Abstract
Background
Ethnicity is an important predictor of coronary artery bypass graft surgery (CABG) outcomes. South Asians (SA), one of the largest ethnic groups with a high burden of cardiovascular disease, are hypothesized to have inferior outcomes after CABG compared to other ethnic groups. Given the paucity and controversy of literature in this area, the objective of this study was to examine the impact of SA versus the general population (GP) on long‐term outcomes following CABG.
Method and Results
Using administrative databases and a surname algorithm, 83 850 patients (SA: 2653, GP: 81 197) who underwent isolated CABG in Ontario, Canada from 1996 to 2007 were identified; mean follow‐up was 9.1±3.9 years. SA were younger (SA: 61.7±9.4, GP: 64.1±10.0 years, standardized difference=0.25) with more cardiac risk factors, including diabetes (SA: 54.1%, GP: 34.9%, standardized difference =0.40). Propensity‐score matching resulted in 2473 matched pairs between SA and GP with all baseline covariates being balanced (standardized difference <0.1). Being a SA compared to the GP was protective against freedom from major adverse cardiac and cerebrovascular events, defined by all‐cause death, myocardial infarction, stroke, or coronary reintervention: Adjusted Cox‐proportional hazard ratio 0.91, 95% CI (0.83–0.99), adjusted‐P=0.04; this was also true for freedom from all‐cause mortality: hazard ratio 0.81, 95% CI (0.72–0.91), adjusted P=0.0004. The adjusted proportion of major adverse cardiac and cerebrovascular events was lower in the SA (SA: 34.7%, GP: 37.8%, McNemar P=0.03), driven largely by all‐cause mortality (SA: 20.4%, GA: 24.3%, McNemar P=0.001).
Conclusions
Contrary to existing notions, our study finds that being a SA is protective with respect to freedom from long‐term major adverse cardiac and cerebrovascular events and mortality after CABG. More studies are required to corroborate and explore causal factors of these findings.
Keywords: coronary artery bypass graft surgery, ethnicity, morbidity/mortality, outcomes research, population studies, propensity score
Subject Categories: Race and Ethnicity, Cardiovascular Surgery, Revascularization, Mortality/Survival, Clinical Studies
Introduction
Ischemic heart disease is one of the leading causes of death worldwide.1, 2 In North America, coronary revascularizations including coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI) are among the most common medical procedures performed.3 South Asians (SA), comprising people originating from India, Pakistan, Sri Lanka, Nepal, and Bangladesh, represent one of the largest ethnic groups in the world4; migration has resulted in a significant number of this ethnic group settling in the Western hemisphere, including Canada, the United States, and the United Kingdom. In Canada, SA are the largest and most rapidly growing visible ethnic group5; it is projected that by 2031, SA will continue to be the largest visible minority in Canada, growing to an estimated 3.2 to 4.1 million.6
Several studies have shown that SA in North America have a higher burden of cardiovascular disease and cardiovascular deaths compared to whites and other ethnic groups.5, 7, 8 Similar findings also exist in other developed regions of the world such as the United Kingdom, where deaths related to coronary disease are higher in SA compared to whites.9 Many factors have been postulated to be linked to these findings including a higher prevalence of diabetes, hypertension, increased small‐size low‐density lipoproteins, increased abdominal visceral fat, and increased prevalence of metabolic syndrome in SA compared to whites.5, 7, 8, 10 Moreover, it has also been shown that SA compared to other ethnic groups tend to have more extensive coronary disease, including higher prevalence of 3‐vessel and left main disease along with systolic dysfunction at time of initial angiography11; there is also widespread belief that SA have smaller‐caliber coronary arteries.12
CABG has become the standard of care for revascularizing patients with advanced severe coronary disease, especially in diabetics.2, 13 A large study involving the Society of Thoracic Surgeons (STS) database showed that ethnicity (whites versus nonwhites) was an independent predictor of operative mortality after CABG.14 Quan et al11 investigated the use of invasive cardiac procedures and suggested that physicians may consider patient ethnicity when recommending procedures including CABG or PCI. With respect to PCI, a recent large retrospective study in the United Kingdom (n=279 256) showed that ethnicity (SA versus whites) was not an independent predictor of mortality (adjusted hazard ratio [HR] 0.99; 95% CI 0.94–1.05, median follow‐up 2.8 years)15; in CABG, however, the evidence has been controversial with regard to whether being SA is an independent predictor of adverse outcomes after CABG.12, 16, 17, 18 These studies are limited by small numbers, lack of adjustment of baseline differences, or absence of long‐term follow‐up. Given that ethnicity may be an important determinant of which procedure is recommended and the notion that SA may have worse outcomes after CABG given the size of their coronary arteries, it would be prudent to conduct a large robust study with long‐term outcomes to determine whether SA ethnicity is truly associated with worse outcomes compared to whites and other ethnic groups. Such evidence can further empower the multidisciplinary heart team to decide whether PCI or CABG would be ideal particularly for individual SA patients.
The primary objective of this article was therefore to determine whether SA compared to the GP is an independent predictor of freedom from long‐term major adverse cardiac and cerebrovascular events (MACCE) after undergoing isolated CABG. The secondary objective was to determine whether being a SA is a predictor of all‐cause mortality. Additional objectives included determining predictors of MACCE in both SAs and the GP along with comparing individual components of MACCE in the unadjusted and the matched cohorts.
Methods
This is a multicenter retrospective propensity‐score matched study using large administrative databases that prospectively collect patient data. These databases are held securely at the Institute of Clinical and Evaluative Sciences, which is a “prescribed entity” under Ontario's health information privacy legislation, which permits this study to be conducted under a waiver of informed consent. The study was approved by the institutional review boards of Sunnybrook Health Sciences Centre and the University of Toronto.
Study Population
All adult patients >20 years of age who underwent isolated CABG across Ontario, Canada between April 1, 1996 and March 31, 2007 were included using the Cardiac Care Network Registry. The time period chosen was based on the earliest complete records of pertinent covariates in the Cardiac Care Network database and permitted acquisition of at least 5 years of follow‐up for each patient. The data of the patients identified were then linked to 3 administrative databases through a unique encrypted patient identifier. These databases included the Ontario Registered Persons Database, used to identify deaths; Office of the Registrar General, Deaths database, used to identify cause of death; and the Canadian Institutes for Health Information Discharge Abstract Database, used in concert with the Cardiac Care Network Registry to determine patient demographic details at time of index CABG and patient outcomes including readmission for a myocardial infarction, re‐intervention, or stroke following the index surgery until June 2012.
Ethnicity
Each patient included in this study was categorized as being a member of the SA population or of the GP (composed of non‐SA, predominantly whites) using the Visible Minority surname list derived by Shah et al.19 Briefly, building on a previously derived SA surname list using Canadian death certificate data,20 surnames from the community telephone directory and encyclopedia of surnames published by the Indian government were added.21 Each name was then reviewed by at least 2 researchers of SA origin. Surnames were excluded if they did not uniquely belong to SA (ie, surnames common to both SA and other ethnic groups, such as “Fernandes”). Disagreements between the 2 researchers were reviewed by a panel of 5 reviewers with SA origin to reach an agreement. The final list included only surnames that were uniquely SA. This list was applied to all members of the Registered Persons Database, a registry of all current and former residents of Ontario. This list was validated against the “gold‐standard” self‐reported ethnicity from the Canadian Community Health Survey, a cross‐sectional national telephone survey conducted by Statistics Canada. The specificity of the Visible Minority surname list for identifying SA ethnicity is 99.7%, sensitivity 50.4%, positive predictive value 89.3%, and negative predictive value 97.2%. The lower sensitivity is primarily a result of excluding surnames that may be common to multiple ethnic groups.19
Statistical Analysis
Propensity‐score analysis
A propensity‐score matched analysis was used to adjust for anticipated baseline confounding variables. A propensity score was calculated for each patient using a logistic regression model to estimate the probability of being a SA. The variables included in this model were time of surgery (stratified into 3 periods of approximately equal intervals: April 1, 1996 to December 31, 1999; January 1, 2000 to December 31, 2003; and January 1, 2004 to March 31, 2007), age (years), creatinine (μmol/L), sex, Canadian Cardiovascular Society Class, left ventricular ejection fraction grade (grade 1: ≥50%, grade 2: 35% to 49%, grade 3: 20% to 34%, grade 4: <20%), left main or multivessel disease (defined as left main disease with or without additional coronary disease, double or triple vessel coronary artery disease including the proximal left anterior descending or 3‐vessel disease without proximal left anterior descending), history of diabetes mellitus, hypertension, acute myocardial infarction (within 30 days prior to operation), any myocardial infarction, hyperlipidemia, smoking, cerebrovascular disease, congestive heart failure, chronic obstructive sleep apnea, dialysis, peripheral vascular disease, previous CABG, and previous PCI. Once the propensity scores were estimated for each patient, each SA patient was matched to 1 patient from the GP cohort in the institution at which the surgery was performed and on the logit of the propensity score using a caliper equal to 0.2 of the SD of the logit of the propensity score.22, 23 Matching was done without replacement, so that each patient occurred at most once in the matched sample.
Baseline demographics
Balance of baseline characteristics between the SA and GP cohort was assessed using standardized differences (STD) of each covariate where a STD <0.1 was considered a negligible difference in the mean or prevalence of a covariate between SA and the GP.24, 25
Outcomes analysis
The primary objective was to determine whether SA patients, following CABG, were at increased risk of long‐term MACCE compared to the GP (MACCE defined by the following: all‐cause mortality, myocardial infarction, coronary reintervention [PCI or re‐CABG], or stroke). The secondary objective was to determine whether SA patients had worse survival (time to all‐cause mortality) compared to GP patients after CABG.
For both of the above objectives, Cox proportional hazards models were fitted using a robust variance estimator in order to account for the matched nature of the sample.26 Effect estimates were presented using HRs and their associated 95% CIs. Kaplan–Meier curves were also estimated for both of the primary objectives. Differences between the survival curves for SA and the GP were tested using a stratified log‐rank test accounting for the matched design; this analysis was also used to generate 30‐day, 1‐year, 5‐year, and 10‐year estimates of freedom from MACCE and all‐cause death stratified by ethnicity.
Given the lack of long‐term data after CABG according to ethnic background, tertiary objectives were to determine the unadjusted crude proportions of MACCE between the SA and GP in addition to determining the predictors of MACCE for each of the original cohorts. For each cohort, a Cox proportional hazards model was used to identify significant predictors. The clinically relevant covariates selected a priori for the models were those included in the propensity model in addition to urgency rating score (a calculated score ranging from 0 to 6, with 0 being the most urgent and 6 being the least).27
Continuous variables were reported as mean±SD. Categorical variables were reported as frequencies and percentages. A 2‐tailed P‐value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Baseline Demographics
Between 1996 and 2006, 83 850 patients underwent isolated CABG across the province of Ontario. Of this cohort, 2653 (3.2%) were SA and 81 197 (96.8%) were part of the GP. As anticipated, these 2 groups were significantly different from each other with respect to many of the baseline covariates at time of the index CABG (Table 1). SA were younger (SA: 61.7±9.4, GP: 64.1±10.0), and had a higher prevalence of cardiac risk factors including diabetes (SA: 1435 [54.1%], GP: 28 332 [34.9%], STD=0.40). The prevalence of smoking, however, was lower in the SA group (SA: 718 [27.1%], GP: 48 138 [59.3%], STD=0.66). The use of propensity‐score matching resulted in the formation of 2473 matched pairs, each comprising 1 SA patient and 1 GP patient (Table 1). Good balance was observed for all the pertinent covariates, with the resulting STD being less than 0.10 for each variable.
Table 1.
Baseline Demographics
| Covariates | Unmatched Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| General Population (N=81 197) | South Asians (N=2653) | St. Diff | General Population (N=2473) | South Asians (N=2473) | St. Diff | |
| Age, y | 64.1±10.0 | 61.7±9.4 | 0.25 | 61.8±10.2 | 61.6±9.5 | 0.02 |
| Creatinine | 97.3±56.0 | 95.2±55.3 | 0.04 | 95.6±51.5 | 95.0±55.5 | 0.01 |
| Urgency rating score | 4.6±1.2 | 4.7±1.1 | 0.08 | 4.6±1.1 | 4.7±1.1 | 0.07 |
| Male, n (%) | 63 186 (77.8) | 2054 (77.4) | 0.01 | 1894 (76.6) | 1910 (77.2) | 0.02 |
| Diabetes mellitus, n (%) | 28 332 (34.9) | 1435 (54.1) | 0.40 | 1334 (53.9) | 1327 (53.7) | 0.01 |
| HTN, n (%) | 58 772 (72.4) | 2063 (77.8) | 0.12 | 1982 (80.1) | 1919 (77.6) | 0.06 |
| History of smoking, n (%) | 48 138 (59.3) | 718 (27.1) | 0.66 | 695 (28.1) | 673 (27.2) | 0.02 |
| Hyperlipidemia, n (%) | 42 369 (52.2) | 1547 (58.3) | 0.12 | 1525 (61.7) | 1438 (58.1) | 0.07 |
| Previous MI, n (%) | 36 570 (45.0) | 1181 (44.5) | 0.01 | 1068 (43.2) | 1108 (44.8) | 0.03 |
| Acute MI, n (%) | 18 730 (23.1) | 662 (25.0) | 0.04 | 619 (25.0) | 623 (25.2) | 0 |
| CHF, n (%) | 11 517 (14.2) | 352 (13.3) | 0.03 | 334 (13.5) | 326 (13.2) | 0.01 |
| CVD, n (%) | 9457 (11.6) | 229 (8.6) | 0.09 | 221 (8.9) | 206 (8.3) | 0.02 |
| PVD, n (%) | 12 110 (14.9) | 205 (7.7) | 0.20 | 185 (7.5) | 189 (7.6) | 0.01 |
| COPD, n (%) | 5821 (7.2) | 97 (3.7) | 0.14 | 81 (3.3) | 87 (3.5) | 0.01 |
| Dialysis, n (%) | 943 (1.2) | 31 (1.2) | 0 | 30 (1.2) | 24 (1.0) | 0.02 |
| Previous PCI, n (%) | 7268 (9.0) | 193 (7.3) | 0.06 | 174 (7.0) | 186 (7.5) | 0.02 |
| Previous CABG, n (%) | 2359 (2.9) | 40 (1.5) | 0.08 | 37 (1.5) | 34 (1.4) | 0.01 |
| Left main disease, n (%) | 19 745 (24.3) | 486 (18.3) | 0.14 | 532 (21.5) | 458 (18.5) | 0.07 |
| MVD, n (%)a | 62 922 (77.5) | 2085 (78.6) | 0.03 | 1945 (78.6) | 1954 (79.0) | 0.01 |
| CCS class, n (%) | 0.03 | 0.02 | ||||
| 1 | 3604 (4.4) | 139 (5.2) | 131 (5.3) | 130 (5.3) | ||
| 2 | 12 365 (15.2) | 451 (17.0) | 432 (17.5) | 428 (17.3) | ||
| 3 | 25 600 (31.5) | 754 (28.4) | 642 (26.0) | 705 (28.5) | ||
| 4 | 38 325 (47.2) | 1283 (48.4) | 1268 (51.3) | 1210 (48.9) | ||
| LVEF grade, n (%) | 0 | 0.05 | ||||
| 1 | 36 442 (44.9) | 1164 (43.9) | 1038 (42.0) | 1106 (44.7) | ||
| 2 | 26 453 (32.6) | 945 (35.6) | 941 (38.1) | 901 (36.4) | ||
| 3 | 12 868 (15.8) | 448 (16.9) | 434 (17.9) | 419 (16.9) | ||
| 4 | 2734 (3.4) | 53 (2.0) | 51 (2.1) | 47 (1.9) | ||
Acute MI indicates any myocardial infarction within 30 days prior to index coronary artery bypass graft (CABG); CCS, Canadian Cardiovascular Society Class; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; HTN, hypertension; LVEF, left ventricular ejection fraction; previous MI, previous myocardial infarction (any MI within 15 years prior to index CABG); PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; St. Diff, standardized difference (less than 0.1 is negligible).
MVD, multivessel disease (defined as left main disease, double or triple vessel disease including the proximal left anterior descending artery [LAD], or 3‐vessel disease without proximal LAD). Hyperlipidemia is defined as documented history of dyslipidemia diagnosed and/or treated by a physician.
Outcomes
The mean duration of follow‐up was 9.1±3.9 years for the overall unmatched cohort, and 9.3±3.5 years for the matched cohort. There were no significant differences with respect to follow‐up duration between the 2 ethnicity groups.
Primary outcome
SA patients, compared to the GP, had a decreased rate of occurrence of MACCE (HR 0.91, 95% CI 0.83–0.99, P=0.04) (Table 2). The Kaplan–Meier curves were also statistically different, with freedom from MACCE being higher in SA: 1 year—SA: 93.9%, GP: 93.8%; 5 years—SA: 84.0%, GP: 83.5%; 10 years—SA: 67.6%, GP: 64.4%; stratified log‐rank P=0.02 (Figure 1A).
Table 2.
Time to Event Analysis for Freedom From MACCE
| Freedom From MACCE for South Asians Compared to the General Population | Hazard Ratio: 0.91, 95% CI ;(0.83–0.99), Adjusted P‐Value for Paired Cox Proportional Hazard Model=0.04 | ||
|---|---|---|---|
| Freedom From MACCE | General Population % (95% CI) | South Asians % (95% CI) | |
| 30 day | 98.1 (97.6–98.6) | 97.5 (96.8–98.0) |
Stratified log‐rank P=0.02 |
| 1 –y | 93.8 (92.8–94.7) | 93.9 (92.9–94.8) | |
| 5 –y | 83.5 (82.0–84.9) | 84.0 (82.5–85.4) | |
| 10 –y | 64.4 (62.3–66.5) | 67.6 (65.5–70.0) | |
MACCE indicates Major Adverse Cardiac and Cerebrovascular Events.
Figure 1.
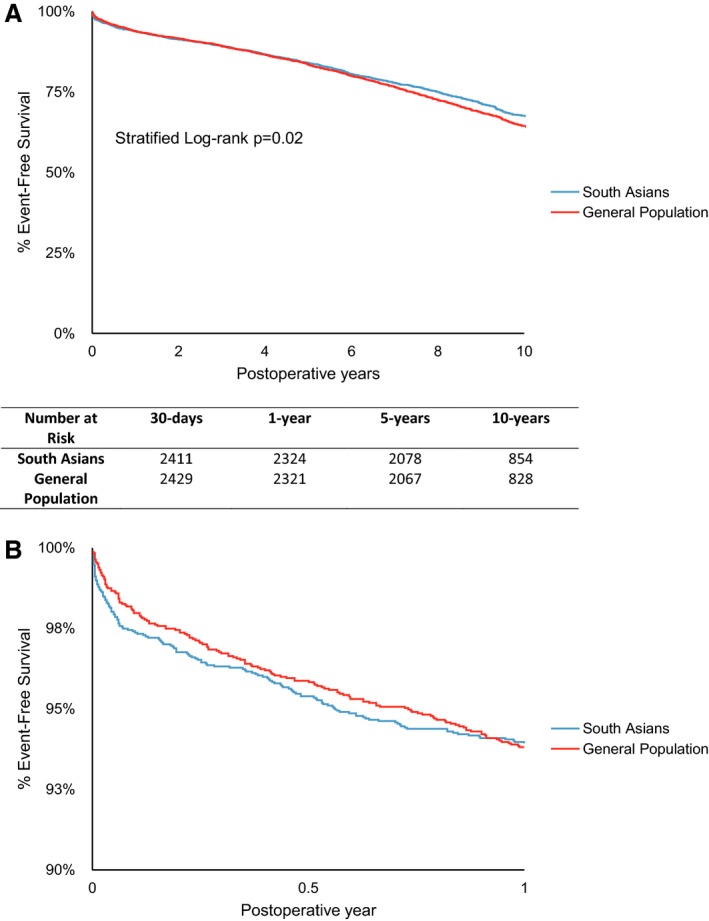
A, Freedom from major adverse cardiac and cerebrovascular events (MACCE). MACCE is defined by all‐cause mortality, myocardial infarction, stroke, or coronary re‐intervention. B, Freedom from MACCE, scale adjusted to highlight the first year. MACCE is defined by all‐cause mortality, myocardial infarction, stroke, or re‐intervention.
Secondary outcome
Being a SA was strongly associated with a decreased risk of all‐cause mortality compared to the GP (HR 0.81, 95% CI 0.72–0.91, adjusted P=0.0004) (Table 3). The adjusted Kaplan–Meier curves were strongly significantly different between the 2 groups: 1 year, SA—97.3%, GP—97.2%; 5 years, SA—92.9%, GP—92.2%; 10 years, SA—83.0%, GP—78.7%; stratified P=0.003 (Figure 2A). Freedom from cardiac mortality between SA and GP was similar (HR 0.82, 95% CI 0.68–1.00, P=0.05).
Table 3.
Time to Event Analysis for Freedom From All‐Cause Mortality After Adjustment Using Propensity Match Analysis
| Freedom From All‐Cause Mortality for South Asians Compared to the General Population | Hazard Ratio: 0.81, 95% CI (0.72–0.91), Adjusted P‐Value for Paired Cox Proportional Hazard Model 0.0004 | ||
|---|---|---|---|
| Freedom From All‐Cause Mortality | General Population % (95% CI) | South Asians % (95% CI) | |
| 30 day | 98.6 (98.1–99.0) | 98.3 (97.7–98.7) |
Stratified log‐rank P=0.003 |
| 1 –y | 97.2 (96.5–97.8) | 97.3 (96.7–97.9) | |
| 5 –y | 92.2 (91.0–93.1) | 92.9 (91.8–93.8) | |
| 10 –y | 78.7 (76.8–80.5) | 83.0 (81.3–84.6) | |
Figure 2.
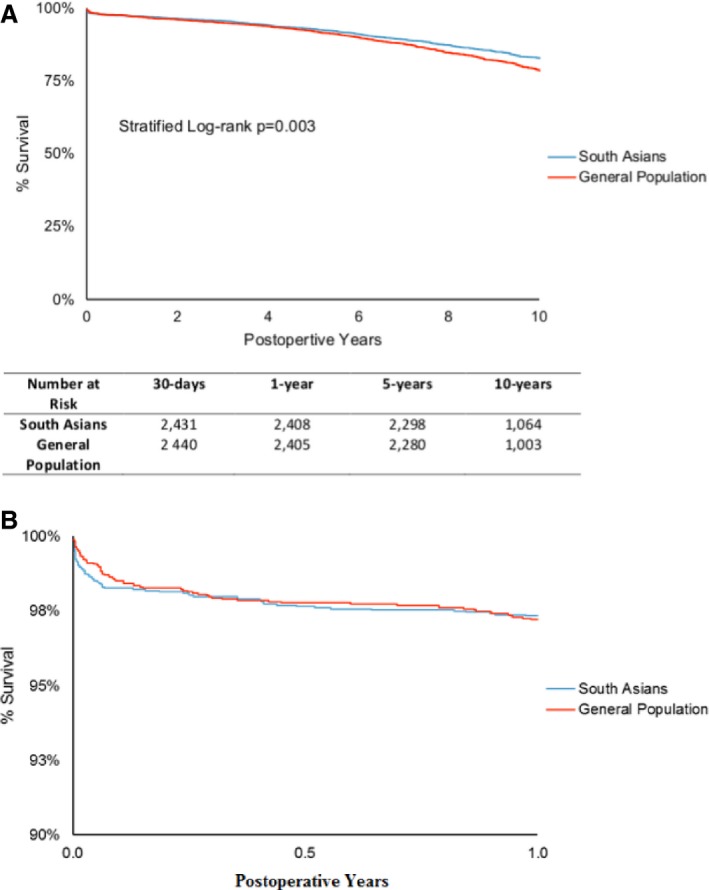
A, Freedom from all‐cause mortality. B, Freedom from all‐cause mortality, scale adjusted to highlight the first year. MACCE is defined by all‐cause mortality, myocardial infarction, stroke, or re‐intervention. MACCE indicates major adverse cardiac and cerebrovascular events.
Predictors
Predictors of freedom from MACCE (determined from the full unmatched cohort) were similar for both the SA and GP (Table 4). Being male was strongly protective in both cohorts (SA: HR 0.80, P=0.007; GP: HR 0.90, P<0.0001). Having diabetes (SA: HR 1.34, P<0.0001; GP: HR 1.36, P<0.0001) and peripheral vascular disease (SA: HR 1.50, P=0.0002; GP: HR 1.39, P<0.0001) were among the strongest risk factors in both cohorts.
Table 4.
Predictors of Freedom From MACCE After CABG in South Asians and the General Population
| Covariates | General Population (n=81 197) | South Asians (n=2653) | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P‐value | Hazard Ratio | 95% CI | P‐Value | |
| Age, y | 1.03 | 1.03 to 1.04 | <0.0001 | 1.03 | 1.02 to 1.03 | <0.0001 |
| Creatinine, μmol/L | 1.00 | 1.00 to 1.00 | <0.0001 | 1.00 | 1.00 to 1.01 | <0.0001 |
| URS | 0.97 | 0.96 to 0.98 | <0.0001 | 0.92 | 0.85 to 0.99 | 0.031 |
| Male | 0.90 | 0.88 to 0.93 | <0.0001 | 0.80 | 0.68 to 0.94 | 0.007 |
| Diabetes | 1.36 | 1.33 to 1.39 | <0.0001 | 1.34 | 1.16 to 1.54 | <0.0001 |
| HTN | 1.17 | 1.14 to 1.20 | <0.0001 | 1.36 | 1.13 to 1.63 | 0.001 |
| History of smoking | 1.16 | 1.13 to 1.19 | <0.0001 | 1.13 | 0.97 to 1.32 | 0.123 |
| Hyperlipidemia | 0.91 | 0.89 to 0.93 | <0.0001 | 0.95 | 0.82 to 1.09 | 0.445 |
| Previous MI | 1.17 | 1.14 to 1.20 | <0.0001 | 1.01 | 0.85 to 1.21 | 0.916 |
| Acute MI | 0.99 | 0.96 to 1.02 | 0.463 | 1.07 | 0.87 to 1.32 | 0.506 |
| CHF | 1.40 | 1.36 to 1.44 | <0.0001 | 1.47 | 1.22 to 1.76 | <0.0001 |
| CVD | 1.46 | 1.41 to 1.50 | <0.0001 | 1.43 | 1.16 to 1.76 | 0.0009 |
| PVD | 1.39 | 1.35 to 1.43 | <0.0001 | 1.50 | 1.22 to 1.86 | 0.0002 |
| COPD | 1.37 | 1.32 to 1.42 | <0.0001 | 1.46 | 1.07 to 1.98 | 0.017 |
| Dialysis | 1.23 | 1.10 to 1.37 | 0.0003 | 0.95 | 0.42 to 2.14 | 0.899 |
| Previous PCI | 1.19 | 1.14 to 1.23 | <0.0001 | 1.31 | 1.02 to 1.69 | 0.035 |
| Previous CABG | 1.30 | 1.23 to 1.37 | <0.0001 | 1.21 | 0.72 to 2.03 | 0.484 |
| Left main disease | 1.08 | 1.05 to 1.11 | <0.0001 | 1.08 | 0.89 to 1.30 | 0.435 |
| MVD | 0.99 | 0.97 to 1.02 | 0.693 | 0.93 | 0.79 to 1.11 | 0.418 |
Acute MI indicates any myocardial infarction within 30 days prior to index coronary artery bypass graft (CABG); CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; HTN, hypertension; MACCE, Major Adverse Cardiac and Cerebrovascular Events; MVD, multivessel disease (defined as left main disease or proximal left anterior descending artery [LAD] and 1 or both circumflex and right coronary or 3‐vessel disease without proximal LAD); previous MI, previous myocardial infarction (any MI within 15 years prior to index CABG); PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; URS, urgency rating score.
Additional outcomes
Overall MACCE was substantially higher in the GP compared to SA in the unmatched cohorts (unmatched SA: 942 [35.5%], GP: 38 288 [47.2%], P<0.001; matched SA: 859 [34.7%], GP: 934 [37.8%], P=0.03) (Table 5). The relationship was similar for all‐cause and cardiac mortality (all‐cause mortality—unmatched SA: 562 [21.2%], GP: 28 338 [34.9%], P<0.001; matched SA: 505 [20.4%], GP: 600 [24.3%], P=0.001; cardiac mortality—unmatched SA: 193 [7.3%], GP: 10 059 [12.4%], P<0.001; matched SA: 172 [7.0%], GP: 207 [8.4%], P=0.06). Proportion of re‐intervention and stroke were similar between the 2 matched groups.
Table 5.
Overall Outcomes for Duration of Follow‐Up
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| General Population (N=81 197) | South Asians (N=2653) | P‐valuea | General Population (N=2473) | South Asians (N=2473) | P‐valueb | |
| Mean follow‐up, y | 9.1±3.9 | 9.3±3.5 | ||||
| MACCEc | 38 288 (47.2%) | 942 (35.5%) | <0.001 | 934 (37.8%) | 859 (34.7%) | 0.03 |
| All‐cause mortality | 28 338 (34.9%) | 562 (21.2%) | <0.001 | 600 (24.3%) | 505 (20.4%) | 0.001 |
| Cardiac‐cause mortality | 10 059 (12.4%) | 193 (7.3%) | <0.001 | 207 (8.4%) | 172 (7.0%) | 0.06 |
| Myocardial infarction | 7275 (9.0%) | 235 (8.9%) | 0.86 | 173 (7.0%) | 216 (8.7%) | 0.02 |
| Stroke | 6080 (7.5%) | 167 (6.3%) | 0.02 | 163 (6.6%) | 155 (6.3%) | 0.63 |
| Re‐interventiond | 8280 (10.2%) | 306 (11.5%) | 0.03 | 263 (10.7%) | 283 (11.4%) | 0.36 |
P‐value from a χ2 statistic for independent data.
P‐value from a McNemar statistic for matched data.
MACCE is major adverse cardiac and cerebrovascular events defined by all‐cause mortality, myocardial infarction, stroke, or re‐intervention following the index coronary artery bypass surgery (CABG).
Re‐intervention is composed of repeat CABG and/or repeat percutaneous intervention.
Discussion
It has been suggested that physicians consider ethnicity when recommending interventions.11 To our knowledge, the current study is one of the largest multicenter administrative database studies to report long‐term results (mean of 9 years) after CABG, comparing SA, an ethnicity with a high burden of cardiovascular disease, to the GP in Canada. We report that SA patients had better long‐term outcomes than the GP, including higher freedom from MACCE, which was driven predominantly by lower all‐cause mortality.
We wanted to determine whether outcomes in SA patients were worse than those in the GP. Indeed, using the crude populations, they are better in the SA than the GP, which is probably the most important result in terms of public policy and heathcare decision making. To reduce inherent biases due to baseline differences in the SA and GP, we performed propensity matching. Using the propensity‐matched patient groups, the SA still had lower MACCE, all‐cause mortality, and cardiac mortality. A limitation of this study in terms of generalizability is that the propensity‐matched sample consisted of patients who resembled SA; however, they had much better outcomes than the crude GP patients, which further reinforces our conclusion.
The currently available evidence in this high‐risk group following CABG is scarce, controversial, and mostly reports early outcomes following surgery and not long‐term outcomes, which was our primary objective. Brister et al16 performed a single‐center propensity score–matched analysis of 917 SA and whites and reported that operative mortality was higher in the SA group (SA: 2.5%, whites: 1.1%, P=0.02) and suggested that being a SA was an independent predictor of early mortality (odds ratio [OR] 3.1, 95% CI 1.4–6.8, P‐value not reported). This study was performed from 1994 to 2003 in a single center in Ontario; as such, we performed a sensitivity analysis, which showed that the HR changed only slightly when excluding the center in Brister's study for mortality (HR 0.83, P=0.01). While some patients did overlap between our current study and that of Brister, our study reports a longer follow‐up.
A study similar to that of Brister was performed in the United Kingdom by Zindrou et al28 (SA: n=436, whites: n=1458), which reported that SA patients had almost twice the 30‐day mortality rate as that of whites. Another UK study by Goldsmith et al17 (SA: n=194, whites: n=190) showed similar in‐hospital morbidity but higher in‐hospital mortality in SA (SA: 6.7%, whites: 2.6%, P=0.06). This study, however, had a higher proportion of SA patients undergoing nonelective surgery, and no difference in mortality was found when stratified by this covariate; in our study, urgency of surgery (defined by an urgency rating score) was similar between the SA and GP cohort (Table 1). In contrast, another study by Elahi and colleagues12 (SA: n=650, whites: n=7226) found similar 30‐day mortality (OR 1.07, P=0.59) and 6‐month mortality (OR 1.1, P=0.31) and concluded that SA ethnicity did not appear to be a strong risk factor for adverse outcomes following CABG compared to whites. The controversial results of early excess mortality in SA patients demonstrated in the previous studies may have resulted due to limited sample size, biases associated with single‐centered practices, or lack of adjustment for baseline differences. In our present study, while the main objective was to assess long‐term outcomes, we found that adjusted freedom from 30‐day mortality was slightly worse with a strong overall P‐value between SA and the GP in the matched cohorts (SA: 1.7%, GP: 1.4%, stratified log‐rank P=0.003). A scale‐adjusted magnified view of the time to event analysis of event‐free survival curve (Figure 1B) and all‐cause mortality (Figure 2B) shows that SA do slightly worse in the early period. We performed a meta‐analysis using the random effects model, adding our crude nonadjusted data to the available literature (excluding Brister's study to prevent double counting since it was a center that was also included in our study) for 30‐day mortality data and found that the odds of mortality was higher being a SA compared to the GP (OR 1.36, 95% CI 0.92–2.04, P=0.13, [Figure S1] for early mortality); although this did not reach statistical significance, the directionality of the OR seems to be consistent with that of our matched cohort (Table 3, 30‐day mortality) and that of Brister's study for the early period after CABG.
In addition to all‐cause mortality, for the first time, we were able to assess cause of mortality (cardiac versus all‐cause mortality). The proportion of cardiac‐related deaths compared to all‐cause mortality was 34% to 35% in both the unmatched and matched GP and the unmatched and matched SA patient groups (Table 5). Cardiac mortality was greater in the unmatched (P<0.001) GP compared to the SA population. Although freedom from cardiac mortality had a protective HR for SA (HR 0.82), its 95% CI crossed 1 (0.68–1.00); while we cannot conclude that cardiac mortality is lower in the SA in the propensity‐matched groups, we can conclude that cardiac mortality is certainly not higher in the SA.
While we have shown that SA patients do well following CABG in the long term, we do not yet know the underlying reasons for these findings. It has been suggested that SA compared to whites have smaller coronary arteries, therefore potentially resulting in poorer outcomes after CABG.9, 29 However, a recent study using quantitative coronary angiography comparing SA and whites contradicted this hypothesis by reporting similar proximal coronary artery sizes and severity of coronary disease.9 These findings could explain why, in our study, SA did not perform poorly after CABG compared to the GP. Alternatively, given the pre‐existing notion that SA patients do poorly because of reduced caliber of coronary arteries, it is possible that SA patients in our study who were accepted for coronary surgery in Ontario were highly selected, and considered to be surgical candidates only if the target artery sizes were suitably large, resulting in better outcomes.
While the above factors are speculative, there are several other factors that could potentially explain why long‐term post‐CABG outcomes for SA patients were better than those for the GP in our study. It has been reported that SAs tend to smoke tobacco less than whites; females of SA origin seldom have a smoking history.5, 7 A long‐term study with a median follow‐up of 20 years showed that persistent smokers had higher risks of all‐cause death and repeat revascularization after CABG.30 While in our study, the higher proportion of smokers present in the GP cohort were adjusted for through matching, it may be possible that following CABG, more patients in the GP continued to smoke, explaining the better outcomes associated with SA patients. Medication adherence is another important factor for secondary prevention of cardiac events after CABG.31 In patients following an acute myocardial infarction, it was reported that SA were as or more likely than Non‐Asians to get prescription of evidence‐based therapies. whites, and Chinese ethnicities.32 Adherence was similar between SA and non‐SA for statins and calcium‐channel blockers; SA were more likely to adhere to β‐blockers and less likely to adhere to angiotensin‐converting enzyme inhibitors compared to their non‐SA counterparts.32 Given the gap in the literature in medication practices specifically following CABG with respect to ethnicity, we can speculate that perhaps a higher medication adherence may be present in the SA compared to the GP following CABG. A third factor is cardiac rehabilitation, which has been associated with early physiologic benefits,33 decreased long‐term mortality,34 and reduced need for hospitalization following CABG.35 While literature investigating cultural influences in cardiac rehabilitation is scarce, a study by Banerjee et al36 showed that while SA patients were less likely to complete the entire 6‐month rehabilitation program compared to whites, they trended to a greater change in maximum metabolic equivalents, and more often reached at least 85% of their target heart rate. While important research has been recently published in this area using qualitative studies identifying enabling and reinforcing factors such as flexible rehabilitation programs and closer physician and family support,37 studies specifically evaluating cardiac rehabilitation practices following CABG are still lacking. Finally, dietary modifications have been shown to be effective, at least in the short term, after CABG.38 While Chiu and colleagues,39 in a large cross‐sectional study of Ontario residents, reported an increased prevalence in inadequate fruit and vegetable consumption along with obesity in SA, to our knowledge there are no studies specifically examining dietary changes following CABG in the context of ethnic differences. We can only speculate that, in parallel with medication adherence, SA patients may also be following a more heart‐healthy diet following CABG.
The major strengths of this study are that it is multicentered, the largest to date on this subject, and statistically robust. We performed a matched analysis using propensity scores that emulates, as closely as possible, a randomized controlled study design with the design phase being the matching process separated by the analysis phase.24 This method of propensity scoring compared to other propensity techniques has been shown to reduce a greater degree of bias due to observed confounding variables40; the matching process was also ideal for this study as there were a large number of control subjects (GP), allowing us to match nearly all (93.2%) of the identified SA cohort.
There were also pertinent limitations in this study. This was an observational study using provincial and national administrative databases. Intraoperative data were largely unavailable in these databases and represents an important limitation. The single‐centered study by Brister et al16 using propensity analysis neutralized many of these factors, including cross‐clamp time, pump time, and mean distal grafts. While we know that our match worked well, as the STD for all measured baseline variables were negligible (including proportion of left main and multivessel disease), we still cannot be certain that intraoperative factors were balanced. Furthermore, while propensity matching performs well in balancing known confounders, it does not account for unknown confounders and treatment‐selection bias, which are inherent in observational studies.22 Similarly, these databases cannot accurately capture lifestyle factors including exercise, diet, smoking habits, and medication compliance. As mentioned above, these components may help to explain why SA patients had better long‐term outcomes than the GP, and therefore dedicated prospective studies in these areas are encouraged. Lastly, we used an ethnic surname list to differentiate SA patients from the GP. One limitation of such a list is surnames obtained through marriage; however, it has been reported that SA are least likely to marry outside their ethnic group.19 Furthermore, while this list is highly specific, it has a moderate sensitivity due to exclusion of surnames that may be common to many ethnicities. While this may have resulted in some names that were misclassified as the GP, this does bias the results conservatively to the null, and therefore the outcome differences may actually be underestimated.
Conclusions
Contrary to the notion that SA have worse outcomes after CABG compared to other ethnic groups, our large propensity‐matched study finds that SA actually do better than the GP in the long‐term after CABG with respect to freedom from MACCE and all‐cause mortality in Ontario. The causes for these findings are likely multifactorial and warrant further investigation. Given that ethnicity may influence physician recommendation for medical procedures, our study, while hypothesis generating, will contribute in this regard; however, further studies are required to corroborate our findings.
Sources of Funding
This work was supported by the Vanier Canada Graduate Scholarship from the Canadian Institute of Health Research (Dr Deb), Tier 1 Canada Research Chair in Health Services Research and an Eaton Scholar Award (Dr Tu), Clinician Scientist Award from the Heart and Stroke Foundation, Ontario Provincial Office (Dr Ko), Career Investigator Award from the Heart and Stroke Foundation, Ontario Provincial Office (Dr Austin), and the Bernard S. Goldman Chair in Cardiovascular Surgery (Dr Fremes).
Disclosures
None.
Supporting information
Figure S1. Meta‐analysis of in‐hospital or 30‐day mortality of South Asians and the general population after coronary artery bypass graft surgery excluding study by Brister et al.1–6
Acknowledgments
We are thankful to Julie Wang, Alice Chong, and Jiming Fang at the Institute of Clinical and Evaluative Sciences, Toronto, Canada, for their assistance in obtaining the necessary data set and guidance in the analysis. We are also grateful to Dr Prateek Lala, from the Hospital for Sick Children, Toronto, Canada, for editing our manuscript.
(J Am Heart Assoc. 2016;5: e003941 doi: 10.1161/JAHA.116.003941)
References
- 1. Lim GB. Public health: global burden of cardiovascular disease. Nat Rev Cardiol. 2013;10:59. [DOI] [PubMed] [Google Scholar]
- 2. Deb S, Wijeysundera HC, Ko DT, Tsubota H, Hill S, Fremes SE. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: a systematic review. JAMA. 2013;310:2086–2095. [DOI] [PubMed] [Google Scholar]
- 3. Gasevic D, Khan NA, Qian H, Karim S, Simkus G, Quan H, Mackay MH, O'Neill BJ, Ayyobi AF. Outcomes following percutaneous coronary intervention and coronary artery bypass grafting surgery in Chinese, South Asian and White patients with acute myocardial infarction: administrative data analysis. BMC Cardiovasc Disord. 2013;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta M, Brister S. Is South Asian ethnicity an independent cardiovascular risk factor? Can J Cardiol. 2006;22:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rana A, de Souza RJ, Kandasamy S, Lear SA, Anand SS. Cardiovascular risk among South Asians living in Canada: a systematic review and meta‐analysis. CMAJ Open. 2014;2:E183–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malenfant EC, Lebel A, Martel L. Projections of the diversity of the Canadian population. 2006 to 2031. Ottawa, Canada: Statistics Canada, and Ministry of Industry; 2010.
- 7. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924–e929. [DOI] [PubMed] [Google Scholar]
- 8. Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H, Hegele RA, McQueen M. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet. 2000;356:279–284. [DOI] [PubMed] [Google Scholar]
- 9. Zindrou D, Taylor KM, Bagger JP. Coronary artery size and disease in UK South Asian and Caucasian men. Eur J Cardiothorac Surg. 2006;29:492–495. [DOI] [PubMed] [Google Scholar]
- 10. Tillin T, Forouhi N, Johnston DG, McKeigue PM, Chaturvedi N, Godsland IF. Metabolic syndrome and coronary heart disease in South Asians, African‐Caribbeans and white Europeans: a UK population‐based cross‐sectional study. Diabetologia. 2005;48:649–656. [DOI] [PubMed] [Google Scholar]
- 11. Quan H, Khan N, Li B, Humphries KH, Faris P, Galbraith PD, Graham M, Knudtson ML, Ghali WA. Invasive cardiac procedure use and mortality among South Asian and Chinese Canadians with coronary artery disease. Can J Cardiol. 2010;26:e236–e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elahi M, Chetty G, Matata B. Ethnic differences in the management of coronary heart disease patients: lessons to be learned in Indo‐Asians. Med Princ Pract. 2006;15:69–73. [DOI] [PubMed] [Google Scholar]
- 13. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130:1749–1767. [DOI] [PubMed] [Google Scholar]
- 14. Hartz RS, Rao AV, Plomondon ME, Grover FL, Shroyer AL. Effects of race, with or without gender, on operative mortality after coronary artery bypass grafting: a study using The Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2001;71:512–520. [DOI] [PubMed] [Google Scholar]
- 15. Jones DA, Gallagher S, Rathod KS, Redwood S, de Belder MA, Mathur A, Timmis AD, Ludman PF, Townend JN, Wragg A. Mortality in South Asians and Caucasians after percutaneous coronary intervention in the United Kingdom: an observational cohort study of 279,256 patients from the BCIS (British Cardiovascular Intervention Society) National Database. JACC Cardiovasc Interv. 2014;7:362–371. [DOI] [PubMed] [Google Scholar]
- 16. Brister SJ, Hamdulay Z, Verma S, Maganti M, Buchanan MR. Ethnic diversity: South Asian ethnicity is associated with increased coronary artery bypass grafting mortality. J Thorac Cardiovasc Surg. 2007;133:150–154. [DOI] [PubMed] [Google Scholar]
- 17. Goldsmith I, Lip GY, Tsang G, Patel RL. Comparison of primary coronary artery bypass surgery in a British Indo‐Asian and white Caucasian population. Eur Heart J. 1999;20:1094–1100. [DOI] [PubMed] [Google Scholar]
- 18. Hadjinikolaou L, Klimatsidas M, Maria Iacona G, Spyt T, Samani NJ. Short‐ and medium‐term survival following coronary artery bypass surgery in British Indo‐Asian and white Caucasian individuals: impact of diabetes mellitus. Interact Cardiovasc Thorac Surg. 2010;10:389–393. [DOI] [PubMed] [Google Scholar]
- 19. Shah BR, Chiu M, Amin S, Ramani M, Sadry S, Tu JV. Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: a validation study. BMC Med Res Methodol. 2010;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheth T, Nargundkar M, Chagani K, Anand S, Nair C, Yusuf S. Classifying ethnicity utilizing the Canadian Mortality Data Base. Ethn Health. 1997;2:287–295. [DOI] [PubMed] [Google Scholar]
- 21. Singh KS. People of India: National Series Volume VIII: Communities, Segments, Synonyms, Surnames and Titles. Delhi, Oxford University Press;1996.
- 22. Deb S, Austin PC, Tu JV, Ko DT, Mazer CD, Kiss A, Fremes SE. A review of propensity‐score methods and their use in cardiovascular research. Can J Cardiol. 2016;32:259–265. [DOI] [PubMed] [Google Scholar]
- 23. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC. A tutorial and case study in propensity score analysis: an application to estimating the effect of in‐hospital smoking cessation counseling on mortality. Multivar Behav Res. 2011;46:119–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cardiac Care Network . CCN software application data definitions & data dictionary. 2013.
- 28. Zindrou D, Bagger JP, Smith P, Taylor KM, Ratnatunga CP. Comparison of operative mortality after coronary artery bypass grafting in Indian subcontinent Asians versus Caucasians. Am J Cardiol. 2001;88:313–316. [DOI] [PubMed] [Google Scholar]
- 29. Sahni D, Jit I. Origin and size of the coronary arteries in the north‐west Indians. Indian Heart J. 1989;41:221–228. [PubMed] [Google Scholar]
- 30. van Domburg RT, Meeter K, van Berkel DF, Veldkamp RF, van Herwerden LA, Bogers AJ. Smoking cessation reduces mortality after coronary artery bypass surgery: a 20‐year follow‐up study. J Am Coll Cardiol. 2000;36:878–883. [DOI] [PubMed] [Google Scholar]
- 31. Hlatky MA, Solomon MD, Shilane D, Leong TK, Brindis R, Go AS. Use of medications for secondary prevention after coronary bypass surgery compared with percutaneous coronary intervention. J Am Coll Cardiol. 2013;61:295–301. [DOI] [PubMed] [Google Scholar]
- 32. Lai EJ, Grubisic M, Palepu A, Quan H, King KM, Khan NA. Cardiac medication prescribing and adherence after acute myocardial infarction in Chinese and South Asian Canadian patients. BMC Cardiovasc Disord. 2011;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jelinek HF, Huang ZQ, Khandoker AH, Chang D, Kiat H. Cardiac rehabilitation outcomes following a 6‐week program of PCI and CABG patients. Front Physiol. 2013;4:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pack QR, Goel K, Lahr BD, Greason KL, Squires RW, Lopez‐Jimenez F, Zhang Z, Thomas RJ. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community‐based study. Circulation. 2013;128:590–597. [DOI] [PubMed] [Google Scholar]
- 35. Hedback B, Perk J, Hornblad M, Ohlsson U. Cardiac rehabilitation after coronary artery bypass surgery: 10‐year results on mortality, morbidity and readmissions to hospital. J Cardiovasc Risk. 2001;8:153–158. [DOI] [PubMed] [Google Scholar]
- 36. Banerjee AT, Gupta M, Singh N. Patient characteristics, compliance, and exercise outcomes of South Asians enrolled in cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2007;27:212–218. [DOI] [PubMed] [Google Scholar]
- 37. Banerjee AT, Grace SL, Thomas SG, Faulkner G. Cultural factors facilitating cardiac rehabilitation participation among Canadian South Asians: a qualitative study. Heart Lung. 2010;39:494–503. [DOI] [PubMed] [Google Scholar]
- 38. Coyan GN, Reeder KM, Vacek JL, Coyan GN, Reeder KM, Vacek JL. Diet and exercise interventions following coronary artery bypass graft surgery: a review and call to action. Phys Sportsmed. 2014;42:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiu M, Maclagan LC, Tu JV, Shah BR. Temporal trends in cardiovascular disease risk factors among white, South Asian, Chinese and black groups in Ontario, Canada, 2001 to 2012: a population‐based study. BMJ Open. 2015;5:e007232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 2009;29:661–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Meta‐analysis of in‐hospital or 30‐day mortality of South Asians and the general population after coronary artery bypass graft surgery excluding study by Brister et al.1–6


