events for which patients were at risk. After linking the mortality and readmission data to adverse events data, 793 unique hospitals remained in the final study sample. AMI indicates acute myocardial infarction; CMS, Centers for Medicare & Medicaid Services; MPSMS, Medicare Patient Safety Monitoring System.
The median numbers of adverse events for which patients were at risk was 36 for AMI.
Statistical Analysis
To describe patient and hospital characteristics and the rates of individual adverse event measures, we classified each hospital into tertiles based on its risk‐standardized occurrence rate of adverse events. We also classified each hospital into tertiles based on its number of adverse events for which patients were at risk and compared the 2 distributions. To quantify the difference in the occurrence of adverse events across hospitals, we computed between‐hospital variation in the occurrence rate of adverse events after accounting for patient characteristics using an odds ratio representing the odds of a patient experiencing an adverse event when treated in a hospital 1 SD above the overall average relative to being treated at a hospital 1 SD below the overall average.38
To evaluate the association between hospital performance on patient safety and hospital performance on mortality, we fitted 2 mixed‐effects models to estimate the: (1) hospital‐specific risk‐standardized mortality rate as a function of the hospital‐specific observed occurrence rate of adverse events and (2) hospital‐specific risk‐standardized mortality rate as a function of the hospital‐specific risk‐standardized occurrence rate of adverse events, with and without adjusting for the hospital characteristics described previously. We repeated these models for the readmission measure as well. Because different states have different patient safety regulations and quality improvement initiatives that may impact hospital performance within a state, all models were fitted with state‐specific random intercepts to account for within‐state and between‐state variation and weighted by the hospital‐specific number of adverse events for which patients were at risk. We conducted a second analysis that included all hospitals regardless of their volume of possible adverse events for which patients were at risk. Analyses were conducted using SAS software (version 9.3; SAS Institute Inc., Cary, NC). The institutional review board (IRB) at Solutions IRB (http://www.solutionsirb.com) determined that the requirement for informed consent could be waived based on the nature of the study.
Results
Study Sample
The final sample included 793 hospitals (Table 3) from the linked MPSMS and CMS data. The MPSMS data included 7019 AMI patients. These patients were at risk for 51 969 adverse events. The hospital median (interquartile range [IQR]) number of adverse events for which patients were at risk was 59 (29) (Figure 1, right panel). The CMS data included 167 734 and 174 457 AMI patients for the mortality and readmission outcomes, respectively. The median (IQR) numbers of patients per hospital for the mortality and readmission measures were 149 (226) and 158 (262) (Figure 1, left and middle panels). Table 4, which is at the hospital level, shows hospital characteristics by the tertiles of hospital's risk‐standardized occurrence rate of adverse events for which patients were at risk. Teaching hospitals and hospitals with a high proportion of patients with diabetes mellitus, obesity, and cerebrovascular disease had higher occurrence rates of adverse events (Table 4).
Figure 1.
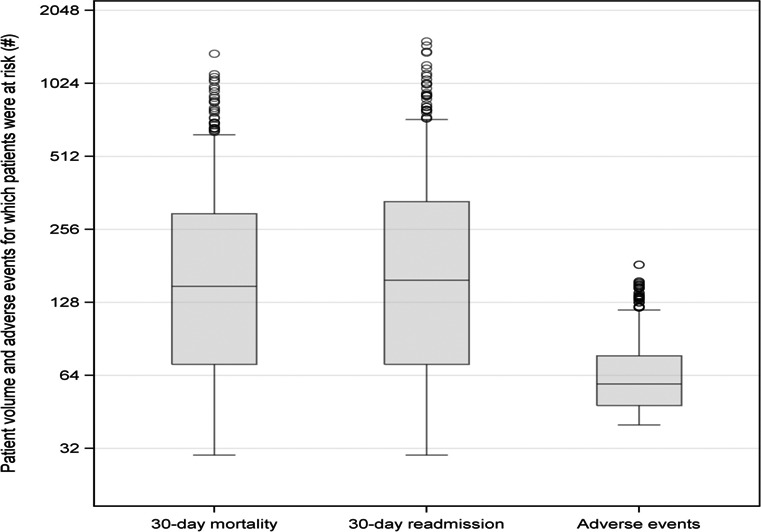
Box and whisker plots of the distributions of hospital‐specific patient volumes and the number of adverse events for which patients were at risk. The length of the box represents the interquartile range (IQR), the horizontal line in the box interior represents the median, the whiskers represent the 1.5 IQR of the 25th quartile or 1.5 IQR of the 75th quartile, and the dots represent outliers. The median (IQR) numbers of patients per hospital for the mortality and unplanned readmission measures were 149 (226) and 158 (262); the median (IQR) number of adverse events for which patients were at risk was 59 (29).
Table 4.
Hospital Characteristics
| Hospital Characteristics | Hospital's Risk‐Standardized Occurrence Rate of Adverse Events for Which Patients Were at Risk by Tertiles (%) | ||
|---|---|---|---|
| First Tertile (1.35–2.82) | Second Tertile (2.83–4.44) | Third Tertile (4.45–19.28) | |
| Total hospitals (n)a | 264 | 265 | 264 |
| Total AMI patients (n) | 2507 | 2279 | 2233 |
| Observed rate of occurrence of adverse events (%) | 0.8 | 3.0 | 7.2 |
| Observed 1 or more adverse events (%) | 4.9 | 19.8 | 37.4 |
| Risk‐standardized rate of occurrence of adverse events, mean (SD) | 2.1 (2.9) | 3.5 (3.6) | 6.7 (19.0) |
| Median (IQR) number of patients for which adverse events were tallied | 8 (4) | 8 (4) | 8 (4) |
| Median (IQR) number of adverse events for which patients were at risk | 57 (27) | 57 (29) | 63 (34) |
| Teaching (%) | 3.4 | 7.6 | 13.6 |
| Accredited by Joint Commission (%) | 86.4 | 86.0 | 85.2 |
| Private and not‐for‐profit (%) | 40.2 | 40.4 | 41.7 |
| Rural setting (%) | 34.5 | 28.3 | 23.9 |
| Perform coronary artery bypass graft surgery (%) | 29.6 | 42.6 | 65.2 |
| Catheterization and/or percutaneous coronary intervention procedures (%) | 42.8 | 59.3 | 75.8 |
| Beds, median (IQR) | 163 (163) | 209 (228) | 251 (248) |
| Nurse‐to‐patient ratio, median (IQR) | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) |
| Overall patient characteristicsb | |||
| Age, y, mean (SD) | 79.8 (8.6) | 79.2 (8.7) | 78.3 (8.4) |
| Female (%) | 53.1 | 50.3 | 46.0 |
| White (%) | 90.6 | 88.5 | 88.1 |
| Black (%) | 5.9 | 7.2 | 7.2 |
| Other race (%) | 3.5 | 4.3 | 4.7 |
| History of heart failure (%) | 52.9 | 49.9 | 47.7 |
| Obesity (%) | 18.5 | 18.3 | 19.5 |
| Coronary artery disease (%) | 97.8 | 98.4 | 98.4 |
| Renal disease (%) | 39.7 | 37.5 | 34.8 |
| Cerebrovascular disease (%) | 26.0 | 24.3 | 24.6 |
| Chronic obstructive pulmonary disease (%) | 30.2 | 27.8 | 25.5 |
| All cancer (%) | 20.3 | 21.7 | 19.4 |
| Diabetes (%) | 41.8 | 40.6 | 43.0 |
| Smoking (%) | 16.3 | 15.5 | 17.3 |
AMI indicates acute myocardial infarction; IQR, interquartile range; MPSMS, Medicare Patient Safety Monitoring System.
Hospitals (n=793) had at least 30 patients for mortality and readmission measures and had at least 40 adverse events for which patients were at risk.
Based on MPSMS abstracted data.
Adverse Events, Mortality, and Readmission Rates
For the occurrence of adverse events, mortality, and readmission, respectively, the ranges (minimum to maximum) of risk‐standardized rates were 10.3% to 19.9%, 14.3% to 21.8%, and 1.4% to 19.3%. Hospital‐specific risk‐standardized occurrence rate of adverse events was widely dispersed across hospitals (Figure 2, right panel). The mean hospital‐specific mortality and readmission rates were 14.7% and 18.0%, respectively (Figure 2, left and middle panels).
Figure 2.
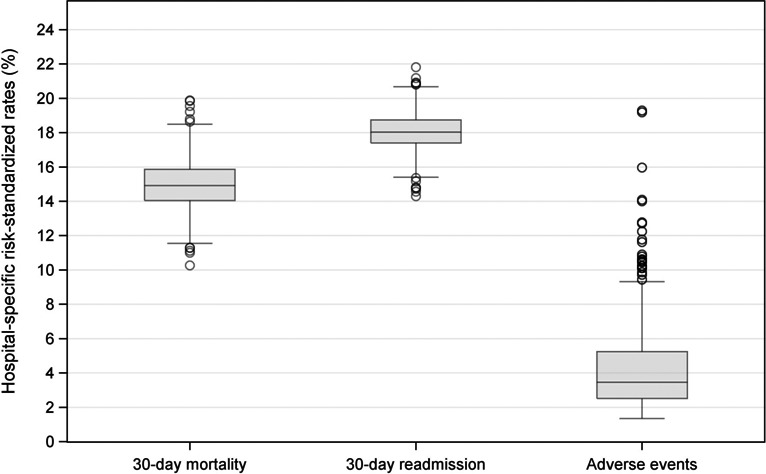
Box and whisker plots of the distributions of hospital‐specific 30‐day all‐cause risk‐standardized mortality and unplanned readmission rates and hospital‐specific risk‐standardized occurrence rates of adverse events for which patients were at risk. The length of the box represents the interquartile range (IQR), the horizontal line in the box interior represents the median, the whiskers represent the 1.5 IQR of the 25th quartile or 1.5 IQR of the 75th quartile, and the dots represent outliers. For mortality, unplanned readmission, and adverse events, respectively, the ranges (minimum to maximum) of risk‐standardized rates were 10.3% to 19.9%, 14.3% to 21.8%, and 1.4% to 19.3%.
Table 5, which is at the patient level, shows the number of patients and adverse event rates for each of the 21 adverse measures as well as the 2 composite adverse event rates measures by the tertiles of hospital's risk‐standardized occurrence rate of adverse events for which patients were at risk. The number of adverse events for which patients were at risk per hospitalization varied by patient characteristics and ranged from 2 to 18. On average, patients were at risk for 7.4 events per hospitalization. Hospitals with a high risk‐standardized occurrence rate of adverse events usually had more patients at risk. For the first, second, and third tertiles, respectively, patients were at risk for 6.7, 7.3, and 8.3 events per hospitalization (Table 5). Nevertheless, ≈32.0% of hospitals in the highest tertile of adverse events for which patients were at risk were in the lowest tertile of risk‐standardized occurrence rate of adverse events. Conversely, 25.8% of hospitals in the lowest tertile of patients at risk had a higher rate of adverse events (Figure 3).
Table 5.
Hospital's Risk‐Standardized Occurrence Rate of Adverse Events for Which Patients Were at Risk by Tertiles, July 1, 2009 to June 30, 2013 (Aged ≥65 Years or Older, Medicare Fee‐for‐Service Patients Discharged With Acute Myocardial Infarction)
| Measurements of Adverse Events | Total Patients (N=7019) | Hospital's Risk‐Standardized Occurrence Rate of Adverse Events for Which Patients Were at Risk by Tertiles (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Tertile (1.35–2.82) | Second Tertile (2.83–4.44) | Third Tertile (4.45–19.28) | ||||||||||
| No. of Patients at Risk | Adverse Event Rate | No. of Patients at Risk | Adverse Event Rate | No. of Patients at Risk | Adverse Event Rate | No. of Patients at Risk | Adverse Event Rate | |||||
| n | % | % (95% CI) | n | % | % (95% CI) | n | % | % (95% CI) | n | % | % (95% CI) | |
| Adverse drug events | ||||||||||||
| Adverse events associated with digoxin | 353 | 5.0 | 1.7 (0.6–3.7) | 131 | 5.2 | 0.0 (0.0–2.7) | 106 | 4.7 | 1.9 (0.2–6.7) | 116 | 5.2 | 3.5 (1–8.7) |
| Adverse events associated with hypoglycemic agents | 2853 | 40.6 | 10.5 (9.4–11.7) | 916 | 36.5 | 2.3 (1.4–3.5) | 900 | 39.5 | 10.0 (8.1–12.2) | 1037 | 46.4 | 18.2 (15.9–20.7) |
| Adverse events associated with IV heparin | 2253 | 32.1 | 11.4 (10.1–12.8) | 641 | 25.6 | 2.0 (1.1–3.4) | 711 | 31.2 | 7.3 (5.5–9.5) | 901 | 40.3 | 21.3 (18.7–24.1) |
| Adverse events associated with low‐molecular‐weight heparin and factor Xa inhibitor | 3156 | 45.0 | 5.2 (4.4–6.0) | 1216 | 48.5 | 0.6 (0.2–1.2) | 1032 | 45.3 | 4.3 (3.1–5.7) | 908 | 40.7 | 12.3 (10.3–14.7) |
| Adverse events associated with warfarin | 669 | 9.5 | 6.3 (4.6–8.4) | 203 | 8.1 | 1.0 (0.1–3.5) | 232 | 10.2 | 5.2 (2.7–8.9) | 234 | 10.5 | 12.0 (8.1–16.8) |
| General events | ||||||||||||
| Hospital‐acquired pressure ulcers | 7019 | 100.0 | 5.4 (4.9–6) | 2507 | 100.0 | 1.6 (1.2–2.2) | 2279 | 100.0 | 5.0 (4.2–6) | 2233 | 100.0 | 10.0 (8.8–11.4) |
| Inpatient falls | 7019 | 100.0 | 0.8 (0.6–1.0) | 2507 | 100.0 | 0.2 (0.1–0.5) | 2279 | 100.0 | 0.8 (0.5–1.3) | 2233 | 100.0 | 1.4 (1–2) |
| Hospital‐acquired infections | ||||||||||||
| Hospital‐acquired antibiotic‐associated Clostridium difficile | 3150 | 44.9 | 0.7 (0.4–1.0) | 1051 | 41.9 | 0.0 (0.0–0.4) | 1009 | 44.3 | 0.7 (0.3–1.4) | 1090 | 48.8 | 1.3 (0.7–2.2) |
| Central line‐associated bloodstream infection | 863 | 12.3 | 1.0 (0.5–2.0) | 151 | 6.0 | 0.0 (0.0–2.4) | 268 | 11.8 | 0.7 (0.1–2.7) | 444 | 19.9 | 1.6 (0.6–3.2) |
| Catheter‐associated urinary tract infection | 2471 | 35.2 | 5.9 (5–6.9) | 776 | 31.0 | 2.3 (1.4–3.6) | 803 | 35.2 | 4.5 (3.2–6.2) | 892 | 39.9 | 10.2 (8.3–12.4) |
| Hospital‐acquired methicillin‐resistant Staphylococcus aureus | 6845 | 97.5 | 0.1 (0–0.2) | 2447 | 97.6 | 0.0 (0.0–0.2) | 2213 | 97.1 | 0.0 (0.0–0.2) | 2185 | 97.9 | 0.3 (0.1–0.6) |
| Hospital‐acquired vancomycin‐resistant Enterococcus | 7002 | 99.8 | <0.01 (0.0–0.1) | 2504 | 99.9 | 0.0 (0.0–0.2) | 2270 | 99.6 | 0.0 (0.0–0.2) | 2228 | 99.8 | 0.0 (0.0–0.2) |
| Postoperative Pneumonia | 431 | 6.1 | 7.4 (5.1–10.3) | 44 | 1.8 | 0.0 (0.0–8) | 119 | 5.2 | 3.4 (0.9–8.4) | 268 | 12.0 | 10.4 (7.1–14.8) |
| Ventilator‐associated pneumonia | 229 | 3.3 | 11.4 (7.6–16.2) | 32 | 1.3 | 0.0 (0.0–10.9) | 64 | 2.8 | 3.1 (0.4–10.8) | 133 | 6.0 | 18.0 (11.9–25.7) |
| Postprocedural events | ||||||||||||
| Adverse events associated with femoral artery puncture for catheter angiographic procedures | 2808 | 40.0 | 2.6 (2–3.2) | 657 | 26.2 | 0.5 (0.1–1.3) | 909 | 39.9 | 2.2 (1.4–3.4) | 1242 | 55.6 | 3.9 (2.9–5.2) |
| Adverse events associated with hip joint replacement | 3 | <0.01 | 66.7 (9.4–99.2) | 0 | 0.0 | N/A | 0 | 0.0 | N/A | 3 | 0.1 | 66.7 (9.4–99.2) |
| Adverse events associated with knee joint replacement | 0 | 0.0 | N/A | 0 | 0.0 | N/A | 0 | 0.0 | N/A | 0 | 0.0 | N/A |
| Contrast nephropathy associated with catheter angiography | 2828 | 40.3 | 13.8 (12.5–15.1) | 659 | 26.3 | 2.4 (1.4–3.9) | 919 | 40.3 | 10.2 (8.3–12.4) | 1250 | 56.0 | 22.3 (20–24.7) |
| Mechanical complications associated with central lines | 1087 | 15.5 | 3.3 (2.3–4.6) | 201 | 8.0 | 0.0 (0.0–1.8) | 351 | 15.4 | 2.0 (0.8–4.1) | 535 | 24.0 | 5.4 (3.7–7.7) |
| Postoperative cardiac events (cardiac and noncardiac surgeries) | 465 | 6.6 | 3.4 (2–5.5) | 50 | 2.0 | 0.0 (0.0–7.1) | 127 | 5.6 | 2.4 (0.5–6.8) | 288 | 12.9 | 4.5 (2.4–7.6) |
| Postoperative venous thromboembolic event | 465 | 6.6 | 0.6 (0.1–1.9) | 50 | 2.0 | 0.0 (0.0–7.1) | 127 | 5.6 | 0.8 (0–4.3) | 288 | 12.9 | 0.7 (0.1–2.5) |
| All events | ||||||||||||
| Occurrence event ratea | 51 969 | 7.4b | 3.8 (3.6–3.9) | 16 745 | 6.7b | 0.8 (0.6–0.9) | 16 718 | 7.3b | 3.0 (2.8–3.3) | 18 506 | 8.3b | 7.2 (6.8–7.5) |
| One or more event per hospitalizationc | 7019 | 100.0 | 20.1 (19.2–21.0) | 2507 | 35.7 | 4.9 (4.1–5.8) | 2279 | 32.5 | 19.8 (18.2–21.4) | 2233 | 31.8 | 37.4 (35.4–39.4) |
N/A indicates not applicable.
The numerator is the number of adverse events that occurred and the denominator is the number of adverse events for which patients were at risk.
Number of adverse events for which patients were at risk per hospitalization.
The numerator is the number of patients who experienced one or more adverse events and the denominator is the number of hospitalizations.
Figure 3.
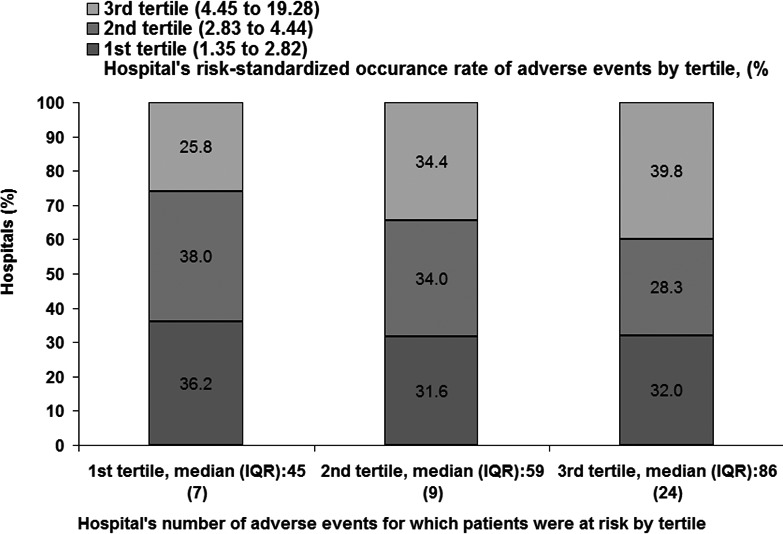
Hospital‐specific risk‐standardized occurrence rates of adverse events by tertile versus hospital‐specific total number of adverse events for which patients were at risk by tertile. IQR indicates interquartile range.
Of the 793 hospitals, the overall occurrence rate of adverse events, in which the numerator is the number of adverse events that occurred and the denominator is the number of adverse events for which patients were at risk, was 3.8%. The overall rate of patients with 1 or more adverse events per hospitalization, in which the numerator is the number of patients who experienced 1 or more adverse events and the denominator is the number of patients in the MPSMS data, was 20.1% (Tables 2 and 5). A change of 1% point in the occurrence rate of adverse events for which patients were at risk was associated with an average change of 4.8% points (95% CI, 4.7–4.9) in the rate of patients with 1 or more adverse events per hospitalization (Figure 4). For teaching and nonteaching hospital groups, respectively, the means (SD) of risk‐standardized hospital‐specific rate of occurrence of adverse events were 5.3 (18.7) and 4.1 (19.2).
Figure 4.
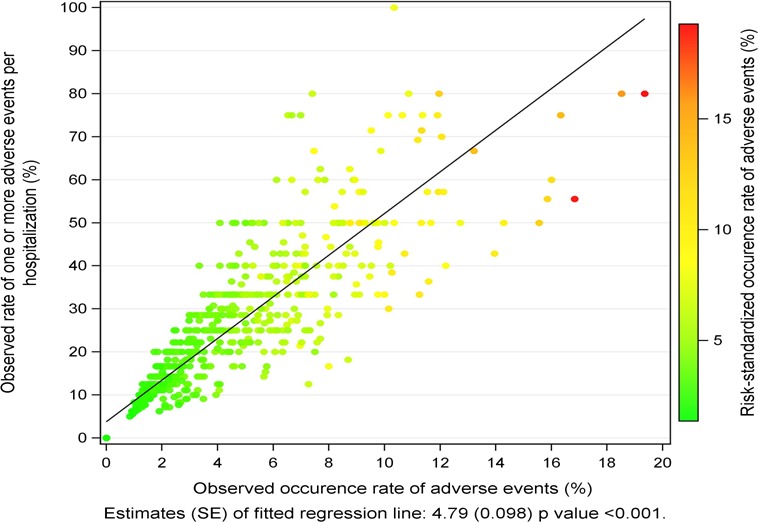
Relationship between the observed rate of patients had 1 or more adverse events and occurrence rate of adverse events for which patients were at risk.
Between‐hospital variation in the risk of adverse events was observed. The odds of an adverse event occurring when treated at a hospital 1 SD above the overall average relative to the odds of an adverse event occurring when treated at a hospital 1 SD below the overall average were 3.86 (95% CI, 3.62–4.12).
Associations Between Adverse Events and Mortality and Readmission Rates
The hospital characteristic‐adjusted associations between the observed occurrence rate of adverse events and risk‐standardized mortality and readmission rates were statistically significant (Figure 5, top panel). The hospital characteristic‐adjusted associations between the risk‐standardized occurrence rate of adverse events and risk‐standardized mortality and readmission rates were also statistically significant (Figure 5, bottom panel). A change of 1% point in the risk‐standardized occurrence rate of adverse events was associated with an average change in the risk‐standardized mortality rate of 4.86% points (95% CI, 0.79–8.94) and an average change in the risk‐standardized readmission rate of 3.44% points (95% CI, 0.19–6.68). Because the overall occurrence rate of adverse events was 3.8%, a 1% point change in this rate translates to a relative change of 26.3%. The second analysis, which included all hospitals regardless of their volume of adverse events for which patients were at risk, did not change the results substantially (Figure 6). A change of 1% point in the risk‐standardized occurrence rate of adverse events was associated with average changes in the risk‐standardized mortality rate of 5.13% points (95% CI, 2.23–8.03) and risk‐standardized readmission rate of 2.51% points (95% CI, 0.21–4.82).
Figure 5.
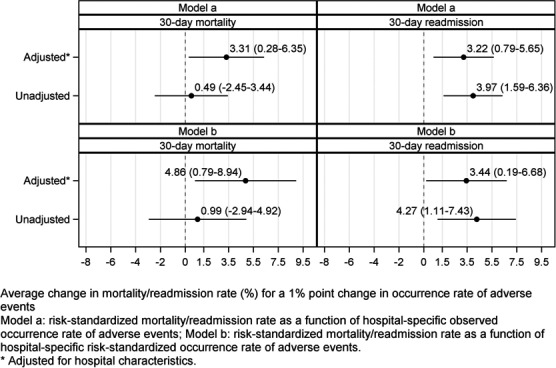
Point estimates and 95% CIs of the associations between the hospital‐specific risk‐standardized 30‐day mortality rate and hospital‐specific risk‐standardized occurrence rate of adverse events and hospital‐specific risk‐standardized 30‐day unplanned readmission rate and hospital‐specific risk‐standardized occurrence rate of adverse events. Hospital characteristics included in the adjusted models were teaching status (teaching vs nonteaching); Joint Commission certification status (yes/no); geographical location (urban vs rural); ownership (private not‐for‐profit vs others); bed size; nurse‐to‐patient ratio; perform cardiac catheterization and/or percutaneous coronary intervention procedures (yes/no); and perform coronary artery bypass graft surgery (yes/no). Model‐a models the risk‐standardized mortality or unplanned readmission rate as a function of hospital‐specific observed occurrence rate of adverse events; model‐b models the risk‐standardized mortality or unplanned readmission rate as a function of hospital‐specific risk‐standardized occurrence rate of adverse events.
Figure 6.
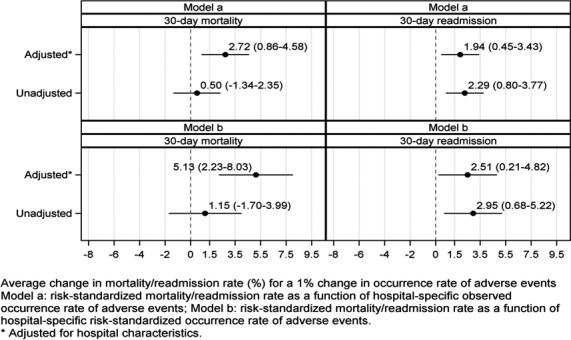
Point estimates and 95% CIs of the associations between hospital performance on mortality and unplanned readmission rates and hospital performance on the occurrence rate of adverse events, regardless of hospitals’ volume of adverse events for which patients were at risk. There were 1592 and 1460 hospitals for the mortality and readmission outcomes, respectively.
Discussion
In this study, we found that hospital performance on patient safety, measured by the hospital‐specific risk‐standardized occurrence rate of adverse events for which patients were at risk, was associated with hospital‐specific risk‐standardized 30‐day all‐cause mortality and unplanned readmission rates for patients with AMI. Our findings are consistent with several possible explanations. Adverse event rates may be a marker of overall hospital quality, including patient safety culture, which is increasingly being recognized as associated with patient outcomes.39, 40, 41, 42, 43 It is possible that some of the interventions to reduce the risk of adverse events could also improve other patient outcomes. For example, improved communication might improve a variety of hospital performance outcomes. It is also possible that changes made at the hospital level that reduce mortality and unplanned readmissions may also improve patient safety performance, as indicated by fewer adverse events.29, 44, 45 Furthermore, patients who experience 1 or more adverse events may be at higher risk for mortality and readmission, specifically attributed to harm caused by the adverse event.19, 27, 28, 29, 30, 31, 32
Our study, based on medical record‐abstracted patient safety information, represents a unique, large, and recent investigation of the associations between hospital performance on patient safety and mortality and readmissions for Medicare patients with AMI in the United States. Previous studies focused on the association between adverse events and mortality or readmission rates at the patient level. This study, however, extends that association to the hospital level by linking hospital performance on patient safety to hospital performance on mortality and readmission rates. In a patient‐level analysis, for example, Lyder et al.27 found that patients who developed pressure ulcers during their hospitalizations had higher 30‐day mortality and readmission rates. Friedman et al.28 studied ≈1.5 million surgical care patients across 1088 hospitals and found that the 30‐day readmission rate was 5% higher for patients with 1 or more in‐hospital adverse events when compared with those with no adverse events. Vorhies et al.30 reported that adverse events were associated with increased readmissions for Medicare fee‐for‐service patients with hip replacements. However, none of these studies measured hospital performance nor were focused on AMI.
We chose to study the hospital level because it is the most actionable, given that improvement efforts require system changes beyond the reach of the individual practitioner and patient. Although the literature has emphasized patient socioeconomic influences on readmissions, our findings suggest that hospital specific factors also play a role. Getting hospitals to invest in those improvement efforts is a major undertaking. By showing an association between patient safety and mortality and readmissions for AMI patients at the hospital level, our study may encourage hospitals to improve their performance on patient safety. Although our findings do not establish a causal relationship, they suggest that patient safety improvement may be afforded a place among other common strategies to reduce mortality and unplanned readmissions. Further research is warranted to determine whether such improvement measurably reduces mortality and unplanned readmissions for other conditions.
Although we found that hospitals with a higher volume of patients at risk were more likely to have a higher adverse event rate, not all hospitals with a higher volume of patients at risk had a higher rate of adverse events. In our study, ≈32% of hospitals with a higher volume of adverse events for which patients were at risk had a lower risk‐standardized occurrence rate of adverse events, and 26% of hospitals with a lower volume of patients at risk had a higher rate of adverse events. Thus, regardless of the hospital's volume of patients at risk, hospitals with a higher adverse event rate have room to improve their care. Furthermore, the treatment pattern itself may provide an opportunity for quality improvement. For example, the overall patient at‐risk rate for the catheter‐associated urinary tract infection indicator was 35%, but we can reduce both the number of adverse events and the number of patients at risk for this indicator by not placing a urinary catheter unless absolutely necessary. A higher volume of patients at risk, may actually indicate overuse of certain health care interventions, and not necessarily reflect case‐mix differences. Having more opportunities to harm is not an adequate rationale to harm more, but should be the impetus to harm less.
Patient safety is arguably indistinguishable from the delivery of quality care.1 Our study illustrates that variation exists in the risk of adverse events across acute care hospitals in the United States. Reducing such variation by focusing on patient safety interventions may favorably impact downstream outcomes, such as mortality and unplanned readmissions. Because the occurrence rate of adverse events was ≈4% and the rate of patients with 1 or more adverse events was 20% for Medicare patients discharged with AMI, an absolute 1% point decrease in the occurrence rate of adverse events is equal to a relative decline of 25% in that rate itself, or an absolute 5% point reduction in the rate of patients with 1 or more adverse events. Ample opportunities for reducing in‐hospital adverse events exist, including promoting transparency and facilitating open discussion to prevent recurring errors,46 implementing and meaningfully using electronic health records,47 adopting and implementing evidence‐based patient safety strategies,48 and, most important, enhancing patient safety culture.49
Our study has several limitations. We focused on adverse events that were both detected and documented during the index hospitalization and were unable to identify events that occurred but were not documented. Restricted by the sample size of the MPSMS data, we were not able to determine whether some of the adverse events have stronger relationships with mortality or unplanned readmission than others. Restricted by the CMS mortality and readmission data, only Medicare fee‐for‐service beneficiaries were included in this study, who were older and more female than the general population of patients with AMI. Limited by the scope of the MPSMS data, only common patient comorbidities were abstracted from the medical records. Therefore, our model to calculate the risk‐standardized occurrence rate of adverse events might not fully account for patient characteristics. Though it is possible that some proportion of the adverse events detected in MPSMS data were not preventable, each of these 21 adverse event measures is characterized as being frequently preventable with the delivery of high‐quality care. We also recognize that some adverse events fall outside those defined in MPSMS. Still, this study distinguishes itself by the breadth and standardization of events measured and its national scope.
Conclusion
For Medicare fee‐for‐service patients discharged with AMI, hospitals with poorer patient safety performance tended to have poorer performance on 30‐day all‐cause mortality and unplanned readmission rates. This strengthens the evidence that mortality and unplanned readmissions reflect the quality of hospital care.
Sources of Funding
This work was supported by contract HHSA290201200003C from the Agency for Healthcare Research and Quality, US Department of Health and Human Services (Rockville, MD). Qualidigm was the contractor. Dr Krumholz is partially funded by grant 1U01HL105270‐02 (Krumholz, Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Dr Normand is partially supported by a grant (R01 GM111339, Normand) from the National Institutes of Health, and Dr Wang is partially supported by the US Environmental Protection Agency (RD‐83490001, Dominici), National Institutes of Health (R21 ES022585‐01, Dominici; R21 ES024012, Zanobetti; R01 GM111339, Normand; R01 ES024332, Zanobetti), and the Agency for Healthcare Research and Quality (K18 HS021991, Dominici).
Disclosures
Dr Krumholz is the recipient of research grants from Medtronic and from Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing, and chairs a cardiac scientific advisory board for UnitedHealth. Drs Krumholz and Normand work under contract to the Centers for Medicare & Medicaid Services to develop and maintain performance measures.
Acknowledgments
The US Department of Health and Human Services, National Heart, Lung, and Blood Institute, National Institutes of Health, and US Environmental Protection Agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. We thank all the previous and current MPSMS team members for their contributions to this work, with a special thanks to the abstractors and other team members at the CMS Clinical Data Abstraction Center. We thank Maliha Tariq, BA, research associate at the Center for Outcomes Research and Evaluation, Yale–New Haven Hospital and Yale University, for her valuable comments. The content of the publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. The authors assume full responsibility for the accuracy and completeness of the ideas presented. Dr Wang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2016;5:e003731 doi: 10.1161/JAHA.116.003731)
References
- 1. Institute of Medicine report, patient safety: achieving a new standard for care. Acad Emerg Med. 2005;12:1011–1012. [DOI] [PubMed] [Google Scholar]
- 2. Leape L, Berwick D. Five years after to Err is human—what have we learned. JAMA. 2005;293:2384–2390. [DOI] [PubMed] [Google Scholar]
- 3. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 4. Berenson RA, Paulus RA, Kalman NS. Medicare's readmissions‐reduction program–a positive alternative. N Engl J Med. 2012;366:1364–1366. [DOI] [PubMed] [Google Scholar]
- 5. Joynt KE, Jha AK. Thirty‐day readmissions—truth or consequences. N Engl J Med. 2012;366:1366–1369. [DOI] [PubMed] [Google Scholar]
- 6. Krumholz H. Posthospitalization syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;382:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krumholz HM, Normand SL. Public reporting of 30‐day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118:1394–1397. [DOI] [PubMed] [Google Scholar]
- 8. Ashton C, Wray N. A conceptual framework for the study of early readmission as an indicator of quality of care. Soc Sci Med. 1996;43:1533–1541. [DOI] [PubMed] [Google Scholar]
- 9. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305:504–505. [DOI] [PubMed] [Google Scholar]
- 10. Van Den Bos J, Rustagi K, Gray T, Halford M, Ziemkiewicz E, Shreve J. The $17.1 billion problem: the annual cost of measurable medical errors. Health Aff. 2011;30:596–603. [DOI] [PubMed] [Google Scholar]
- 11. The Agency for Healthcare Research and Quality Patient Safety Challenge Grants. Available at: http://www.ahrq.gov/research/findings/index.html. Accessed May 15, 2016.
- 12. Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD. The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295:324–327. [DOI] [PubMed] [Google Scholar]
- 13. McCannon CJ, Hackbarth AD, Griffin FA. Miles to go: an introduction to the 5 million lives campaign. Jt Comm J Qual Patient Saf. 2007;33:477–484. [DOI] [PubMed] [Google Scholar]
- 14. Jencks SF, Huff ED, Cuerdon T. Change in the quality of care delivered to Medicare beneficiaries, 1998–1999 to 2000–2001. JAMA. 2003;289:305–312. [DOI] [PubMed] [Google Scholar]
- 15. Heidenreich PA, Hernandez AF, Yancy CW, Liang L, Peterson ED, Fonarow GC. Get With The Guidelines program participation, process of care, and outcome for Medicare patients hospitalized with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:37–43. [DOI] [PubMed] [Google Scholar]
- 16. Peterson ED, Roe MT, Rumsfeld JS, Shaw RE, Brindis RG, Fonarow GC, Cannon CP. A call to ACTION (acute coronary treatment and intervention outcomes network): a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:491–499. [DOI] [PubMed] [Google Scholar]
- 17. Krumholz HM, Wang Y, Chen J, Drye EE, Radford MJ, Havranek EP, Masoudi FA, Nallamothu BK, Spertus JA, Ross JS, Curtis JP, Lichtman JH, Han LF, Rapp MT, Straube BM, Normand SLT. Reduction in acute myocardial infarction mortality in the United States: risk‐standardized mortality rates from 1995–2006. JAMA. 2009;302:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J, Normand SLT, Wang Y, Drye EE, Schreiner GC, Krumholz HM. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee‐for‐service beneficiaries: progress and continuing challenges. Circulation. 2010;121:1322–1328. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Eldridge N, Metersky ML, Verzier N, Meehan TP, Pandolfi M, Foody JM, Ho S‐Y, Galusha D, Kliman R, Sonnenfeld N, Krumholz HM, Battles J. National trends in patient safety for four common conditions, 2005 to 2011. N Engl J Med. 2014;370:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. U.S. Department of Health & Human Services . New HHS data shows major strides made in patient safety, leading to improved care and savings. Available at: http://innovation.cms.gov/Files/reports/patient-safety-results.pdf. Accessed May 16, 2016.
- 21. Krumholz HM, Normand S‐LT, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke: 1999–2011. Circulation. 2014;130:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hospital compare. Available at: http://www.medicare.gov/hospitalcompare/search.html?AspxAutoDetectCookieSupport=1. Accessed May 16, 2016.
- 23. Centers for Medicare & Medicaid Services, readmissions reduction program. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed May 16, 2016.
- 24. Agency for Healthcare Research and Quality, Re‐Engineered Discharge (RED) Toolkit. Available at: http://www.ahrq.gov/professionals/systems/hospital/red/toolkit/. Accessed May 16, 2016.
- 25. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. [DOI] [PubMed] [Google Scholar]
- 26. Krumholz HM, Lin Z, Keenan PS, Chen J, Ross JS, Drye EE, Bernheim SM, Wang Y, Bradley EH, Han LF, Normand SLT. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lyder CH, Wang Y, Metersky ML, Verzier N, Hunt DR. Hospital‐acquired pressure ulcers: results from the national Medicare patient safety monitoring system study. J Am Geriatr Soc. 2012;60:1603–1608. [DOI] [PubMed] [Google Scholar]
- 28. Friedman B, Encinosa W, Jiang HJ, Mutter R. Do patient safety events increase readmissions? Med Care. 2009;47:583–590. [DOI] [PubMed] [Google Scholar]
- 29. HHS Office of Inspector General . Adverse events in hospitals: national incidence among Medicare beneficiaries. November 2010. OEI‐06‐09‐00090. Available at: http://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf. Accessed May 16, 2016.
- 30. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26:119–123. [DOI] [PubMed] [Google Scholar]
- 31. Rosen AK, Loveland S, Shin M, Shwartz M, Hanchate A, Chen Q, Kaafarani HM, Borzecki A. Examining the impact of the AHRQ Patient Safety Indicators (PSIs) on the Veterans Health Administration: the case of readmissions. Med Care. 2013;51:37–44. [DOI] [PubMed] [Google Scholar]
- 32. Glance LG, Kellermann AL, Osler TM, Li Y, Mukamel DB, Lustik SJ, Eaton MP, Dick AW. Hospital readmission after noncardiac surgery: the role of major complications. JAMA Surg. 2014;149:439–445. [DOI] [PubMed] [Google Scholar]
- 33. Hunt DR, Verzier N, Abend SL, Lyder C, Jaser LJ, Safer N, Davern P. Fundamentals of Medicare patient safety surveillance: intent, relevance and transparency. AHRQ's compendium of patient safety research advances in patient safety: from research to implementation. Available at: http://www.ahrq.gov/downloads/pub/advances/vol2/Hunt.pdf. Accessed May 16, 2016. [PubMed]
- 34. Krumholz HM, Wang Y, Mattera JA, Wang Y‐F, Han LF, Ingber MJ, Roman S, Normand S‐LT. An administrative claims model suitable for profiling hospital performance based upon 30‐day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–1692. [DOI] [PubMed] [Google Scholar]
- 35. Krumholz HM, Wang Y, Mattera JA, Wang Y‐F, Han LF, Ingber MJ, Roman S, Normand S‐LT. An administrative claims model suitable for profiling hospital performance based upon 30‐day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. [DOI] [PubMed] [Google Scholar]
- 36. Bratzler W, Normand SLT, Wang Y, O'Donnell WJ, Metersky M, Han LF, Rapp MT, Krumholz HM. An Administrative claims model for profiling hospital 30‐day mortality rates for pneumonia patients. PLoS One. 2011;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Normand SLT, Wang Y, Krumholz HM. Assessing surrogacy of data sources for institutional comparisons. Health Serv Outcomes Res Methodol. 2007;7:79–96. [Google Scholar]
- 38. Zaslavsky AM. Statistical issues in reporting quality data: small samples and casemix variation. Int J Qual Health Care. 2001;13:481–488. [DOI] [PubMed] [Google Scholar]
- 39. Dicuccio MH. The relationship between patient safety culture and patient outcomes: a systematic review. J Patient Saf. 2015;11:135–142. [DOI] [PubMed] [Google Scholar]
- 40. Tourani S, Hassani M, Ayoubian A, Habibi M, Zaboli R. Analyzing and prioritizing the dimensions of patient safety culture in emergency wards using the TOPSIS technique. Glob J Health Sci. 2015;7:40709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohr DC, Eaton JL, McPhaul KM, Hodgson MJ. Does employee safety matter for patients too? Employee safety climate and patient safety culture in health care. J Patient Saf. 2015; doi: 10.1097/PTS.0000000000000186. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42. Hansen LO, Williams MV, Singer SJ. Perceptions of hospital safety climate and incidence of readmission. Health Serv Res. 2011;46:596–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mardon RE, Khanna K, Sorra J, Dyer N, Famolaro T. Exploring relationships between hospital patient safety culture and adverse events. J Patient Saf. 2010;6:226–232. [DOI] [PubMed] [Google Scholar]
- 44. 2013 annual progress report to congress: national strategy for quality improvement in health care: submitted by the U.S. Department of Health and Human Services. Available at: http://www.ahrq.gov/workingforquality/nqs/nqs2013annlrpt.htm. Accessed May 16, 2016.
- 45. Curry LA, Spatz E, Cherlin E, Thompson JW, Berg D, Ting HH, Decker C, Krumholz HM, Bradley EH. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? A qualitative study Ann Intern Med. 2011;154:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kachalia A. Improving patient safety through transparency. N Engl J Med. 2013;369:1677–1679. [DOI] [PubMed] [Google Scholar]
- 47. Institute of Medicine . Health IT and Patient Safety: Building Safer Systems for Better Care. Washington, DC: National Academies Press; 2012. [PubMed] [Google Scholar]
- 48. Shekelle PG, Pronovost PJ, McDonald KM, Carayon P, Farley DO, Neuhauser DV, Saint S, Shekelle PG, Pronovost PJ, McDonald KM, Carayon P, Farley DO, Neuhauser DV. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158(5 Pt 2):365–368. [DOI] [PubMed] [Google Scholar]
- 49. Botwinick L, Bisognano M, Haraden C. Leadership Guide to Patient Safety. IHI Innovation Series White Paper. Cambridge, MA: Institute for Healthcare Improvement; 2006. Available at: www.IHI.org. Accessed May 16, 2016. [Google Scholar]


