Abstract
OBJECTIVE
The purpose of this study is to determine whether Hounsfield numbers of soft tissues on unenhanced abdominal CT of the same patient vary on repeat scans done on two different manufacturers’ MDCT scanners.
MATERIALS AND METHODS
A database search was performed to identify patients older than 18 years who underwent unenhanced CT of the abdomen and pelvis performed both on a Volume CT (GE Healthcare) and a Definition AS Plus (Siemens Healthcare) 64-MDCT scanner within 12 months of each other. After excluding those patients for whom Hounsfield unit measurements would be affected by mitigating factors, 48 patients (mean age, 58.8 years) were identified. Hounsfield unit measurements were obtained in nine different soft-tissue anatomic locations on each scan, and the location of these sites was kept identical on each scan pair. Data were analyzed to evaluate Hounsfield unit differences between these scanners.
RESULTS
In general, there was a low consistency in the Hounsfield unit measurements for each of these sites on scans obtained by the two scanners, with the subcutaneous fat in the left posterolateral flank showing the lowest correlation (intraclass correlation coefficient, 0.198). There were differences in the Hounsfield unit measurements obtained in all anatomic sites on scans obtained by both scanners. Mean Hounsfield unit measurements obtained on the Definition AS Plus scanner were lower than those obtained on the Volume CT scanner, with the intriguing exception of the anterior midline subcutaneous fat Hounsfield unit measurements, which were higher on the Definition AS Plus scanner. All differences were statistically significant (p < 0.05).
CONCLUSION
Hounsfield unit measurements for unenhanced abdominal soft tissues of the same patient vary between scanners of two common MDCT manufacturers.
Keywords: Hounsfield numbers, MDCT
Attenuation of the x-ray beam in CT depends on the thickness of the anatomy traversed and the composition (physical density and atomic number) of the tissues in the path of the traversed beam [1]. After image reconstruction, each pixel in the CT image is assigned a number, referred to as the Hounsfield or CT number, which is a rescaled normalized function of the linear attenuation coefficient [1, 2]. The CT Hounsfield scale is calibrated such that the Hounsfield unit value for water is 0 HU and that for air is −1000 HU at all tube energies used [1, 2]. These thus represent the two fixed points on the Hounsfield unit scale.
Hounsfield number measurements thus allow a quick and simple method to characterize certain tissue types on abdominal CT. Consequently, characterization of cystic lesions, adrenal nodules, hepatic steatosis, and the nature of intraperitoneal fluid is commonly performed by measuring the Hounsfield unit values. However, it has been reported since the early days of CT that Hounsfield number accuracy may be influenced by several factors, including the convolution kernel, reconstruction artifacts, beam hardening, spectral energy, and scatter, as well as variations in patient size, shape, and position in scanner [3–5]. This variability has also been shown more recently on modern-day CT scanners as well, using an anthropomorphic body phantom [6]. To our knowledge, only a single study has evaluated the Hounsfield unit variability in human tissues on different scanners, and that study was done for adrenal nodules [7]. Despite this, radiologists continued to rely on Hounsfield unit values to make important clinical decisions, especially with respect to characterizing renal lesions and adrenal nodules.
In our practice, we noted several instances of variability in the Hounsfield numbers of renal lesions and adrenal nodules on repeat unenhanced abdominal CT examinations of the same patient performed on scanners from different manufacturers. The purpose of this study thus was to retrospectively determine whether CT Hounsfield numbers of soft tissues on unenhanced abdominal CT of the same patient vary on repeat scans done on two different manufacturers’ MDCT scanners.
Materials and Methods
Patients
The institutional review board approved this single-institution HIPAA-compliant study, and a waiver of informed consent was obtained because of its retrospective nature. A search of our radiology database was performed for the interval of October 1, 2009, to September 30, 2012, to identify patients older than 18 years who had undergone unenhanced CT of the abdomen and pelvis on both a Volume CT (GE Healthcare) and a Definition AS Plus (Siemens Healthcare) 64-MDCT scanner, within 12 months of each other. For the instances where a patient had more than two unenhanced CT scans on these scanners in a 12-month period, the scan pair with the shortest interscan interval was chosen.
Seventy-three patients were identified. Patients with metallic spinal hardware (n = 6), severe scoliosis or arms on the sides of the abdomen or both (n = 6), decubitus orientation in the CT gantry on one of the scans in the scan pair (n = 1), diffuse anasarca on one or both scans in the scan pair (n = 6), distortions from prior surgery (n = 3), residual contrast enhancement of tissues from a recent contrast-enhanced CT (n = 1), acutely ruptured abdominal aortic aneurysm on one of the scans in the pair (n = 1), or peak kilovoltage other than 120 kVp on one or both scans in the scan pair (n = 1) were excluded because the artifacts and other factors would confound the Hounsfield unit measurements in these patients. There were multiple other comitigating factors in a few of these excluded patients, such as metallic coils in the kidney (n = 1), metastatic disease to the liver (n = 2), and nephrostomy tube in the kidney (n = 3). Forty-eight subjects thus met the inclusion criteria of this study. The clinical indication for these CT examinations was a history of suspected renal stones in 32, abdominal pain in 12, and bladder carcinoma, renal cell carcinoma, small-bowel obstruction, and abdominal aortic aneurysm with weight loss in one patient each.
To evaluate for a possible variation in the CT Hounsfield numbers of soft tissues between two MDCT scanners of different manufacturers, we selected patients who had undergone an unenhanced CT scan on both scanners in a 12-month period. Unenhanced CT examinations were chosen for this study because CT Hounsfield numbers on contrast-enhanced CT are affected by a variety of factors, such as tube voltage; the volume, concentration, and rate of injection of injected contrast medium; and patient physiologic factors, such as the heart rate, ejection fraction, total blood volume, hydration status, and so forth.
CT Technique
On the Volume CT scanner, scans were performed using a detector configuration of 64 × 0.625, beam collimation of 40 mm, 120 kV, variable tube currents using automated dose modulation with a noise index of 30 or 34 (on 1.25-mm reconstructed images), pitch of 1.375, and a gantry rotation speed of 0.5 second. On the Definition AS Plus scanner, scans were performed using a detector configuration of 128 × 0.6, beam collimation of 38.4 mm, 120 kV, variable tube currents using automated dose modulation with a reference tube current of 220 mA, pitch of 0.8, and a gantry rotation speed of 0.5 second. The Definition AS plus scanner generates 128 axial slices by oversampling the data in the z-axis, but has 64 physical detector rows and hence is closely comparable to the Volume CT 64-MDCT scanner with respect to the detector geometry. All patients were introduced into the CT gantry in the supine position with the feet entering first. All patients were accurately centered in the axial plane in the CT gantry using the horizontal laser light in the CT gantry. Reviewed images were reconstructed using a filtered back projection technique at a thickness of either 3.0 or 5.0 mm, using a standard body filter on the Volume CT scanner and a kernel of B40f on the Definition AS Plus scanner. Three of the 96 scans were obtained with oral contrast agent. Both scanners are calibrated regularly to maintain Hounsfield unit constancy according to the specifications of the manufacturer, using phantoms supplied by the respective manufacturers.
Measurements
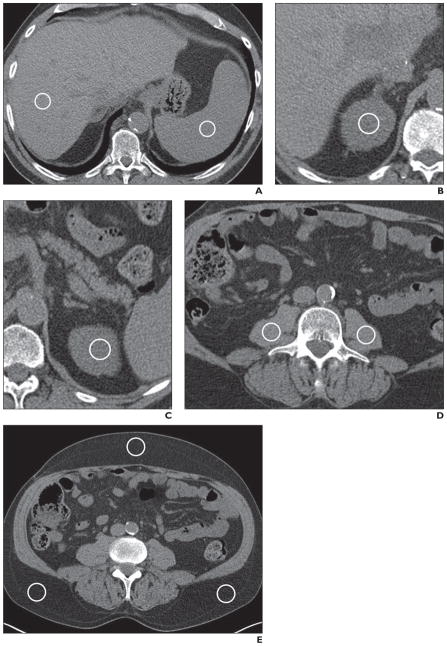
All ROIs were placed on the CT images on a radiology PACS workstation (iSite version 3.6, Philips Healthcare) by a radiologist with over 14 years of experience, in consensus with a second radiologist with over 8 years of experience. A third radiologist, with over 35 years’ experience, reviewed all of the images with the saved ROIs to validate correct placement of the ROIs according to the chosen criteria. After circular ROIs with an area ranging from 0.8 to 2.0 cm2 were placed, Hounsfield unit measurements were obtained in nine separate anatomic sites: the right lobe of the liver, the spleen, the right and left renal cortex in the polar regions (mostly the upper poles), the right and left psoas muscles, the subcutaneous fat in the right and left posterolateral flanks, and the subcutaneous fat in the midline anterior abdominal wall (Figs. 1A–1E). The ROIs were carefully placed in areas that lacked heterogeneity, and the anatomic locations of the ROIs in each site were kept as similar as possible on the initial and repeat scans, allowing minor variations in some cases due to different orientation of the organs or the patient in the scanner on the repeat scan. In the liver, ROIs were not placed on visible portal or hepatic vein branches. In the solid organs, ROIs were not placed on focal lesions, such as cysts, masses, focal fat, scar tissue, or calcification. In the psoas muscles, ROIs were not placed on fat fascicles. In the subcutaneous fat, ROIs were not placed in areas of stranding, edema, or prior injection sites.
Fig. 1. CT images show soft-tissue sites where circular ROIs were placed for measuring Hounsfield unit values.
A, Image shows ROIs (circles) in right lobe of liver and spleen.
B, Image shows ROI (circle) in right renal cortex.
C, Image shows ROI (circle) in left renal cortex.
D, Image shows ROIs (circles) in right and left psoas muscles.
E, Image shows ROIs in subcutaneous fat in right and left posterolateral flank (bottom circles) and in midline anterior abdominal wall (top circle).
In three patients, Hounsfield unit measurements of the liver were not obtained because there was obvious hepatic steatosis on subjective visual evaluation. In four patients, Hounsfield unit measurements of the right renal cortex were not obtained because of severe hydronephrosis and parenchymal atrophy, pyelonephritis, atrophy of the kidney, and the presence of perirenal hematoma after a nephrostomy tube placement, respectively. In one patient, Hounsfield unit measurements of the right kidney could not be obtained because the kidney was absent. In one patient, Hounsfield unit measurements of the right and left psoas muscles could not be obtained because of severe atrophy of the psoas muscles. In one patient, Hounsfield unit measurements of the subcutaneous fat in the midline anteriorly were not obtained because of postoperative stranding in the fat. Thus, 22 Hounsfield unit measurements could not be made and a total of 842 Hounsfield unit measurements were obtained in the 48 patients.
Statistical Methods
All data were recorded in a spreadsheet (Excel 2011, Microsoft) with anonymized patient identifiers. For each of the anatomic locations (right lobe of the liver, spleen, right renal cortex, left renal cortex, right psoas muscle, left psoas muscle, right posterolateral flank subcutaneous fat, left posterolateral flank subcutaneous fat, and anterior midline subcutaneous fat), the intraclass correlation coefficient (ICC) was used to assess the consistency of the CT Hounsfield measurements taken by the two scanners. For each of these anatomic locations, the two-sided paired Student t test or Wilcoxon signed rank test, when appropriate, was used to compare the CT Hounsfield measurements taken by the two different scanners. In addition, repeated-measures ANOVA was used to compare the CT Hounsfield measurements taken by the two scanners to adjust for patient age and sex.
A p value less than 0.05 was considered statistically significant. All analyses were performed with SAS (version 9.2, SAS Institute).
Results
Summary Statistics
The mean age of the patients was 58.8 years (median, 60 years; range, 24–95 years; SD, 16.0 years). There were 25 women (52.1%; mean age, 56.1 years) and 23 men (47.9%; mean age, 61.8 years). The average interval between scans was 143.4 days (SD, 122.9 days; range, 5–361 days). The initial scan was performed on a Definition AS Plus scanner in 25 patients (52.1%).
Hounsfield Unit Variability
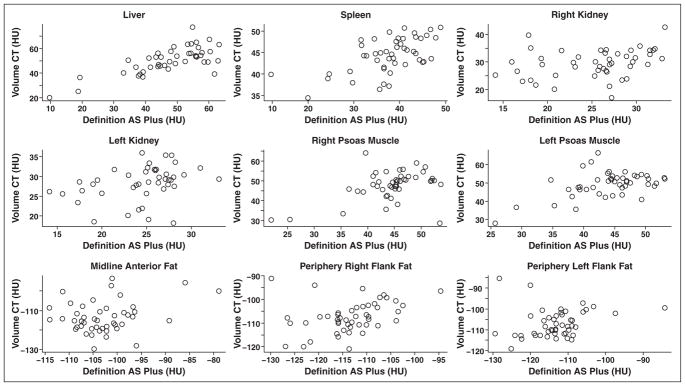
Scatterplot graphs of the Hounsfield unit measurements in the nine anatomic sites for scans obtained on the two scanners are shown in Figure 2. ICCs obtained to evaluate the consistency of the Hounsfield unit measurements in each of these sites on scans obtained on the two scanners are shown in Table 1. This shows a low consistency for most of the sites, with Hounsfield unit measurements in the right lobe of the liver showing the highest correlation (ICC = 0.736) and those in the subcutaneous fat in the left posterolateral flank showing the lowest correlation (ICC = 0.198). Interestingly, there is a small difference in correlations for the right and left posterolateral subcutaneous fat (ICC = 0.198 and 0.323, respectively).
Fig. 2.
Scatterplot graphs of Hounsfield unit measurements taken by Definition AS Plus (Siemens Healthcare) and Volume CT (GE Healthcare) 64-MDCT scanners for nine different anatomic soft-tissue locations studied.
TABLE 1.
Intraclass Correlation Coefficients (ICCs) for the Consistency of the Hounsfield Unit Measurements Taken by the Volume CT (GE Healthcare) and Definition AS Plus (Siemens Healthcare) 64-MDCT Scanners for Each of the Nine Anatomic Sites
| Anatomic Site | ICC |
|---|---|
|
| |
| Liver | 0.736 |
| Spleen | 0.489 |
| Kidney (right) | 0.31 |
| Kidney (left) | 0.334 |
| Psoas muscle (right) | 0.454 |
| Psoas muscle (left) | 0.485 |
| Midline anterior fat | 0.248 |
| Periphery flank fat (right) | 0.323 |
| Periphery flank fat (left) | 0.198 |
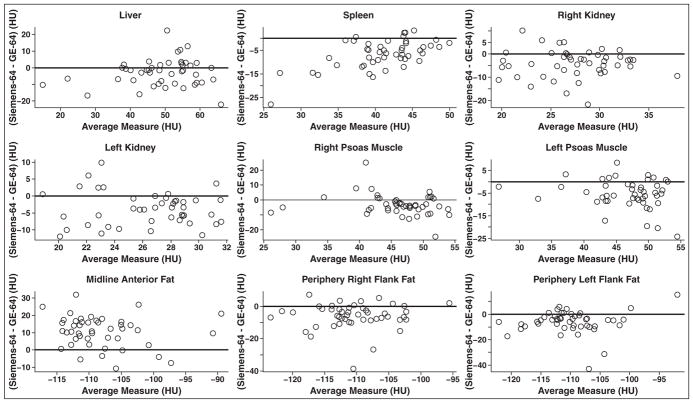
Two-sided paired Student t test or Wilcoxon signed rank test, when appropriate, showed that there were differences in the Hounsfield unit measurements obtained in all anatomic sites on scans obtained by the scanners (Table 2). Furthermore, these differences were statistically significant in all sites measured without adjusting for patient age or sex (Table 3). Repeated-measures ANOVA showed that the differences remained statistically significant after adjusting for patient age and sex (Table 3). In all the anatomic sites, the mean and median Hounsfield unit measurements obtained by the Definition AS Plus scanner were lower than those obtained by the Volume CT scanner, with the intriguing exception of the anterior midline subcutaneous fat Hounsfield unit measurements, which were higher on the Definition AS Plus scanner. Scatterplot graphs in Figure 3 show the differences in the Hounsfield unit measurements between the two scanners for all the anatomic sites. The difference (mean and median) in Hounsfield unit measurements obtained by the two scanners was highest for the anterior midline subcutaneous fat (10.5 and 11.0 HU, respectively). Data for the Hounsfield unit measurements obtained by the two scanners and the differences between these measurements on the scanners for all the anatomic sites are detailed in Tables 2 and 3.
TABLE 2.
Data for Hounsfield Unit Values Obtained by the Definition AS Plus (Siemens Healthcare) and Volume CT (GE Healthcare) 64-MDCT Scanners for the Nine Anatomic Soft-Tissue Sites Studied
| Scanner, Anatomic Site | No. of Scans | Hounsfield Unit Measurements
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Standard Error | Median | Minimum | Maximum | Interquartile Range | ||
|
| ||||||||
| Definition AS Plus | ||||||||
| Right lobe liver | 45 | 47.5 | 11.8 | 7.8 | 49.0 | 9.6 | 63.2 | 14.5 |
| Spleen | 48 | 37.7 | 7.3 | 1.1 | 38.5 | 11.9 | 49.0 | 8.3 |
| Right renal cortex | 47 | 25.4 | 5.1 | 0.7 | 26.6 | 14.1 | 33.2 | 8.4 |
| Left renal cortex | 44 | 24.5 | 4.1 | 0.6 | 25.2 | 14.1 | 33.1 | 4.4 |
| Right psoas muscle | 47 | 44.4 | 6.1 | 0.9 | 45.3 | 21.9 | 53.7 | 5.0 |
| Left psoas muscle | 47 | 44.0 | 5.7 | 0.8 | 44.6 | 25.7 | 53.1 | 7.1 |
| Midline anterior subcutaneous fat | 47 | −102.9 | 6.7 | 1.0 | −103.7 | −114.1 | −78.9 | 7.3 |
| Right posterolateral flank subcutaneous fat | 48 | −113.4 | 7.0 | 1.0 | −113.3 | −129.9 | −94.6 | 6.4 |
| Left posterolateral flank subcutaneous fat | 48 | −113.1 | 7.5 | 1.1 | −113.1 | −129.4 | −84.2 | 7.1 |
| Volume CT | ||||||||
| Right lobe liver | 45 | 50.4 | 10.7 | 1.6 | 50.6 | 20.0 | 77.2 | 11.5 |
| Spleen | 48 | 44.3 | 4.1 | 1.1 | 44.7 | 34.4 | 50.9 | 6.2 |
| Right renal cortex | 47 | 29.5 | 5.1 | 0.7 | 29.8 | 17.0 | 42.6 | 6.9 |
| Left renal cortex | 44 | 28.3 | 4.4 | 0.7 | 28.9 | 18.1 | 36.0 | 5.2 |
| Right psoas muscle | 47 | 47.7 | 7.3 | 1.1 | 49.2 | 28.5 | 64.2 | 6.3 |
| Left psoas muscle | 47 | 49.4 | 6.8 | 1.0 | 50.6 | 27.9 | 66.6 | 6.4 |
| Midline anterior subcutaneous fat | 47 | −113.4 | 7.9 | 1.2 | −115.0 | −129.7 | −93.5 | 9.8 |
| Right posterolateral flank subcutaneous fat | 48 | −107.4 | 6.7 | 1.0 | −107.8 | −121.0 | −91.1 | 7.5 |
| Left posterolateral flank subcutaneous fat | 48 | −107.0 | 6.7 | 1.0 | −108.5 | −119.2 | −84.4 | 9.5 |
Note—All values have been rounded off to one decimal place.
TABLE 3.
Differences in Hounsfield Unit Values Between Definition AS Plus (Siemens Healthcare) and Volume CT (GE Healthcare) 64-MDCT Scanners for the Nine Anatomic Soft Tissue Sites Studied
| Anatomic Site | No. of Scans | Difference in Hounsfield Unit Measurements Between Scanners
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Standard error | Median | Minimum | Maximum | Interquartile Range | pa | pb | ||
|
| ||||||||||
| Right lobe liver | 45 | −2.9 | 8.2 | 1.2 | −1.6 | −22.3 | 22.5 | 10.7 | 0.023 | 0.022 |
| Spleen | 48 | −6.6 | 6.0 | 0.9 | −6.1 | −28.0 | 3.4 | 7.6 | < 0.0001c | < 0.0001 |
| Right renal cortex | 47 | −4.1 | 6.0 | 0.9 | −3.1 | −21.8 | 10.2 | 7.4 | < 0.0001 | < 0.0001 |
| Left renal cortex | 44 | −3.8 | 4.9 | 0.7 | −3.9 | −12.0 | 9.9 | 6.6 | < 0.0001 | < 0.0001 |
| Right psoas muscle | 47 | −3.3 | 7.0 | 1.0 | −4.0 | −24.6 | 25.0 | 7.6 | < 0.0001c | 0.003 |
| Left psoas muscle | 47 | −5.4 | 6.4 | 0.9 | −5.3 | −24.3 | 8.5 | 6.9 | < 0.0001c | < 0.0001 |
| Midline anterior subcutaneous fat | 47 | 10.5 | 9.0 | 1.3 | 11.0 | −11.0 | 32.0 | 10.0 | < 0.0001 | < 0.0001 |
| Right posterolateral flank subcutaneous fat | 48 | −6.0 | 7.9 | 1.1 | −5.4 | −38.8 | 7.3 | 6.9 | < 0.0001c | < 0.0001 |
| Left posterolateral flank subcutaneous fat | 48 | −6.0 | 9.0 | 1.3 | −4.6 | −42.9 | 15.4 | 7.9 | < 0.0001c | < 0.0001 |
Without adjustment for age or sex obtained using either the two-sided paired Student t test or two-sided Wilcoxon signed rank test.
After adjustment for age or sex.
Two-sided Wilcoxon signed rank test.
Fig. 3.
Scatterplot graphs of average Hounsfield unit measurements obtained by both Definition AS Plus (Siemens Healthcare) and Volume CT (GE Healthcare) 64-MDCT scanners compared to difference in Hounsfield unit measurements, calculated as Definition AS Plus value minus Volume CT value, between two scanners for each anatomic location studied. Black solid line is placed at difference of 0 HU.
Discussion
Each voxel in a CT image is assigned a Hounsfield number, which gives a quantitative value of the tissue attenuation in that voxel [1]. Although the unreliability of CT Hounsfield numbers as absolute values has been reported since the early days of CT [3–5], characterization of some masses is commonly performed by measuring this Hounsfield value, which is simple and easy to perform. Hounsfield unit criteria that allow the distinction of cystic from noncystic lesions, characterization of adrenal adenomas, and detection of hepatic fat on unenhanced CT are well established and widely used [8–13]. This requires that the measured Hounsfield value be both accurate and consistent across different scanners. For every CT scanner, Hounsfield unit accuracy is checked at regular intervals, and machines are calibrated such that the CT value for water generally does not deviate more than 2 HU from the reference value of 0 HU [2].
Our study shows that, in general, there was a low consistency in the Hounsfield unit values measured in different soft-tissue anatomic sites in the abdomen on unenhanced CT scans obtained on two different manufacturers’ MDCT scanners used in our radiology department. The mean Hounsfield unit values in these soft tissues were noted to be lower on the Definition AS Plus scanner than on the Volume CT scanner in all sites except the midline anterior subcutaneous fat; these differences were statistically significant. These variations in CT numbers, albeit small, can have implications for the Hounsfield unit characterization of tissues on unenhanced CT, chiefly renal masses and adrenal nodules. The results of our in vivo study in human subjects corroborate the observations of Birnbaum et al. [6], who noted in a study of an anthropomorphic body phantom that CT attenuation values vary significantly between MDCT scanners of different manufacturers, among different generations of the same manufacturer’s scanners, and with individual combinations of MDCT scanner and convolution kernels [6].
Clinical Implications
Unenhanced CT is done for a number of clinical indications, such as the evaluation and follow-up of urolithiasis and intraabdominal hemorrhage, for patients undergoing CT colonography, or for patients who are unable to receive injected iodinated contrast agent because of renal insufficiency or allergy. Incidental renal and adrenal lesions are commonly encountered on these unenhanced CT examinations [14].
Although most incidental renal masses are benign, renal cell carcinomas (RCCs) are sometimes identified as incidental findings [14]. A discriminatory threshold of 20 HU, derived from experience with conventional nonhelical single-detector CT scanners, has historically been used to help differentiate simple renal cysts from solid lesions [8]. A homogeneous low-attenuation lesion without thick septations or calcifications in the kidney on an unenhanced or a single-phase enhanced CT ranging from 0 to 20 HU is thus considered to represent a benign cyst [9]. In the study by Birnbaum et al. [6], the mean Hounsfield unit values found for a renal cyst with an assigned value of 0 HU in an anthropomorphic body phantom were 0.0, 1.9, −0.9, 9.0, and 9.7 HU, respectively, when measured on Siemens Healthcare 4-,16-, and 64-MDCT scanners (reconstructed using B40f kernel); a GE Healthcare 4-MDCT scanner (reconstructed using a standard body kernel); and a Philips Healthcare 4-MDCT scanner (reconstructed using a B kernel) [6].
Furthermore, it has been reported that the papillary type of RCC, which can account for 13–15% of diagnosed RCCs [15], tends to be homogeneous [16] and can have a low attenuation on unenhanced CT [17]. Yamada et al. [17] reported the mean attenuation of two different subtypes of papillary RCC to be 34.6 HU (n = 12) and 38.4 HU (n = 8), respectively, on the unenhanced phase. However, the scanner types used in that study were not specified. Furthermore, it has been recently reported that a discriminatory threshold of 70 HU can be used to distinguish a hyperdense cyst from a solid renal neoplasm on unenhanced CT [18]. All patients in that study were scanned on two different generations of a single manufacturer’s MDCT scanners (GE Healthcare LightSpeed 4- and 16-MDCT scanners). According to that study, a homogeneous renal mass measuring 70 HU or more at unenhanced CT would almost always represent a high-attenuation renal cyst, not requiring any further investigation [18].
Thus, in current practice, a renal lesion with an attenuation of 20–70 HU is considered indeterminate and needs further evaluation. Although there is a paucity of data on the unenhanced CT attenuation of RCCs, we have on occasion encountered cases of proven RCCs with Hounsfield unit values closely approaching or in the range of a simple cyst on unenhanced CT. It therefore is clear that if using absolute Hounsfield numbers for characterization of renal lesions on unenhanced CT, even a small variability of Hounsfield unit numbers can potentially result in mis-characterization of renal lesions as being benign or malignant.
A second clinical application where Hounsfield unit variability can influence diagnostic decisions is when characterizing an adrenal nodule on unenhanced CT. Incidental adrenal lesions are commonly detected on CT. Lesion characterization is important particularly in patients with cancer to distinguish a benign adenoma from a metastatic lesion. CT densitometry of adrenal nodules exploits the fact that up to 70% of adrenal adenomas contain abundant intracellular fat, which results in a low attenuation on unenhanced CT, whereas almost all malignant lesions do not [19]. Lee et al. in 1991 [20] reported that unenhanced CT densitometry could effectively differentiate adrenal adenomas from nonadenomatous lesions (mean values of −2.2 and 28.9 HU, respectively), subsequently corroborated by Korobkin et al. [10]. A pooled analysis in 1998 of 10 prior studies concluded that a threshold of 10 HU would result in a sensitivity and specificity of 71% and 98%, respectively, for the diagnosis of an adrenal adenoma on unenhanced CT [21]. Since then, the 10-HU threshold has been used to diagnose benign lipid-rich adenomas on unenhanced CT. This threshold has thus been established from experience with both conventional nonhelical and helical non-MDCT scanners. Using this established criterion, all lesions with a value of 10 HU or less on unenhanced CT would be considered benign and those with a value of 10 HU or higher would be considered indeterminate, regardless of scanner type. An ex vivo study of adrenal explants in 2004, however, reported differences in CT density of adrenal tumors on different scanners (one 4-MDCT and two single-detector scanners) [22]. A subsequent study on human subjects reported only slight differences in attenuation of adrenal nodules on repeat scans of the same patient obtained with different MDCT scanners [7]. Although our study does not focus on measuring adrenal nodules, the differences in Hounsfield unit measurements noted on our study were larger than reported in this study. Also, contrary to our results and those of Birnbaum et al. [6], there was a tendency for lower Hounsfield unit values on GE Healthcare scanners compared with Siemens Healthcare scanners.
Another application of unenhanced CT is the detection of hepatic fat. Nonalcoholic hepatic liver disease is common, with an estimated prevalence of 3–24% in the general population [23]. Measuring liver Hounsfield unit values on unenhanced CT provides a fast, simple, objective, and noninvasive assessment of liver fat content. Kodama et al. [24] reported the use of a single absolute value of 40 HU or less on unenhanced CT as a simple method of estimating liver fat, correlating with a pathologic fat content of 30 HU or more. All scans in their study were obtained on a GE Healthcare 4-MDCT scanner. Others have evaluated differences in hepatic and splenic attenuation to detect and quantify hepatic fat on unenhanced CT [25, 26]. Although no study, to our knowledge, has compared the effect of scanner type on evaluation of hepatic fat content using absolute unenhanced CT numbers, the results of this study suggest that using the spleen as an internal control may be preferable to the use of an absolute CT value for estimating liver fat content.
Possible Reasons for Hounsfield Number Variability
Postulates to explain this variability of Hounsfield numbers between scanners may possibly include the following differences between scanners of different manufacturers: amount of scatter radiation that reaches the detectors, variation in the spectra of the x-ray beam due to different degrees of filtration before the beam exits the tube housing, differences in convolution kernels used by different manufacturers [6], and differences in CT calibration methods between vendors. Of these, the effect of scatter radiation on CT number accuracy has been known since the early days of CT [27, 28]. Furthermore, this may be amplified on the modern “higher row” MDCT scanners that use a cone beam [29]. Patient factors such as variations in positioning of patients can also affect the CT numbers on repeat scans of the same patient. Finally, phantoms used for CT calibration are usually homogeneous cylindric objects and do not conform to the shape of the human body and composition of human tissues, nor do they take into account the unique and different habitus of each patient. Differences in tube current are not known to affect the Hounsfield unit values, because only tube current affects the noise parameter [6].
Limitations
Our study has some limitations, partly because of its retrospective nature. Ours is a single-center study that compares MDCT scanners of only two different CT manufacturers. It does not compare Hounsfield unit variability among different generations of MDCT scanners from the same manufacturer and CT scanners from other manufacturers. Although we cannot extrapolate our results to all scanners in use today, we think that the variability in CT attenuation that we observed is likely applicable to several MDCT scanners. The methods used in this study could, however, be extended to address some of these questions in future studies. Neither our study nor any previous study, to our knowledge, has compared Hounsfield unit variability of modern MDCT scanners with conventional helical and nonhelical scanners from which most of the CT densitometric data come. Our study group is small given that stringent inclusion and exclusion criteria were required in our study. Finally, attenuation was measured only for soft tissues (including fat) on unenhanced CT, where Compton interactions account for the majority (91% and 94%, respectively) of the x-ray-tissue interactions [1]. We did not study variations in attenuation of bone and soft tissues after injection of iodinated contrast medium, where the photoelectric effect predominates and accounts for the attenuation. The results of our study thus cannot be extrapolated to contrast-enhanced CT.
Conclusion and Recommendations
In conclusion, our study shows that Hounsfield unit measurements of unenhanced soft tissues in the abdomen vary between MDCT scanners of two different manufacturers. In view of our results and those of prior investigators, we think that established absolute Hounsfield unit thresholds that are currently used for CT characterization of renal lesions and adrenal nodules on unenhanced CT can result in mischaracterization of these lesions. We propose that, for tissue characterization on un-enhanced CT that depends on absolute CT attenuation values, either the use of dedicated calibration phantoms or scanner- and convolution kernel–specific Hounsfield unit thresholds may need to be investigated for the modern MDCT scanners in clinical use. For renal and adrenal masses, the risk of misdiagnosis is greater for a malignant than for a benign lesion. Although Hounsfield characterization of tissues remains and will continue to be an important and effective decision-making tool in clinical CT, it is important for radiologists to be aware of these variations. Caution is thus advised when using absolute CT numbers to characterize masses on unenhanced CT and, if appropriate and warranted, further characterization using other methods should be considered. As with any other test result, CT Hounsfield unit value measurements should not be interpreted in a vacuum. Clinical data, together with other morphologic characteristics and the relative risk-benefit and cost of additional workup, must be considered in individual cases.
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (grant UL1 TR000002).
References
- 1.Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The essential physics of medical imaging. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 2.Kalender WA. Computed tomography: fundamentals, system technology, image quality, applications. 3. Erlangen, Germany: Publicis Publishing; 2011. [Google Scholar]
- 3.Levi C, Gray JE, McCullough EX, Hattery RR. The unreliability of CT numbers as absolute values. AJR. 1982;139:443–447. doi: 10.2214/ajr.139.3.443. [DOI] [PubMed] [Google Scholar]
- 4.Zerhouni EA, Spivey JF, Morgan RH, Leo FP, Stitik FP, Siegelman SS. Factors influencing quantitative CT measurements of solitary pulmonary nodules. J Comput Assist Tomogr. 1982;6:1075–1087. doi: 10.1097/00004728-198212000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Hunter TB, Pond GD, Medina O. Dependence of substance CT number on scanning technique and position within scanner. Comput Radiol. 1983;7:199–203. doi: 10.1016/0730-4862(83)90099-9. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum BA, Hindman N, Lee J, Babb JS. Multidetector row CT attenuation measurements: assessment of intra- and interscanner variability with an anthropomorphic body CT phantom. Radiology. 2007;242:109–119. doi: 10.1148/radiol.2421052066. [DOI] [PubMed] [Google Scholar]
- 7.Hahn PF, Blake MA, Boland GW. Adrenal lesions: attenuation measurement differences between CT scanners. Radiology. 2006;240:458–463. doi: 10.1148/radiol.2402042120. [DOI] [PubMed] [Google Scholar]
- 8.Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1–10. doi: 10.1148/radiology.158.1.3510019. [DOI] [PubMed] [Google Scholar]
- 9.Silverman SG, Israel GM, Herts BR, Richie JP. Management of the incidental renal mass. Radiology. 2008;249:16–31. doi: 10.1148/radiol.2491070783. [DOI] [PubMed] [Google Scholar]
- 10.Korobkin M, Brodeur FJ, Yutzy GG, et al. Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. AJR. 1996;166:531–536. doi: 10.2214/ajr.166.3.8623622. [DOI] [PubMed] [Google Scholar]
- 11.Boland GW, Blake MA, Hahn PF, Mayo-Smith WW. Incidental adrenal lesions: principles, techniques, and algorithms for imaging characterization. Radiology. 2008;249:756–775. doi: 10.1148/radiol.2493070976. [DOI] [PubMed] [Google Scholar]
- 12.Lee SW, Park SH, Kim KW, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–485. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 13.Boyce CJ, Pickhardt PJ, Kim DH, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR. 2010;194:623–628. doi: 10.2214/AJR.09.2590. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor SD, Pickhardt PJ, Kim DH, Oliva MR, Silverman SG. Incidental finding of renal masses at unenhanced CT: prevalence and analysis of features for guiding management. AJR. 2011;197:139–145. doi: 10.2214/AJR.10.5920. [DOI] [PubMed] [Google Scholar]
- 15.Reuter VE. The pathology of renal epithelial neoplasms. Semin Oncol. 2006;33:534–543. doi: 10.1053/j.seminoncol.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Herts BR, Coll DM, Novick AC, et al. Enhancement characteristics of papillary renal neoplasms revealed on triphasic helical CT of the kidneys. AJR. 2002;178:367–372. doi: 10.2214/ajr.178.2.1780367. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, Endo M, Tsuboi M, et al. Differentiation of pathologic subtypes of papillary renal cell carcinoma on CT. AJR. 2008;191:1559–1563. doi: 10.2214/AJR.07.3181. [DOI] [PubMed] [Google Scholar]
- 18.Jonisch AI, Rubinowitz AN, Mutalik PG, Israel GM. Can high-attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced CT? Radiology. 2007;243:445–450. doi: 10.1148/radiol.2432060559. [DOI] [PubMed] [Google Scholar]
- 19.Korobkin M, Giordano TJ, Brodeur FJ, et al. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology. 1996;200:743–747. doi: 10.1148/radiology.200.3.8756925. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Hahn PF, Papanicolau N, et al. Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology. 1991;179:415–418. doi: 10.1148/radiology.179.2.2014283. [DOI] [PubMed] [Google Scholar]
- 21.Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR. 1998;171:201–204. doi: 10.2214/ajr.171.1.9648789. [DOI] [PubMed] [Google Scholar]
- 22.Stadler A, Schima W, Prager G, et al. CT density measurements for characterization of adrenal tumors ex vivo: variability among three CT scanners. AJR. 2004;182:671–675. doi: 10.2214/ajr.182.3.1820671. [DOI] [PubMed] [Google Scholar]
- 23.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(suppl 1):S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 24.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 25.Limanond P, Raman SS, Lassman C, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276–280. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 26.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 27.Joseph PM, Spital RD. The effects of scatter in x-ray computed tomography. Med Phys. 1982;9:464–472. doi: 10.1118/1.595111. [DOI] [PubMed] [Google Scholar]
- 28.Kanamori H, Nakamori N, Inoue K, Takenaka E. Effects of scattered X-rays on CT images. Phys Med Biol. 1985;30:239–249. doi: 10.1088/0031-9155/30/3/004. [DOI] [PubMed] [Google Scholar]
- 29.Endo M, Tsunoo T, Nakamori N, Yoshida K. Effect of scattered radiation on image noise in cone beam CT. Med Phys. 2001;28:469–474. doi: 10.1118/1.1357457. [DOI] [PubMed] [Google Scholar]