Summary
Background
Artemisinin resistance in Plasmodium falciparum lengthens parasite clearance half-life during artemisinin monotherapy or artemisinin-based combination therapy. Absence of in-vitro and ex-vivo correlates of artemisinin resistance hinders study of this phenotype. We aimed to assess whether an in-vitro ring-stage survival assay (RSA) can identify culture-adapted P falciparum isolates from patients with slow-clearing or fast-clearing infections, to investigate the stage-dependent susceptibility of parasites to dihydroartemisinin in the in-vitro RSA, and to assess whether an ex-vivo RSA can identify artemisinin-resistant P falciparum infections.
Methods
We culture-adapted parasites from patients with long and short parasite clearance half-lives from a study done in Pursat, Cambodia, in 2010 (registered with ClinicalTrials.gov, number NCT00341003) and used novel in-vitro survival assays to explore the stage-dependent susceptibility of slow-clearing and fast-clearing parasites to dihydroartemisinin. In 2012, we implemented the RSA in prospective parasite clearance studies in Pursat, Preah Vihear, and Ratanakiri, Cambodia (NCT01736319), to measure the ex-vivo responses of parasites from patients with malaria. Continuous variables were compared with the Mann-Whitney U test. Correlations were analysed with the Spearman correlation test.
Findings
In-vitro survival rates of culture-adapted parasites from 13 slow-clearing and 13 fast-clearing infections differed significantly when assays were done on 0–3 h ring-stage parasites (10·88% vs 0·23%; p=0·007). Ex-vivo survival rates significantly correlated with in-vivo parasite clearance half-lives (n=30, r=0·74, 95% CI 0·50–0·87; p<0·0001).
Interpretation
The in-vitro RSA of 0–3 h ring-stage parasites provides a platform for the molecular characterisation of artemisinin resistance. The ex-vivo RSA can be easily implemented where surveillance for artemisinin resistance is needed.
Funding
Institut Pasteur du Cambodge and the Intramural Research Program, NIAID, NIH.
Introduction
After the WHO’s recommendation1 to use artemisinin-based combination therapies (ACTs) for the treatment of Plasmodium falciparum malaria, the burden of this disease declined substantially.2 As with earlier antimalarial drugs,3 parasite resistance to artemisinin and its derivatives has emerged in southeast Asia. Since the first reports in 2008 from Battambang4 province and 2009 from Pailin5 province, both in western Cambodia, artemisinin-resistant P falciparum malaria has been reported elsewhere in western Cambodia,6,7 western Thailand,8 southern Burma,9 and southern Vietnam.10 Artemisinin resistance threatens malaria control, treatment, and elimination efforts worldwide.11,12 To prevent the spread of artemisinin-resistant parasites throughout southeast Asia and to Africa, rapid detection of new artemisinin resistance foci and implementation of containment interventions are a top priority.13
Although artemisinin resistance has not been precisely defined, it is recognised as a relatively slow parasite clearance rate in patients receiving an artemisinin or ACT.14 The parasite clearance half-life can be estimated from frequent parasite density counts in patients with initial parasite densities of 10 000 parasites per μL of blood or greater (ie, ≥0·2% parasitaemia).15 In regions of low malaria transmission, like Cambodia, parasite clearance studies require screening of thousands of febrile individuals over entire transmission seasons to enrol the few patients (<5%) who meet inclusion criteria and agree to several days of hospitalisation. Such studies are thus logistically and financially demanding, as well as inconvenient for patients and their families. There is therefore an urgent need to develop in-vitro and ex-vivo assay readouts that correlate with parasite clearance half-life.
In-vitro readouts (ie, those obtained from culture-adapted parasite lines in the laboratory) might be useful in elucidating the molecular basis of artemisinin resistance by providing robust phenotypes for genome-wide association studies or the experimental validation of candidate molecular markers. Ex-vivo readouts (ie, those obtained from uncultured parasite isolates collected directly from patients in the field) might be useful in mapping the geographical spread or worsening of artemisinin resistance in real-time, thus providing actionable information for national malaria control programmes. So far, consistent and significant correlations between half-lives and readouts from any in-vitro or ex-vivo artemisinin susceptibility assay (eg, elevated IC50 value—the drug concentration that inhibits parasite growth by 50%) have not been shown.4-6 One potential reason for this observation is that parasites in these assays are exposed to very low concentrations of dihydroartemisinin (the active metabolite of all artemisinins) for 48–72 h, whereas parasites in vivo are exposed to much higher concentrations of dihydroartemisinin for only 1–2 h.
Artemisinin resistance in drug-selected P falciparum lines has been associated with decreased susceptibility of ring-stage parasites16-18 and, in some lines, mature trophozoite-stage parasites as well.16,19 Using a novel in-vitro assay (ring-stage survival assay; RSA),20 we recently measured the susceptibility of 0–12 h post-invasion rings to a pharmacologically relevant exposure (700 nM for 6 h) to dihydroartemisinin. We noted a 17-times higher survival rate of culture-adapted parasite isolates from Pailin province, a region of artemisinin resistance in western Cambodia, compared with those from Ratanakiri province, a region of artemisinin sensitivity in eastern Cambodia. We do not know how this geographical dichotomy relates to the clinical artemisinin resistance phenotype, because ring-stage parasites from patients with known parasite clearance kinetics have not yet been tested in the RSA.
We aimed to assess whether an in-vitro RSA can distinguish culture-adapted P falciparum isolates from patients with slow-clearing or fast-clearing infections, to investigate the stage-dependent susceptibility of parasites to dihydroartemisinin in the in-vitro RSA, and to assess whether an ex-vivo RSA can identify artemisinin-resistant P falciparum infections in patients with malaria. To mimic the in-vivo exposure of circulating, ring-stage parasites to pharmacologically relevant doses of dihydroartemisinin, we exposed synchronised, ring-stage parasites to brief, high-dose pulses of this drug in the in-vitro RSA. We similarly exposed ring-stage parasites obtained directly from patients in the ex-vivo RSA.
See Online for appendix
Methods
Study design, patients, and drug treatment
We did two clinical studies in Cambodia to measure therapeutic responses to artesunate. One study was done in 2009–10 in Pursat province (western Cambodia; registered with ClinicalTrials.gov, number NCT00341003),6 where artemisinins have been used for 35 years and artemisinin resistance is well established, and the other in 2012 in Pursat province and also in Preah Vihear (northern Cambodia) and Ratanakiri provinces (eastern Cambodia; registered with ClinicalTrials.gov, number NCT01736319), where ACTs were first used in 2000 and artemisinin resistance has not yet been reported. The studies were done in referral hospitals in each province. The Cambodian National Ethics Committee for Health Research and the US National Institute of Allergy and Infectious Diseases Institutional Review Board approved both studies.
The 2009–10 study in Pursat was previously reported.6 Patients were treated with oral doses of 4 mg/kg artesunate at 0 h, 24 h, and 48 h, and then 15 mg/kg mefloquine at 72 h and 10 mg/kg mefloquine at 96 h.
In the 2012 study, children older than 1 year and non-pregnant adults with uncomplicated falciparum malaria (parasite density ≥10 000 and ≤200 000 parasites per μL of blood) were enrolled if written informed consent was obtained from patients or parents or guardians of children. Patients with severe malaria, Plasmodium vivax infection, haematocrit less than 25%, antimalarial drug use in the past 7 days, or known allergy to artemisinins or piperaquine were excluded. Patients were treated with oral doses of Duo-Cotecxin (containing 40 mg dihydroartemisinin and 320 mg piperaquine per tablet; Holleypharma, China) at 0 h, 24 h, and 48 h. The doses were based on bodyweight: half a tablet (<10 kg), one tablet (10–19 kg), one and a half tablets (20–29 kg), two tablets (30–39 kg), and three tablets (≥40 kg).
Parasite density count, staging, and clearance
In the 2009–10 study, thick blood films were made from samples before the first dose of artesunate (0 h) and then every 6 h until asexual parasitaemia was undetectable.6 In the 2012 study, blood films were made at 0 h, 2 h, 4 h, 6 h, 8 h, and 12 h, and then every 6 h until parasitaemia was undetectable. Parasite developmental stages at 0 h were estimated as tiny or large rings on the basis of morphological criteria (appendix). After patients completed the study, parasite clearance curves were derived from parasite density counts. The parasite clearance half-life (ie, the time for parasite density to decrease by 50%) was calculated from the slope constant with the parasite clearance estimator.15,21 The half-life was deemed interpretable when the R2 value of the slope regression line was greater than 0·8.
In-vitro parasite adaptation
In the 2009–10 study, blood samples were collected into acid-citrate-dextrose vacutainers (Becton-Dickinson, Franklin Lakes, NJ, USA) at 0 h. Parasitised erythrocytes were cryopreserved in Glycerolyte 57 (Baxter Healthcare Corp, Deerfield, IL, USA)22 immediately or after short-term cultivation, and stored in liquid nitrogen until use.
Isotopic in-vitro sensitivity testing
The in-vitro sensitivity of culture-adapted parasites to artesunate and dihydroartemisinin (obtained from the Worldwide Antimalarial Resistance Network) was assessed with a 48 h isotopic test20 with drug concen trations ranging from 0·1 nM to 102·4 nM for artesunate, and from 0·0625 nM to 64 nM for dihydroartemisinin. The quality of in-vitro assays was monitored with the P falciparum 3D7 line. Results were expressed as the inhibitory concentrations IC50 and IC90, defined as the drug concentrations at which 50% or 90% of 3H-hypoxanthine (Amersham, Les Ulis, France) incorporation was inhibited compared with drug-free controls. IC50 and IC90 values were established by non-linear regression with ICEstimator software.23,24
For more on the ICEstimator see http://www.antimalarial-icestimator.net/index.htm
In-vitro survival assays
Culture-adapted parasites were synchronised twice with 5% sorbitol (Sigma-Aldrich, Singapore) at 40 h intervals. Synchronous 10–12 nuclei schizonts were incubated for 15 min at 37°C in RPMI-1640 supplemented with 15 U/mL of sodium heparin (Rotexmedica, Luitre, France) to disrupt agglutinated erythrocytes, purified on a 35%/75% Percoll (Sigma-Aldrich) discontinuous gradient, washed in RPMI-1640, and cultured for 3 h with fresh erythrocytes. Cultures were treated with 5% sorbitol to eliminate remaining schizonts, adjusted to 2% haematocrit and 1% parasitaemia by adding uninfected erythrocytes, and dispensed (2 mL per well in a 24-well culture plate) into two parallel cultures. The RSA0–3 h was done immediately with 0–3 h postinvasion rings, the RSA9–12 h with 9–12 h postinvasion rings, and the trophozoite-stage survival assay (TSA18–21 h) with 18–21 h postinvasion trophozoites.
In each assay, parasites were exposed to 700 nM dihydroartemisinin or 0·1% dimethyl sulfoxide for 6 h, washed with 12 mL RPMI-1640 to remove drug, resuspended in complete medium (RPMI-1640, 0·5% Albumax II, 2% heat-inactivated B+ plasma, 50 μg/mL gentamicin), and cultured at 37°C in a tri-gas atmosphere (5% CO2, 5% O2, 90% N2). Thin blood smears were prepared and stained with 10% Giemsa (Merck KGaA, Darmstadt, Germany) for 20 min. Survival rates were assessed microscopically by counting the proportion of viable parasites that developed into second-generation rings or trophozoites with normal morphology at 66 h (RSA0–3 h), 57 h (RSA9–12 h), and 48 h (TSA18–21 h) after drug removal. For each sample, roughly 10 000 erythrocytes were assessed independently by two microscopists (BW and CA) from whom each other’s data and half-lives were masked. When the difference between survival rates was greater than 20%, a third microscopist (DM), from whom the data were also masked, assessed the slides.25 Mean parasite counts were calculated and survival rates expressed as ratios of viable parasitaemias in dihydroartemisinin-exposed and dimethyl sulfoxide-exposed samples.
Ex-vivo survival assay
In the prospective 2012 study, ex-vivo RSAs were done on parasites directly from consecutively enrolled patients in Pursat, Preah Vihear, and Ratanakiri. 2 mL of venous blood were collected into acid-citrate-dextrose vacutainers before the first Duo-Cotecxin dose and processed within 24 h. Plasma was removed and the blood washed three times in RPMI-1640. If the parasitaemia was greater than 1%, it was adjusted to 1% by adding uninfected erythrocytes. Ex-vivo RSAs were done as above except that complete medium did not contain human plasma, parasites were not experimentally synchronised, and three different atmospheres were tested in parallel: tri-gas, 5% CO2, and candle jar. These atmospheres were used to assess whether ex-vivo RSAs can produce interpretable results in field-based or under-resourced settings where gas cylinders and gas-mixing incubators might not be available or affordable. Smears made 66 h after drug removal were assessed and survival rates calculated as described above. Results were viewed as interpretable if the parasitaemia in the sample exposed to dimethyl sulfoxide was higher than the starting parasitaemia.
Parasite genotyping
DNA was extracted from 200 μL of whole blood collected in 2010 at 0 h and from corresponding culture-adapted parasites just before in-vitro assays using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA). Parasite genotyping was done as described.26 12 single-nucleotide polymorphisms were assessed with a PCR ligase detection reaction fluorescence microspheres assay (appendix).
Statistical analysis
Data were analysed with Microsoft Excel and MedCalc version 12 (Mariakerke, Belgium). Quantitative data were expressed as median (IQR). Stage-dependent patterns of survival were expressed as the difference between RSA0–3 h and TSA18–21 h (Δ). Continuous variables were compared with the Mann-Whitney U test. Correlations were analysed with the Spearman correlation test. Ex-vivo RSA values that were obtained in three atmospheric conditions were compared with one-way repeated-measures ANOVA with Bonferroni correction for p values (Friedman test). We deemed significant p values of less than 0·05.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
From the 89 patients enrolled, we selected 18 fast-clearing and 20 slow-clearing parasites representing the lower and upper quartiles of the half-life distribution and adapted them to culture as described.20 Assays were ultimately done on parasites from 13 fast-clearing and 13 slow-clearing infections; the other 12 selected parasites were excluded from the study because they did not adapt to culture, did not have a corresponding half-life value that was interpretable, or did not show an identical genotype to the parasite originally obtained from the patient (appendix).
Parasites were collected in Pursat in 2010 (appendix), and used in three stage-specific survival assays (figure 1). In the RSA for 0–3 h rings (RSA0–3 h), the median survival rate of slow-clearing parasites was 47 times greater than that of fast-clearing parasites (table, figure 2). By contrast, 9–12 h rings and 18–21 h trophozoites from fast-clearing and slow-clearing infections showed no significant difference in survival (table, figure 2). The stage-dependent survival patterns differed between fast-clearing and slow-clearing parasites (figure 2, appendix). Specifically, the survival rates of slow-clearing parasites decreased with parasite stage, whereas those of fast-clearing parasites increased with parasite stage (Δ=9·9% [IQR 1·7 to 14·4] vs –0·3% [–1·1 to 0·4]; p=0·007). In an isotope-based sensitivity assay that monitored replication of parasites exposed to drug for 48 h,27 fast-clearing and slow-clearing parasites did not differ significantly in IC50 and IC90 values for artesunate or dihydroartemisinin (table; appendix).
Figure 1. Dihydroartemisinin survival assays.
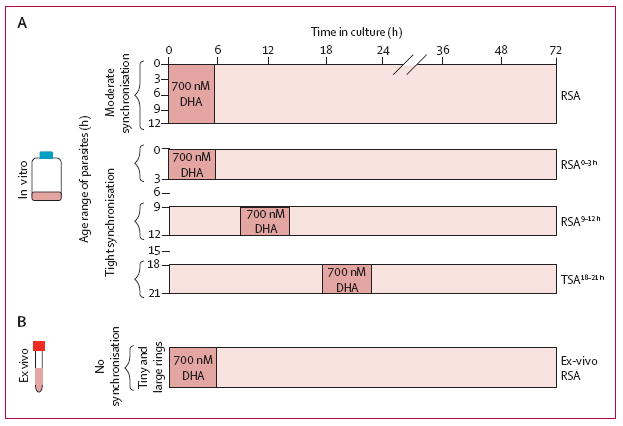
Synchronisation and timing of DHA exposure (A) for four in-vitro survival assays—RSA, previously described by Witkowski and colleagues,20 RSA0–3 h, RSA9–12 h, and TSA18–21 h—done on culture-adapted Plasmodium falciparum isolates. During their 48 h cycle of intraerythrocytic development, parasites circulate as ring-stages (0–18 h) and then sequester by specifically adhering to the endothelium of microvessels, where they mature into trophozoites (18–36 h) and schizonts (36–48 h). Because of sequestration, clinical studies assess the clearance rate of circulating ring-stage parasites only. In individual patients, the actual age-distribution of parasites circulating in peripheral blood is unknown and can vary from patient to patient. The timing of dihydroartemisinin exposure for the ex-vivo survival assay (B) done on circulating, ring-stage parasites (0–18 h) obtained directly from the blood of patients with uncomplicated malaria. This assay thus measures the dihydroartemisinin susceptibility of the parasite isolate at the same developmental stage and at the same time as the in-vivo parasite clearance study.
DHA=dihydroartemisnin. RSA=ring-stage survival assay. TSA=trophozoite-stage survival assay.
Table.
Parasite survival in in-vitro assays using culture-adapted isolates
| Fast-clearing parasites (short half-life) | Slow-clearing parasites (long half-life) | p value | |
|---|---|---|---|
| RSA0–3 h | 0·23 (0·14–2·93, 0·01–51·39) | 10·88 (4·75–13·91, 0·16–29·14) | 0·007 |
| RSA9–12 h | 1·07 (0·77–1·70, 0·06–10·00) | 2·12 (1·46–3·55, 0·33–8·00) | 0·06 |
| TSA18–21 h | 0·99 (0·48–2·20, 0·16–4·10) | 1·16 (0·78–2·05, 0·38–5·30) | 0·54 |
| Dihydroartemisinin IC50 | 0·71 (0·58–0·94, 0·29–1·20) | 0·79 (0·62–0·11, 0·42–1·51) | 0·44 |
| Dihydroartemisinin IC90 | 2·60 (2·28–3·30, 1·54–4·49) | 2·46 (1·78–3·02, 1·48–4·40) | 0·36 |
| Artesunate IC50 | 1·00 (0·84–1·47, 0·28–1·71) | 1·11 (0·98–1·84, 0·83–2·50) | 0·20 |
| Artesunate IC90 | 3·32 (2·52–3·94, 2·30–5·80) | 3·02 (2·38–3·86, 1·99–6·38) | 0·70 |
Data are median (IQR, range). Percentage survival in RSA0–3 h, RSA9–12 h, and TSA18–21 h, and IC50 and IC90 dihydroartemisinin and artesunate in isotope-based sensitivity assays. p values for significance from Mann-Whitney test. RSA=ring-stage survival assay. TSA=trophozoite-stage survival assay.
Figure 2. In-vitro survival after exposure to dihydroartemisinin.
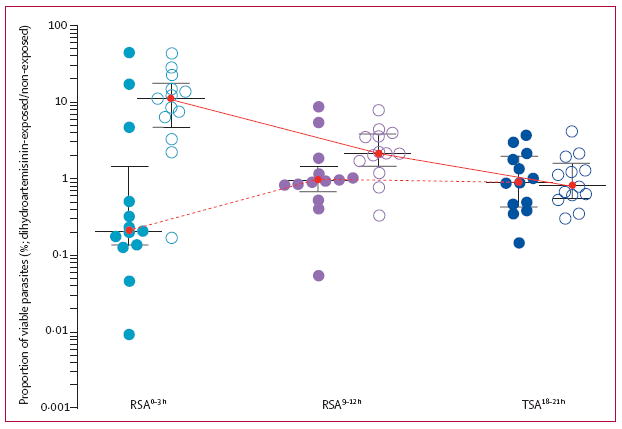
Results are expressed as the proportion of viable Plasmodium falciparum parasites after a 6 h exposure of 0–3 h rings (RSA0–3 h), 9–12 h rings (RSA39–12 h), and 18–21 h trophozoites (TSA18–21 h) to 700 nM dihydroartemisinin compared with dimethyl sulfoxide. These assays were done on culture-adapted parasite isolates obtained from 13 patients with fast-clearing infections (filled circles) and 13 patients with slow-clearing infections (open circles) in Pursat in 2010. The horizontal lines represent the medians and whiskers the IQRs. The solid lines show stage-dependent survival pattern of parasites from slow-clearing infections and the dotted lines the stage-dependent survival pattern of parasites from fast-clearing infections. RSA=ring-stage survival assay. TSA=trophozoite-stage survival assay.
In patients with falciparum malaria, the age distribution of circulating ring-stage parasites is heterogeneous, ranging from 0 h to 18 h at the time of clinical presentation;28 that is, ring-stage parasites are not necessarily tightly synchronised at the 0–3 h age of development. We therefore sought to assess whether an ex-vivo RSA could distinguish fast-clearing from slow-clearing parasites that have been neither culture-adapted nor experimentally synchronised.
In the prospective 2012 study, 30 (83%) of 36 patients had interpretable half-life values and tri-gas survival rates (appendix), which correlated significantly (figure 3). Parasite survival rates did not differ between the three atmospheres (n=26; p=0·30, Friedman test). The ex-vivo RSA accurately identified artemisinin-resistant infections where they have not been previously described (figure 3, appendix). In Preah Vihear, for example, one parasite with a 12·2% survival rate had an 8·17 h half-life, whereas the other six parasites with a median 0·70% survival rate (IQR 0·18–2·0) had a median 2·28 h half-life (1·89–3·52). In Ratanakiri, one parasite with a 38·3% survival rate had a 9·06 h half-life, whereas the other ten parasites had a median 0·40% survival rate (0·26–1·48) and a median 2·28 h half-life (1·90–2·64). Our findings suggest that artemisinin-resistant P falciparum has spread or independently emerged in northern and eastern Cambodia, a possibility that can now be confirmed with the in-vitro RSA0–3 h.
Figure 3. Correlation of in-vivo parasite clearance half-lives and ex-vivo dihydroartemisinin survival rates.
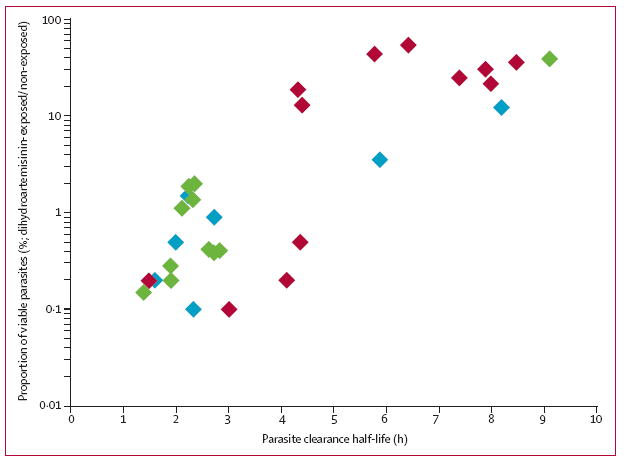
Ex-vivo ring-stage survival assays (RSAs) were done on parasite isolates obtained directly from patients with malaria in Pursat, Preah Vihear, and Ratanakiri in 2012. Results from the ex-vivo RSAs are expressed as the proportion of viable parasites after a 6 h exposure to 700 nM dihydroartemisinin compared with dimethyl-sulfoxide-exposed controls. Results from the parasite clearance studies are expressed as the parasite clearance half-life in hours. The proportion of viable parasites in ex-vivo RSAs correlated significantly with the parasite clearance half-life (r=0·74, 95% CI 0·50–0·87; p<0·0001) in Pursat (red), Preah Vihear (blue), and Ratanakiri (green).
Discussion
P falciparum isolates from slow-clearing and fast-clearing infections in Cambodia respond differently to a 6 h, 700 nM exposure to dihydroartemisinin. In the RSA0–3 h, rings of slow-clearing parasites had much higher survival rates than those of fast-clearing parasites. In the ex-vivo RSA, survival rates correlated with parasite clearance half-lives. Importantly, the ex-vivo RSA accurately identified slow-clearing infections in Cambodian provinces where they have not yet been described. To our knowledge, these are the first reported in-vitro and ex-vivo dihydroartemisinin susceptibility data that correlate with in-vivo parasite clearance half-lives (panel). These data qualify the in-vitro RSA0–3 h as a new laboratory test for elucidating the mechanism of artemisinin resistance through molecular studies. These studies might include genome-wide association studies,29 associating RSA0–3 h survival rates with whole-genome single-nucleotide polymorphism data, phenotypic screening of parasite progeny clones obtained from genetic crosses between artemisinin-sensitive and artemisinin-resistant parental lines, phenotypic characterisation of the different artemisinin-resistant parasite subpopulations circulating in western Cambodia,29 and validation of candidate molecular markers through genetic manipulation of parasites.
Our findings also suggest that the ex-vivo RSA is a feasible, convenient method for detecting the spread and emergence of artemisinin resistance in areas where it has not yet been reported (eg, eastern Cambodia), or the worsening of artemisinin resistance where it is entrenched (eg, western Cambodia). Both types of findings from ex-vivo RSAs might inform national malaria control programmes to expand or intensify containment measures. In a screen and confirm approach to support such efforts, we propose that the ex-vivo RSA be used in the field to screen for artemisinin-resistant parasites. Any parasite showing dihydroartemisinin resistance in this assay can then be adapted to short-term culture in a laboratory, genotyped to ensure its identity to the clinical parasite isolate obtained from a patient, and tested to confirm dihydroartemisinin resistance with the in-vitro RSA0–3 h.
For both artemisinin-sensitive and artemisinin-resistant parasites, we show stage-dependent heterogeneity of dihydroartemisinin susceptibility in ring forms. In artemisinin-sensitive parasites, 0–3 h rings were more susceptible to dihydroartemisinin than were 9–12 h rings. This finding with clinical parasite isolates is consistent with the recent report that 2–4 h rings of artemisinin-sensitive laboratory lines are specifically hypersensitive to dihydroartemisinin.18 However, in artemisinin-resistant parasites, 0–3 h rings were less susceptible to dihydroartemisinin than were 9–12 h rings. We tentatively conclude that the susceptibility of Cambodian parasites to dihydroartemisinin is controlled predominantly at the 0–3 h stage of parasite development. This interpretation, and our finding that trophozoites are mostly susceptible to dihydroartemisinin irrespective of half-life, is consistent with mathematical modelling predictions30 and transcriptomics data31 from studies of ring-stage parasites.
Half-lives and RSA0–3 h survival rates were discordant in four patients (figure 2, appendix). Three patients (1007, 1006, and 1009) had fast-clearing infections with parasites showing survival rates of 5·3%, 19·3%, and 51·4%, and a resistant stage-dependent pattern (Δ=1·2%, 17·3%, and 50·2%, respectively). Their patterns differed from those of fast clearing-infections (Δ=–0·7% vs 17·3%; p=0·01), being similar to those from slow-clearing-infections (Δ=10·3% vs 17·3%; p=0·56; appendix). To explain this discordance, we postulated that these three parasites had already developed into dihydroartemisinin-susceptible, late ring-stage parasites in the patients’ blood at the time of the first artesunate dose.
To assess this possibility, we reviewed the initial blood smears from these patients and estimated the relative age of their ring-stage parasites just before treatment with artesunate (appendix). In thin blood smears made at 0 h, we noted that these three discordant patients indeed had a two-times lower proportion of tiny rings compared with the 12 concordant patients from the slow-clearing group (ie, those having slow-clearing infections with dihydroartemisinin-resistant parasites; 42·4% vs 75·0%; p=0·03). Higher proportions of large, older rings could account for shorter than expected half-lives because these forms are more susceptible to dihydroartemisinin than tiny, young rings. Overall, our findings suggest that the relative abundance of tiny and large rings at the time of the first artemisinin dose affects the parasite clearance half-life, and that accurate ex-vivo staging of parasites is crucial for classifying treatment outcome. In one patient (896), having a slow-clearing infection with a parasite showing a survival rate of 0·2%, and a sensitive stage-dependent pattern (Δ=−1·4%; appendix), we cannot rule out an inadequate immune response to infection32 or insufficient plasma concentrations of artemisinins.
For the standard operating procedures for ex-vivo and in-vitro RSA see http://www.wwarn.org/toolkit/procedures/invitro
The RSA0–3 h survival rate might be crucially informative in ongoing parasite genetics studies31,33,34 aimed at identifying loci under artemisinin selection, because it is unaffected by in-vivo variables (eg, pharmacokinetics, haemoglobin type, and acquired immunity) that might affect the parasite clearance half-life. Although this phenotype might also be a useful readout in studies to define and validate molecular markers for tracking artemisinin-resistant parasites in the field, the RSA0–3 h is a laborious assay. By contrast, the ex-vivo RSA saves weeks of effort (results are available in 3 days), and avoids the confounding effects of parasite clone elimination and metabolic changes that might accompany the culture adaptation of parasites. In addition to implementing methods that more precisely establish the age of rings, FACS-based or ELISA-based analysis of parasite viability should improve the throughput of dihydroartemisinin-susceptibility studies. Until such methods are developed and validated, we propose the simple ex-vivo RSA as a highly informative surveillance approach for the identification of artemisinin-resistant parasites in areas where slow parasite clearance is suspected. Investigating the relation between RSA survival rates ex vivo and parasite recrudescence rates in vivo might be useful in assessing the clinical effect of artemisinin resistance.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed with the terms “artemisinin resistant malaria”, limited our search to clinical trials, and used no date or language restrictions. This process produced 56 publications. Any in-vitro or ex-vivo drug assays done in these studies involved the continuous exposure of Plasmodium falciparum parasites to very low concentrations of artemisinins during the entire lifecycle of their blood-stage development. Results of these assays have not consistently correlated with clinical efficacy.4-6 Only four recent publications describe new assays that were specifically designed to measure P falciparum susceptibility to artemisinins.17-20 Klonis and colleagues18 described an assay working with laboratory-adapted parasites that were artemisinin-sensitive. Witkowski and coworkers17 and Teuscher and colleagues19 described assays working with laboratory-adapted parasites and their drug-selected counterparts that became resistant to artemisinins. Witkowski and colleagues20 described a more relevant study using parasites from western and eastern Cambodia, where parasites are commonly resistant and sensitive to artemisinin respectively. None of these studies were designed to test whether in-vitro susceptibility data correlated with in-vivo efficacy data (ie, parasite clearance rates after artemisinin treatment, the currently accepted clinical phenotype); therefore, these assays could not be clinically validated.
Interpretation
We report for the first time novel in-vitro and ex-vivo ring-stage survival assays (RSAs) that detect artemisinin-resistant, slow-clearing P falciparum infections in patients with malaria. In both assays, early ring-stage parasites are exposed to a pharmacologically relevant pulse of dihydroartemisinin and their survival measured 72 h later. With parasites adapted to culture in the laboratory, the in-vitro RSA can be used to discover the molecular mechanisms of artemisinin resistance, to investigate the mode of action of artemisinins, and to identify artemisinin-resistant parasite strains for testing next-generation antimalarial drugs. The ex-vivo RSA with parasites obtained directly from patients with malaria can be easily implemented in field-based settings to monitor the worsening of artemisinin resistance where it is highly prevalent (eg, western Cambodia), and to map its spread or independent emergence elsewhere in the Greater Mekong Subregion. Also, this simple test might readily be established at sentinel sites in sub-Saharan Africa, where the arrival of artemisinin-resistant P falciparum is expected to be especially devastating. The ex-vivo RSA can thus provide crucial surveillance data to the national malaria control programmes of all countries threatened by artemisinin-resistant malaria.
Acknowledgments
We thank Robert Gwadz, Savuth Koeuth, François Nosten, Eng Ly Pech, Thomas Wellems, and Chongjun Zhou for their efforts in support of this work. This study was funded by the Intramural Research Program, NIAID, NIH, and by grants from Institut Pasteur du Cambodge (Institut Pasteur, International Division and Banque Natixis) and Laboratoire d’excellence IBEID (Agence Nationale de la Recherche, France). BW is supported by a postdoctoral fellowship from the International Division, Institut Pasteur, and DM by the French Ministry of Foreign Affairs.
Footnotes
Contributors
BW, CA, PL, JMA, SK, SD, CMC, WRJT, OM-P, RMF, and DM contributed to study design. NK genotyped parasites. BW, CA, and PC did the in-vitro and ex-vivo drug assays. SSr, SM, CS, BS, and SSu gathered clinical data. BW, CA, OM-P, RMF, and DM analysed data and wrote the report.
Conflicts of interest
We declare that we have no conflicts of interest.
Contributor Information
Benoit Witkowski, Malaria Molecular Epidemiology Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia.
Chanaki Amaratunga, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Nimol Khim, Malaria Molecular Epidemiology Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia.
Sokunthea Sreng, National Centre for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia.
Pheaktra Chim, Malaria Molecular Epidemiology Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia.
Saorin Kim, Malaria Molecular Epidemiology Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia.
Pharath Lim, Malaria Molecular Epidemiology Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia; Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA; National Centre for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia.
Sivanna Mao, Sampov Meas Referral Hospital, Pursat, Cambodia.
Chantha Sopha, Makara 16 Referral Hospital, Preah Vihear, Cambodia.
Baramey Sam, Ratanakiri Referral Hospital, Ratanakiri, Cambodia.
Jennifer M Anderson, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Prof Socheat Duong, National Centre for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia.
Char Meng Chuor, National Centre for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia.
Walter R J Taylor, Service de Médecine Internationale et Humanitaire, Hôpitaux Universitaires de Genève, Geneva, Switzerland.
Seila Suon, National Centre for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia.
Odile Mercereau-Puijalon, Parasite Molecular Immunology Unit, Institut Pasteur, Paris, France.
Rick M Fairhurst, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Didier Menard, Malaria Molecular Epidemiology Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia.
References
- 1.WHO. Antimalarial drug combination therapy. Geneva: World Health Organization; 2001. [Google Scholar]
- 2.WHO. World malaria report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 3.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, et al. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–18. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 4.Noedl H, Se Y, Schaecher K, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 5.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaratunga C, Sreng S, Suon S, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–58. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaratunga C, Mao S, Sreng S, et al. Slow parasite clearance rates in response to artemether in patients with severe malaria. Lancet Infect Dis. 2013;13:113–14. doi: 10.1016/S1473-3099(12)70347-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phyo AP, Nkhoma S, Stepniewska K, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–66. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyaw MP, Nyunt MH, Chit K, et al. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS One. 2013;8:e57689. doi: 10.1371/journal.pone.0057689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hien TT, Thuy-Nhien NT, Phu NH, et al. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J. 2012;11:355. doi: 10.1186/1475-2875-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enserink M. Malaria’s drug miracle in danger. Science. 2010;328:844–46. doi: 10.1126/science.328.5980.844. [DOI] [PubMed] [Google Scholar]
- 12.Dondorp AM, Fairhurst RM, Slutsker L, et al. The threat of artemisinin-resistant malaria. N Engl J Med. 2011;365:1073–75. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Emergency response to artemisinin resistance in the Greater Mekong subregion: regional framework for action 2013–2015. Geneva: World Health Organization; 2013. [Google Scholar]
- 14.White NJ. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegg JA, Guerin PJ, White NJ, et al. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui L, Wang Z, Miao J, et al. Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum. Mol Microbiol. 2012;86:111–28. doi: 10.1111/j.1365-2958.2012.08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witkowski B, Lelievre J, Barragan MJ, et al. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother. 2010;54:1872–77. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klonis N, Xie SC, McCaw JM, et al. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc Natl Acad Sci USA. 2013;110:5157–62. doi: 10.1073/pnas.1217452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teuscher F, Chen N, Kyle DE, et al. Phenotypic changes in artemisinin-resistant Plasmodium falciparum lines in vitro: evidence for decreased sensitivity to dormancy and growth inhibition. Antimicrob Agents Chemother. 2012;56:428–31. doi: 10.1128/AAC.05456-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witkowski B, Khim N, Chim P, et al. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother. 2012;57:914–23. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WWARN. [Aug 21, 2013];Parasite clearance estimator. https://www.wwarn.org/toolkit/data-management/parasite-clearance-estimator.
- 22.Moll K, Ljungström I, Perlmann H, et al., editors. [Aug 21, 2013];Methods in malaria research. (5). (version 5.2 revision). http://www.mr4.org/Portals/3/Methods_In_Malaria_Research-5theditionv5-2.pdf.
- 23.Le Nagard H, Vincent C, Mentre F, et al. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput Methods Programs Biomed. 2011;104:10–18. doi: 10.1016/j.cmpb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Kaddouri H, Nakache S, Houze S, et al. Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from Africa by using a plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob Agents Chemother. 2006;50:3343–49. doi: 10.1128/AAC.00367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Malaria microscopy quality assurance manual—version 1. Geneva: World Health Organization; 2009. [Google Scholar]
- 26.Daniels R, Volkman SK, Milner DA, et al. A general SNP-based molecular barcode for Plasmodium falciparum identification and tracking. Malar J. 2008;7:223. doi: 10.1186/1475-2875-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desjardins RE, Canfield CJ, Haynes JD, et al. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–18. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silamut K, White NJ. Relation of the stage of parasite development in the peripheral blood to prognosis in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1993;87:436–43. doi: 10.1016/0035-9203(93)90028-o. [DOI] [PubMed] [Google Scholar]
- 29.Miotto O, Almagro-Garcia J, Manske M, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45:648–55. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saralamba S, Pan-Ngum W, Maude RJ, et al. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 2010;108:397–402. doi: 10.1073/pnas.1006113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mok S, Imwong M, Mackinnon MJ, et al. Artemisinin resistance in Plasmodium falciparum is associated with an altered temporal pattern of transcription. BMC Genomics. 2011;12:391. doi: 10.1186/1471-2164-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopera-Mesa TM, Doumbia S, Chiang S, et al. Plasmodium falciparum clearance rates in response to artesunate in Malian children with malaria: effect of acquired immunity. J Infect Dis. 2013;207:1655–63. doi: 10.1093/infdis/jit082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheeseman IH, Miller BA, Nair S, et al. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336:79–82. doi: 10.1126/science.1215966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takala-Harrison S, Clark TG, Jacob CG, et al. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in southeast Asia. Proc Natl Acad Sci USA. 2012;110:240–45. doi: 10.1073/pnas.1211205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


