Abstract
Previous work from our group has shown that chronic homotypic stress (repeated restraint – RR) increases microglial morphological activation in the prefrontal cortex (PFC), while chronic heterotypic stress (chronic variable stress – CVS) produces no such effect. Therefore, we hypothesized that stressor modality would also determine the susceptibility of the PFC to a subsequent inflammatory stimulus (low dose lipopolysaccharide (LPS)). We found that RR, but not CVS, increased Iba-1 soma size in the PFC after LPS injection, consistent with microglial activation. In contrast, CVS decreased gene expression of proinflammatory cytokines and Iba-1 in the PFC under baseline conditions, which were not further affected by LPS. Thus, RR appears to promote microglial responses to LPS, whereas CVS is largely immunosuppressive. The results suggest that neuroimmune changes caused by CVS may to some extent protect the PFC from subsequent inflammatory stimuli. These data suggest that modality and/or intensity of stressful experiences will be a major determinant of central inflammation and its effect on prefrontal cortex-mediated functions.
1. Introduction
Stress-induced changes in the CNS immune environment are thought to play a major role in the development of stress related disorders, such as depression (Kreisel et al 2014; Leonard 2005; Hayley et al 2003; Grippo et al 2005; Wager-Smith & Markou 2011). Stress influences CNS immune responses via a variety of mechanisms, including glucocorticoid (Frank et al 2012) or β-adrenergic receptor signaling (Porterfield et al 2012). Microglia, the major immune cells of the brain, show dynamic responses to stress that contribute to behavioral and hormonal consequences (Walker et al 2013). Stress can have differential effects on microglial function, inducing pro- or anti-inflammatory responses. In many stress-responsive brain regions, acute stress increases microglial numbers and markers of microglial activation (Kreisel et al 2014; Johnson et al 2002; Sugama et al 2007; Jankord et al 2010). However, under persistent chronic unpredictable stress, microglial activation can become greatly reduced and the cells can even undergo apoptosis (Kreisel et al 2014). Reversing either the acute or chronic effects of stress on microglia can attenuate the development of stress-induced depression-like behavior (Kreisel et al 2014). Furthermore, antidepressant treatment can reverse both acute stress-induced microglial proliferation (Kreisel et al 2014) and chronic stress-induced immunosuppression (Basso et al 1993) and microglial decline (Kreisel et al 2014).
Conversely, there are a variety of other chronic stressors (repeated restraint stress, repeated social defeat, and chronic intermittent cold stress) that are known to enhance microglial activation in a variety of stress-responsive brain regions (Tynan et al 2010; Gadek et al 2012; Wohleb et al 2012; Reader et al 2015; Porterfield et al 2012; Girotti et al 2011). This enhanced microglial activation may potentiate central immune responses to subsequent inflammatory challenge, called priming. Stress primes CNS immune responses by enhancing microglial activation and prolonging cytokine release to a subsequent stressor or immune challenge, such as exposure to lipopolysaccharide (LPS). These enhanced microglial responses primed by stress contribute to the behavioral and hormonal consequences of stress (Hinwood et al 2012; Gadek et al 2012; Wohleb et al 2012; Grippo et al 2005).
Previous work by our group has shown that chronic stress modality can affect microglial activation in the prefrontal cortex (PFC) (Kopp et al 2013). The PFC is a major stress regulatory brain region, and PFC dysfunction is implicated in numerous stress related disorders, including major depression (Drevets et al 1997; Drevets et al 2008; Mayberg et al 2005; Lemogne et al 2012). Importantly, our work suggests that the characteristics of different stress regimens may differentially affect PFC inflammation: chronic homotypic stress (repeated restraint stress) increases Iba-1 coverage in the PFC whereas chronic heterotypic stress (chronic variable stress) does not affect PFC Iba-1 percent area (Kopp et al 2013). The present study was designed to explore possible differences in chronic stress modality by testing for prolonged or sustained PFC microglial activation following LPS. We compared the effects of repeated restraint stress versus chronic variable stress on PFC microglial morphology and cytokine expression after subsequent peripheral immune challenge with a low dose of lipopolysaccharide (LPS). Our results indicate that repeated restraint stress enhances Iba-1 cell activation by LPS (indicated by increased Iba-1 soma size), while chronic variable stress blunts PFC cytokine and Iba-1 gene expression despite LPS challenge, demonstrating differential effects of chronic stress modality on PFC inflammatory tone. These findings highlight the effects of different chronic stress protocols on the PFC immune environment, which likely will have important implications for understanding PFC behaviors that are linked to the development of stress-related disorders.
2. Methods
2.1 Subjects
Adult male Sprague-Dawley rats, purchased from Harlan (Indianapolis IN USA), were acclimated to the colony room for 1 week. The colony room was humidity and temperature controlled with a 12 hour light cycle (lights on at 6:00am, lights off at 6:00pm). Rats were housed two per cage in clear polycarbonate cages, with corncob bedding, with food and water available ad libitum. Male rats, weighing 270–300 g when the experiment began, were randomly placed into one of three groups: unhandled control (CON, n = 18), chronic variable stress (CVS, n = 18), and repeated restraint (RR, n = 18). All procedures were carried out in compliance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
2.2 Stress regimens
For CVS, subjects were given two stressors per day for 14 consecutive days, with each stressor lasting for 1 h or less (except for overnight stressors). Morning and afternoon stressors were spaced by at least 2 h, with the morning stressor taking place any time between 08:00 – 11:300 h and afternoon stressor between 13:30 – 17:00 h. Stressors were scheduled in an unpredictable manner and included: cold swim (10 min at 16 – 18 °C), hypoxia (30 min with 92%N2/8%O2), open field in a guinea pig cage with bright light (5 min), elevated plus maze (5 min), cart transport (15 min), rotational shaker (1 h at 100rpm), cold room (1 h at 4°C), overnight crowding (6 per rat cage), and overnight single housing in a mouse cage. For RR, subjects were placed in Plexiglas® restraint cylinders in their home cage for 30 min every morning at 10:00 h. The RR regimen also lasted 14 consecutive days, concurrent with the CVS regimen. Animals in the CON group were unhandled.
2.3 LPS administration
On the morning of the 15th day, 12 of the 18 subjects from each group were given an intraperitoneal (i.p.) injection of 50μg/kg of lipopolysaccharide (LPS, Sigma Aldrich St. Louis MO USA) dissolved in sterile saline (1mL/kg injection volume). The remaining 6 of 18 subjects from each group received an equal volume i.p. injection of vehicle (sterile saline (SAL)).
2.4 Experimental Time points
Six animals from each stress group were killed via rapid decapitation 6 hours after injection on the 15th day (n = 6 each for 6h CON LPS, 6h RR LPS, 6h CVS LPS). There were no SAL groups at the 6h time point. On the morning of the 16th day (24 h post LPS/SAL injection), 6 animals from each group were killed (n = 6 each for 24h CON SAL, 24h RR SAL, 24h CVS SAL, 24h CON LPS, 24h RR LPS, 24 CVS LPS). Our primary measure was the 24h time point, since microglial activation to LPS can be detected immunohistochemically 24h after LPS injection (Wang et al 2009, Kongsui et al 2015) and we sought to simultaneously determine whether stress altered cytokine and microglial mRNA expression for a more prolonged period after LPS. As a secondary measure, we collected tissue at the 6h time point to determine whether stress altered early increases in proinflammatory cytokine expression following LPS.
2.5 Tissue Extraction
Following decapitation, trunk blood was collected into tubes with EDTA for corticosterone radioimmunoassay (RIA). Brains were promptly removed and hemisected. One half of each brain was flash frozen in isopentane at −30°C and stored at −80°C for qPCR. The other half was post-fixed for immunohistochemistry, using a protocol that was graciously provided by Dr. Arshad Khan and described in Khan & Watts 2004. Briefly, hemisected brains were placed in semi-frozen 4% paraformaldehyde (PFA) fix with sodium acetate buffer (pH 6.0, 1–4°C) for 24 h, followed by 4% PFA fix with sodium borate (pH 9.5, 4°C) for 2 days, 4% PFA/20% glycerol buffered with 0.1M sodium phosphate overnight, and finally transferred to 30% sucrose/0.1M sodium phosphate buffer at 4°C until sectioning with a microtome.
2.6 Quantitative PCR
Using a cryostat, the prelimbic (PL) and infralimbic (IL) divisions of the prefrontal cortex were micropunched (1mm each) and placed together in RNAlater® solution (Ambion) at −80°C. PL and IL punches were pooled to ensure sufficient RNA yield, since brains were hemisected. To prepare for RNA isolation, punches were thawed and manually homogenized with pestles. The RNA isolation was performed using a kit from Ambion, according to manufacturer’s instructions, with DNA decontamination steps at the end. RNA was quantified by low range RiboGreen® Assay (Invitrogen), with detected fluorescence measured as RNA quantity. RNA was reverse transcribed to cDNA (Invitrogen). Primers for genes PTGES, IL6, TANK, TNFa, IL18, IL1B, IL10, AIF1, ITGAM, and RPL32 were purchased from Invitrogen (see Table 1). RT PCR was performed individually for each primer on all samples with Taqman® 10μl fast reaction, according to instructions from the Invitrogen kit. Samples were run in duplicate. Average CT values above 37 were considered not expressed, and data not analyzed. Two punches included the corpus callosum, and were excluded. Therefore, for all qPCR data, n = 6 for 6h CON, 6h RR, 6h CVS, 24h CON LPS, 24h RR LPS, 24h CVS LPS, 24h CVS SAL and n = 5 for 24h CON SAL and 24h RR SAL. The data were analyzed by calculating expression relative to L32, finding ΔΔCT values, and expressing all values as % expression relative to the 24h unstressed saline treated group (24 CON SAL).
Table 1.
qPCR: List of primer names, primer reference number, accession number, and exon boundary.
| Target | Gene name | Accession # | Ref # | Exon boundary |
|---|---|---|---|---|
| IL10 | Interleukin 10 | NM_012854.2 | Rn00563409_m1 | 4 – 5 |
| ITGAM (CD11b) | Integrin alpha M (complement component 3 receptor 3 subunit) | NM_012711.1 | Rn00709342_m1 | 1 – 2 |
| TNF | Tumor necrosis factor | NM_012675.3 | Rn99999017_m1 | 2 – 3 |
| IL6 | Interleukin 6 | NM_012589.2 | Rn01410330_m1 | 3 – 4 |
| IL1b | Interleukin 1 beta | NM_031512.2 | Rn00580432-m1 | 5 – 6 |
| PTGES (mPGES-1) | Prostaglandin E synthase | NM_021583.3 | Rn00572047_m1 | 2 – 3 |
| TANK | TRAF family member associated NFKB activator (1, 2) |
NM_001164073.1 NM_145788.2 |
Rn00595794_m1 | 7 – 8 |
| IL18 | Interleukin 18 | NM_019165.1 | Rn01422083_m1 | 3 – 4 |
| AIF1 (Iba-1) | Allograft inflammatory factor 1 | NM_017196.3 | Rn00574125_g1 | 5 – 6 |
| RPL32 | Ribosomal protein L32 | NM_013226.2 | Rn00820748_g1 | 4 – 4 |
2.7 Immunohistochemistry
We used ionized calcium binding adaptor molecule 1 (Iba-1) as a marker of microglia. Iba-1 is restricted to microglia and macrophages, and mediates membrane motility for phagocytosis (Ohsawa et al 2000). Consequently, Iba-1 is not detectable in neurons, astrocytes, or oligodendrocytes (Ito et al 1988). For immunohistochemistry, hemisected brains were removed from 30% sucrose, frozen to a dry ice stage, and sectioned with a sliding microtome. The 35μm sections were stored at −20°C in cryoprotectant (0.1 M phosphate buffer, 30% sucrose, 1% polyvinylpyrrolidone, 30% ethylene glycol). Sections were removed from cryoprotectant and rinsed 5 × 5 min in 50mM potassium phosphate buffered saline (KPBS, pH 7.2) at room temperature (RT). The remaining steps were also done at RT. After rinsing, sections were incubated for 1 h in blocking solution (0.1% bovine serum albumin (BSA), 0.2% Triton-X 100, 50mM KPBS). Next, sections were incubated overnight in primary anti-Iba-1 polyclonal rabbit antibody (1:1500, Synaptic Systems, Goettingen, Germany) and primary anti-NeuN monoclonal mouse antibody (1:200, Millipore, Temecula CA USA), diluted in blocking solution. On the second day, sections were rinsed 5 × 5 min with KPBS before 1 h incubation with Cy 3 donkey anti-rabbit (Jackson Immuno Research, West Grove, PA USA) and Alexa 488 goat anti-mouse (Molecular Probes, Eugene OR USA). These fluorescent secondary antibodies were each diluted 1:500 in 0.1% BSA with 50mM KPBS. Finally, sections were rinsed 4 × 5 min in KPBS and mounted onto slides. After drying, slides were immersed in Nano-H2O for 1 min and coverslipped for imaging, using Fluka mounting medium (Sigma Aldrich, St. Louis MO USA).
2.8 Imaging and Histological Analysis of Microglia
Using Zeiss Axiovision 4.6 software and the Paxinos & Watson rat brain atlas (Paxinos & Watson 1997), the medial prefrontal cortex (PFC, AP + 3.5, DV − 3.0 to − 5.0, ML ± 0.25 to 1.0) was imaged and analyzed by an individual blind to the experimental conditions. The Alexa 488 NeuN label was used to discriminate PFC cell layers, while the Cy 3 Iba-1 label was used to analyze Iba-1 positive cells. At 40× magnification, Cy 3 Z-stacks were taken from layers 5/6 of the prelimbic (PL) and infralimbic (IL) divisions of the PFC. These individual Z-stacks were used to create consecutive projection images, each 7μm in thickness. The Iba-1 positive cells were manually counted in the projection images for each division of the PFC. Iba-1 percent area was measured in the PL and IL with a percent area tool on Axiovision software as previously described in Kopp et al 2013. Condensed images were also examined using Image J software (NIH open access) to quantify soma perimeter, a parameter used for assessing Iba-1 cell activation. The perimeter of the soma was measured using the ImageJ software tool “Freehand line”, as described in Cutando et al 2013. Iba-1 cells were only included for analysis if the whole cell was present and not overlapping any other cells in the image. Contrast was increased to clearly see the edges of each cell. Afterwards, the soma perimeter was measured using the ImageJ software tool “Measure” under the “Analyze” option. An equal number of measurements (3 cells per animal for each region (PL and IL)) were taken in each area for each animal, then averaged and converted to μm based on the scale set in the Image J program (150 pixels/inch).
2.9 Corticosterone Radioimmunoassay (RIA)
Plasma was collected from blood samples centrifuged at 4°C for 20 min at 1800 × g and stored at −80°C. Samples were thawed on ice and plasma corticosterone levels were measured with RIA 125I kit (MP Biomedicals, Solon OH USA).
2.10 Statistical Analysis
Data are graphed as mean ± SE. Two-way repeated measures ANOVA was used for body weight changes and two-way ANOVAs were used for analyzing the remaining data. Critical significance level was set at p < 0.05 and Fisher’s Least Significant Difference (LSD) post-hoc test was used with stress group (CON, RR, CVS) and treatment (LPS, SAL; with two-way ANOVAs) as between-subjects factors. For the 6h vs 24h comparison, stress group (CON, RR, CVS) and time (6h, 24h) were between-subjects factors. Any data that failed the assumption of equal variance or normality were transformed by log10 or square root, but the graphs represent the untransformed values. Figure legends indicate which data were transformed. Analysis was carried out with Sigma Stat (Systat Software, San Jose CA USA).
3. Results
3.1 Stress regimens and body weight
There was a significant interaction between day and stress on body weight [F(1, 153) = 21.399; p < 0.001] (Fig. 1). At the beginning of the study on day 0, there were no significant differences between groups. But by day 4, RR and CVS animals gained less weight than CON animals, and also weighed less on days 8 and 15 (p < 0.05, Fisher’s LSD post-hoc).
Figure 1.
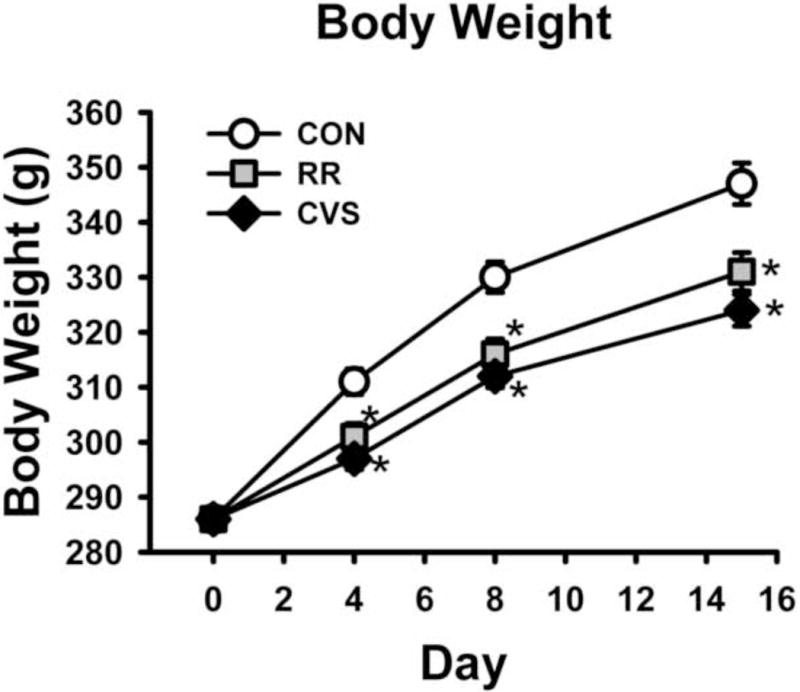
Animals subjected to CVS or RR had decreased body weight gain. *p < 0.001 vs CON
3.2 Corticosterone (CORT) levels
From trunk blood samples taken 24 h post LPS, there was a main effect of LPS treatment on CORT concentration [F(1, 30) = 17.502; p < 0.001] (Fig. 2) but no stress main effect or stress × LPS treatment interaction. The post-hoc analysis revealed that the LPS CON group had significantly higher CORT concentration than the SAL CON group, and that LPS RR group had significantly higher CORT levels than the SAL RR group (p < 0.05, Fisher’s LSD post-hoc). The LPS CVS group did not have significantly higher CORT than the SAL CVS group.
Figure 2.
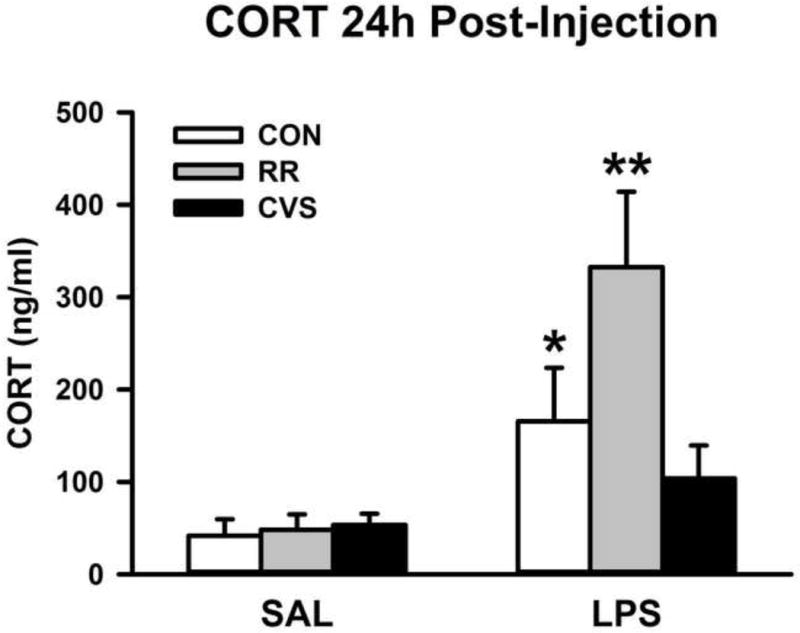
LPS increased morning circulating CORT in the CON and RR groups. *p < 0.01 vs CON SAL, **p < 0.001 vs RR SAL (Data were log10 transformed for statistical analysis, but figure depicts untransformed values)
3.3 Iba-1 Immunohistochemistry
For Iba-1 soma size in the IL PFC, there was a significant interaction between stress and LPS treatment [F(2, 29) = 3.946; p = 0.031] (Fig. 3A). LPS increased Iba-1 soma perimeter, but only in the animals exposed to RR (p < 0.05, Fisher’s LSD post-hoc). In the LPS treated animals, Iba-1 soma perimeter was increased in the RR group (p < 0.05, Fisher’s LSD post-hoc) relative to the CON LPS group and RR LPS group (Fig. 3A). This effect was not seen in the PL PFC (Fig. 3B) and there were no significant differences in Iba-1 positive cell counts in the IL PFC (Fig. 3C) or PL PFC (Fig. 3D). Representative images of Iba-1 immunohistochemistry are shown in Fig. 4. There were no differences in Iba-1 percent area.
Figure 3.
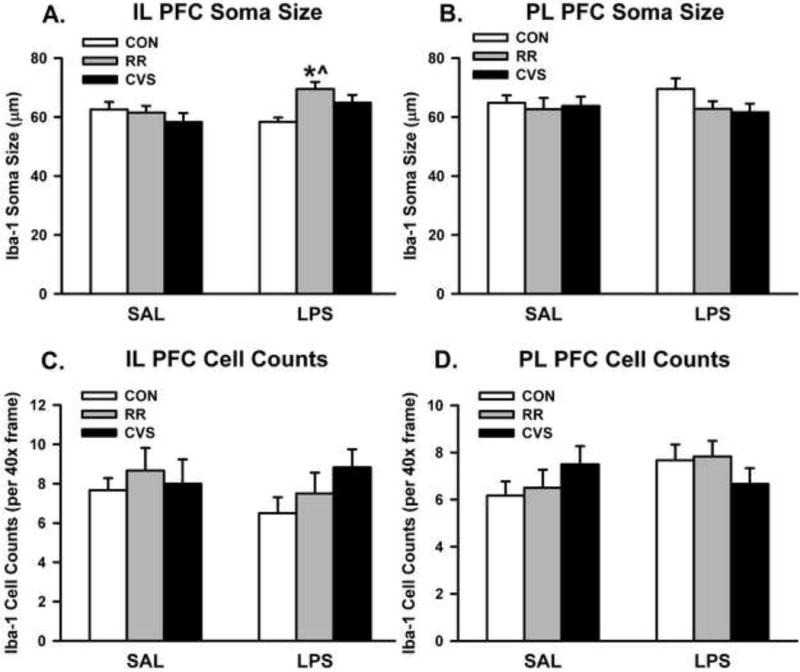
(A) Iba-1 soma perimeter was increased in the IL PFC in RR animals treated with LPS. (B) There were no differences in soma perimeter in the PL PFC and (C – D) there were no differences in cell counts. *p < 0.01 vs CON LPS; ^ p < 0.05 vs RR SAL
Figure 4.
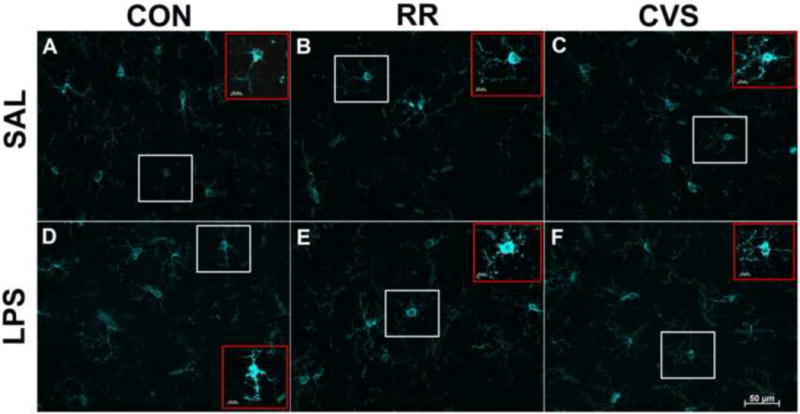
Representative 40× images of Iba-1 positive cells in the IL PFC, layers 5/6. White boxes depict examples of cells selected for analysis. Red boxes (insets) depict the same respective cell per figure with enhanced zoom and contrast, showing soma perimeter with red dashed line. Scale bar in inset represents 20μm. In the LPS treated groups (D–F), only the RR LPS group (E) had increased soma size relative to the RR SAL group (B). The CON SAL (A) did not differ from CON LPS (D), and CVS SAL (C) did not differ from CVS LPS (F).
3.4 Microglia gene expression: CD11b (ITGAM) and Iba-1 (AIF1)
There was a main effect of LPS treatment on ITGAM mRNA expression in the medial PFC (IL and PL combined) [F(1, 28) = 5.895; p = 0.022] (Fig. 5A). LPS treated animals had higher ITGAM expression than SAL treated animals, with post hoc revealing that the LPS CVS group had significantly higher ITGAM expression than the SAL CVS group (p < 0.05, Fisher’s LSD post-hoc). Then there was a significant main effect of stress on AIF1 mRNA levels in the medial PFC [F (2, 28) = 14.646; p < 0.001] (Fig. 5B). CVS animals had decreased AIF1 expression, with the SAL CVS group significantly lower than both SAL CON and SAL RR and the LPS CVS significantly lower than the LPS CON (p < 0.05, Fisher’s LSD post-hoc). There was no main effect of LPS treatment on AIF1 mRNA expression. In comparing microglial gene expression at the 6h vs. 24h time point, there was a main effect of stress on AIF1 expression [F(2, 30) = 13.517; p < 0.001], but no effect of time (Figure 7A). Animals exposed to CVS had lower AIF1 mRNA expression than both the RR and CON groups at 6h and lower AIF1 mRNA expression than the CON group at 24h(p < 0.05, Fisher’s LSD post-hoc) (Figure 7A).
Figure 5.
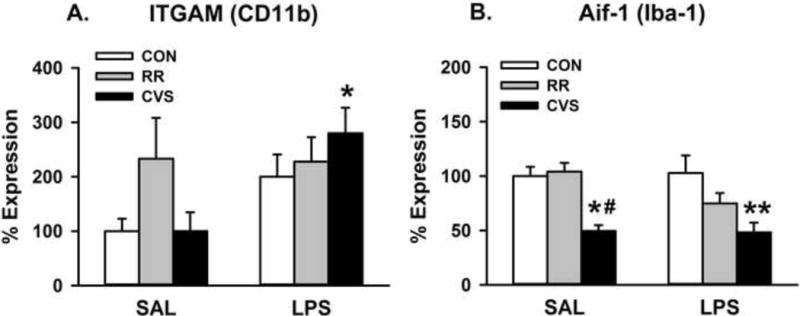
Microglial gene expression at the 24h time point. (A) LPS increased CD11b gene expression specifically in the CVS LPS group. *p < 0.01 vs CVS SAL. (B) CVS animals showed decreased gene expression of Aif1 at 24h post-injection, independent of LPS. *p = 0.002 vs CON SAL, # p < 0.001 vs RR SAL, **p < 0.001 vs CON LPS
Figure 7.
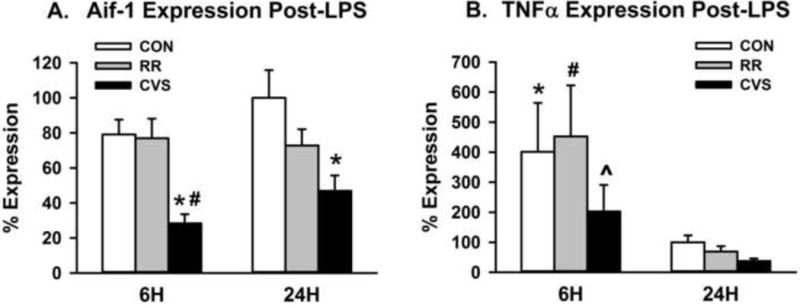
Comparison of gene expression at 6h vs. 24h time point. (A) Animals exposed to CVS had decreased Iba-1 gene expression (Aif-1) both 6h and 24h after LPS injection *p < 0.005 vs respective CON group, # p < 0.005 vs 6h RR (B) Animals injected with LPS had increased TNFα mRNA expression 6h after injection versus 24h after injection. *p < 0.05 vs 24h CON, # p < 0.005 vs 24h RR, ^ p < 0.01 vs 24h CVS
3.5 Proinflammatory cytokine gene expression
There was a main effect of stress on IL-1β mRNA expression in the medial PFC [F(2, 28) = 3.866; p = 0.033] (Fig. 6A). CVS animals had significantly lower IL-1β mRNA expression than their respective unstressed controls, both in the SAL and LPS groups (p < 0.05, Fisher’s LSD post-hoc) (Fig. 6A). Similarly, there were main effects of stress on TNFα expression [F(2, 28) = 8.129; p = 0.002] (Fig. 6C) and IL-18 expression [F(2, 28) = 3.534; p = 0.043] (Fig. 6D). CVS-exposed animals had significantly lower TNFα mRNA expression than their respective unstressed controls, both in the SAL and LPS groups (p < 0.05, Fisher’s LSD post-hoc) (Fig. 6C). Animals exposed to CVS also had lower IL-18 mRNA expression, with the CVS SAL group being significantly lower than the RR SAL group and the CVS LPS group being significantly lower than the CON SAL group (p < 0.05, Fisher’s LSD post-hoc) (Fig. 6D). There were no significant effects of LPS treatment or stress on IL-6 expression (Fig. 6B). In comparing cytokine expression at the 6h vs. 24h time point, there was a main effect of time on TNFα expression [F(1, 30) = 23.474; p < 0.001] (Figure 7B). Independent of stress group, animals had significantly higher TNFα mRNA expression at 6h compared to their respective stress group 24h after LPS injection (p < 0.05, Fisher’s LSD post-hoc) (Figure 7B). There was again a main effect of stress on IL-18 expression [F(2, 30) = 3.765; p = 0.035], but no effect of time. Animals exposed to CVS had lower IL-18 mRNA expression than the unstressed controls at the 24h time point (p < 0.05, Fisher’s LSD post-hoc) (data not shown). In comparing the 6h vs. 24h time point, there were no significant differences in IL-1β or IL-6 expression.
Figure 6.
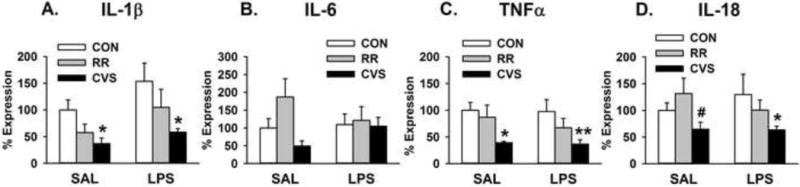
Proinflammatory cytokine gene expression at the 24h time point. (A) CVS animals showed decreased proinflammatory cytokine expression of IL-1β. *p < 0.05 vs respective CON group (B) There were no significant differences in IL-6 expression. (C) CVS animals displayed decreased TNFα expression. *p < 0.05 vs CON SAL, **p = 0.005 vs CON LPS (D) CVS animals also showed decreased IL-18 expression. *p < 0.05 vs CON LPS, # p < 0.05 vs RR SAL (Data for IL-1β and TNFα were square root and log10 transformed, respectively, but figure depicts untransformed values)
4. Discussion
Previous work has shown that exposure to a repeated restraint stress experience induces changes in medial PFC microglial morphology that reflect an activated state (Kopp et al 2013; Tynan et al 2010), while CVS fails to induce these changes (Kopp et al 2013). The present study demonstrates that RR exposure increases Iba-1 soma size in the IL PFC with LPS injection, while CVS downregulates Iba-1 gene expression and proinflammatory cytokine gene expression in the medial PFC, consistent with differential effects of stressor regimens on central immune responses. Moreover, CVS-induced downregulation of pro-inflammatory gene expression appears to be an enduring phenomenon independent of superimposed peripheral immune stimulation by LPS. These divergent effects of homotypic versus heterotypic chronic stress on PFC inflammatory responses suggest that microglia may be highly selective in their responsivity to various stress stimuli, particularly chronic insults.
Acute restraint stress activates neurons of the paraventricular nucleus of the hypothalamus (PVN) and subsequently increases circulating glucocorticoids. Acute restraint with water submersion increases microglial labeling in the hypothalamus, thalamus, and hippocampus (Sugama et al 2007). Repeated restraint increases microglial labeling throughout a broad range of limbic brain regions, including the prefrontal cortex (Tynan et al 2010). With repeated restraint, there is some habituation of the HPA axis by the end of a 14 day regimen (Viau & Sawchenko 2002). Interestingly, acute and repeated restraint stress activate distinctly different neural populations of the PVN (Viau & Sawchenko 2002) and there is significant habituation of immediate early gene responses in stress regulatory brain regions to chronic restraint in comparison to a single acute restraint session (Melia et al 1994, Stamp & Herbert 1999, Perrotti et al 2004). This supports the notion that the neurobiology of repeated restraint stress is different from an independent acute restraint exposure.
The chronic variable stress (CVS) model is a heterotypic stress regimen that prevents habituation and promotes increased basal glucocorticoid secretion (Herman et al 1995, Radley & Sawchenko 2015). CVS recapitulates pathological features of chronically stressed people, indicated by increased HPA activation seen in stress-related disorders such as depression and anxiety (Herman et al 1995, Chrousos 2009). Individual stressors of the CVS regimen, such as cold exposure, acutely activate the HPA axis and activate microglia in stress-responsive brain regions (Sugama et al 2011). However, as with acute vs repeated restraint, cumulative exposure to CVS is likely very different from exposure to any one stressor of the regimen alone, since chronic variable stress induces substantial reorganization of stress regulatory circuits (Jankord & Herman 2008).
Chronic stress (CVS) has clear peripheral immunosuppressive actions, as indicated by decreased T lymphocyte percentage and sensitivity (Basso et al 1993). Centrally, CVS reduces microglial activation and can induce microglial apoptosis in the hippocampus (Kreisel et al 2014). Our data also suggests that chronic stress in the form of CVS may constrain pro-inflammatory activity in the PFC. The difference between the two paradigms may be related to habituation of the HPA axis. The CVS paradigm induces greater cumulative glucocorticoid exposure than RR since animals do not habituate (Herman et al 1995; Ulrich-Lai et al 2006; Flak et al 2009; Flak et al 2012; Radley & Sawchenko 2015), which may serve as a mechanism to limit inflammatory responses. Lower concentrations of corticosteroids increase pro-inflammatory activities of microglia through the microglial mineralocorticoid receptor (MR), while higher concentrations of corticosteroids inhibit microglial activation through the microglial glucocorticoid receptor (GR) (Tanaka et al 1997). While our current data do not allow us to directly comment on glucocorticoid load or HPA axis sensitivity, the differences we see may be due to stress habituation. Repeated restraint induces significant habituation of the HPA axis that is mediated by MR and not GR (Cole et al 2000). The habituating nature of the RR paradigm may predominantly activate microglial MR to enhance microglial activity whereas the non-habituating nature of the CVS paradigm may activate microglial GR to inhibit microglial activity.
Interestingly, RR increases Iba-1 soma perimeter with LPS challenge specifically in the infralimbic (IL) but not prelimbic (PL) division of the PFC. Previous studies have shown that RR or LPS alone increase indices of microglial activation in both the PL and IL (Kopp et al 2013, Tynan et al 2010, Kongsui et al 2015). However, these studies used a much higher dose of LPS (Kongsui et al 2015) and took tissue much sooner after cessation of RR than the present study (Kopp et al 2013, Tynan et al 2010). It is possible that the previously published effects of RR alone return to baseline 36h after cessation of the RR regimen. The IL is sensitive to RR-induced molecular changes in pathways relevant to depression (Barreto et al 2012), so our current data suggest that it is possible that the IL is more sensitive to a lower dose of LPS and more prolonged effects of RR. This would suggest a larger role for the IL vs PL in chronic stress-induced depression-like behaviors that are potentially mediated by increased microglial activation. In contrast, the CVS regimen causes decreased Iba-1 and proinflammatory cytokine gene expression in the face of LPS injection. For gene expression, IL and PL were pooled to allow sufficient RNA yield. Further studies are necessary to determine the exact role of IL vs PL in chronic stress-induced suppression of proinflammatory molecules.
The timing of our effects also highlights the putative divergent actions of CVS and RR. Here we see evidence of a RR-induced increase in microglial activation at the protein level 24 h after LPS injection, indicated by increased Iba-1 soma perimeter. For our present study, we were interested in evaluating the effect of prior chronic stress on sustained PFC inflammatory responses 24h after LPS challenge. At the mRNA level at the 24h time point, expression of Iba-1 and proinflammatory cytokines IL-1β, TNFα, and IL-18 are reduced in the CVS groups, both with LPS and saline treatment. In contrast, RR does not affect proinflammatory cytokine gene expression. Previous studies indicate that stress-induced potentiation of cytokine responses to LPS challenge occur within 1h of injection at the protein level (Johnson et al 2002), suggesting that the LPS effects on gene expression may have dissipated by the 6 and 24 hour time point. However, the CVS-induced downregulation of proinflammatory cytokine gene expression in the saline group is present even though the final stressor of the CVS paradigm was 36 hours prior to tissue collection. This cytokine downregulation is maintained in the face of LPS injection and Iba-1 gene expression is decreased in the CVS group even at the 6h time point. Together, this suggests that CVS promotes a tonic suppression of CNS immune activity, possibly via GR-mediated genomic effects of glucocorticoids.
While CVS decreases PFC Iba-1 gene expression and proinflammatory cytokine expression, an increase in LPS-evoked CD11b expression was observed in the CVS group. This suggests that despite a decrease in some brain inflammatory markers in the PFC with CVS, the animals are still capable of mounting a CNS response to peripheral LPS challenge. This is supported by the data showing that even animals exposed to CVS have elevated TNFα expression 6h after LPS. Although not reaching significance, the TNFα expression 6h after LPS is lower in the CVS group compared to the CON and RR groups.
The present data further demonstrate divergent effects of chronic homotypic stress (repeated restraint, RR) and chronic heterotypic stress (chronic variable stress, CVS) on immune status in the PFC following subsequent immune challenge with LPS. Taken together, our findings suggest that stressor modality or habituation help to determine the nature of chronic stress-induced immune consequences in the PFC. By understanding how the PFC immune environment responds to a variety of chronic stress regimens, we can determine whether these responses play a neuroprotective role in stressor habituation or a neurotoxic role in the development of stress related disorders.
Highlights.
Repeated restraint increases PFC Iba-1 soma size with subsequent LPS injection.
CVS decreases PFC proinflammatory cytokine gene expression despite LPS injection.
PFC immune responses are highly dependent on chronic stress modality.
Acknowledgments
The authors would like to acknowledge Dr. Arshad Khan for sharing the post-fixation protocol and Maureen Fitzgerald for assistance in executing that protocol. Additionally, we would like to thank Cristiane Busnardo PhD, Rachel Morano, and Abigail Thompson for helping to execute details of the experiment that required assistance. And finally, we would like to acknowledge the University of Cincinnati Neuroscience Graduate Program, funding awarded to BLS (T32 NS007453, T32 DK059803) and our funding to JPH (R01 MH049698 and R01 MH069860).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barreto RA, Walker FR, Dunkley PR, Day TA, Smith DW. Fluoxetine prevents development of an early stress-related molecular signature in the rat infralimbic medial prefrontal cortex. Implications for depression? BMC Neurosci. 2012 Oct 18;13:125. doi: 10.1186/1471-2202-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Depiante-Depaoli M, Cancela L, Molina V. Seven-day variable-stress regime alters cortical beta-adrenoceptor binding and immunologic responses: reversal by imipramine. Pharmacol Biochem Behav. 1993 Jul;45(3):665–72. doi: 10.1016/0091-3057(93)90522-u. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009 Jul;5(7):374–81. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J Neuroendocrinol. 2000 Oct;12(10):1034–42. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- Cutando L, Busquets-Garcia A, Puighermanal E, Gomis-González M, Delgado-García JM, Gruart A, Maldonado R, Ozaita A. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Invest. 2013 Jul;123(7):2816–31. doi: 10.1172/JCI67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997 Apr 24;386(6627):824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008 Sep;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009 Nov 10;517(2):156–65. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur J Neurosci. 2012 Aug;36(4):2547–55. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012 Feb;26(2):337–45. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gądek-Michalska A, Tadeusz J, Rachwalska P, Spyrka J, Bugajski J. Effect of repeated restraint on homotypic stress-induced nitric oxide synthases expression in brain structures regulating HPA axis. Pharmacol Rep. 2012;64(6):1381–90. doi: 10.1016/s1734-1140(12)70935-0. [DOI] [PubMed] [Google Scholar]
- Girotti M, Donegan JJ, Morilak DA. Chronic intermittent cold stress sensitizes neuro-immune reactivity in the rat brain. Psychoneuroendocrinology. 2011 Sep;36(8):1164–74. doi: 10.1016/j.psyneuen.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hayley S, Merali Z, Anisman H. Stress and cytokine-elicited neuroendocrine and neurotransmitter sensitization: implications for depressive illness. Stress. 2003 Mar;6(1):19–32. doi: 10.1080/1025389031000091167. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995 Feb;61(2):180–90. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012 Jun;22(6):1442–54. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex. 2013 Aug;23(8):1784–97. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998 Jun 1;57(1):1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008 Dec;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Zhang R, Flak JN, Solomon MB, Albertz J, Herman JP. Stress activation of IL-6 neurons in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2010 Jul;299(1):R343–51. doi: 10.1152/ajpregu.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002 Aug;16(4):461–76. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Keller SE, Weiss JM, Schleifer SJ, Miller NE, Stein M. Suppression of immunity by stress: effect of a graded series of stressors on lymphocyte stimulation in the rat. Science. 1981 Sep 18;213(4514):1397–400. doi: 10.1126/science.6973822. [DOI] [PubMed] [Google Scholar]
- Khan A, Watts AG. Intravenous 2-deoxy-D-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen-activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinology. 2004;145(1):351–9. doi: 10.1210/en.2003-0539. [DOI] [PubMed] [Google Scholar]
- Kongsui R, Johnson SJ, Graham BA, Nilsson M, Walker FR. A combined cumulative threshold spectra and digital reconstruction analysis reveal structural alterations of microglia within the prefrontal cortex following low-dose LPS administration. Neuroscience. 2015 Dec 3;310:629–40. doi: 10.1016/j.neuroscience.2015.09.061. [DOI] [PubMed] [Google Scholar]
- Kopp BL, Wick D, Herman JP. Differential effects of homotypic vs. heterotypic chronic stress regimens on microglial activation in the prefrontal cortex. Physiol Behav. 2013 Oct 2;122:246–52. doi: 10.1016/j.physbeh.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014 Jun;19(6):699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012 Jan;136(1–2):e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005 Oct;20(Suppl 3):S302–6. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994 Oct;14(10):5929–38. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005 Mar 3;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J Cell Sci. 2000 Sep;113(Pt 17):3073–84. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney, Australia: Academic Press; 1997. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004 Nov 24;24(47):10594–602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield VM, Gabella KM, Simmons MA, Johnson JD. Repeated stressor exposure regionally enhances beta-adrenergic receptor-mediated brain IL-1β production. Brain Behav Immun. 2012 Nov;26(8):1249–55. doi: 10.1016/j.bbi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress. J Comp Neurol. 2015 Dec 15;523(18):2769–87. doi: 10.1002/cne.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015 Mar 19;289:429–42. doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94(4):1313–22. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007 May 25;146(3):1388–99. doi: 10.1016/j.neuroscience.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Kitani H, Hashimoto M. Cold stress induced morphological microglial activation and increased IL-1β expression in astroglial cells in rat brain. J Neuroimmunol. 2011 Apr;233(1–2):29–36. doi: 10.1016/j.jneuroim.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: the two receptors mediate differential effects of corticosteroids. Glia. 1997 May;20(1):23–37. [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010 Oct;24(7):1058–68. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006 Nov;291(5):E965–73. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol. 2002 Apr 15;445(4):293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Wager-Smith K, Markou A. Depression: a repair response to stress-induced neuronal microdamage that can grade into a chronic neuroinflammatory condition? Neurosci Biobehav Rev. 2011 Jan;35(3):742–64. doi: 10.1016/j.neubiorev.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013 Oct;14(11):1262–76. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, van Hoecke M, Tang XN, Lee H, Zheng Z, Swanson RA, Yenari MA. Pyruvate protects against experimental stroke via an anti-inflammatory mechanism. Neurobiol Dis. 2009 Oct;36(1):223–31. doi: 10.1016/j.nbd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012 Sep;37(9):1491–505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


