Abstract
Purpose
Decisions about treatment for women with metastatic breast cancer are usually based on the estrogen (ER), progesterone (PgR), and human epidermal growth factor receptor 2 (HER2) status of the primary tumor. Retrospective data suggest that discordance between primary and metastatic lesions leads to detrimental outcome. This prospective study investigated receptor status of primary tumors and metastases in the same patient and assessed the impact of discordance on patient management and survival.
Patients and Methods
Biopsies of suspected metastases were analyzed for ER, PgR, and HER2. Primary tumors and metastases were analyzed using similar methodology. The treating oncologist indicated a treatment plan before and after biopsy to determine whether the result influenced management. Patients were followed up for progression or death.
Results
Of 121 women undergoing biopsy, 80% could be analyzed for receptor status. Discordance in ER, PgR, and HER2 between the primary and the metastasis was 16%, 40%, and 10%, respectively. Biopsy led to a reported change of management in 14% of women (95% CI, 8.4% to 21.5%). Fine-needle aspiration and biopsy of bone led to reduced ability to analyze receptors. After a median follow-up of 12 months, there were no trends for an association between receptor discordance and either time to treatment failure or overall survival.
Conclusion
Biopsy of metastases is technically feasible. Clinicians alter immediate management in one of seven patients on the basis of results of the biopsy, and discordance is not then associated with detrimental effects on outcome. Tissue confirmation should be considered in women with breast cancer and suspected metastatic recurrence.
INTRODUCTION
Discordance in tumor characteristics between primary and metastatic breast cancer has been described for more than 30 years,1,2 but data describing such discordance have been considered unreliable.3 Therefore, practice guidelines recommend that decisions regarding systemic therapy for women with metastatic disease be based on the properties of the primary breast cancer,4 and confirmatory biopsy of suspected metastatic lesions is not recommended consistently.
When compared with the primary tumor, expression of the estrogen (ER) and progesterone (PgR) receptors in metastatic breast cancer can be discordant in up to 40% of women.5 Lower rates of discordance are described for human epidermal growth factor receptor 2 (HER2).6 Most studies describing such discordance are retrospective and have limitations, including selection bias and use of different techniques to evaluate receptors in the primary tumor and metastatic tissue. Such studies cannot evaluate success rates of biopsy of metastatic lesions and cannot accurately inform the impact of receptor discordance on clinical management.
Our group undertook a pilot prospective study in which 35 women with suspected new metastases underwent biopsy; we found that 40% had discordance of receptors, and this led to a change in management in 20% of patients.7 Other prospective studies include high proportions of women with operable, locoregional recurrences and have not evaluated the effects of discordance on patient survival.8 Retrospective analyses of primary and recurrent breast cancers suggest that receptor discordance is associated with poorer survival,9–11 perhaps as a result of the use of inappropriate targeted therapy or the selection of tumors with a more unstable phenotype and therefore more aggressive behavior.
The present study builds on our pilot to address prospectively the success rates of biopsy of metastatic lesions in women with distant metastatic disease when a change in treatment is contemplated. We evaluated whether such biopsies altered management and examined the impact of receptor discordance on disease progression and survival in a prospective cohort of patients. We hypothesized that in the presence of discordance, if treatment is modified according to results of the metastatic biopsy, no detrimental effect of outcome would be observed.
PATIENTS AND METHODS
Study Population
This prospective cohort study took place at a single large cancer hospital. Women with recurrent or progressive metastatic breast cancer were eligible. Availability of archival primary tumor was mandatory. There were no restrictions relating to the number of prior lines of systemic therapy. Exclusion criteria included operable locoregional recurrence with no evidence of metastatic disease, clotting disorder precluding biopsy, rapidly progressive disease, or history of nonbreast second malignancies. The study was approved by the local research ethics board.
Trial End Points
The primary end point of this study was the proportion of patients in whom results of the metastatic biopsy led to a change in management. The secondary goals were to define the discordance rates in ER, PgR, and HER2 between primary and metastatic tissue; assess procedural success rate, risks, and patient satisfaction with performing a metastatic biopsy; and evaluate time to treatment failure (TTF) and overall survival (OS).
Trial Design
Eligibility was assessed and consent obtained. The treating oncologist completed a questionnaire, before obtaining a biopsy from a metastatic lesion, to determine their treatment plan and the proposed start date. Once biopsy results were available, oncologists were again surveyed to determine whether their treatment plan had changed based on the biopsy results and to determine the actual start date of treatment. Procedural success rate was assessed as the number of matched histopathologic examinations of primary and metastasis as a proportion of biopsies undertaken. Delay of therapy was evaluated as the duration between the proposed and actual treatment start dates. Patient satisfaction was assessed using questionnaires, which were administered to all consenting women at the time of clinic follow-up; these evaluated adverse effects resulting from the biopsy procedure and willingness to recommend metastatic biopsy to other women (Appendix Fig A1, online only). Women were followed up, and investigations were carried out at the clinician's discretion; the protocol did not stipulate frequency of assessment. TTF was calculated as the duration between biopsy and first documented evidence of progressive disease or death as a result of any cause. OS was defined as the duration from biopsy to death as a result of any cause. A post hoc, exploratory analysis of the effect of discordance on survival after diagnosis of metastatic disease was also conducted.
Biopsy Procedures
For superficial metastases, fine-needle aspiration (FNA), core, punch, or excisional biopsy was performed using palpation guidance only. For internal lesions, the most amenable site of biopsy was determined in consultation with an interventional radiologist, and FNA, core biopsy, or aspiration of fluid was carried out under radiologic guidance. When aspiration was undertaken, smears of tumor cells were prepared. Samples were fixed in 10% formalin within 20 minutes of the biopsy and processed using the same protocol as for other tissues (including formalin fixation for ≥8 hours). To optimize analysis of receptor expression, biopsies of metastatic bone lesions were not decalcified whenever possible.12
Tissue Processing
A single pathologist (N.M.) and a single cytopathologist (W.G.) evaluated all biopsies. Confirmation of malignancy and evaluation of hormone receptor and HER2 expression were analyzed from all available samples and compared with corresponding results for the primary tumor. All primary and metastatic tissues were analyzed using the same standardized methodology. Primary tumor tissue that was not reported in a central, university-affiliated laboratory or that did not use the latest antibodies and/or hybridization techniques was reanalyzed. Pathologists were not systematically blinded to the receptor status of the primary tumor.
ER staining was carried out using the Ventana SP1 antibody (Ventana Medical Systems, Tucson, AZ), and PgR staining was carried out using Novocastra Clone 16 (Novocastra, Newcastle upon Tyne, United Kingdom). A positive result was defined as ≥1% of tumor cell nuclei staining positively with any intensity.13,14 HER2 expression was assessed exclusively by fluorescent in situ hybridization (FISH) using the PathVysion HER-2 DNA Probe Kit (Vysis, Downers Grove, IL). HER2 and CEP17 signals were enumerated from 60 tumor nuclei. In borderline cases, an additional 60 nuclei were enumerated. The threshold for overexpression was an HER2/CEP17 ratio of more than 2.2. To allow consistent comparison with FISH performed on primary tumors, HER2 FISH on cytology specimens was performed on paraffin sections of pelleted cells. For bone metastases, material not requiring decalcification was used for FISH. To assess receptor discordance, all results were dichotomized into either positive or negative using the methods described previously. Quantitative changes in receptor expression were analyzed descriptively.
Statistical Analyses
Pilot data suggested that biopsy was associated with a change in therapy in approximately 20% of patients.7 Assuming this estimate, 121 patients were required to obtain an accuracy of ±7.5% in the proportion of patients having a change in management. All data were presented descriptively as medians or proportions. Two subgroup analyses were prespecified: the assessment of receptor discordance by number of lines of prior therapy and duration between initial assessment of the primary breast tumor and biopsy of a metastasis. Differences between subgroups were tested by an interaction test.15 Two post hoc subgroup analyses assessed whether the site of metastatic biopsy or the receptor profile of the original tumor influenced receptor discordance. TTF and OS were estimated using the Kaplan-Meier product limit method, and subgroups were compared using the log-rank statistic. Hazard ratios (HR) and their CIs were computed using the Mantel-Haenszel method. Two-sided tests with P values less than .05 were considered statistically significant. Corrections were not made for multiple comparisons.
RESULTS
Patient Characteristics
Over a 2-year period, 151 women were approached, 137 consented, and 121 underwent biopsy. A flow diagram is shown in Figure 1, and patient demographics are shown in Table 1.
Fig 1.
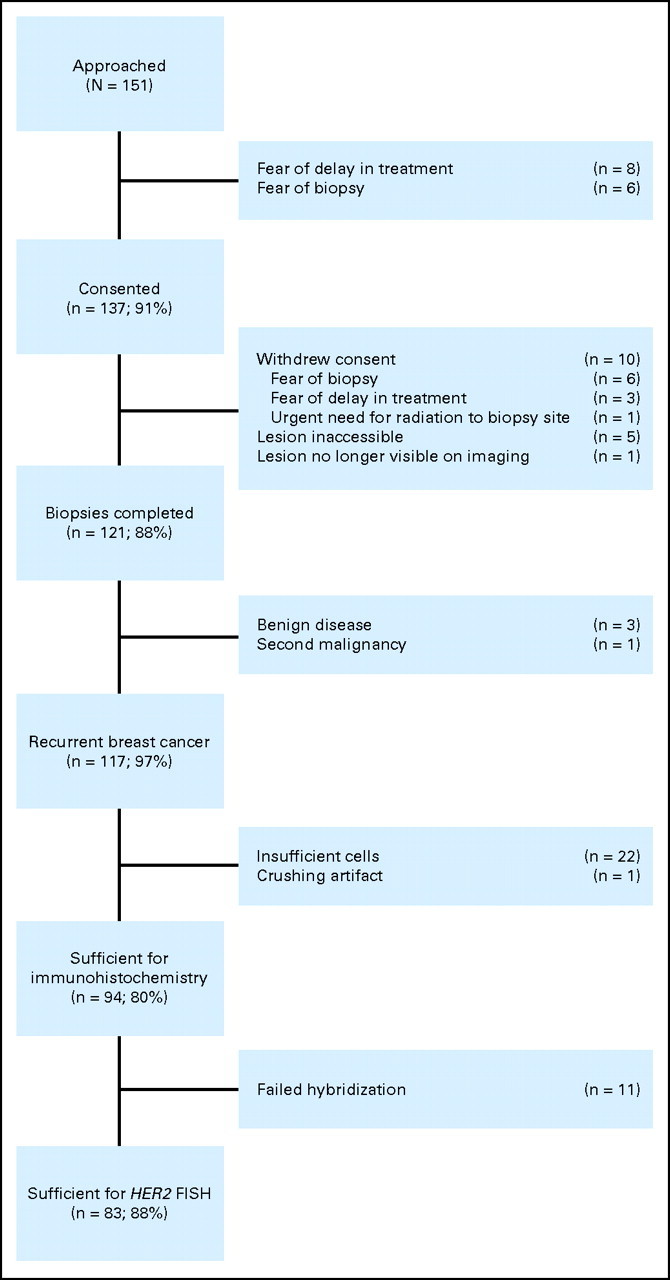
Flow diagram for the study. FISH, fluorescent in situ hybridization.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | All Biopsied Patients (n = 121) |
Concordant Group* (n = 53) |
Discordant Group* (n = 41) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | |||||||
| Median | 59 | 58.5 | 59 | .20 | |||
| Range | 29-83 | 35-83 | 36-72 | ||||
| Adjuvant treatment | |||||||
| Chemotherapy | 63 | 52.1 | 20 | 36.4 | 21 | 51.2 | .19 |
| Endocrine therapy | 90 | 75 | 28 | 50.9 | 17 | 41.5 | .50 |
| Trastuzumab | 5 | 4.1 | 2 | 3.6 | 2 | 4.9 | .85 |
| Advanced disease status | |||||||
| Newly diagnosed metastatic | 56 | 46.3 | 11 | 20.0 | 7 | 17.1 | |
| 1 prior line of treatment in metastatic setting | 21 | 17.4 | 15 | 27.3 | 10 | 24.4 | .65 |
| ≥2 lines of treatment in metastatic setting | 44 | 36.4 | 29 | 52.7 | 24 | 58.5 | |
| Duration of metastatic disease, months | |||||||
| Median | 35 | 18 | 24 | .35 | |||
| Range | 0-274 | 0.5-79 | 0.5-108 | ||||
| Palliative treatment | |||||||
| Median lines of endocrine therapy | 2 | 1 | 1 | .10 | |||
| Median lines of chemotherapy | 1 | 1 | 0 | .26 | |||
Includes only those patients with availability of matched primary and metastatic tissue.
Procedural Success Rate
The sites of biopsy and the analyzable yield for determination of receptor status are shown in the Appendix Table A1 (online only). In total, 117 (96.7%) of the 121 biopsies confirmed recurrent breast cancer. In three women (2.5%), biopsies showed benign disease, and their follow-up imaging showed either resolution or stability of the index lesion. In one participant (0.8%), a second malignancy (basal cell carcinoma) was discovered. Determination of ER and PgR by immunohistochemistry (IHC) was possible in 94 women (80.3%). Reasons for inability to perform IHC are shown in Figure 1. FNA, paracentesis, and thoracocentesis were less likely to provide sufficient cells for cytologic examination (13 [59.1%] of 22 insufficient samples) than core biopsies. Core biopsies from bone and bone marrow trephines were also less likely to yield adequate tissue (eight [36.4%] of 22 insufficient samples). Of the 94 biopsies in which IHC was undertaken, HER2 FISH analysis was successful in 83 (88.3%).
Potential Risks
Biopsy was associated with a median delay to commencing treatment of 15 days (range, 2 to 56 days). There was one serious adverse event related to biopsy: bleeding from a punch biopsy of the skin leading to admission. This resolved with conservative measures, and the patient was discharged the next day.
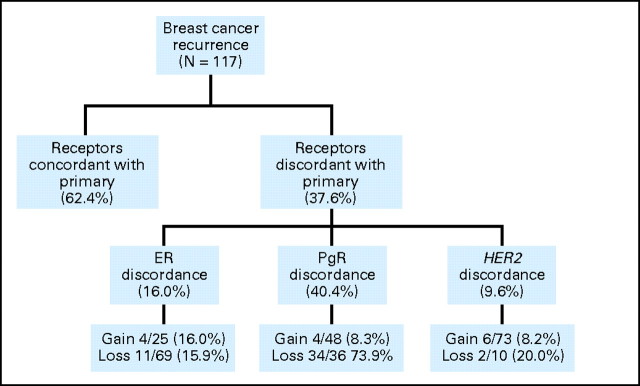
Receptor Discordance
Discordance in one or more receptors between the original pathology report of the primary and the metastatic biopsy was found in 37.6% of women (Fig 2). The most common change was loss of PgR. Quantitative changes in ER and PgR are shown in Figure 3.
Fig 2.
Changes in estrogen receptor (ER), progesterone receptor (PgR), and HER2 between the original pathology report of the primary tumor and the metastasis.
Fig 3.
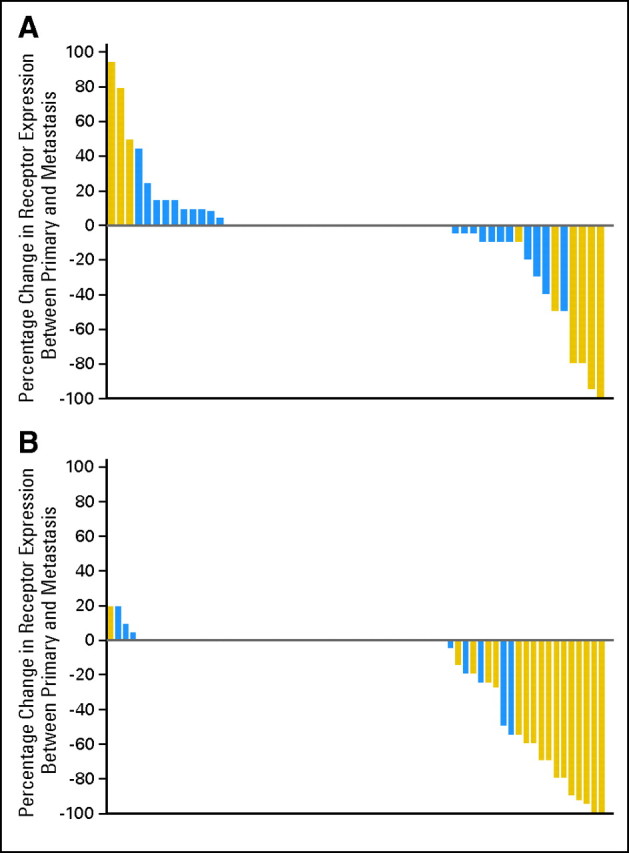
Waterfall plot showing absolute change in hormone receptor expression. A positive score confirms increased expression of receptor from the primary to the metastasis; a negative score confirms reduced expression. (A) Estrogen receptor; (B) progesterone receptor. Blue, concordance with primary; gold, discordance with primary.
Of the 94 patients in whom metastases could be analyzed for receptor status, 52 primary tumors (55%) were reanalyzed and compared with the original pathology report. The median time from initial analysis of the primary to metastatic biopsy was 81 months (range, 9 to 182 months) in those reanalyzed, whereas it was 20 months (range, 0.5 to 35 months) in those not reanalyzed. For three tumors (5.8%), the initial report suggested ER-negative disease, and the reanalysis found ER-positive disease. For six tumors (11.5%), there was discordance in PgR (four negative-positive and two positive-negative pairs). For two tumors (3.8%), there was discordance in HER2, with one changing from positive to negative and the other from negative to positive. These findings are similar to those reported in large studies comparing local and central reports of receptor status.16,17
True receptor discordance was not seen in any patients with triple-negative breast cancer: two of 23 women reported to have triple-negative tumors on the basis of their original pathology showed apparent receptor discordance in one or more receptors, but reanalysis of the primary tumor of these women confirmed ER-positive disease consistent with that in the metastasis.
Change in Therapy
Seventeen women (14%; 95% CI, 8.4% to 1.5%) had a change in treatment as compared with the prebiopsy therapeutic plan. Changes in management included the addition of trastuzumab in women with gain of HER2 overexpression (n = 6), the use of chemotherapy in place of endocrine therapy in those with loss of ER (n = 5), no change to previous treatment in those with benign disease or second primary (n = 4), and provision of endocrine therapy in place of chemotherapy for those gaining ER (n = 2). When loss of ER led to use of chemotherapy, clinical factors such as response to prior lines of endocrine therapy were considered in conjunction with results of the biopsy. There was no evidence that probability of change in treatment was influenced by the number of prior lines of treatment received or the time interval between evaluation of primary breast cancer and metastasis (Table 2). There was no evident difference in the likelihood of change in therapy between different sites of metastatic biopsy.
Table 2.
Proportion of Women With a Change in Originally Planned Therapy by Subgroup
| Subgroup | No. | % | Test of Interaction P |
|---|---|---|---|
| All patients | 17 | 14.0 | |
| Lines of therapy | |||
| Newly metastatic | 7 | 12.5 | .72 |
| 1 prior line of therapy in metastatic setting | 2 | 9.5 | |
| ≥ 2 prior lines of therapy in metastatic setting | 8 | 18.2 | |
| 2 lines (n = 14) | 2 | 14.3 | |
| 3 lines (n = 8) | 1 | 12.5 | |
| 4 lines (n = 4) | 1 | 25 | |
| 5 lines (n = 4) | 1 | 25 | |
| ≥6 lines (n = 14) | 3 | 21.4 | |
| Duration from primary breast cancer diagnosis and biopsy | |||
| First quartile (<35 months) | 4 | 11.4 | .15 |
| Second quartile (36-67 months) | 4 | 15.4 | |
| Third quartile (68-118 months) | 7 | 24.1 | |
| Fourth quartile (>118 months) | 2 | 6.5 |
Patient Satisfaction
Nine women had deteriorated clinically and were unable to complete questionnaires, four women could not be contacted, and 18 women declined to complete the questionnaire. Of 90 women completing the questionnaire, 31 (34%) described anxiety prebiopsy and 53 (59%) described pain postbiopsy; 29 women (32%) described mild, 17 (19%) moderate, and seven (8%) severe pain. Seventy-nine women (87.8%) recommended metastatic biopsy to others with breast cancer.
Survival Analysis
Follow-up was available for all 94 women for whom primary and metastatic tissue could be compared. At a median follow-up of 12 months, 77 patients (80%) had experienced disease progression, and 38 patients (40%) had died. Discordance in receptors between metastases and the primary tumor was not associated with differences in either TTF or OS (Fig 4). Median TTF was 6.3 and 6.5 months for the concordant and discordant groups, respectively. The HR for the discordant group was 0.88 (95% CI, 0.55 to 1.41; P = .59). Median OS was 27.6 and 30.2 months for the concordant and discordant groups, respectively (HR = 0.94; 95% CI,0.49 to; P = .85). Post hoc analysis also showed that receptor discordance was associated with a numerically shorter, but nonsignificant difference in duration between diagnosis of metastatic disease and death (period encompassing both pre- and postbiopsy, median 50.6 v 57.8 months, HR = 1.36; 95% CI, 0.67 to 2.78; P = .40). Statistical power to detect differences in these analyses is low. In patients with primary tumors that were ER and PgR positive, loss of PgR expression in the metastasis (n = 15) was associated with worse TTF on endocrine therapy compared with those that maintained PgR expression (n = 15; 12-month treatment failure rates 73% and 53%, respectively).
Fig 4.
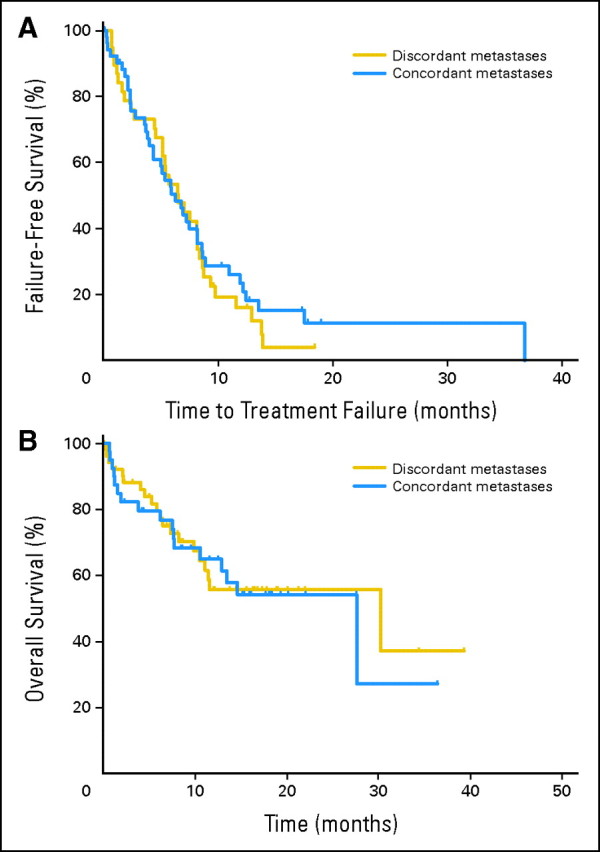
Survival by discordance. (A) Time to treatment failure. (B) Overall survival.
DISCUSSION
The choice of systemic therapy in advanced breast cancer is dependent on the appropriate targeting of ER, PgR, and HER2 receptors. The receptor status of metastatic disease is usually assumed to be the same as that of the primary tumor, but there is evidence for discordance in receptor status between primary and metastatic tumor (reviewed in Lindstrom et al18). Few studies have determined the success rates of biopsy of metastatic lesions or the impact of discordance on patient management or survival. Therefore, the use of metastatic biopsy is contentious.3,19
Our results show that biopsy of metastatic sites is technically feasible and that hormone receptors and HER2 expression can be determined from most biopsies. Receptor discordance was more common with hormone receptors than with HER2. Loss of PgR expression was the most common change, but this rarely had any impact on choice of therapy. Changes in therapy were reported in approximately 14% of women. These findings are similar to those published previously.7,8,20,21 No baseline factors were identified that increased the likelihood of change in therapy, although triple-negative tumors tended not to be discordant. There was also some discordance between the initial pathology report and the reanalysis of archival primary tissue, and in two women, change in therapy could have been guided by re-evaluation of the primary tumor.
Changes in receptor profile should be interpreted with caution, as variations in tissue processing can lead to erroneous results. Inadequate fixation can lead to false-negative results for ER expression.22,23 Inadequate sampling of a heterogeneous cancer can also lead to inaccurate results. Clinicians should consider responses to previous therapy before withholding targeted therapy in women with loss of receptor expression. Failure to detect tumors changing from receptor negative to positive is likely to have a greater impact on treatment decisions than failure to detect tumors changing from receptor positive to negative.24
Discordance between primary and metastatic lesions was not associated with apparent differences in TTF or OS if treatment was modified accordingly. The power of our study to detect such differences was, however, low. Poor survival associated with receptor discordance in retrospective studies9–11 may be due to inappropriate use of targeted therapy in discordant cases. Biopsy of metastatic lesions may predict sensitivity to endocrine therapy: women with ER-positive and PgR-positive primaries, who were treated with endocrine therapy after their biopsy, had a higher likelihood of being progression-free at 12 months if PgR was maintained in the metastasis.
Delaying systemic therapy to await biopsy of a metastatic lesion and its characterization is a concern. In this study, the median delay associated with biopsy was 15 days (range, 2 to 56 days). This was caused partly by batching of samples for HER2 FISH analysis. Approximately one in three patients described prebiopsy anxiety, and nearly 60% described pain associated with their biopsy. Procedure-related complications were rare. Acceptability of metastatic biopsy was high, with almost 90% willing to recommend metastatic biopsy to other women with breast cancer.
Approximately 80% of biopsies from metastases could be analyzed for ER, PgR, and HER2; FNA and biopsy of bone and bone marrow were associated with lower yield. Higher sensitivity from FNA biopsy might have been obtained if immediate sample preparation and evaluation were available.25 Such evaluation might also allow expert triage of tumor material for ancillary studies and rigorous standardization of specimen preparation. Rebiopsy of patients in whom initial attempts did not provide adequate tissue was not routinely carried out in the present study. Such practice would almost certainly increase overall yield, but further delay commencing therapy.
Biopsy of metastatic disease does have limitations. Although improvements in interventional radiology mean that most tissue is now accessible by minimally invasive methods, the choice of the location for biopsy is usually determined by the most accessible site. Such practice can lead to sampling bias, although postmortem data suggest that heterogeneity of hormone receptors among different metastatic sites is rare.26 Bone is the most common site of metastasis in breast cancer,27 and biopsies of bone metastases provide a lower analyzable yield for IHC than other tissues.28 These factors limit the applicability of our results in some patients.
This study has shortcomings. It assessed the impact of biopsy on clinicians' choice of therapy rather than improvement in outcome. It is very difficult to conduct a randomized trial in this setting, and this was felt to be the most feasible design. Although strict protocols were followed in the handling of the biopsies, the handling of the primary specimens was generally not known. Any suboptimal fixation may have led to incorrect receptor analysis, both for the original analysis of the primary and at the time of reanalysis. Primary tumor samples reported in a central laboratory were not reanalyzed, and comparison with biopsies of metastases was therefore subject to interobserver variability. This reflects clinical practice and should not therefore detract from the impact of the results. Finally, follow-up imaging was conducted at the clinician's discretion rather than at fixed intervals. This is pragmatic, but may lead to uncertainty in the definition of TTF.
In conclusion, this prospective study shows that biopsy of metastases appears beneficial. Clinicians alter immediate management in one of seven patients, and if treatment is modified according to results, discordant and concordant cases have similar outcome. A decision to biopsy a metastatic site should be based on patient and tumor-related factors, and clinicians should consider carefully the method and site of biopsy to maximize analyzable yield.
Supplementary Material
Acknowledgment
We thank Julie Napolskikh, Htway Maung, and Aurora DeBorja for trial coordination.
Appendix
Table A1.
Sites of Biopsy and Analyzable Yield of Each Site
| Site | No. | % | Method of Sampling | No. | % | Analyzable Yield* |
|
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| Lymph node | 25 | 20.7 | US-guided core biopsy | 2 | 8 | 17 | 68 |
| US-guided FNA | 8 | 32 | |||||
| Unguided FNA | 15 | 60 | |||||
| Cutaneous | 24 | 19.8 | Unguided FNA | 2 | 8.3 | 24 | 100 |
| Unguided punch biopsy | 21 | 87.5 | |||||
| Excisional biopsy | 1 | 4.2 | |||||
| Bone | 20 | 16.5 | CT-guided core biopsy | 18 | 90 | 15 | 75 |
| CT-guided FNA | 2 | 10 | |||||
| Liver | 19 | 15.7 | US-guided core biopsy | 19 | 100 | 19 | 100 |
| Soft tissue† | 10 | 8.3 | US-guided core biopsy | 3 | 30 | 7 | 70 |
| CT-guided core biopsy | 2 | 20 | |||||
| Unguided FNA | 5 | 50 | |||||
| Bone marrow | 9 | 7.4 | Trephine biopsies | 9 | 100 | 6 | 66.7 |
| Paracentesis | 7 | 5.8 | Pleural drainage | 4 | 57.1 | 5 | 71.4 |
| Ascitic drainage | 3 | 43.9 | |||||
| Lung | 5 | 4.1 | CT-guided FNA | 5 | 100 | 4 | 80 |
| CNS | 2 | 1.7 | Lumbar puncture | 2 | 100 | 2 | 100 |
Abbreviations: CT, computed tomography; FNA, fine-needle aspiration; US, ultrasound.
Analyzable yield is the proportion of biopsies for which analysis for ER, PgR, and HER2 could be completed.
Soft tissue includes subcutaneous tissue, muscle, and peri-osseous tumor.
Footnotes
See accompanying editorial on page 575 and article on page 593; listen to the podcast by Dr Davidson at www.jco.org/podcasts
Supported by the Canadian Breast Cancer Foundation–Ontario Chapter.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: Ontario Cancer Trials-OCT1194.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Eitan Amir, Orit Freedman, Farrah Kassam, Christine Simmons, George Dranitsaris, Andreas Laupacis, Ian F. Tannock, Mark Clemons
Financial support: Mark Clemons
Administrative support: Ian F. Tannock, Mark Clemons
Provision of study materials or patients: Ian F. Tannock, Mark Clemons
Collection and assembly of data: Eitan Amir, Naomi Miller, William Geddie, Orit Freedman, Maria Oldfield, Mark Clemons
Data analysis and interpretation: Eitan Amir, George Tomlinson, Ian F. Tannock, Mark Clemons
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Brennan MJ, Donegan WL, Appleby DE. The variability of estrogen receptors in metastatic breast cancer. Am J Surg. 1979;137:260–262. doi: 10.1016/0002-9610(79)90159-4. [DOI] [PubMed] [Google Scholar]
- 2.Holdaway IM, Bowditch JV. Variation in receptor status between primary and metastatic breast cancer. Cancer. 1983;52:479–485. doi: 10.1002/1097-0142(19830801)52:3<479::aid-cncr2820520317>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Pusztai L, Viale G, Kelly CM, et al. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist. 2010;15:1164–1168. doi: 10.1634/theoncologist.2010-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Practice Guidelines in Oncology: Breast Cancer v. 1.2010. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
- 5.Li BD, Byskosh A, Molteni A, et al. Estrogen and progesterone receptor concordance between primary and recurrent breast cancer. J Surg Oncol. 1994;57:71–77. doi: 10.1002/jso.2930570202. [DOI] [PubMed] [Google Scholar]
- 6.Regitnig P, Schippinger W, Lindbauer M, et al. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J Pathol. 2004;203:918–926. doi: 10.1002/path.1592. [DOI] [PubMed] [Google Scholar]
- 7.Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20:1499–1504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson AM, Jordan LB, Quinlan P, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: The Breast Recurrence in Tissues Study (BRITS) Breast Cancer Res. 2010;12:R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–1958. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson E, Lindstrom LS, Wilking U, et al. Discordance in hormone receptor status in breast cancer during tumor progression. J Clin Oncol. 2010;28(suppl):116s. abstr 1009. [Google Scholar]
- 11.Wilking U, Karlsson E, Skoog L, et al. HER2 status in a population-derived breast cancer cohort: Discordances during tumor progression. Breast Cancer Res Treat. 2011;125:553–561. doi: 10.1007/s10549-010-1029-2. [DOI] [PubMed] [Google Scholar]
- 12.Bussolati G, Leonardo E. Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J Clin Pathol. 2008;61:1184–1192. doi: 10.1136/jcp.2007.047720. [DOI] [PubMed] [Google Scholar]
- 13.Diaz LK, Sneige N. Estrogen receptor analysis for breast cancer: Current issues and keys to increasing testing accuracy. Adv Anat Pathol. 2005;12:10–19. doi: 10.1097/00125480-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–372. [PubMed] [Google Scholar]
- 16.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 17.Badve SS, Baehner FL, Gray RP, et al. Estrogen- and progesterone-receptor status in ECOG 2197: Comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol. 2008;26:2473–2481. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 18.Lindstrom L, Howell S, Astrom G, et al. Controversies in the management of metastatic breast cancer: Biologic evaluation of breast cancer—Should metastases be biopsied? Am Soc Clin Oncol Ed Book. 2010:e7–e12. [Google Scholar]
- 19.Khasraw M, Brogi E, Seidman AD. The need to examine metastatic tissue at the time of progression of breast cancer: Is re-biopsy a necessity or a luxury? Curr Oncol Rep. 2011;13:17–25. doi: 10.1007/s11912-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 20.Curigliano G, Bagnardi V, Viale G, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol. doi: 10.1093/annonc/mdq751. [epub ahead of print on February 22, 2011] [DOI] [PubMed] [Google Scholar]
- 21.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodi Z, Chakrabarti J, Lee AH, et al. The reliability of assessment of oestrogen receptor expression on needle core biopsy specimens of invasive carcinomas of the breast. J Clin Pathol. 2007;60:299–302. doi: 10.1136/jcp.2006.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann GB, Fahey VD, Feleppa F, et al. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol. 2005;23:5148–5154. doi: 10.1200/JCO.2005.02.076. [DOI] [PubMed] [Google Scholar]
- 24.Amir E, Clemons M. Should a biopsy be recommended for confirmation of metastatic disease in women with breast cancer? Lancet Oncol. 2009;10:933–935. doi: 10.1016/S1470-2045(09)70295-5. [DOI] [PubMed] [Google Scholar]
- 25.Kocjan G, Chandra A, Cross P, et al. BSCC Code of Practice: Fine needle aspiration cytology. Cytopathology. 2009;20:283–296. doi: 10.1111/j.1365-2303.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu JM, Fackler MJ, Halushka MK, et al. Heterogeneity of breast cancer metastases: Comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Amir E, Ooi WS, Simmons C, et al. Discordance between receptor status in primary and metastatic breast cancer: An exploratory study of bone and bone marrow biopsies. Clin Oncol (R Coll Radiol) 2008;20:763–768. doi: 10.1016/j.clon.2008.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.