EXECUTIVE SUMMARY
Cancer care in the United States remained a mixed picture in 2015. Declining mortality rates, growing numbers of survivors, and exciting progress in treatment were set against the backdrop of increasingly unsustainable costs and a volatile practice environment. In this third annual State of Cancer Care in America report, the American Society of Clinical Oncology (ASCO) describes both challenges and opportunities facing the US cancer care system.
Balancing Progress and Challenges
In 2015, the US cancer care system developed new, more sophisticated therapies, expanded screening capabilities, and improved mortality for many types of cancer. However, there remains much room for improvement.
Progress in cancer care. This year, the US Food and Drug Administration (FDA) added 15 new drugs and biologic therapies to its list of more than 180 approved anticancer agents and expanded use for 12 previously approved treatments.1 2015 also marked introduction into the US market of the first product deemed biosimilar to an existing biologic product, paving the way for nonbrand products in the biologic drug sphere. Precision medicine was highlighted by President Obama as an important strategy for improving patient outcomes, and immunotherapy gained momentum within the cancer community. Thanks to these and other developments, patients with cancer face better treatment prospects than ever before.
Growing demand, stubborn mortality, and persistent inequities. An estimated 589,430 Americans died as a result of cancer in 2015.2 Mortality rates for some cancers, such as bladder cancer, brain cancer, and melanoma, have remained steady over the past decade, and pancreatic and liver cancer mortality rates have increased.3 Cancer incidence and mortality rates continue to vary substantially by race and ethnicity. For the first time in 2015, breast cancer incidence rates were higher for African American women than for any other racial group—a troubling development, because African American women with breast cancer are diagnosed at a younger age and have higher mortality rates than other women.4 These trends serve as a reminder that more effort is needed to improve outcomes for all patients with cancer.
Increasing complexity of care. This year’s report focuses on three areas that affect the complexity of cancer and its treatment: (1) cancer screening, (2) implementing precision medicine treatments, and (3) the aging of the US population. Screening programs have been successful in reducing morbidity and mortality in certain types of cancer, such as breast, colorectal, and cervical cancers. For many other cancers, the risk–benefit considerations are not so straightforward. The complexity involved in implementing cancer screening is based on the need to avoid over- and underscreening, as well as to make appropriate screening decisions when the evidence is ambiguous as to the potential health benefits for the patient. Precision medicine offers notable advantages to patients in need of expanded treatment options. However, physicians and patients are struggling to manage overwhelming amounts of information about risks and benefits of genetic testing—and its role in selecting treatment. Finally, an aging population means there will be an increasing number of patients whose cancer will be complicated by other chronic diseases.
Continued commitment to research funding and innovation needed. Advances in the scientific understanding and treatment of cancer have led to improved patient outcomes and quality of life. However, federal investment in research has not kept pace with this increasingly complex disease. Additionally, health information technology infrastructure must evolve to support innovative research designs, such as those using big data to gain rapid insight into patient outcomes and experiences.
Cancer Care Access and Affordability: Ensuring All Patients Can Receive Current and Developing Therapies
The Affordable Care Act (ACA) has expanded access to health insurance for millions of Americans, but many remain un- or underinsured. However, the cost of drugs, uneven implementation of the law, and other access barriers have placed care out of reach for many.
Insurance coverage. The ACA has extended insurance coverage to millions of Americans, and evidence suggests that this coverage is improving access to affordable care and having a positive impact on health.5,6
Variations in enrollment and coverage. Although the ACA has increased millions of people’s access to health insurance, approximately 35 million nonelderly adults remained uninsured in 2015.7 An additional 31 million individuals are underinsured because their deductible and/or out-of-pocket costs are high relative to their household income.8 Coverage across insurers and plans also remains inconsistent.
Rising drug prices and increasing burden for patients. Although the rate of growth in cost slowed temporarily during the recent economic downturn, health care spending has once again picked up speed—and costs associated with cancer care are rising more rapidly than costs in other medical sectors.9,10 For patients with cancer, two issues of critical importance are: (1) cost of cancer drugs and (2) increased patient burden associated with rising deductibles and cost shifting. A recent survey found that 24% of Americans say they have a hard time paying for prescription drugs, and 72% view the prices of prescription drugs as unreasonable.11
Oncology Practice and Workforce Trends: Engines for Discovery and Care Delivery Are at Risk
The 2016 ASCO Census and ASCO Practice Trends surveys suggest no abatement of volatility in the oncology practice environment. Economic pressures, market dynamics, and shifts in payment policy have combined to place many independent community practices in jeopardy. These trends and an increasingly constrained workforce raise concerns about how the US cancer care system will be able to respond to the projected surge in demand for cancer care in the coming years.
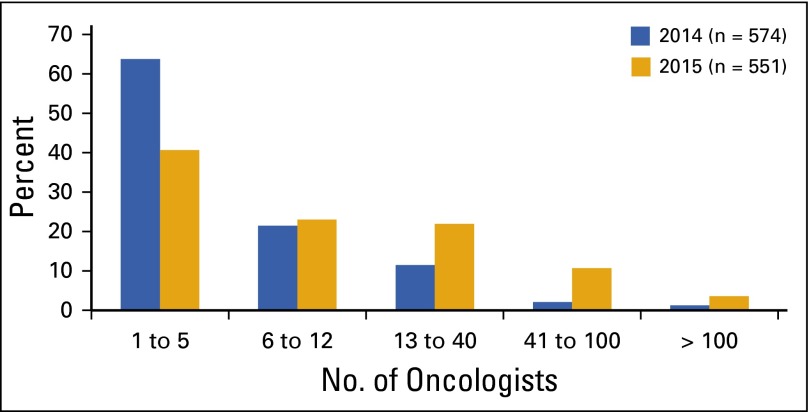
Oncologist workforce remains stable despite growth in demand. The size of the overall oncology workforce has remained relatively stable, with more than 11,700 hematologists and/or medical oncologists providing cancer care in the United States. However, specialists continue to age and largely practice in metropolitan areas, trends that could adversely affect the ability to meet demand for cancer services across the country.
The cancer care team. Increasing emphasis on use of the medical home model for delivery of care is driving greater emphasis on team-based care by health providers from a variety of backgrounds and specialties, including—but not limited to—primary care physicians, urologists, gynecologists, pathologists, pharmacists, genetic counselors, mental health specialists, pain and palliative care specialists, and advanced practice providers.
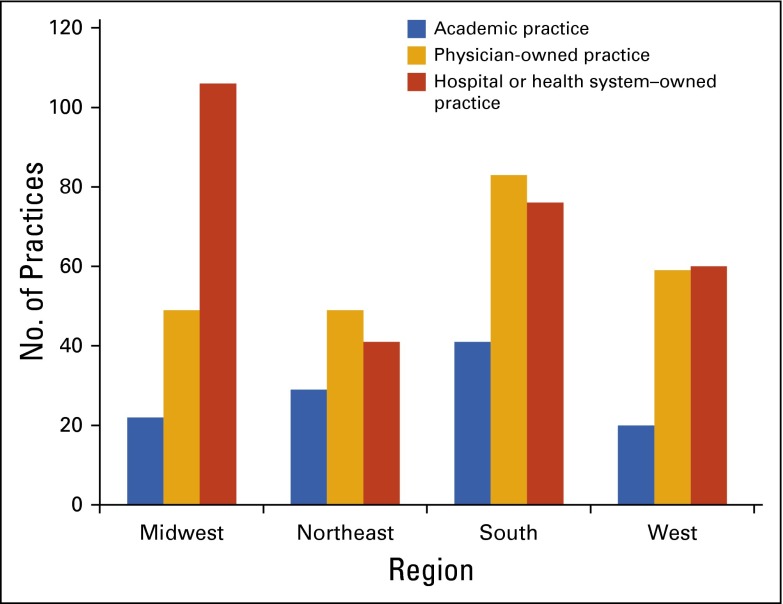
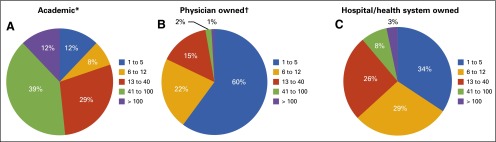
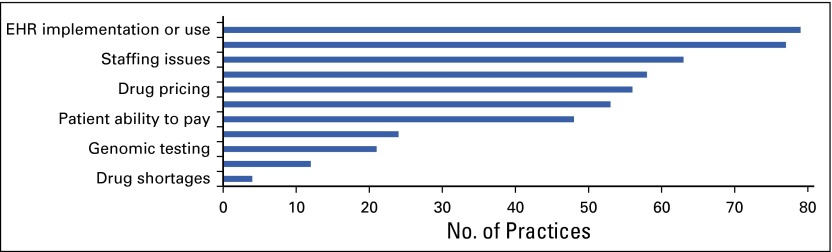
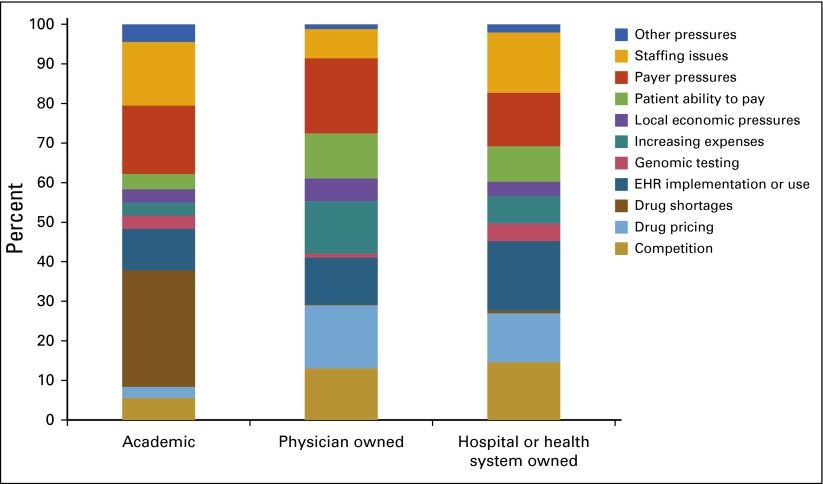
Census and Practice Trends surveys. ASCO surveyed a representative sample of academic, physician-owned, and hospital or health system–owned census respondents to gain further insight into high-priority and emerging topics of concern, including practice pressures, alternative payment models, clinical pathways, electronic health records (EHRs), and cost of care.
EHR implementation a top practice pressure. Nearly half (45%) of ASCO Practice Trends survey respondents cited EHR implementation or use as a priority practice pressure, surpassing all other pressures in 2015.
New Strategies for Delivering High-Quality, High-Value Cancer Care
As pressures to control costs escalate, payers and other stakeholders are pursuing new payment and care delivery models that lower spending while preserving quality. This report provides an overview of initiatives proposed and ongoing in 2015 to help relieve these pressures, namely in the areas of payment reform, value optimization, and performance improvement.
Payment reform; increased financial flexibility, high-quality care expected. A historic development for the US health care system was the April 2015 decision by the US Congress to repeal the sustainable growth rate (SGR) formula. The decision, part of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), effectively reversed required payment cuts and replaced the SGR with a plan to return stability to the reimbursement of physician services by the Medicare program. MACRA also encourages physicians to participate in new payment models that provide more flexible options for reimbursing physician services in exchange for increased accountability in delivering high-quality care.
New payment and care models under evaluation. A number of alternative payment models have been developed to improve care and reduce costs. These models move away from fee-for-service payments to other payment approaches that increase accountability for both quality and total cost of care and that emphasize population health management, as opposed to payment for specific services. This report highlights specific alternative payment models being explored or currently in development for cancer care, including the new Centers for Medicare & Medicaid Services (CMS) Oncology Care Model (OCM), the ASCO Patient-Centered Oncology Payment (PCOP) model, and many efforts undertaken by private and public institutions. It also explores the implications of clinical pathway use in oncology practices.
Initiatives targeting high-value cancer care. The topic of health care costs is being incorporated into many medical education programs in the United States. In addition, many organizations, practices, and researchers are launching initiatives intended to address rising health care costs by making the value of various treatment choices more transparent to patients and their clinicians.
Strategies to measure and improve performance in cancer care. As payment systems shift incentives from volume to value, quality-monitoring programs are increasingly important mechanisms to protect from over- or underuse and to distinguish performance among practices and providers. New emphasis on performance measurement and reporting ushered in with MACRA also put new pressure on EHRs and other health information technologies to support compliance.
Conclusions and Recommendations
In 2016, the collective wisdom of stakeholders throughout the oncology community will be needed to overcome challenges and ensure every patient with cancer receives high-value, high-quality care. ASCO recommends prioritizing the following as means of addressing the challenges described in this report:
Ensure all publicly funded insurance programs offer consistent and appropriate benefits and services for patients with cancer.
Congress should mandate that private and public health insurance plans provide parity in benefits and coverage for intravenous cancer drugs and orally administered or self-injectable cancer drugs.
Congress should address ongoing disparities in Medicaid by modifying Medicaid coverage requirements to include coverage of clinical trials and removing disparities in benefits between Medicaid programs established before and after the ACA.
Professional organizations should remain engaged with their members to track ACA implementation effects and trends and work with policymakers to address issues preventing access to high-quality, high-value care for patients with cancer.
Test multiple innovative payment and care delivery models to identify feasible solutions that promote high-quality, high-value cancer care.
Professional organizations should develop innovative care models that can be tested by the Center for Medicare & Medicaid Innovation (CMMI) and private payers as they seek better ways to incentivize and support high-quality, high-value patient care.
Congress should aggressively monitor implementation of MACRA to ensure (1) that the Administration works with professional organizations to test multiple payment models of care and (2) the Administration provides a clear path for implementation of payment models shown to provide positive results for patients, providers, and payers.
Advance health information technology that supports efficient, coordinated care across the cancer care continuum.
Congress should require that health information technology vendors create products that promote interoperability.
Policymakers should ensure that patients with cancer, oncologists, and other oncology providers do not bear the cost of achieving interoperable EHRs and that companies refrain from information blocking.
Professional organizations and other stakeholders should work with federal officials to ensure that health care providers have the information necessary to be prudent purchasers and users of health information technology systems.
Recognize and address the unsustainable trend in the cost of cancer care.
Congress should work with stakeholders to pursue solutions that will curb unsustainable costs for patients, providers, and the health care system.
Payers should design payment systems that incentivize patient-centered, high-value care and invest in infrastructure that supports a viable care delivery system.
Professional organizations should develop and disseminate clinical guidelines, tools, and resources such as Choosing Wisely to optimize patient care, reduce waste, and avoid inappropriate treatment.
Professional organizations should promote shared decision making between patients and physicians and the development of high-value treatment plans consistent with patients’ needs, values, and preferences.
ASCO will continue to: (1) track and evaluate the ever-shifting landscape in cancer care over the coming year, (2) support cancer care providers as they negotiate these growing pressures, and (3) work with policymakers to ensure that changes in the system support access to high-quality, high-value care for all patients.
INTRODUCTION
In March 2014, ASCO published its inaugural State of Cancer Care in America report, with the goal of increasing awareness among policymakers and the larger cancer community about improvements and current challenges in the delivery of high-quality cancer care, as well as about emerging issues that are likely to require future attention. This report is now an annual publication. It provides a comprehensive look at demographic, economic, and oncology practice trends that will affect cancer care in the United States each year.
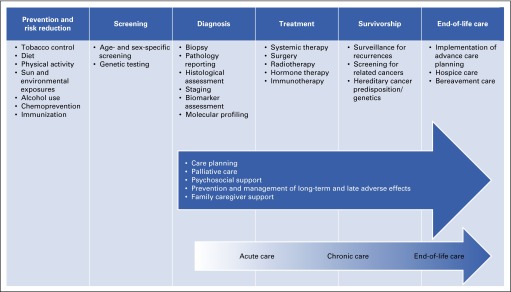
There were more than 14 million cancer survivors in 2014, and 1.7 million new diagnoses were expected in 2015.2,12 The impact of the disease is much broader than patients with cancer; friends and family members are also deeply affected. Many individuals serve as caregivers and provide social support to patients with cancer. In addition, people who have not been diagnosed with cancer participate in screening and prevention programs. The quality of cancer care across the care continuum must meet the needs of patients, families, and healthy individuals (Fig 1).
FIG 1.
Domains of the cancer care continuum. NOTE. Blue arrow identifies components of high-quality cancer care that span the cancer care continuum from diagnosis through end-of-life care. Graded arrow is another way of conceptualizing the time from diagnosis through end-of-life care. Data adapted.13
This year’s analysis of the state of cancer care reveals many promising advances. There are improvements in the development of precision medicine and immunotherapies, the ability to use big data to answer pressing research questions, and alternative payment models that have the potential to enhance the quality and value of cancer care. In addition, many patients with cancer have better access to care with the ongoing implementation of the ACA.
The 2016 State of Cancer Care in America report also describes ongoing concerns about the cancer care delivery system and its ability to take advantage of advances in treatment and care delivery. Additional efforts are needed to ensure that the results of research are translated into practice; that the reimbursement system rewards high-quality, high-value care; that patients have access to affordable care; and that disparities in patients’ access to care are reduced.
To organize the 2016 State of Cancer Care in America report, ASCO adapted the conceptual framework of the Institute of Medicine (IOM) for improving the quality of cancer care (Box 1).13 Chapter 1 focuses on trends in cancer research and the delivery of evidence-based care. Chapter 2 reviews the accessibility and affordability of cancer care, primarily from the perspective of patients. Chapter 3 explores the current landscape in the oncology workforce and oncology practices. Chapter 4 provides an overview of alternate payment models and efforts to improve the value of cancer care. The report concludes with a set of policy recommendations for the coming year.
Box 1. Framework for Organization of State of Cancer Care in America Report.
ASCO builds on the Institute of Medicine framework for “patient-centered, evidence-based, high-quality cancer care.”13 The components of the modified ASCO framework include:
Engaged patients: A system that supports all patients in making informed medical decisions consistent with their needs, values, and preferences in consultation with their clinicians who have expertise in patient-centered communication and shared decision making.
Adequately staffed, trained, and coordinated workforce: A system that provides competent, trusted, interprofessional cancer care teams that are aligned with patients’ needs, values, and preferences and coordinated with patients’ care teams and their caregivers.
Research to develop new therapies and evidence of effectiveness: A system that provides a robust infrastructure to support clinical research, such as clinical trials and comparative-effectiveness research, and uses evidence-based scientific research to inform medical decisions.
Learning health care information technology system for cancer: A system that uses advances in information technology to enhance the quality and delivery of cancer care, patient outcomes, innovative research, quality measurement, and performance improvement.
Translation of evidence into clinical practice, quality measurement, and performance improvement: A system that rapidly and efficiently incorporates new medical knowledge into clinical practice guidelines and clinical pathways, measures and assesses progress in improving the delivery of cancer care and publicly reports performance information, and develops innovative strategies for further improvement.
Accessible, equitable, and affordable cancer care: A system that is accessible to all patients and treats them equitably and that uses new payment models to align reimbursement to reward care teams for providing patient-centered, high-quality, high-value care and eliminating wasteful interventions.
High-value cancer care: A system that allows patients and their care teams to assess the value of various treatment options based on a transparent process; collective understanding; patient needs, values, and preferences; and accepted definition of what value in cancer care means.
New this year is the focus on a particular cancer treatment—immunotherapy—as a means of illustrating many of the themes of the report, their inter-relationship, and their impact on both outcomes of care and the overall patient experience. Advances in immunotherapy illustrate the importance of resolving issues in the health care delivery system (Box 2).
Box 2. Immunotherapy in Cancer.
Immunotherapies present an exciting opportunity to deliver a new, highly effective treatment to patients with cancer. These treatments are helping patients with many types of cancer, including cancers that previously have had no effective interventions. In fact, ASCO identified immunotherapy as the advancement of the year in its Clinical Cancer Advances 2016 report. This class of cancer treatment works by boosting the body’s natural defenses against cancerous cells.
However, the path toward achieving the full clinical benefit of immunotherapies faces many challenges. These challenges are indicative of the general obstacles facing the provision of high-quality care by the cancer care delivery system more broadly. The challenges to the cancer care delivery system—as highlighted in this report—are reviewed in the context of immunotherapy as follows:
Innovation Challenges
US investments in biomedical research have led to exciting and potentially transformative discoveries of many immunotherapies, including a new class of therapeutic agents called immune checkpoint inhibitors. Three drugs in this class—pembrolizumab, nivolumab, and ipilimumab—have now been approved by the US Food and Drug Administration for a total of three indications. Promising approaches to immunotherapy, including cellular and vaccine therapies, require further research investment to bring them to fruition. Immunotherapy research requires collaboration across several scientific and medical disciplines and new approaches to clinical trial design. The focus on individual response to disease and treatment means that immunotherapy research must be conducted in many subgroups of patients (ie, patients with different races and ethnicities, ages, and comorbidities); there is also a need for ongoing monitoring of use after approval in real-world populations.
Like many precision medicine treatments, immunotherapy can be optimized by providing clinicians with better tools for identifying and delivering treatments to the right patients—and for avoiding treatments when patients are unlikely to benefit. However, realizing this valuable benefit requires research to identify biologic molecules in tissues, cells, or blood that signal tumor susceptibility to attack by the immune system. Research is also needed to develop laboratory tests that can detect these molecules. The relatively low participation by patients with cancer in clinical trials and the continuing challenges with reimbursement make the rapid development of this promising field an ongoing challenge.
Clinical Challenges
It is difficult for clinicians to remain up to date on the huge volume of new information emerging from research on immunotherapy, especially given the differences among immunotherapies in toxicity profiles and modes of administration when compared with other kinds of cancer treatments. For example, patients may experience autoimmune reactions that are unfamiliar to clinical oncologists, and progression of their cancer might seem to occur before a therapeutic response. Without knowledge of the unique features of immunotherapy, clinicians and patients may prematurely abandon the therapy before achieving benefit. Additional clinician education will be necessary to fill this knowledge gap. The novelty and significant cost of immunotherapies raise particular concerns regarding quality and access to care. Special focus will be needed to ensure they are available equitably, regardless of geography, practice type, or patient characteristic (eg, race or ethnicity or insurance status) and according to prevailing standards of care.
Value and Quality Challenges
High unit cost and inconsistent reimbursement policies across payers hinder patients’ access to immunotherapies. Emerging data suggest that using drugs in combination and at higher doses increases efficacy, making the prospect of an unsustainable financial burden—for both individual patients and the system—more likely.14,15 For example, a combination of nivolumab and ipilimumab was approved for melanoma in October 2015, with an annual cost of more than $250,000 per patient.16 Former President Jimmy Carter, who was diagnosed with advanced melanoma in August 2015, announced in December that he is cancer free after immunotherapy with pembrolizumab, which costs $150,000 per year.17 It is unclear if patients or payers can afford these treatments or whether the health system is able to offer them and remain financially sound. Efforts to reform payment and identify high-value treatments will be essential to integrating immunotherapies into routine practice in a thoughtful manner.
1. BALANCING PROGRESS AND CHALLENGES
The demand for cancer services continues to grow as the US population ages and grows. At the same time, new drugs, technologies, and clinical advancements are improving quality of care and survival for those diagnosed with cancer—noteworthy successes that themselves influence demand. This chapter reviews major areas where the United States has made progress in improving quality of cancer care in the past year and identifies ongoing challenges that will require attention moving forward.
PROGRESS IN CANCER CARE
In 2015, the United States made significant improvements in cancer care, as evidenced by declining incidence and mortality rates for many types of cancer, the growing number of new drugs and technologies available to patients, and advancements in precision medicine.
Declining Cancer Incidence and Mortality Rates
The overall number of newly diagnosed patients with cancer in the United States continues to increase, in large part because of a growing and aging population. However, cancer incidence (the rate of cancer per 100,000 individuals) has dropped significantly in the past decade.3 For all cancers combined, the incidence rate declined between 2003 and 2012, with an annual percent change of 0.7%. Over this period, incidence rates for many common cancers declined even more, including rates for prostate, lung, colorectal, cervical, and stomach cancers.3 Prevention efforts, including smoking cessation, infection control, and refined cancer screening processes, have contributed to these rate reductions.18
Cancer mortality has declined an average of 1.5% annually over the past decade, with even greater annual declines in mortality rates for the four most common cancers—breast, prostate, lung, and colorectal cancers.3 Many factors have contributed to these reductions, including expanded treatment options, improved therapeutic outcomes, and prevention efforts.19 As a result, the number of cancer survivors in the United States is expected to grow from 14.5 million in 2014 to 19 million by 2024.12
Growing Number of New Drugs and Technologies
People with cancer have access to a wider array of treatment options than ever before. In 2015, the FDA added 15 new drugs and biologic therapies to its list of more than 180 approved anticancer agents (Table 1) and expanded use for 12 previously approved treatments.1
Table 1.
| Generic Name | Brand Name | Cancer Type | Precision Therapy† | Target | Oral or Injection |
|---|---|---|---|---|---|
| Alectinib | Alecensa | NSCLC | Yes | ALK | Oral |
| Elotuzumab | Empliciti | Multiple myeloma | Yes | SLAMF7 (CS1/CD319/CRACC) | Injection |
| Necitumumab | Portrazza | Squamous NSCLC | Yes | EGFR (HER1/ERBB1) | Injection |
| Ixazomib | Ninlaro | Multiple myeloma | Yes | Proteasome | Oral |
| Daratumumab | Darzalex | Multiple myeloma | Yes | CD38 | Injection |
| Osimertinib | Tagrisso | NSCLC | Yes | EGFR | Oral |
| Cobimetinib | Cotellic | Melanoma | Yes | MEK | Oral |
| Trabectedin | Yondelis | Liposarcoma or leiomyosarcoma | No | Injection | |
| Irinotecan liposome | Onivyde | Pancreatic adenocarcinoma | No | Injection | |
| Trifluridine/tipiracil | Lonsurf | Colorectal cancer | No | Oral | |
| Sonidegib | Odomzo | Basal cell carcinoma | Yes | Smoothened (SMO) | Oral |
| Dinutuximab | Unituxin | Neuroblastoma | Yes | B4GALNT1 (GD2) | Injection |
| Panobinostat | Farydak | Multiple myeloma | Yes | HDAC | Oral |
| Lenvatinib | Lenvima | Thyroid cancer | Yes | VEGFR2 | Oral |
| Palbociclib | Ibrance | Breast cancer, ER positive, HER2 negative | Yes | CDK4, CDK6 | Oral |
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; ER, estrogen receptor; FDA, US Food and Drug Administration; HDAC, histone deacetylase; HER, human epidermal growth factor receptor; NSCLC, non–small-cell lung cancer; VEGFR2, vascular endothelial growth factor receptor 2.
Listed in chronologic order, from most recent to least recent.
Refers to therapies that are directed at discrete molecular targets.
An important development for 2015 came with FDA approval of filgrastim-sndz, the first biosimilar product licensed in the United States. Filgrastim-sndz and its reference product filgrastim help generate WBCs that fight infection in patients receiving chemotherapy. The ACA established a process in 2010 for the FDA to approve products as either biosimilar or interchangeable with biologic reference products, similar to generic versions of drugs. Biologic products are complex molecules that are created within living material, so the process to demonstrate similarity of the products is challenging. Manufacturers of biosimilar products must submit data to the FDA demonstrating that a product is “highly similar” to the reference product, with “no clinically meaningful differences in terms of safety and effectiveness.”20(p1) An interchangeable product has to meet additional standards and may be substituted for the reference product by a pharmacist without prescriber consultation.
The naming convention for biosimilar and interchangeable products has yet to be determined. In draft guidance published in August 2015, the FDA proposed adding a suffix of four randomly generated letters to the nonproprietary biologic as a means of identifying its biosimilar status.21 The FDA used as an example filgrastim-sndz (which has a suffix that corresponds to its manufacturer, Sandoz [Holzkirchen, Germany]). The biosimilar would be named filgrastim-bflm (a nonmeaningful suffix). In addition, the FDA asked for public comment on whether interchangeable products should have a unique suffix or should match the reference product. ASCO submitted a letter opposing the use of nonmeaningful suffixes,22 encouraging the FDA to use a naming convention that ensures safety and does not add any administrative burden for physicians and pharmacists. The WHO opposes the FDA mixing nonproprietary names and proprietary claims. Pharmacists, insurers, and group purchasing organizations are also opposed to the FDA proposal because of confusion that may deter use of biosimilar products. The trade group for brand manufacturers supports the use of suffixes but urged the FDA to make them meaningful.
The FDA has also approved screening and diagnostic tests that have the potential to improve outcomes for patients with cancer. In 2015, three new molecular diagnostic tests were approved: (1) the ALK (D5F3) CDx Assay (Ventana Medical Systems, Tucson, AZ), which identifies the anaplastic lymphoma kinase protein in non–small-cell lung cancer tissue specimens to determine whether crizotinib treatment will be beneficial; (2) the cobas KRAS Mutation Test (Roche Molecular Diagnostics, Pleasanton, CA), which detects KRAS gene mutations to ascertain effectiveness of colorectal cancer treatments cetuximab and panitumumab; and (3) the PD-L1 IHC 28-8 pharmDx test (Dako, Glostrup, Denmark), which measures programmed death-ligand 1 protein in nonsquamous non–small-cell lung cancer tissue samples to assess the potential benefit of nivolumab.25 In addition, a newly approved screening test, MAMMOMAT inspiration with tomosynthesis (Siemens Healthcare, Erlangen, Germany), uses cross-sectional images along with conventional two-dimensional mammography to enhance the accuracy of breast cancer detection and diagnosis.
Achieving the Promise of Precision Medicine
Scientific and medical communities continue to prioritize precision medicine as a means to significantly improve patient outcomes. This priority was highlighted by President Obama in his 2015 State of the Union address and further strengthened by his $215 million commitment to increase research funding with the Precision Medicine Initiative.26 In cancer care, precision or targeted therapies work by exploiting the molecular underpinnings of cancer. The precision of cancer treatments has become more sophisticated with each passing year. Therapies that attack multiple genetic drivers of cancer in combination or harness the body’s own immune system to attack tumor cells have improved outcomes for patients with difficult-to-treat cancers. Of all the newly FDA-approved cancer therapies listed in Table 1, 12 (62.5%) are classified as precision therapies. With the aid of evidence-driven diagnostic testing, physicians can often identify targeted treatments most likely to work for individual patients with cancer—and avoid treatments that are unlikely to help, thereby saving time and sparing patients and families from potential toxicities and costs.
Immunotherapy has been a particularly fruitful application of precision medicine and has garnered momentum within the cancer community. One method of activating the immune system to fight cancer is to prevent brakes or checkpoints from suppressing immune response. Three immunotherapy drugs that work in this manner, so-called immune checkpoint inhibitors—pembrolizumab, nivolumab, and ipilimumab—were approved in recent years for treating melanoma and are showing promise in other cancers. In 2015, nivolumab and pembrolizumab both received FDA approval for treatment of non–small-cell lung cancer based on compelling clinical research findings.1,27 Other clinical trials have shown notable benefits for patients with liver cancer, head and neck cancer, stomach cancer, bladder cancer, and Hodgkin lymphoma. In 2015, data were also released showing that some patients with melanoma benefit from use of immune checkpoint inhibitors in combination.28 Immunotherapy holds great promise to improve the care and quality of life for patients with cancer, but it also illustrates many of the challenges that persist in today’s cancer care delivery system (Box 2).
CHALLENGES IN CANCER CARE
Despite significant progress made in 2015 toward improving the quality of cancer care, many ongoing challenges persist. These include the rising demand for and complexity of cancer care, immovable mortality rates for some cancers, ongoing health disparities, and static funding for cancer research.
Rising Rates for Some Types of Cancer
Nearly 1.7 million new cancer cases were diagnosed in 2015, bringing the total number of Americans living with cancer to 14.5 million.2 The overall number of newly diagnosed patients with cancer is expected to grow by 45% between 2010 and 2030.29
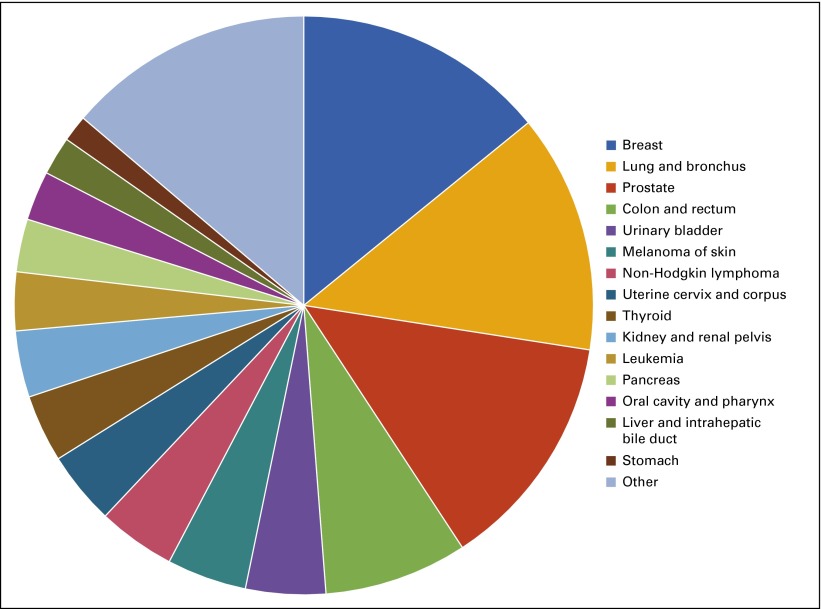
Among new cancer cases, nearly half (46%) are diagnosed as one of the four most common cancers: (1) breast, (2) prostate, (3) lung, or (4) colon cancer (Fig 2).2,3 A recent analysis shows that cases of breast cancer—the most commonly occurring cancer—will increase by 50% between 2011 and 2030.30
FIG 2.
Distribution of common cancer diagnoses, 2015.
Between 2003 and 2012, melanoma, thyroid cancer, kidney cancer, liver cancer, and pancreatic cancer incidence rates significantly increased.3 By one recent projection, thyroid cancer will replace colon cancer as the fourth leading cancer diagnosis by 2030, in large part because of better thyroid cancer detection.19 As overall cancer incidence increases and shifts between cancer types in the coming years, demand for screening and treatment services will change, challenging the cancer care delivery system. In addition, the millions of Americans who develop and survive cancer will require long-term care and monitoring to detect and treat recurrence or new cancers. They may also require support for long-term physical, emotional, psychosocial, and financial adverse effects as a result of their treatment.
Because patients with cancer are living longer, an increasing proportion of new cancer cases occur in patients with a previous history of cancer. Approximately 19% of cancers diagnosed from 2005 to 2009 were not first cancers, compared with only 9% diagnosed from 1975 to 1979.31 Patients diagnosed with second cancers may experience heightened distress and may face new barriers to treatment, including lifetime tolerability limits for particular radiotherapy and chemotherapy regimens.
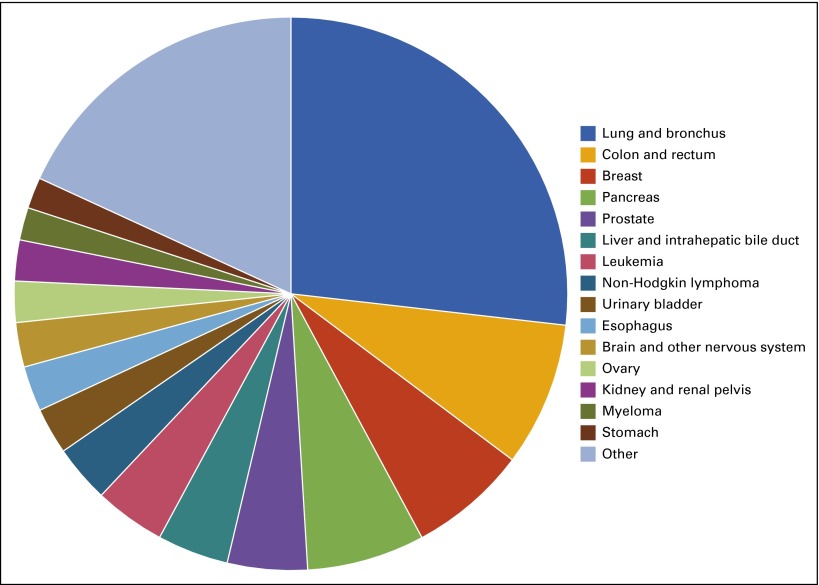
Despite the progress made toward reducing mortality for many types of cancer, cancer remains the second leading cause of death in the United States, accounting for nearly a quarter of deaths. An estimated 589,430 Americans died as a result of cancer in 2015, with lung cancer causing more than a quarter (27%) of these deaths (Fig 3).2 Mortality rates for some cancers, such as bladder cancer, brain cancer, and melanoma, have remained steady over the past decade, and pancreatic and liver cancer mortality rates have increased.3 These trends serve as a reminder that more effort is needed to improve outcomes for all patients with cancer.
FIG 3.
Distribution of common cancer deaths, 2015.
Ongoing Health Disparities
Substantial disparities in cancer incidence and mortality remain a feature of the current health care system. In particular, there are striking differences in cancer incidence and mortality rates across racial and ethnic groups. According to recent data from the National Cancer Institute (NCI), African Americans are 3% more likely to develop cancer than whites and are 18% more likely to die as a result of cancer.3 These discrepancies can be more pronounced for certain cancers and between men and women.
An October 2015 report from the American Cancer Society (ACS) found that racial disparities in breast cancer are on the rise.4 According to this analysis, although breast cancer incidence has remained relatively stable among white women, the rate among African American women—historically lower than that of whites—has risen this year to surpass those of all other racial groups. Because African American women with breast cancer are diagnosed at a younger age and have higher mortality rates than white women, these new data are concerning—especially in light of recent progress made in breast cancer outcomes.
Racial and ethnic health disparities arise from a complex set of factors that include education, socioeconomic status, health insurance status, health behaviors, and presence of environmental and behavioral risk factors. It is difficult to tease apart racial or ethnic differences that are biologically based from differences related to interactions between race or ethnicity and environmental and social variables that limit access to care. The ACS breast cancer study hypothesizes that increases in breast cancer rates in African American women may be a result of rising obesity and changing reproductive behaviors, but it also notes that black women are more likely to have an aggressive type of cancer that may have a genetic basis.4
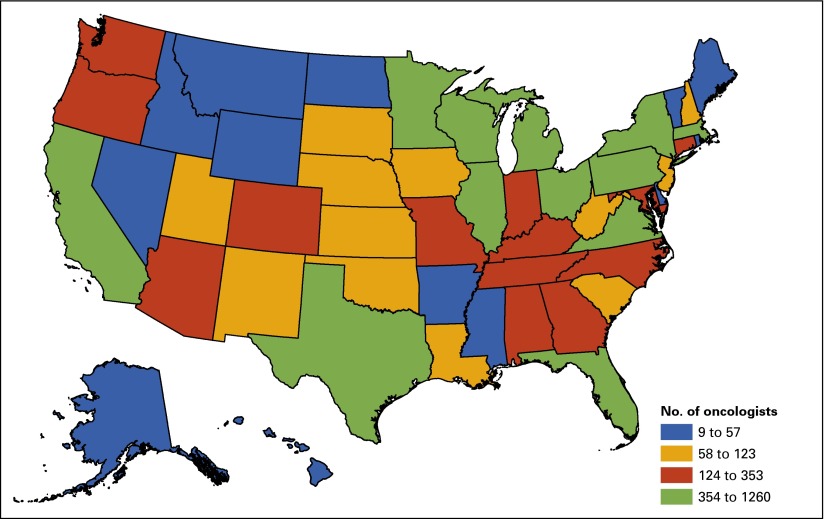
Geographic location also plays a role in cancer incidence and survival.32 Some geographic disparities in cancer incidence and outcomes may be exacerbated by state decisions on Medicaid expansion and differing levels of care available to poor and minority residents, as well as environmental risk factors. Distribution of oncologists across the United States, relative to where patients reside, may present access issues that further affect disparities in outcome. In the face of continuing barriers to access, especially for vulnerable populations, ASCO has continued its efforts to provide the oncology workforce with resources that increase awareness of cancer disparities and actions to address these disparities. Visit www.asco.org/healthdisparities for more information.
Increasing Complexity of Cancer Care
A major challenge to improving quality of cancer care is its complexity. Patients differ in personal characteristics (eg, age, genetic makeup, and physical health), cancer diagnosis, prognosis, and treatment preferences. This section of the report focuses on three examples of cancer care complexity that have received recent public attention: (1) cancer screening, (2) implementing precision medicine treatments, and (3) the aging of the US population, resulting in patients with cancer who are older and have more comorbidities than younger cohorts.
Cancer screening
Cancer screening programs are aimed at detecting cancer early, before symptoms are present. Early detection can help patients avoid the need for aggressive treatment and improve overall outcomes. The complexity involved in implementing cancer screening is based on the need to avoid over- and underscreening, as well as to make appropriate screening decisions when the evidence is ambiguous as to the potential health benefits to the patient. Furthermore, because screening tools are not perfectly sensitive or specific, they can lead to false-positive or false-negative results.
Screening capabilities and subsequent intervention options vary tremendously by cancer type. For breast, colorectal, and cervical cancers, there is clear evidence that routine screening among appropriate age groups, followed by intervention, significantly improves survival. A recent systematic review found that colorectal cancer screening (colonoscopy and sigmoidoscopy) programs reduced cancer mortality by 46% to 66% in observational studies analyzed.33
For many other cancers, the risk–benefit considerations are not so straightforward. For instance, the ACS, which has taken an aggressive stance on mammography screening for breast cancer in past years, updated its guidelines in October 2015 to recommend screening at later ages and with less frequency than previously recommended. Also in 2015, the US Preventive Services Task Force (USPSTF) released draft updates to its breast cancer screening guidelines, reaffirming that screening before age 50 years for women at average risk is not supported by current clinical evidence.34
The USPSTF has also reviewed evidence on prostate, bladder, skin, and oral cancer screening and decided not to publish screening recommendations for these cancers because of inconclusive findings.35 Inconsistencies in guidelines and inconclusive data make it difficult for patients and physicians to make appropriate screening decisions. Furthermore, because the ACA hinges preventive services coverage on USPSTF guidelines, some stakeholders have expressed concern that screening will not be offered to all individuals who could potentially benefit.36
In addition, there are risks to patients’ health from both over- and underscreening. Patients who are underscreened risk the possibility of a cancer not being detected early in its disease trajectory, thus potentially experiencing worse outcomes. Current guidelines recommend screening for breast, colon, and colorectal cancers in susceptible populations. However, a recent analysis of 2013 national survey data conducted by the Centers for Disease Control and Prevention uncovered the following age-adjusted findings, which are lower than the Centers for Disease Control and Prevention Healthy People 2020 national targets for screening in these three areas—suggesting many patients may be underscreened37:
72.6% of women age 50 to 74 years reported recent mammography.
80.7% of women age 21 to 65 years reported a recent Pap test.
58.2% of respondents age 50 to 75 years reported recent colorectal screening tests.
Conversely, one of the major risks of overscreening is that a test may detect benign tumors and malignant tumors unlikely to become clinically significant during a patient’s natural lifespan. Patients who undergo these screening procedures can experience nontrivial health consequences, including emotional distress and unnecessary or detrimental treatment, as well as financial burden.
Implementing precision medicine
Precision medicine has enormous potential to improve the quality of cancer care. However, there are some challenges to achieving these benefits in clinical practice. Testing for specific individual genetic mutations such as EGFR in lung cancer and BRAF V600E in melanoma has become commonplace in oncology practice. There is also growing interest in multiplex genetic testing, where tumors are evaluated for changes in several cancer-related genes at once. Little is known about how multiplex genetic information is used by physicians and patients.
A recent survey found that 22% of physicians at a major cancer center had low confidence in their own knowledge of genomics; however, 42% were willing to disclose uncertain findings of genetic testing to patients.38 Some institutions have implemented molecular tumor boards to provide education and clinical guidance, but guidelines or other decision support will be important as the practice of multiplex genetic testing becomes more widespread.39 In addition, as more practices begin providing immunotherapies, clinicians will need information about safety concerns associated with this class of drugs and the appropriate management of serious adverse effects.
Changing demographics
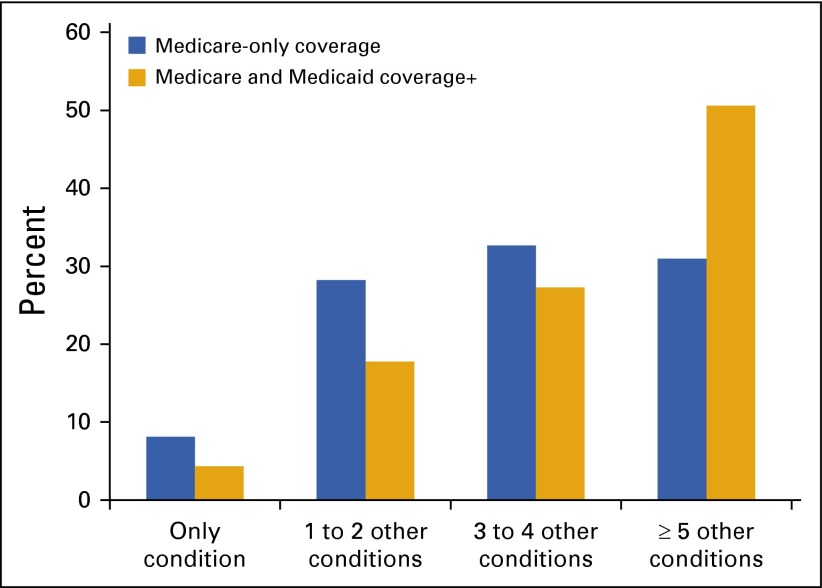
An important and growing demographic in cancer is the number of elderly Americans. The majority of new cancer cases are diagnosed among those age 65 years or older.40 This population is also susceptible to other chronic diseases, such as diabetes, heart disease, and Alzheimer’s. Chemotherapy and other cancer treatments can further increase the risk of developing chronic disease symptoms, especially those related to cardiovascular health. Among Medicare beneficiaries with breast, colorectal, lung, or prostate cancer, 91.9% had one or more other chronic conditions (Fig 4).41 Medicare–Medicaid dual eligible beneficiaries have an even higher prevalence of comorbidities (95.7%; Fig 4).
FIG 4.
Distribution of chronic disease comorbidities among Medicare beneficiaries with cancer. (*) Other conditions include: Alzheimer’s disease, related disorders, or senile dementia; arthritis (including rheumatoid and osteoarthritis); asthma or atrial fibrillation; autism spectrum disorders; chronic kidney disease; chronic obstructive pulmonary disease; depression; diabetes (excluding diabetic conditions related to pregnancy); heart failure; hyperlipidemia (high cholesterol); hypertension (high blood pressure); ischemic heart disease; osteoporosis; schizophrenia or other psychotic disorders; and stroke or transient ischemic attack.
The growing number of patients with cancer and serious chronic conditions presents new challenges, because providers must assess and manage comorbidities in treatment planning, medication prescribing and adherence (eg, awareness of contraindications), and coordination of care with primary care physicians or other chronic disease specialists. With so many patients with cancer having multiple conditions, it will be essential to consider this complexity in assessing workforce and practice needs.
The difficulties of caring for an aging population are further complicated by the fact that the elderly are often under-represented in cancer clinical trials. In general, only approximately 3% of US patients with cancer participate in clinical trials—and these patients tend to be younger, healthier, and less racially and ethnically diverse than the overall population of patients with cancer.42 Thus, our evidence base for treating older patients with cancer is quite limited. In 2015, ASCO released a policy statement advocating for increased research on older adults with cancer, a population disproportionately affected by cancer (Box 3).
Box 3. Statement on Improving Evidence Base for Treating Older Adults With Cancer.
ASCO convened a subcommittee of experts to consider the role for and engagement of older adults in clinical research. The following recommendations were issued43:
Use clinical trials to improve the evidence base for treating older adults with cancer.
Leverage research designs and infrastructure for generating evidence on older adults with cancer.
Increase US Food and Drug Administration authority to incentivize and require research involving older adults with cancer.
Increase clinicians’ recruitment of older adults with cancer to clinical trials.
Use journal policies to improve researchers’ reporting on the age distribution and health risk profiles of research participants.
The full statement is available online at http://www.asco.org/sites/www.asco.org/files/older_adults_asco_statement_2015.pdf.
Funding for Cancer Research
The progress in immunotherapy and other areas of cancer research highlighted in this chapter was made possible by research investments from previous decades. Sustained funding of the National Institutes of Health (NIH) and the NCI is critical to continuing progress against cancer through the development and delivery of new cancer therapies to patients. However, as cancer care demand and complexity increase, research funding at the federal level has failed to keep pace.
The need for increased research funding was recognized in a rare instance of bipartisan agreement within the US Congress. In July 2015, the House of Representatives passed the 21st Century Cures Act (H.R. 6) by a wide margin. The bill included $10 billion ($2 billion per year for fiscal years 2016 to 2020) in mandatory spending for the NIH to focus on precision medicine and young investigators and $550 million ($110 million per year for fiscal years 2016 to 2020) in mandatory spending for the FDA. ASCO supports H.R. 6 and is working collaboratively with the Senate to achieve passage of the legislative initiatives.
In late 2015, the House and Senate approved funding increases for 2016 for the NIH, including $200 million for the new Precision Medicine Initiative and an increase of $264 million (5.3%) for the NCI. This increase in appropriations came after a decade-long decline in NIH funding in real dollars, potentially signifying a regenerated commitment to medical research.
The cancer community also makes more out of available funding by leveraging clinical data to study outcomes of patients receiving treatment in practice. There is a growing recognition that much can be learned from the experiences of the millions of patients receiving cancer care throughout the United States. The availability of EHR data, combined with the computing capability of informatics and big data systems, presents an invaluable opportunity for rapid-learning systems, in which real-world clinical data are collected and analyzed to help guide clinical decision making. However, the current interoperability of many EHRs poses a significant barrier to realizing the vision of using big data to its full potential.
In the era of precision medicine, only small subsets of patients may have the appropriate molecular targets for specific targeted therapies. Clinical trials testing such new therapies have to be designed to accrue sufficient numbers of patients to assess treatment efficacy and safety. There have also been accounts of exceptional responders to cancer therapies—patients who respond uncharacteristically well to treatments.44 Cataloging and studying these responses may lead to new uses of drugs currently available on the market.
CONCLUSION
Advances in science and technology, including great promise and tangible advances in precision medicine (which includes targeted and immunotherapies), have contributed to substantial progress in cancer detection and treatment. However, patients with certain tumor types and from some demographic groups are not benefiting fully from the advances in cancer care. In addition, although precision medicine is offering promising new avenues for cancer treatment, it is also challenged by a vast array of unanswered questions. Sustained research funding and infrastructure are required to seize opportunities to improve treatment options for patients. This should include support for research using informatics and big data systems that can talk to one another and provide clinical decision support to ensure rapid learning and optimal patient care.
2. CANCER CARE ACCESS AND AFFORDABILITY: ENSURING ALL PATIENTS CAN RECEIVE CURRENT AND DEVELOPING THERAPIES
Nationwide efforts to expand the accessibility and affordability of health care have benefited millions of Americans—but not all patients have benefited equally. This chapter discusses recent changes in access and affordability in cancer care, highlighting both positive trends and ongoing concerns for the oncology community. The chapter begins with a brief overview of the state of health insurance in the United States, followed by a summary of the benefits of the ACA as it applies to access to treatment of current and future patients with cancer. The chapter also details ongoing problems with design and implementation of the law, as well as implications of these concerns for cancer care. The final section examines the cost of cancer care, with an emphasis on financial burdens to patients.
HEALTH INSURANCE AND ACCESS TO CANCER CARE
For the past 50 years, the federal government has engaged in efforts to improve accessibility and affordability of health care for US patients, mainly through its three landmark insurance programs: (1) Medicare, (2) Medicaid, and (3) the ACA.45 As 2015 marked the 50th anniversary for Medicare and Medicaid, the ACA reached its fifth anniversary and continues toward full implementation. These laws have had profound effects on the US health care system and the state of cancer care. Although progress is incomplete, the programs provide health insurance for millions of Americans and have a profound impact on how patient care is organized and delivered.
Medicare and Medicaid, the oldest and largest government-run health care programs in the nation, were established in 1965 to address the high rate of uninsured among the most vulnerable populations in the nation: older adults and low-income individuals. Today, they cover 111 million people (one in three Americans) and account for 39% of national health care spending, including a large proportion of the cost of cancer care.45
Medicare, the federal insurance program for the elderly and permanently disabled, is widely credited with achieving almost universal access to health care for older adults while making it affordable for enrollees.45 Current enrollment includes 45 million older adults and 9 million adults with permanent disabilities. The Medicare Payment Advisory Commission projects an increase from today’s current 54 million to more than 80 million beneficiaries by 2030 as the baby-boom generation ages into the program.46 Because the majority of patients with cancer and cancer survivors are older adults, Medicare provides a critical support system for cancer care.
Medicaid is administered through a federal partnership with individual states and has historically provided coverage to low-income children and adults. The program has undergone considerable changes since first implemented, including a recent expansion of coverage related to provisions in the ACA. Medicaid now enrolls approximately 70 million people annually and provides the majority of insurance coverage for people with limited incomes, including pregnant women and children, elderly adults, and people who are disabled.45 It is also an important source of coverage for patients with cancer.47
The ACA is the most recent federal health care program, aiming to reach the millions of uninsured Americans who are not eligible for Medicare and/or Medicaid and who do not receive health insurance through employers. The remainder of this section discusses important 2015 developments in cancer care related to ACA implementation, including areas of progress and areas of concern.
ACA: Ongoing Progress
The ACA is now in its fifth year of operation. It has completed three open-enrollment periods for Medicaid and marketplace coverage. The following sections discuss two important changes related to the law: (1) expanded insurance coverage and (2) expanded benefits for cancer services.
Expanded insurance coverage
The ACA has extended insurance coverage to millions of Americans, and evidence suggests that this coverage is improving access to affordable care and having a positive impact on health.5,6 Recent data show the number of uninsured has decreased by approximately 17 million people since implementation.48 In 2015, uninsured rates fell in all states, with the largest declines occurring in states with expanded Medicaid programs.6 Families with incomes at or below 138% of the federal poverty level experienced the biggest increase in insurance coverage. Hispanics and African Americans experienced greater declines in uninsured rates than whites.6 These findings suggest that the ACA is fulfilling its goal of increasing access to health insurance for people who were previously uninsured, with important coverage gains in minority and poor populations.
One of the major assumptions underlying the ACA was that expanding people’s access to insurance would affect their health behaviors, use of health care services, and health outcomes. New data indicate that people who obtained coverage through the ACA marketplace and Medicaid expansion are using their plans to access care that was previously unaffordable, are increasingly accessing medicine and providers, and are largely satisfied with their coverage regardless of insurance type.45,47,48
These findings suggest that the ACA has increased patients’ access to a spectrum of health care services, which may eventually improve overall health. A recent study comparing pre-ACA and early 2015 national survey responses found that self-reported health status had improved over time, with 3.5% fewer respondents citing poor to fair health.6 As more time passes, it will be important to examine the impact of the law on health outcomes, including cancer.
The law may also help to decrease long-standing health disparities related to lack of insurance, because Medicaid enrollees have better access to care and fewer unmet health needs than the uninsured. However, not all insurance plans are equally likely to reduce disparities (as described under the section on areas of concern).
Benefits for patients with cancer
The ACA includes a number of provisions that benefit patients with cancer, including:
Prohibition against coverage exclusions based on pre-existing conditions. This means that insurers cannot deny health insurance to individuals because they have a history of cancer.
Elimination of lifetime caps and annual spending limits. This provision is designed to prevent insurance companies from stopping payment for services once a specified dollar amount has been reached. Because treatment of cancer may involve both high annual costs and health care costs that extend over many years, this benefit is likely to reduce cancer-related bankruptcies for patients with cancer and their families.
Expansion of required coverage for routine health care services and basic levels of care, including ambulatory services, mental health services, and rehabilitative services that become critical once the acute stage of cancer treatment has been completed. Coverage also includes chronic disease care, an important benefit for cancer survivors, and palliative and hospice services for care at the end of life.
Coverage of routine costs associated with clinical trial participation. Most insurance policies are required to allow patients with cancer to enroll in clinical trials as a basic covered service.
Coverage of essential screenings, including tests used to detect cancer, at no cost to the patient. Because people without insurance are likely to forgo routine health screenings, this coverage is likely to promote earlier detection of cancer.
Coverage for wellness and preventive care, such as weight loss and smoking cessation—two strategies critical for reducing the risk of cancer onset or progression.
Provision allowing young adults to remain on their parents’ insurance policies until age 26 years. This provision provides continuity of services for individuals diagnosed with cancer as children and helps to decrease the number of uninsured young adults who are not eligible for employer-sponsored insurance.
An estimated 160,000 people with cancer will be part of the 16 million Americans who gain coverage through Medicaid and CHIP by 2019.47 Data on the direct impact of these benefits on cancer care are limited. However, the expansion of access through the ACA to a wide spectrum of health care services should expand access to critical services for patients with cancer and their families. In one recent study, researchers observed an increase in early-stage cervical cancer diagnoses among women age 25 years or younger after the ACA insurance expansion began, suggesting that newly insured women were taking advantage of preventive services.49
ACA: Ongoing Concerns
Although the ACA has increased millions of people’s access to health insurance, serious policy concerns about limitations of the law continue to drive the health care debate. This section highlights four broad areas of ongoing concern: (1) persistent gaps in health care coverage, (2) incomplete Medicaid expansion, (3) marketplace coverage gaps, and (4) clinical trial coverage.
Persistent gaps in health care coverage
Although millions of individuals have gained access to insurance through the ACA, coverage expansion is slowing, and millions of Americans remain uninsured or underinsured.5,48,50 The Congressional Budget Office estimates that approximately 35 million nonelderly adults were without health insurance in 2015.7 The uninsured include low-income people living in states that did not expand Medicaid, people without employer-based health coverage who chose not to purchase health insurance in the marketplaces, and undocumented immigrants who are not addressed by the law.5,51 An additional 31 million individuals were deemed underinsured because their deductible and/or out-of-pocket costs were high relative to their household income.8 Slightly more than 50% of underinsured adults had insurance through their employers, with the remainder having marketplace, Medicare, or Medicaid policies.8
Without health insurance, patients with cancer face tremendous obstacles to receiving the care they need, from prevention through treatment and survivorship and care at the end of life. Insured patients with cancer are diagnosed earlier, have a better chance of survival, enjoy greater financial stability, and experience a higher quality of life. For these reasons, expanding access to health insurance to more Americans remains a critical issue in cancer care, even after passage of the ACA.52
Incomplete Medicaid expansion
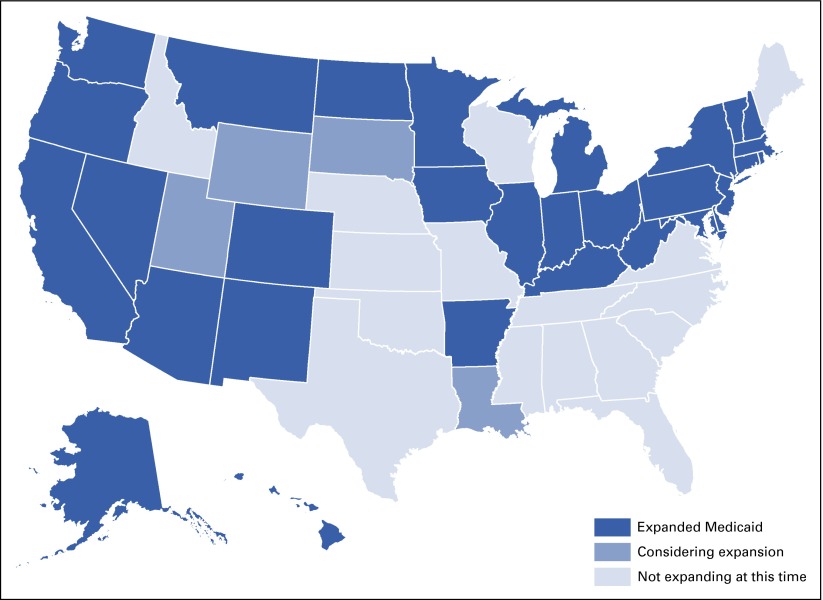
The US Supreme Court ruled in 2012 that states did not have to expand Medicaid, and this decision may have contributed to the continuing high rates of uninsured. Only 30 states and Washington, DC, had expanded Medicaid by the end of 2015 (Fig 5).53 In states that did not expand Medicaid, the rates of uninsured are more than twice those of the states that did expand. Differing decisions on Medicaid expansion have also created significant inequities in health coverage among the states and will likely exacerbate regional disparities in cancer detection rates, quality of care, and outcomes.47
FIG 5.
Status of state Medicaid expansion decisions.
Even within expansion states, there are issues of differences in benefits for individuals who enrolled in plans created before the ACA and those who enrolled under ACA policies. Many of the poorest, most vulnerable recipients receiving coverage through state-run programs may be in plans that do not meet ACA standards for prevention and screening, treatment, or survivorship care.
Policymakers are also concerned about the quality of care offered to Medicaid recipients compared with privately insured patients. Low physician and hospital reimbursement rates in many states limit the pool of providers who are willing to accept Medicaid patients or limit the range of services offered to beneficiaries.45 For example, a recent study found that women with Medicaid were less likely than women with private insurance to be referred for preventive services, such as breast examinations and Pap tests.54 High drug copays are also common in many pre-expansion Medicaid programs. In addition, lack of mandated access to clinical trials also limits the range of treatment options for Medicaid recipients in some states (as discussed in the section on clinical trial coverage).47
To address these concerns, ASCO has made Medicaid reform a high priority and, in 2014, published an extensive list of policy recommendations, with the goal of ensuring access to high-quality, high-value cancer care for Medicaid beneficiaries. Visit www.asco.org/Medicaid for details.
Marketplace coverage gaps
In 2015, the Supreme Court ruling in King v Burwell upheld federal subsidies for marketplace plans, preserving subsidies for more than 6 million Americans.55 However, recent reports that marketplace cooperatives in many states are in financial difficulty or closing raise concern that potential enrollees will face a limited selection of more expensive private insurance plans.56 Individuals without coverage may continue to delay recommended health screenings that detect cancer, decide to forego needed treatments, or face financial hardship while living with cancer.
Clinical trial coverage
The ACA created the first federal law requiring private insurers to cover routine care costs for patients participating in clinical trials. The law applies to a wide range of private and federal insurance plans, including Medicare but excluding Medicaid and some private insurance companies. Although the coverage mandate is now in statute, the federal government has not yet issued regulations to guide implementation, and coverage may remain problematic for patients currently enrolling in trials.57 A 2014 survey of sites conducting clinical trials found that 63% experienced denial of coverage for patients with cancer, even in states that had laws in place mandating coverage.57 These findings reinforce concerns that patients seeking treatment in clinical trials may be denied mandated coverage of state-of-the-art treatment programs until federal guidelines for implementation and enforcement are in place.
Patients with cancer who are covered under Medicaid policies also are likely to be denied coverage for clinical trial participation. Because Medicaid was excluded from the clinical trial mandate, states are not required to include this coverage in their Medicaid policies, and not all states provide such coverage for their Medicaid beneficiaries.58 This creates a significant coverage disparity among states, affecting the most vulnerable poor and minority residents who form the majority of Medicaid recipients.47 To ensure that all Medicaid enrollees have access to the treatment options offered by clinical trials, ASCO has included clinical trial coverage in its list of policy suggestions for Medicaid reform.59 Certain other policies were also excluded from the mandate, and people who are insured under these grandfathered policies also have no guaranteed coverage for the costs associated with clinical trial participation.60
A final concern surrounding clinical trial participation is related to coverage for phase I clinical trials. These trials often represent the first-in-human studies of new treatments and are used to both determine dosage and schedule and obtain evidence of benefit for new treatments.60 In today’s trials of targeted therapy, phase I trials often provide great benefit. Private insurance companies are required to cover participation in phase I to IV trials under the ACA, but participation is limited under Medicaid, Medicare, and some grandfathered health plans. ASCO recently updated its policy statement on the importance of phase I trials in cancer treatment and research to ensure patients have the option of participating in these trials (Box 4).
Box 4. Statement on Phase I Clinical Trial Coverage.
In December 2014, ASCO updated its position on the conduct of phase I clinical trials based on input from the Cancer Research Committee, a panel of experts with various roles in clinical research—including patient advocacy. Concerning insurance coverage of phase I clinical trials, ASCO makes the following recommendations:
The Centers for Medicare & Medicaid Services should recognize that phase I cancer clinical trials meet the therapeutic intent requirement of the National Coverage Determination for routine patient costs in clinical trials.
State Medicaid programs should reimburse for routine patient costs associated with clinical trials, including phase I trials.
Visit http://www.asco.org/sites/www.asco.org/files/phase_1_trials_in_cancer_research-_asco_policy_statement.pdf for the full statement.
RISING COST OF CANCER CARE
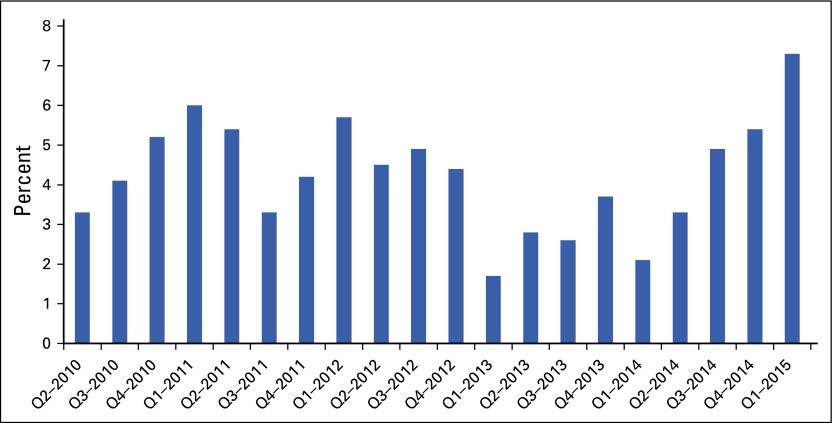
The rising cost of health care presents a fiscal challenge to the United States. Although the rate of growth in cost temporarily slowed during the recent economic downturn, health care spending has once again picked up speed.9 As seen in Figure 6, health service expenditures grew from 2.1% in the first quarter of 2014 to 7.3% in the first quarter of 2015.61 The reasons for this upturn seem to be higher overall rates of use of health services and a greater number of individuals insured by the ACA.
FIG 6.
Health services spending growth in recent quarters (Qs).
Costs associated with cancer care are rising more rapidly than costs in other medical sectors. Given current rates of growth, cancer-related costs may reach as high as $173 billion by 2020.10 The reasons for the high cost of cancer care are varied, including development of new technologies and treatments, consolidation of oncology practices into hospital-based practices where care costs more, and rising drug prices. Costs associated with newly insured patients, expanded prevention and screening programs, and growing populations of new patients with cancer and survivors will also likely contribute to future cost increases.
Despite spending far less on cancer care, many countries achieve similar or better cancer outcomes than the United States.62 Furthermore, health care costs in the United States have severe financial implications for patients with cancer and survivors and may place care out of the financial reach of many patients, despite the gains in insurance access discussed previously. Affordability of cancer care remains a significant policy concern for ASCO in 2016. Two issues of critical importance for patients with cancer are: (1) cost of cancer drugs and (2) increased patient burden associated with rising deductibles and cost shifting.
Cost of Cancer Drugs
Although drug costs represent only a small portion of overall cancer care costs in the United States, they receive outsized attention because of their alarming price tags and substantial price increases in recent years. A single cancer drug can cost nearly $300,000 per year.63 Cancer drugs account for seven of the 10 most expensive drugs reimbursed through Medicare Part B (Table 2). For the Medicare Part D prescription program, CMS paid a total of $1.35 billion for the cancer drug lenalidomide in 2013—making it one of the 10 most expensive drugs that year despite being used by many fewer beneficiaries than competing drugs on list.65
Table 2.
Ten Most Expensive Medicare Part B Payments for Drugs Delivered in Physician Office and at Home
| HCPCS | Name | Dose (mg) | Average Sales Price per Dose | Total Medicare Annual Payment | FDA-Approved Indication |
|---|---|---|---|---|---|
| J2778 | Ranibizumab injection | 0.1 | $396.43 | $1,310,751,832 | Macular degeneration, macular edema, diabetic macular edema |
| J0178 | Aflibercept injection (ophthalmic) | 1 | $980.50 | $1,239,918,536 | Macular degeneration, macular edema, diabetic macular edema, diabetic retinopathy |
| J9310 | Rituximab injection | 100 | $708.68 | $852,588,010 | Non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, Wegener’s granulomatosis and microscopic polyangiitis |
| J1745 | Infliximab injection | 10 | $74.34 | $785,929,255 | Crohn’s disease, pediatric Crohn’s disease, ulcerative colitis, pediatric ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis |
| J2505 | Injection, pegfilgrastim | 6 | $3,387.93 | $641,285,763 | Febrile neutropenia |
| J9035 | Bevacizumab injection | 10 | $66.65 | $593,988,145 | Metastatic colorectal cancer; NSCLC; glioblastoma; renal cell carcinoma; cervical cancer; epithelial ovarian, fallopian tube, or primary peritoneal cancer |
| J0897 | Denosumab injection | 1 | $14.45 | $505,871,083 | Skeletal-related events, giant cell tumor of bone, hypercalcemia of malignancy |
| J9355 | Trastuzumab injection | 10 | $82.49 | $289,275,777 | Breast cancer, gastric or gastroesophageal junction adenocarcinoma |
| J9305 | Pemetrexed injection | 10 | $60.27 | $287,737,319 | NSCLC, mesothelioma |
| J9041 | Bortezomib injection | 0.1 | $46.08 | $283,007,272 | Multiple myeloma, mantle cell lymphoma |
NOTE. Drugs in bold font are used to treat patients with cancer. Pricing data reflect fourth quarter 2014 payment rates, which corresponding to second quarter 2014 manufacturer reports. Final column lists FDA-approved indications, but Medicare may also provide reimbursement for additional, so-called off-label, uses. Data adapted.64
Abbreviations: FDA, US Food and Drug Administration; HCPCS, Healthcare Common Procedure Coding System; NSCLC, non–small-cell lung cancer.
Precision therapies are particularly expensive and are being used by an increasing number and proportion of patients with cancer. As noted during a plenary session at the 2015 ASCO Annual Meeting, the immunotherapy treatment ipilimumab is “approximately 4,000 times the cost of gold.”66,67
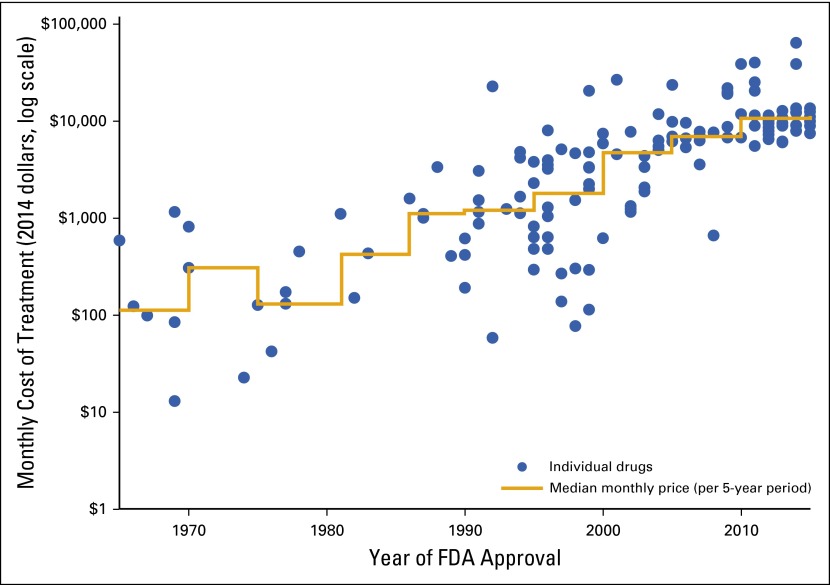
Rising drug costs are a concern for both the health care system and patients with cancer and their families. Figure 7 illustrates the growth in the monthly cost of drugs over time. Even more concerning, studies are beginning to show that many of these drugs provide greater benefit when delivered at higher doses or in combination, which could result in annual price tags into the millions.28,66 Escalation in individual unit cost of new drugs—along with the growth in the number of such drugs introduced to the market—raises serious concerns about sustainability for patients and the overall health care system.
FIG 7.
Median monthly costs for new cancer drugs at time of US Food and Drug Administration (FDA) approval, 1965 to 2015 (data from Peter Bach, Memorial Sloan Kettering Cancer Center, New York, NY).
Drug manufacturers attribute high drug costs to the cost of drug development and the limited markets for specialty drugs, but they do not publicly disclose their margins of profit—making it difficult for payers and patients to assess their return on investment. Moreover, US prices are much higher than prices charged for the same medications in other countries.68 For example, an independent academic study presented at the 2015 European Cancer Congress found that those in the United States pay more than twice the price paid by Europeans for one class of cancer drugs.68
Costs for some generic and first-generation therapy drugs have also increased over time, despite lack of any further investment after widespread adoption. One recent analysis found that more than half of all retail generic drugs increased in price between 2013 and 2014.69 In August 2015, Turing Pharmaceuticals (New York, NY) stirred controversy when it purchased daraprim—a 62-year-old drug used to fight complications in patients whose immune systems have been weakened by HIV, pregnancy, or chemotherapy—and changed its price from $13.50 to $750 per pill—a 5,000% increase.70 The move generated outcry from Congress, along with several presidential candidates, and triggered the rapid development of an affordable alternative.71,72
In cancer care, creation of a market for biosimilar products was expected to help lower drug prices by increasing patient access to lower-cost biologic therapies. Economic analysis by the RAND Corporation (Santa Monica, CA) projected $44.2 billion in savings from 2014 to 2024 as a result of biosimilar products across all therapeutic classes but noted the savings could range from $13 billion to $66 billion, largely depending on the amount of competition.73 However, filgrastim-sndz, the first biosimilar approved in the United States, was introduced to market in 2015 at a 15% discount from the reference product—a decrease much smaller than expected from generic drugs.74 Although additional biosimilar products are in development, it is unclear how many biosimilar products will come to market and whether they will result in the dramatic price decreases brought by generic drugs. The price of a cancer drug may have little to do with its demonstrated efficacy—and newly approved cancer drugs that have not been shown to improve long-term survival continue to be marketed with high price tags.75
The price of many cancer drugs also forces patients and families to make difficult financial choices about whether to forego or curtail needed treatments. Some estimates suggest that 10% to 20% of patients with cancer may not take prescribed treatments because of the cost and that they are more likely to declare bankruptcy than those without cancer.63,76 Patients without health insurance are especially vulnerable to high drug costs, but costs can be unaffordable even for those with insurance when high deductibles transfer more of the cost of treatment to the insured. The result is that out-of-pocket drug expenses for patients with health insurance can run $25,000 or more per year.63
Not surprisingly, drug prices are unpalatable to patients and providers, and consumers are increasingly focused on escalating drug prices in the United States. A recent survey found that 24% of Americans say that they have a hard time paying for prescription drugs, 72% view the prices of prescription drugs as unreasonable, and 74% believe that Americans pay more for drugs than their European, Canadian, and Mexican counterparts.11 Concerned for their patients and for the solvency of the US cancer care system, physicians are becoming more vocal about the issue of drug costs. In October 2015, 118 oncologists published a commentary underscoring the need to prioritize high-value cancer care, proposing the following actions as possible solutions63:
Creating a post-FDA drug approval review mechanism to propose a fair price for new treatments based on the value to patients and heath care.
Allowing Medicare to negotiate drug prices.
Allowing the Patient-Centered Outcomes Research Institute, created through the ACA initiatives to evaluate the benefits of new treatments, and similar organizations to include drug prices in their assessments of treatment value.
Allowing importation of cancer drugs across borders for personal use (eg, prices in Canada are approximately half those in the United States).
Passing legislation to prevent drug companies from delaying access to generic drugs (ie, pay for delay).13
Reforming the patent system to make it more difficult to prolong product exclusivity unnecessarily (ie, patent evergreening).
Encouraging organizations that represent cancer specialists and patients (eg, ASCO, American Society of Hematology, American Association for Cancer Research, ACS, and National Comprehensive Cancer Network [NCCN]) to consider the overall value of drugs and treatments in formulating treatment guidelines.
Chapter 4 outlines current initiatives aimed at combating escalating drug and care delivery costs.
Rising Deductibles and Cost Shifting
Out-of-pocket spending is becoming an increasing concern for patients and their families. As health care costs grow, payers are shifting more of the financial burden for care to consumers. The result is that Americans are paying substantially more for insurance and the cost of care than ever before.
Tiered premiums and cost shifting through higher deductibles and copays have become more prevalent in employer-based plans as more companies offer high-deductible plans designed to help keep premiums low.48,77 The number of employers offering only high-deductible plans has grown dramatically in recent years, from 13% in 2012 to 25% in 2015.78
Recent analyses indicate that private insurance deductibles have increased steadily over the past 5 years, rising six times more rapidly than income.51 Although insurance companies claim that higher deductibles reduce unnecessary health care expenditures, evidence suggests that they may cause employees to postpone or forego needed care or to make decisions about which services to use based on what they can afford.79 A recent survey found that as the average annual in-network deductible increased from $680 to $1,200 between 2009 and 2015, more US consumers reported foregoing care (29% v 40%).78 Workers with high-deductible plans have lower rates of physician visits and laboratory tests, as well as fewer mammograms and cervical cancer screenings, even when their plans include coverage for preventive care.80
One area of particular concern for patients with cancer is the inconsistency between copays for oral cancer medications and intravenous drugs. Because oral cancer drugs are not covered under Medicare Part D or other pharmacy benefit programs, patients may experience higher out-of-pocket costs for these than for infused therapy. In June 2015, Congress introduced the Cancer Drug Coverage Parity Act (H.R. 2739 and S. 1566) to prohibit differential pricing for oral therapies. In the absence of a federal law, many states have taken action. As of October 2015, 40 states and Washington, DC, had passed oral parity legislation.81 ASCO is advocating for and closely monitoring the progress of both federal and state legislation.
Policy premiums have also increased significantly, as has the total cost of a policy, and these costs are expected to increase in 2016.51 Some states have approved sizable insurance premium increases (up to 36%) for the coming year.80
The tradeoff between lower monthly premiums and higher deductibles is also a feature of the plans available on the ACA marketplaces. Marketplace plans are offered on four tiers that differ significantly in cost, with the lower-tier bronze and silver plans associated with lower premiums but higher deductibles. The result is that consumers are asked to make difficult choices about how much health care coverage they can afford and how much they can afford to pay out of pocket before insurance coverage takes over. Special provisions of the law provide federal cost-sharing subsidies to help make the plans more affordable for many people with incomes above the poverty level. However, many of the currently uninsured may be unaware of this option or are not well informed about how to select a plan that is eligible for subsidy. A 2015 study found that 2.2 million enrollees did not receive cost-sharing subsidies because they selected plans with low monthly premiums that did not qualify for assistance.82
Marketplace plans are also containing costs by narrowing networks of providers. A 2015 analysis of marketplace plans found 41% of silver plan physician networks to be small or extra small (including 10% to 25% and < 10% of local, office-based physicians, respectively).83 Narrow networks are concerning for patients with cancer—especially those with childhood and rare cancers—because their plans may not include clinicians with the appropriate expertise to treat their disease or access to NCI-designated cancer centers.83
As the US insurance pool widens and health care costs continue to rise, insurers will continue resorting to cost-sharing mechanisms to keep their costs at bay. Many of these cost-sharing mechanisms are aimed at cutting down on unnecessary treatments and procedures—but in cancer care, they may discourage patients from seeking the care they need.
CONCLUSION
The US health care system continues to transition, and cancer care in 2015 is reflective of this time of change and uncertainty. Since its implementation, the ACA has expanded access to coverage through health marketplaces and Medicaid to millions of previously uninsured children and adults. It has also strengthened coverage protections and broadened requirements for covered services in multiple ways that translate into improved access to prevention and screening, treatment including clinical trials, survivorship services, and palliative and end-of-life care. Data supporting the success of these measures for patients with cancer are not yet available, but the ACA has expanded the cancer-related benefits available to Medicare and Medicaid beneficiaries and moved the country closer to providing equitable care for all patients with cancer. However, coverage remains incomplete, rising costs present barriers to care that affect even those with insurance coverage, and the system continues to experience large disparities in care that affect people at all points along the cancer continuum.
3. ONCOLOGY PRACTICE AND WORKFORCE TRENDS: ENGINES FOR DISCOVERY AND CARE DELIVERY ARE AT RISK
Across the United States, oncology practices continue to reconfigure in response to internal and external pressures, including economic constraints, administrative burdens, and proliferation of cost-containment measures imposed by payers. This chapter describes the state of the current oncology workforce, with a focus on medical oncologists and hematologists, and highlights issues facing oncology practices across the country.
ONCOLOGY WORKFORCE
Information on the number, demographics, and geographic distribution of oncologists in the United States comes from the ASCO Workforce Information System (WIS), described in detail in the Appendix (online only). This section of the report also features information about other disciplines involved in cancer care and examines ways for providers to work across disciplines to deliver seamless, patient-centered care.
Access to Oncologists
In 2015, more than 11,700 hematologists and/or medical oncologists provided patient care to people living with cancer in the United States (Table 3). Although other oncology subspecialities are mentioned in this article, this report primarily focuses on medical oncology and hematology. Medical oncologists treat cancers that occur primarily in body organs and tissues, and hematologists focus on cancers of the blood and other blood diseases.
Table 3.
Numbers of Physicians in Oncology Specialties
| Oncology Specialty* | Masterfile | Physician Compare | |
|---|---|---|---|
| Total No. | No. in Direct Patient Care† | ||
| Medical oncology and/or hematology‡ | 14,215 | 11,894 | 11,726 |
| Gynecologic oncology | 511 | 456 | 959 |
| Pediatric hematology or oncology | 2,429 | 1,787 | Not relevant |
| Radiation oncology | 4,810 | 4,391 | 4,313 |
| Surgical oncology | 466 | 429 | 840 |
Oncologists are classified according to their primary specialty designation within each data source.
Based on AMA Masterfile84 information from physicians who report direct patient care as their primary professional activity.
Physicians who report medical oncology, hematology/oncology, or hematology as their primary specialty.
Estimates from Table 3 come from two data sources: the American Medical Association (AMA) Physician Masterfile and the CMS Physician Compare data set.84,85 Because the two data sets account for a similar number of oncologists engaged in patient care (within 1.4% difference for hematologists and oncologists), ASCO draws information from both sources to paint a complete picture of today’s workforce.
The AMA Masterfile identified more than 22,000 oncologists working in the fields of medical oncology and hematology, gynecologic oncology, pediatric hematology and oncology, radiation oncology, and surgical oncology (Table 3). Of these, nearly 19,000 (84.5%) cited direct patient care as their primary professional activity. The remaining 16% spent the majority of their time on administration, research, and teaching, among other activities. A total of 14,215 work in the subspecialties of medical oncology and/or hematology, with 11,894 (83.7%) primarily focusing on patient care. This represents 1.1% growth in the number of hematologists and oncologists engaged in patient care over the past year.
In a similar timeframe, CMS Physician Compare identified approximately 17,800 oncologists providing active patient care, including 11,726 working in hematology or oncology (Table 3). Physician Compare comprises records from all providers who billed for Medicare services within the prior 12 months. Therefore, it does not account for pediatric specialties.
Aging Workforce
As is true for most of medicine, the aging workforce remains a concern. Among oncologists active in patient care, a growing segment is nearing retirement at age 64 years or older (17.7%).84 Older oncologists continue to outnumber the 13.9% of oncologists younger than age 40 years who have recently entered the field. The median age of oncologists engaged in patient care (51 years) has remained stable over the last few years, although age varies widely by state, with Kansas having the youngest oncologists (median age, 46.5 years) and West Virginia having the oldest oncologists (median age, 57 years; Fig 8). In Delaware, nearly a third (32.1%) of the workforce is nearing retirement at age 64 years or older. Oncologists are not significantly different in age than other types of physicians practicing in the United States (mean, 52.6 v 52.7 years; P = .77).84
FIG 8.
Median age of oncologists by state.
Women in Oncology
Overall, women made up 31% of practicing oncologists in 2015. Gynecologic and surgical oncology have higher female participation according to Physician Compare (40% and 37%, respectively), whereas radiation oncology has lower participation (26%). The majority (51%) of pediatric hematologists or oncologists are female according to the AMA. Nearly half (45%) of oncologists younger than age 40 years are women (Fig 9).84 Among hematology and oncology fellowship programs, 46% of trainees are women.86
FIG 9.
Distribution of oncologists by age and sex.
Workforce Diversity
The physician workforce, and the hematology and oncology workforce in particular, continues to struggle with racial and ethnic minority representation. For example, although the US Census estimates approximately 17% of the population is Hispanic, only 5.8% of practicing oncologists are Hispanic.87,88 In training programs, 5.3% of oncology fellows are Hispanic, whereas 7.6% of all residents and fellows and 7.8% of internal medicine residents are Hispanic (Fig 10A).86 The hematology and oncology field also has lower rates of Hispanics than the other large internal medicine subspecialty fellowships, including cardiology (7.1%), gastroenterology (8.7%), and infectious disease (13%).86 African Americans, despite comprising 13% of the population, represent only 2.3% of practicing oncologists and 3.7% of oncology fellows.86-88 The percentage of all residents and fellows who are African American is 5.7%, and the percentage among internal medicine residents is 6% (Fig 10B).86 Among the major internal medicine subspecialty fellows, the hematology and oncology field has the lowest participation of African Americans. These disparities become even more significant as the burden of cancer shifts among racial and ethnic groups, especially in African Americans (Chapter 1).
FIG 10.
Percentage of residents and fellows who are (A) Hispanic or Latino and (B) black or African American.
In a 2015 article titled “Critical Shortage of African American Medical Oncologists in the United States,” study authors note that medical school graduation rates are especially low among African Americans and that the growth rate over time is much slower than among other nonwhite races and ethnicities.89 Recruiting and retaining greater numbers of racial and ethnic minorities in oncology is one essential step toward improving access to high-quality, effective, affordable, and compassionate cancer care for the underserved. Collaborative efforts across the entire educational system are needed to boost interest in and exposure to medical professions in diverse populations. Visit www.asco.org/diversity to learn about ongoing ASCO initiatives to enhance diversity in oncology.
Geographic Access to Care
Geographic distribution of US oncologists remains uneven. According to the ASCO WIS, half (50.0%) of hematologists and oncologists practice in eight states: California, New York, Texas, Florida, Pennsylvania, Massachusetts, Ohio, and Illinois (Fig 11A). Together, these states account for 40 million US residents who are 55 years of age or older (the population from which 77% of new cancer cases arise).40 Wyoming has the fewest (n = 17) oncologists practicing, and Nevada has the fewest oncologists per 100,000 residents age 55 years or older (Fig 11B).
FIG 11.
No. of oncologists (A) overall and (B) per 100,000 residents age 55 years or older.
Oncologists are concentrated in metropolitan areas throughout the United States. This presents significant access challenges for the more than 59 million US patients residing in rural areas.90 Although more than 11% of Americans live in rural parts of the country, Physician Compare data show that only 5.6% of oncologists provide service in these areas.
Research is beginning to show the effects of geography on cancer outcomes. Geographic disparities in cancer care can be particularly pronounced for cancers that benefit from screening and early detection, such as colorectal cancer. A 2015 spatial analysis of county-level colorectal cancer mortality identified three hotspots of high mortality rates: the lower Mississippi Delta, west central Appalachia, and eastern North Carolina and Virginia.91 Together, these hotspots spanned more than 200 counties across 12 states. Patients residing in these hotspot areas were 40% more likely to die as a result of their cancer than nonhotspot residents. Although travel distance was found to contribute to these findings, they were largely attributed to differences in racial or ethnic makeup, income, and education. The study authors pointed to screening interventions as a method to reduce the wide variation in cancer outcomes.
Another 2015 study of patients with colorectal cancer found that those who traveled longer distances (> 50 miles) were less likely to receive adjuvant chemotherapy in accordance with evidence-based guideline recommendations.92 In a related analysis, patients with rectal cancer who traveled more than 50 miles for care were less likely to receive of radiation therapy in accordance with guidelines.93
Because cancer care is complex, requires frequent patient visits for treatment and monitoring, and involves providers from many disciplines, geographic access to care may be even more challenging for patients with cancer than for other rurally located patients. To address geographic disparities in care, oncology practices are trying new virtual methods of outreach to patients. For example, a recent study of rural patients with hepatocellular carcinoma found that tumor evaluation by virtual tumor board improved the timeliness and comprehensiveness of multidisciplinary evaluation and decreased travel burden.94 Virtual tools and improved care coordination can expand access to high-quality care for patients living far from major cancer centers.
Other Disciplines With Important Contributions to Cancer Care
From diagnosis to survivorship, cancer care is delivered by health providers from a variety of backgrounds and specialties, including—but not limited to—primary care physicians, urologists, gynecologists, pathologists, pharmacists, genetic counselors, mental health specialists, pain and palliative care specialists, and advanced practice providers. Nonprofessionals also play a large role, particularly family caregivers.
This new section of the report highlights recent workforce data available on other disciples that contribute to care for people with cancer. This year’s report features recent data on advanced practice providers, genetic counselors, and primary care providers.
Advanced practice providers
Advanced practice providers, including nurse practitioners (NPs) and physician assistants (PAs), play important roles in the delivery of cancer care in the United States. Services range from ordering chemotherapy to providing pain and symptom management to organizing or providing routine primary care services for active patients with cancer and survivors. In 2015, US oncology practices reported widespread use of NPs and PAs (Fig 12). Nearly three quarters of ASCO Census practices (73.1%) reported employing advanced practice providers—up substantially from the 52% of 2014 census practices. Altogether, practices used a total of 5,419 advanced practice providers (3,913 NPs and 1,506 PAs). The practices with advanced practice providers used an average of 0.44 advanced practice providers per oncologist.
FIG 12.
Nurse practitioners (NPs) and physician assistants (PAs) by practice setting.
To better understand the significant contributions made by advanced practice providers working in oncology, ASCO is partnering with other professional societies to investigate the size and nature of the advanced practice provider workforce in oncology, as well as to catalog the range of services provided. Results will be available in 2017.
Genetic counselors
As noted earlier, there is growing emphasis on precision medicine and the genetic testing that identifies patients who can benefit from targeted therapies. Genetic counselors play a key role in explaining implications and results of genetic testing to healthy individuals and patients with cancer. Traditionally, genetic counselors have focused on hereditary characteristics that put people at higher risk of developing cancer. With greater understanding of cancer development at the molecular level, genomic tests to identify alterations within a cancer cell, and drugs that target these alterations, genetic counselors play an increasingly important role in helping patients and oncologists understand treatment options.
Access to genetic counseling is an important part of patient-centered cancer care; however, the supply of these professionals is limited. The American Board of Genetic Counseling certified 3,766 genetic counselors in 2014, of whom 18% practiced in oncology.95 By this estimate, approximately 680 genetic counselors were available throughout the country to treat patients in a cancer care setting.
To expand the reach of this limited workforce, some oncology practices are leveraging telemedicine to provide access to genetic counseling. For instance, recent demonstration projects in Idaho, Maine, and North Carolina revealed that cancer genetic counseling telemedicine initiatives can lower costs, add convenience for patients, and maintain high patient satisfaction.96-98
Primary care providers
Primary care providers are often the first to detect a patient’s cancer and are involved in a patient’s care long after completion of active cancer treatment. However, a recent study revealed that some primary care providers feel unprepared to care for cancer survivors.99 Moreover, the US primary care workforce is facing a well-documented shortage that is expected to worsen in the wake of the ACA. In 2013, for instance, the National Center for Health Workforce Analysis (part of the US Department of Health and Human Services) projected a shortage of as many as 20,400 primary care physicians by 2020.100
To help care teams provide seamless, patient-centered care to patients transitioning out of active treatment, ASCO partnered with two primary care organizations—the American Academy of Family Physicians and the American College of Physicians—in planning an inaugural Cancer Survivorship Symposium. The symposium took place from January 15 to 16, 2016, in San Francisco, California. Visit www.asco.org/survivorship for more information on the ASCO activities surrounding survivorship care.
ASCO will continue to monitor workforce studies and initiatives across disciplines in an effort to better understand the workforce available to meet the needs of people with cancer. This will enable the society to identify opportunities to collaborate with other health care professions to help ensure delivery of coordinated care.
Interprofessional education and practice
The increasing demand for cancer and other health care services brought on by the growing and aging population is straining the health care workforce at large. New methods of care delivery, including team-based care and smarter use of technology, will be essential to meeting demand in future years. Collaborative care enabled by technology can improve both quality and efficiency of cancer care; it can also enhance patient-centered care, delivering services necessary from the patient standpoint, not from that of the physician or medical specialty.
In 2015, the IOM published a report titled “Measuring the Impact of Interprofessional Education (IPE) on Collaborative Practice and Patient Outcomes,” which called for better alignment between health professional training and collaborative practice—particularly in this time of health system redesign.101 To deliver seamless, patient-centered care, oncology providers from all disciplines must work together. Better care coordination can be achieved by: (1) improving the oncology training environment to reflect team-based practice, (2) continuing education among individuals currently in the workforce, and (3) redesigning practices and health systems to provide integrated care.
Recognizing the importance of interprofessional training, the Accreditation Council of American Medical Graduates requires of hematology and medical oncology fellowship programs that fellows “work in inter-professional teams to enhance patient safety and improve patient care quality.”102(p23) A recent article highlighted the development of a geriatric oncology curriculum by a team comprising a geriatrician, a medical oncologist, an oncology pharmacist, a nurse practitioner, and two oncology chief fellows.103 The curriculum is currently in pilot testing, with evaluation in place to focus on three areas of educational need: geriatric assessment, pharmacology, and psychosocial knowledge skills. Demonstration projects such as this will be important as graduate medical training moves in this direction.
In oncology practice, formal efforts are taking place to educate providers in team-based care. In 2014, the Agency for Healthcare Research and Quality funded Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS) as an evidence-based repository of tools available to practices to improve communication among multidisciplinary teams. Some oncology practices have begun to implement TeamSTEPPS, although evaluation results are not yet available. In another example, an academic practice in South Carolina implemented a multidisciplinary breast clinic model and found patients to be highly satisfied with the care they received.104 It also identified quality improvement targets for the clinic, such as increased emphasis on provider communication about psychosocial issues.
In 2015, ASCO partnered with the NCI to launch Teams in Cancer Care Delivery to apply the science of team-based care to oncology (Box 5).
Box 5. ASCO and National Cancer Institute Team in Cancer Care Delivery.
ASCO and the National Cancer Institute have formed a collaboration to investigate team practice arrangements in oncology, aiming to serve the following goals:
Bring together scientists and clinicians working on issues relevant to the effectiveness of teams involved in cancer care delivery to inform the research agenda for teamwork, team effectiveness, and team performance in an oncology care setting and an era of health reform.
Provide the clinical oncology community with practical strategies for how to organize effective health care teams.
Identify areas to build the foundation for team research in cancer care delivery, such as taxonomy, operational definitions, and measurement.
Clinicians, researchers, and patients will author manuscripts and presentations involving concepts of team-based care and clinical scenarios addressing a point in the cancer continuum, evidence-based concept, and cancer type. In September 2015, 23 teams were selected to participate in the project. Results of their work will be presented during a February 2016 conference, with accompanying articles published in Journal of Oncology Practice.
For more information, visit www.asco.org/teams.
The concept of integrated care is also penetrating the oncology community. Recently, the WHO offered the following working definition for integrated care as it pertains to management of complex, chronic disease: “Initiatives seeking to improve outcomes for those with (complex) chronic health problems and needs by overcoming issues of fragmentation through linkage or coordination of services of different providers along the continuum of care.”105(p6) The Veterans Administration and Kaiser Permanente are two large-scale examples of integrated care systems active in the United States. At the 2015 ASCO Annual Meeting, Harvard Business School professor Dr Michael Porter described the integrated care unit, a model of care with the following key attributes106,107:
Organized around the patient medical condition or set of closely related conditions.
Involves a dedicated, multidisciplinary team that devotes a significant portion of time to the condition.
Providers involved are members of or affiliated with a common organizational unit.
Takes responsibility for the full cycle of care for the condition, encompassing outpatient, inpatient, and rehabilitative care as well as supporting services (eg, nutrition, social work, and behavioral health).
Incorporates patient education, engagement, and follow-up as integral to care.
Uses a single administrative and scheduling structure.
Colocated in dedicated facilities.
Care is led by a physician team captain and a care manager who oversee each patient’s care process.
Measures outcomes, costs, and processes for each patient using a common information platform.
Providers function as a team, meeting formally and informally on a regular basis to discuss patients, processes, and results.
Accepts joint accountability for outcomes and costs.
Dr Porter demonstrated the application of the integrated practice unit in head and neck cancer, where a patient would visit a head and neck center for his or her major point of care. This center would provide easy access to the many providers and services needed for that patient, including medical, surgical, radiation, and dental oncologists, as well as pathologists and speech and swallowing specialists. The center would also facilitate access to primary care, social work, smoking cessation, plastic surgery, and other services essential to head and neck cancer care throughout its full continuum. Establishing such a system, argued Dr Porter, would help economize care delivery while also focusing care on outcomes important to the patient.106
As multidisciplinary care expands in the coming years, it will be increasingly important to monitor logistic toxicities experienced by patients attempting to navigate their cancer care.108 Logistic toxicities refer to administrative burdens borne by patients and can include processing medical bills and completing insurance paperwork. Such responsibilities may lose precedence in the wake of active cancer treatment, especially as patients juggle recurring appointments with multiple providers.
ONCOLOGY PRACTICE LANDSCAPE
The way that oncologists and other providers organize—within practices, health systems, and institutions—substantially affects their ability to meet the growing demand for cancer care services. Although the oncology workforce remains relatively static, the practice environment is highly responsive to changes in the cancer care delivery system.
In this section of the report, ASCO reviews findings from its 2015 Oncology Census of US practices and introduces a supplemental strategy for keeping a pulse on the business and policy issues affecting them: the ASCO Trends Survey.
ASCO Oncology Census
Since 2012, ASCO has conducted an annual census of US oncology practices to better understand and respond to current economic and care delivery issues (Appendix). In 2015, practices completed a brief survey about size, number and location of additional sites, distribution of oncologic specialties, practice setting, and number of new patients. A total of 674 respondents from across the country participated in this year’s census, representing nearly 2,200 practice sites. More than 13,400 oncologists work in these practices (Fig 13), a 30% increase from last year’s participation. These oncologists—from the specialties of hematology, medical oncology, gynecologic oncology, pediatric hematology and oncology, surgical oncology, and radiation oncology—account for 71% of the nearly 19,000 practicing oncologists represented in the Masterfile, suggesting that the census was successful in reaching a broad swath of practices operating today in the United States. The census practices saw more than 1.1 million new patients with cancer in 2015, approximately 67% of the 1.7 million cancers that were diagnosed in 2015.
FIG 13.
Distribution of oncologists represented by the ASCO Census, 2015.
The 2015 ASCO Oncology Census asked respondents to classify the ownership arrangements of their employment setting according to the following options:
Academic practice (including full-time academic practices and practices with an academic affiliation and state-funded institutions).
Physician-owned practice or group (including multisite network).
Practice, group, or outpatient department owned by a hospital or health system.
Government (federal [eg, public health system, military, or Veterans Administration] or state).
Industry (ie, pharmaceutical companies).
Locum tenum or retired.
Nearly half (44.6%) of the clinical practice respondents described their setting as hospital or health system owned (Fig 14). The dominant practice arrangement in the Midwest was hospital or health system owned, with 60% of practices reporting this category. Physician-owned practices were most prevalent in the Northeast and South, whereas hospital or health system– and physician-owned practices were evenly distributed in the West. Academic practices were relatively evenly distributed across all regions.
FIG 14.
Distribution of practice setting by region.
Large practices continued to increase their share of the market. Practices reporting more than 12 oncologists grew from 14% in 2013 to 36% in 2014, whereas small practices (one to five oncologists) dropped from 64% in 2013 to 41% in 2014 (Fig 15). The distribution of midsize practices (six to 12 oncologists) remained relatively stable between 2014 and 2015 (21% v 23%). These trends were observed across all three practice settings: academic, physician owned, and hospital or health system owned. Although different practices were included in the two census rounds, it is likely that much of this change represents the closing and/or acquisition of small practices by larger entities.
FIG 15.
Practice size, 2014 to 2015, according to the ASCO Census. NOTE. Practices that reported practice size for one of multiple practice sites were excluded from this analysis.
Among 2015 respondents, the practice size distribution was similar by regional location, but practice size varied widely by practice setting (Fig 16). Physician-owned practice respondents were smaller than academic and hospital or health system–owned practices, with 60% of practices employing five or fewer oncologists. The majority (51%) of academic practices had more than 40 oncologists. Hospital or health system–owned practices were more evenly distributed between small, medium, and large practices.
FIG 16.
Practice size by practice setting: (A) academic (n = 91), (B) physician owned (n = 218), and (C) hospital or health system owned (n = 242). (*) Including full-time academic practices, practices with an academic affiliation, and state-funded institutions. (†) Including multisite networks.
ASCO Trends Survey
ASCO surveyed a representative sample of academic, physician-owned, and hospital or health system–owned census respondents to gain further insight into high-priority and emerging topics of concern (Appendix). Questions focused on top pressures, alternative payment models, clinical pathways, EHRs, and cost of care.
Practices were asked to rank their top three practice pressures. As illustrated in Fig 17, EHR implementation or use was selected most frequently, by 45% of respondents, followed by payer pressures (44%) and staffing issues (36%). Previous ASCO surveys on practice pressures did not include an EHR-related response option, so there are no historical data to use as a comparison point. However, these findings are consistent with results from a 2014 survey that indicated dissatisfaction with EHR systems among members of the AMA, American College of Physicians, and American Academy of Family Physicians.109 Only one third of respondents indicated they were satisfied or very satisfied with their EHR—a 30% decrease from a similar survey that was conducted 5 years earlier. More than 40% of respondents indicated that their EHR made it difficult or very difficult to improve efficiency and posed productivity challenges.
FIG 17.
Top three pressures cited by practices (n = 177). EHR, electronic health record.
Practice pressures varied considerably by practice setting, with academic practices selecting drug shortages most often as the primary pressure (Fig 18). Physician-owned practices focused on payer pressures, and hospital or health system–owned practices focused on EHR implementation. Among academic practice respondents, staffing issues and payer pressures were also of concern. Physician-owned practices reported drug pricing, competition, and patient ability to pay as other important pressures. Meanwhile, hospital practices identified staffing issues, payer pressures, and competition as notable pressures. With increased focus on payment reform, narrower provider networks, and efforts to reduce costs, it is not surprising that payer pressures ranked highly across all practice settings. It is notable that drug shortages barely registered as a concern among physician-owned and hospital or health system–owned settings, but this issue was highly ranked by academic practices. This difference may be related to how shortages are affecting the conduct of research or may indicate that physician and hospital or health system–owned practices have other purchasing or distribution mechanisms.
FIG 18.
Top three pressures by practice type. EHR, electronic health record.
In 2015, ASCO launched PracticeNET, a rapid learning system that will allow practices to share and gain insights to enhance their business operations and quality of care (Box 6).
Box 6. PracticeNET.
PracticeNET is an initiative of the newly formed ASCO Clinical Affairs Department, which is dedicated to providing services, education, and resources to support oncology practices. In 2015, ASCO began recruiting practices to join PracticeNET. Participating practices will submit business, operational, administrative, and quality data on a monthly basis and will receive quarterly reports measuring their practice and individual physicians against the database of PracticeNET participants.
For more information, visit www.asco.org/practicenet.
CONCLUSION
To operate in today’s care environment, oncologists and oncology practices must find new ways to deliver care to an increasing number of patients with cancer and survivors amid perpetually changing economic and administrative demands. Oncology providers must work in teams and reach beyond the traditional borders of their practice locations to achieve the multidisciplinary care needed to treat the whole patient with cancer—especially in rural locations where specialty services are scarce. Oncology practices are currently struggling to deliver care while facing significant pressures, including EHR implementation, payer pressures, staffing issues, competition, and drug pricing. Through its continued original data collection efforts, including the WIS, ASCO Oncology Census, and ASCO Trends Survey, ASCO maintains current information on the oncology workforce and practice environment to identify new and persistent gaps in the delivery of patient care and to steer its policy priorities.
4. NEW STRATEGIES FOR DELIVERING HIGH-QUALITY, HIGH-VALUE CANCER CARE
As pressures to control cost escalate, payers and other stakeholders are pursuing new payment and care delivery models that lower spending while preserving quality. The ACA was a strong catalyst for many of these efforts, but another significant impetus for new payment strategies appeared earlier this year: the 2015 MACRA. In addition to reforms at the system level, MACRA introduces changes at the provider level, where there is an increasing emphasis on reducing variation in care and demonstrating quality improvement.
The first three chapters of the 2016 State of Cancer in America report identify a cancer care system facing increased demand and complexity, unsustainable costs, and an uncertain practice environment. This chapter provides an overview of initiatives proposed and ongoing in 2015 to help relieve these pressures, namely in the areas of payment reform, value optimization, and performance improvement.
PAYMENT REFORM: INCREASED FINANCIAL FLEXIBILITY AND HIGH-QUALITY CARE EXPECTED
A historic development for the US health care system was the April 2015 decision by Congress to repeal the SGR formula. Over the 13-year lifespan of the SGR, Congress spent nearly $170 billion in short-term fixes to avoid reimbursement cuts imposed by this flawed Medicare payment formula.110 The 2015 decision effectively reversed required payment cuts and replaced the SGR with a plan to return stability to the reimbursement of physician services by Medicare.
The bipartisan resolution was passed as part of MACRA.111 MACRA encourages physicians to participate in new payment models that provide more flexible options for reimbursing physician services in exchange for increased accountability in delivering high-quality care. Other key components of MACRA (HR. 2) include improvements in the ability of Medicare to reimburse for care coordination and advance care planning.
New Payment and Care Models Under Evaluation
A number of alternative payment models have been developed to improve care and reduce costs. These models move away from fee-for-service payments to other payment approaches that: (1) increase accountability for both quality and total cost of care and (2) emphasize population health management, as opposed to payment for specific services. In January 2015, Health and Human Services Secretary Sylvia M. Burwell announced the goal of tying 30% of Medicare payments to alternative payment models by the end of 2016 and extending the proportion to 50% by 2018.112
Alternative payment models under evaluation include:
Accountable care organizations. Groups of physicians, hospitals, and other health care providers that come together to care for an assigned population of patients.
Bundled payments or episodes of care. A single payment that is shared among all providers contributing to a given episode of care.113
Clinical pathways. Recommended care processes for specific clinical situations designed to reduce costly variation in care.
Patient-centered medical homes. Physician-led teams that take responsibility for the full range of services patients need, including coordination of care with other providers.
Highlights of specific alternative payment models being explored or currently in development for cancer care are provided in the following section.
CMS OCM
The CMMI, an office of CMS that supports the development and testing of new payment models, has expanded its focus to specialty care. In 2015, the CMMI launched the OCM, a multipayer demonstration project using episode-based payments designed to promote quality and coordination of care for patients receiving chemotherapy.114 OCM participants must use a certified EHR system, provide round-the-clock access to practitioners, create comprehensive patient care plans, use patient navigators, and engage in continuous quality improvement. The demonstration will cover most cancer types, and participants must assume risk for all spending by their patients.
Medicare limited the OCM demonstration to 100 practices and anticipates notifying successful applicants by early 2016. The CMMI also invited other payers, including commercial insurance plans and state Medicaid agencies, to participate. In late 2014, ASCO submitted comments on the OCM, urging CMS to explore more substantial reforms that better reflect the work provided by oncology care teams.115 Among the concerns of ASCO was that the OCM holds oncologists accountable for services beyond their control.
ASCO PCOP model
In 2014, ASCO introduced the PCOP model of Medicare reimbursement for oncology care. After receiving comments from the public, ASCO published a revised version of the model in March 2015. Key components of the PCOP model include higher, more flexible payments for treatment planning and care management and accountability for delivering high-quality, high-value care.
The PCOP model introduces four new payments for specific clinical services, in addition to the existing services that practices currently bill to payers.116 These include:
New patient treatment planning.
Care management during treatment.
Care management during active monitoring after treatment.
Participation in clinical trials.
The PCOP model differs from shared savings payment systems in that individual practices are not required to reduce spending to receive these additional payments. Instead, in exchange for these new payments, practices are expected to take accountability for providing high-quality, evidence-based care through four measures:
Reducing emergency department visits and hospital admissions resulting from treatment complications.
Following evidence-based guidelines and using lower-cost options where they have shown equivalence to higher-cost options.
Providing high-quality end-of-life care.
Providing care consistent with ASCO quality standards.
In addition to this basic PCOP model, optional advanced versions of the PCOP model have also been proposed that allow greater flexibility to practices based on their individual circumstances (Box 7).
Box 7. Patient-Centered Oncology Payment Model116: Overview of Options.
The ASCO Patient-Centered Oncology Payment model offers practices three modes of participation:
Basic option: Offers four new non–visit-based payments to oncology practices in return for accountability in key aspects of treatment selection and patient care.
Option A: Incorporates consolidated payments for oncology practice services; new billing codes are created for episodes of care to replace existing evaluation and management and infusion billing codes. This option offers more flexibility to determine how to best deliver services to patients.
Option B: Establishes virtual budgets for oncology care; designed to provide greater flexibility and accountability regarding the cost and quality of care. Creates monthly budgets designed to cover services delivered by the oncology practice and those delivered by other providers.
For more information, visit www.asco.org/paymentreform.
Patient-centered medical home for cancer care
The patient-centered medical home, originally created to enhance communication, coordination, and accountability in the primary care setting, has also been applied to oncology and other specialties. On the basis of standards developed in collaboration with ASCO and other specialty organizations, the National Committee for Quality Assurance created the Patient-Centered Specialty Practice Recognition Program.117 The standards address multiple facets of care, including coordination of referrals, access and communication, planning and managing care, shared decision making, tracking and coordination of care and testing, and measurement and improvement of performance.117
Both private and public payers have begun to evaluate the implementation of a patient-centered medical home in cancer care. A pilot program tested the concept in five oncology practices in southeastern Pennsylvania.118 Site visits conducted 6 to 9 months into the project revealed a variation in the uptake of the various medical home components identified by the National Committee for Quality Assurance, with the most frequently attained functions being referral coordination and care management and the least frequently attained functions being tracking and follow-up on tests and referrals, coordination of transitions of care with hospitals and emergency departments, and measurement and improvement of performance quality.
Several barriers to implementation were identified: the absence of specific processes to identify and obtain tests and referral results; lack of a quality improvement team; and lack of centralized, integrated EHRs. Opportunities for improvement were also identified, including health information technology, standardization of symptom management protocols, patient education and financial counseling, and care team communication.118 Researchers also emphasized that engagement of both the practice leadership and involved physicians is important for making the change to a patient-centered medical home model.
The medical home concept is also being assessed in the 3-year COME HOME (Community Oncology Medical Home) project, which includes community oncology practices across seven US states.119 The program is funded by the CMMI with a $20 million grant, representing the largest oncology-related award. Primary tenets of the COME HOME program include triage pathways, improved clinic access, and use of evidence-based care.119 Specific program components include120:
Use of EHRs.
Best-practice care, including triage pathways (decision support tools for first responder telephone operators and triage nurses) and clinical pathways (for diagnosis and treatment of common cancers).
Team-based care.
Active disease management with enhanced patient education.
Enhanced access to practice, including 24-7 triage telephone line, same-day appointments, evening and weekend clinic hours, direct hospital admission, and limiting of patient handoffs.
Enhanced care (on-site or nearby laboratory, pharmacy, and imaging capabilities).
Financial support through direct budgeting for infrastructure.
Data are now available from the first year of the COME HOME program, and preliminary findings show that after five quarters, the program was associated with significant reductions in hospitalizations and emergency department visits as well as in the total cost of care.119
Additionally, the Community Oncology Alliance is working with the Commission on Cancer to develop an oncology medical home accreditation program.121 Applicant organizations are evaluated in five domains that promote medical home principles: (1) patient engagement, (2) expanded access, (3) evidence-based medicine, (4) comprehensive team-based care, and (5) continuous quality improvement. In 2015, the Community Oncology Alliance invited the following practices to participate in a pilot round122:
Oncology Hematology Care, Cincinnati, Ohio
Center for Cancer and Blood Disorders, Fort Worth, Texas
Dayton Physicians Network, Ohio
Austin Cancer Centers, Texas
Oncology Hematology Associates of Springfield, Georgia
Northwest Georgia Oncology Centers, Georgia
Space Coast Cancer Center, Florida
Hematology-Oncology Associates of Central New York, New York
New Mexico Oncology Hematology Consultants, New Mexico
Maine Center for Cancer Medicine & Blood Disorders, Maine
The accreditation program is expected to expand to 50 additional practices by 2016.122
Commercially sponsored models
The top five US health care insurers are all now involved in alternative payment projects for cancer care, some of which have been developed in conjunction with large oncology centers. In 2012, 21st Century Oncology (Fort Myers, FL) and Humana (Louisville, KY) signed an agreement to establish episodic payments for external-beam radiation therapy services. Episodes were defined as beginning at consultation and ending 90 days after treatment. The agreement included 13 common cancer diagnoses that were each assigned a fixed payment to account for all direct radiotherapy expenses.123
A 2014 report of the experience highlighted some positive outcomes after a year of implementation, including a greater than 98% rate of physician compliance with recommended treatments, increased use of clinically appropriate hypofractionation, projected cost savings by Humana from reduced administrative needs, and reported increases in patient satisfaction in ease of insurance approval.123 Humana reported that additional time was needed to assess the effects of episodic payment on overall spending and patient outcomes.
In early 2015, Kaiser Permanente (Oakland, CA), which uses a prepaid payment model, announced its involvement with a Department of Health and Human Services initiative to “support the adoption of alternative payment models through traditional Medicare plans.”124 The program, called the Health Care Payment Learning and Action Network, aims to bring together partners in the private, public, and nonprofit sectors to promote the transition to alternative payment models.124 ASCO will report on updates, especially as related to cancer care applications, as they become available.
In 2014, UnitedHealthcare (Minnetonka, MN) and MD Anderson Cancer Center (Houston, TX) announced the launch of a pilot program of bundled payments for treatment of patients with head and neck cancers.125 The model includes prepriced payments for the tests, treatments, follow-up care, and supportive services that MD Anderson deems appropriate for this cancer type. The head and neck program was undertaken after a 2010 UnitedHealthcare pilot program of episode payments saw total costs of care reduced by a third.126 Although the 2010 initiative included incentives for reducing chemotherapy costs, it saw a paradoxic increase in chemotherapy costs. However, there was still an overall cost reduction of 34%.
Anthem Blue Cross Blue Shield (BCBS; Indianapolis, IN) is involved in several ongoing bundled payment cancer programs. Horizon BCBS of New Jersey (Newark, NJ) has partnered with Regional Cancer Care Associates (Hackensack, NJ) to create an episode-of-care program for patients receiving treatment for breast cancer.127 The program uses real-time data that customize breast cancer treatment based on molecular subtype. There is also an emphasis on value-based reimbursement, because participating oncologists who meet quality and efficiency goals may be eligible for shared savings. Partnerships have also been established between Fox Chase Cancer Center (Philadelphia, PA) and Horizon BCBS (for breast and kidney cancers) and between Mobile Surgery International (Johnsbury, VT) and BCBS of Florida (Jacksonville, FL; for radical prostatectomy for patients with early-stage prostate cancer).128 In April 2015, Aetna (Hartford, CT) announced a collaboration with the Moffitt Cancer Center (Tampa, FL) to create an oncology medical home with the goals of caring for the whole person; using evidence-based, personalized medicine that focuses on quality and safety; offering coordinated and integrated care; and providing enhanced access to care, including open scheduling, expanded hours, and new communication tools.129
Clinical pathways
Clinical pathways are tools used to support delivery of evidence-based health care, with the ultimate goals of improving quality and reducing cost. In the past few years, there has been a proliferation of pathway options and requirements. Although recent studies by companies sponsoring clinical pathways suggest cost savings, pathways are creating confusion and an administrative burden for practices. In some instances, different payers may specify different clinical pathways for the same clinical scenario.
In January 2016, ASCO issued recommendations to improve the development of oncology pathways and processes, allowing the demonstration of pathway concordance in a manner that promotes evidence-based, high-value care, respecting input from patients, payers, and providers (Box 8).130
Box 8. Statement on Clinical Pathways.
In 2014, ASCO established a task force of clinical experts and other stakeholders to confront the issue of clinical pathway proliferation in oncology. In January 2016, ASCO released the following recommendations130:
A collaborative, national approach is necessary to remove the unsustainable administrative burdens associated with the unmanaged proliferation of oncology pathways.
Oncology pathways should be developed through a process that is consistent and transparent to all stakeholders.
Oncology pathways should address the full spectrum of cancer care, from diagnostic evaluation through medical, surgical, and radiation treatments and include imaging, laboratory testing, survivorship, and end-of-life care.
Oncology pathways should promote the best possible evidence-based care in a manner that is updated continuously to reflect the rapid development of new scientific knowledge, as well as insights gained from clinical experience and patient outcomes.
Oncology pathways should recognize patient variability and autonomy, and stakeholders must recognize that 100% concordance with oncology pathways is unreasonable, undesirable, and potentially unsafe.
Oncology pathways should be implemented in ways that promote administrative efficiency for both oncology providers and payers.
Oncology pathways should promote education, research, and access to clinical trials.
Robust criteria must be developed to support certification of oncology pathway programs. Pathway programs should be required to qualify based on these criteria, and payers should accept all oncology pathway programs that achieve certification through such a process.
Pathway developers, users, and private and governmental funding agencies should support research to understand pathway impact on care and outcomes.
Visit jop.ascopubs.org/lookup/doi/10.1200/JOP.2015.009134 to access the full statement.
In the 2015 ASCO Trends Survey (Chapter 3), 117 oncology practices (62%) reported adhering to clinical pathways. Of those, 31% reported adhering to more than one pathway program.
Implications for Oncology Practices
Although alternative payment models are being developed and piloted in a variety of circumstances by an array of entities, there is not universal access to such programs across the oncology community. A recent ASCO survey of oncology practices found that only 15% were paid to some degree by demonstrating use of alternative payment models (Chapter 3 provides ASCO Trends Survey details). Academic practices reported higher use of alternative payment models (19%), whereas 18% of private practices and 8% of hospital-based practices reported using them. With the clear emphasis by MACRA on alternative payment models, payers—including Medicare—will need to work with the medical community to increase the number and scope of opportunities for practices to participate in innovative solutions in this arena.
Value Initiatives
Cancer care is becoming increasingly unaffordable for patients, health care practices, and payers (Chapter 2 discusses the rising cost of cancer care). The topic of health care cost is being incorporated into many medical education programs in the United States. Medical students and residents are now learning about the importance of cost, value, and effectiveness and how to talk with patients and colleagues about these issues.131 In addition, many organizations, practices, and researchers are launching initiatives intended to address rising health care costs by making the value of various treatment choices more transparent to patients and their clinicians.
ASCO published its conceptual framework for assessing the value of different cancer treatment options in 2015.132 The framework, which will ultimately form the basis of a user-friendly tool that can be used in the clinical setting to support shared decision making, was designed to compare the potential value of a new treatment regimen with the standard of care for a specific indication and setting (advanced v potentially curable treatment; Box 9).
Box 9. Framework for Assessing Value in Cancer Care132.
The conceptual framework proposed by ASCO for assessing value in cancer care is based on data from prospective, randomized trials and incorporates multiple components:
Clinical benefit: Accounts for survival improvement or other clinical benefits associated with a treatment.
Toxicity: Reflects the relative toxicity of a new treatment versus a comparator regimen.
Bonus points: Applicable to advanced disease; accounts for improvement in cancer-related symptoms or treatment-free interval.
Direct cost: Includes drug acquisition cost and patient costs.
Clinical benefit, toxicity, and bonus points are combined to yield the net health benefit (NHB) score. The NHB score and cost information are presented independently, allowing the user to gauge the relative value of a treatment.
The full framework can be accessed at www.asco.org/value.
In late 2015, the NCCN published the first of its Evidence Blocks—a new visually represented multipart score that accounts for the efficacy, safety, quality of evidence, consistency of evidence, and affordability of a drug or regimen.133 Evidence Blocks were designed to facilitate conversations between patients and their physicians when making treatment decisions. As of October, the NCCN had published Evidence Blocks for chronic myelogenous leukemia and multiple myeloma.
There are also several other value initiatives aimed at drug costs. For example, Dr Peter Bach, director of the Center for Health Policy at Memorial Sloan Kettering Cancer Center (New York, NY), developed DrugAbacus, an online interactive calculator designed to help users calculate the appropriate price of a drug based on various components of the value of the drug. The tool currently includes a sample of available drugs and considers only the first indication of a drug.134 Express Scripts (St Louis, MO) has also announced a plan starting in 2016 to offer an “indication-based formulary” for certain medications, in which the price of a therapy would change based on its demonstrated efficacy in a given indication.135
Moreover, state legislatures are stepping in, taking measures to limit the cost of specialty drugs, which include many cancer therapies. At least seven states have passed laws limiting coinsurance payments for patients participating in private health plans.136
At the federal level, there is a call for Congress to authorize Medicare Part D to negotiate with manufacturers regarding the price of drugs. Medicare Part D is the largest federal drug program, covering more than 39 million individuals in 2015.137 According to a 2015 report, Medicare Part D pays 198% of the median costs for the same branded drugs compared with the 31 Organization for Economic Cooperation and Development countries.138 Even within the United States, Medicare pays more than other federal organizations—an average of 73% more than Medicaid and 80% more than the Veterans Benefits Administration for brand-name drugs. It has been estimated that Medicare acquisition of brand-name drugs at prices available to Medicaid or the Veterans Benefits Administration could yield $15.2 billion or $16 billion in savings, respectively, each year.138
Strategies to Measure and Improve Performance in Cancer Care
As payment systems shift incentives from volume to value, quality monitoring is more important than ever. Routine measurement and assessment of patient data are two ways of determining that these reforms are not negatively affecting patients. Meaningful quality measures and robust health information technology are critical to providing rapid—and accurate—performance feedback to providers.
Quality measures
Quality measurement and improvement are central elements in virtually every payment reform model proposed in 2015. Under MACRA, for example, CMS has streamlined and fortified its requirements for quality reporting within the Medicare program. Meanwhile, organizations such as the Commission on Cancer, the National Quality Forum, the Agency for Healthcare Research and Quality, and ASCO have published or endorsed quality metrics spanning a variety of cancer services, including appropriate use of treatments and procedures, palliative care, and end-of-life care. This section covers the key data collection requirements under MACRA and a brief update on the Quality Oncology Practice Initiative (QOPI), the ASCO quality measurement program.
MACRA merit-based incentive payment system
The 2015 MACRA legislation includes a plan through which providers’ performance scores on various quality measures will drive either penalties or bonuses. Starting in 2019, parts of three existing incentive payment programs—the Physician Quality Reporting System (PQRS), the value-based payment modifier, and meaningful use of EHRs—will be combined into a single program called the Merit-Based Incentive Payment System.139 The three existing programs are currently implemented as follows:
The PQRS. The PQRS was established by CMS as a mechanism for physicians and other health care providers to report information about the quality of care provided. Beginning in 2015, CMS required providers to participate in the PQRS to obtain full Medicare reimbursement.
The value-based payment modifier. Created under the ACA, the program provides for differential payments to health care providers based on the quality and cost of care delivered to beneficiaries enrolled in the Medicare fee-for-service program. Value modifier adjustments for 2018 will be based on 2016 performance numbers, after which the program will be integrated into the Merit-Based Incentive Payment System.140
Meaningful use of EHRs. Through the CMS meaningful use program, providers must show that they have adopted EHRs in “meaningful ways to help improve the quality and safety of the nation’s healthcare system.”141 Starting in 2015, Medicare-eligible professionals and hospitals were required to meet meaningful use requirements to avoid financial penalty.141 According to the ASCO Trends Survey, as of 2015, three quarters (76%) of oncology practice respondents had satisfied the criteria to meet meaningful use level one, which focuses on data capture and sharing, and 55% had attested to meaningful use level two, which emphasizes advanced clinical processes (Chapter 3 provides survey details).
ASCO QOPI
Through QOPI, ASCO is assisting oncology practices across the country in their quality measurement and assessment efforts. In 2015, CMS approved participation in QOPI as a method for meeting the PQRS requirements. This approval will aid in the transition of practices toward satisfying the MACRA requirements and achieving enhanced reimbursement for services under Medicare.
More than 1,000 US practices have registered for QOPI since its launch in 2006, representing approximately 7,000 oncologists. In three recent rounds of data collection (fall 2013 through spring 2015), 994 practices submitted more than 32,000 patient records for assessment of compliance with quality measures. In the last 5 years, QOPI has expanded dramatically to accommodate practices from a broad range of sizes, settings, and geographic locations. Of the 994 recent QOPI participants, many are offering important interdisciplinary services, including access to social workers (56%), dieticians and nutritionists (52%), and genetic counselors (39%).
New enhancements and measures continue to be added to QOPI. In 2015, for example, QOPI launched its eQOPI initiative, a reporting pathway that allows practices to extract data electronically from their EHRs. eQOPI will reduce the manual abstraction burden of participating in the program and facilitate data importation into the PQRS. Also in 2015, QOPI began pilot testing measures intended to assess value in cancer care delivery. These measures, modeled on the ASCO Choosing Wisely lists of treatments and procedures whose common use and clinical value are not supported by available evidence, include142-144:
Chemotherapy administered to patients with a metastatic solid tumor with performance status of 3 or 4 or undocumented performance status.
Positron emission tomography, computed tomography, or radionuclide bone scan ordered by a practice within 2 months after diagnosis of early-stage prostate cancer with low risk of metastasis.
Granulocyte colony-stimulating factor administered to patients with less than 20% risk for this complication.
Role of health information technology in achieving high-quality care
Several strategies have recently been undertaken to improve the quality of cancer care through greater collaboration among health care professionals and better use of health information technology. In 2015, the IOM published a report titled “Improving Diagnosis in Health Care,” which outlines specific recommendations to address the goal of reducing delays in diagnosis.145 Some of the proposed improvements could also positively affect other points along the cancer care continuum.145
Among the IOM recommendations was a push for standards of interoperability among different EHRs. Currently, different providers may use different health information technology systems that are not compatible, creating a barrier that can inhibit the sharing of information necessary for optimal cancer care. The IOM recommends that these standards of interoperability be met by 2018. ASCO has lauded this proposal by the IOM and has also released a position statement on interoperability in conjunction with a briefing to Congress (Box 10).146
Box 10. Position Statement on Interoperability.
In September 2015, in conjunction with a Capitol Hill briefing, ASCO released a position paper supporting the interoperability of electronic health record (EHR) systems and warned against the practice of information blocking, in which a party intentionally interferes with the exchange of EHR information. ASCO also made the following related recommendations:
Congress should pass legislation to ensure interoperability is adopted.
Congress should pass legislation removing barriers to interoperability.
Policymakers should ensure that the costs associated with ensuring interoperability are not subsidized by patients or health care providers.
Officials should work with ASCO and other stakeholders to help educate health care providers about interoperability of EHR systems and the issue of information blocking.
Visit http://www.asco.org/sites/www.asco.org/files/position_paper_for_clq_briefing_09142015.pdf for the full statement.
Interoperability among EHRs also has the potential to enable big data efforts, not only to improve care but also to speed progress toward more effective treatments. In 2012, ASCO began work on CancerLinQ, a rapid learning health care system designed to harness big data as a means of improving the quality and value of cancer care. The system will securely process real-world patient data directly from EHRs and provide immediate quality feedback and clinical decision support to health care providers. CancerLinQ will also analyze incoming patient characteristics, treatments, and outcomes in aggregate, paving the way for new insights and further improvement in cancer care.
In 2015, ASCO announced a collaboration with SAP (Walldorf, Germany), a global leader in information technology, to move CancerLinQ from early concept to reality.147 The first version of CancerLinQ was launched in late 2015 and now holds approximately 500,000 patient records.
CONCLUSION
The cancer care delivery system is exploring a variety of strategies for improving the quality and value of cancer care. These include alternative payment models, tools to support shared decision making and selection of high-value treatments, and rapid feedback to practices on performance and patient outcomes.
All of these strategies depend heavily on technology that can support information sharing across health care delivery settings and providers. For this reason, interoperability and other health information technology issues will be a top priority for oncology stakeholders—and medicine in general—in the coming year.
CONCLUSIONS AND RECOMMENDATIONS
The ASCO analysis of the current state of cancer care reveals many promising advances. Areas of progress include innovation in precision medicine, immunotherapies, and use of big data to answer pressing clinical questions; improved access to cancer care services; better care coordination; and renewed interest in establishing payment and care delivery models that ensure high-value, affordable care.
However, many challenges in cancer care remain unaddressed, and the cancer care delivery system is ill equipped to take full advantage of the advancements summarized in this report. Evidence-based solutions are needed to ensure that promising research is adequately supported; that the reimbursement system rewards high-quality, high-value care; that patients have access to affordable care; and that disparities and gaps in patients’ access to care are reduced.
This report builds on the strategies outlined in last year’s State of Cancer Care in America report for addressing the most pressing challenges. Many of the obstacles to high-quality cancer care are ongoing and difficult to overcome, and therefore, the path for addressing them cannot be achieved in a 1-year timeline. In addition, this report includes a new recommendation that addresses the rising cost of cancer care.
The following list details areas where, over the past year, there have been improvements in policies to advance the quality of cancer care—as well as specific action items for the coming year where additional attention is needed.
Ensure All Publicly Funded Insurance Programs Offer Consistent and Appropriate Benefits and Services for Patients With Cancer
A primary goal of the ACA was to make health care more accessible and affordable for all Americans—an objective that is closely aligned with the ASCO goal of providing access to high-quality care for all individuals with cancer. However, not all Americans have benefited equally from implementation of the law. Coverage remains incomplete, increasing cost shifting to patients, and rising costs present barriers to care that affect even those with insurance coverage. Furthermore, the system continues to foster large disparities in care that affect people at all points along the cancer continuum (Chapter 2). For 2016:
Congress should mandate that private and public health insurance plans provide parity in benefits and coverage for intravenous cancer drugs and orally administered or self-injectable cancer drugs.
Congress should address ongoing disparities in Medicaid by modifying Medicaid coverage requirements to include coverage of clinical trials and removing disparities in benefits between Medicaid programs established before and after the ACA.
Professional organizations should remain engaged with their members to track ACA implementation effects and trends and work with policymakers to address issues preventing access to high-quality, high-value care for patients with cancer.
Test Multiple Innovative Payment and Care Delivery Models to Identify Feasible Solutions That Promote High-Quality, High-Value Cancer Care
The ACA has been an impetus for payers to initiate testing of many new models of care delivery and payment, with the goals of maintaining or improving the quality of care and lowering costs (Chapter 4). These aims were reinforced in 2015 through MACRA, which repealed the SGR formula for reimbursing physician services under Medicare. Although MACRA provides strong incentives for physicians to participate in new payment models and an emphasis on specialty-specific alternative payment models, the CMMI has shown little inclination to test oncology care models beyond its own OCM demonstration, which is limited in scope. Furthermore, although the CMMI and CMS have authority to translate positive results of demonstration projects into the national Medicare program, there is no clear process for this to occur. For 2016:
Congress should aggressively monitor implementation of MACRA to ensure (1) that the Administration works with professional organizations to test multiple payment models of care and (2) that the Administration provides a clear path for implementation of payment models shown to provide positive results for patients, providers, and payers.
Professional organizations should develop innovative care models that can be tested by the CMMI and private payers as they seek better ways to incentivize and support high-quality, high-value patient care.
Advance Health Information Technology That Supports Efficient, Coordinated Care Across the Cancer Care Continuum
It is in the best interest of patients, caregivers, clinicians, and the health care system if cancer care is coordinated across care settings. Health information technology is a key tool for achieving coordination. However, health information technology systems must be interoperable so that providers can communicate efficiently, exchange data, and make effective use of the full scope of available electronic health information. For 2016:
Congress should require that health information technology vendors create products that promote interoperability.
Policymakers should ensure that patients with cancer, oncologists, and other oncology providers do not bear the cost of achieving interoperable EHRs and that companies refrain from information blocking.
Professional organizations and other stakeholders should work with federal officials to ensure that health care providers have the information necessary to be prudent purchasers and users of health information technology systems.
Recognize and Address the Unsustainable Trend in the Cost of Cancer Care
Costs associated with cancer care are rising more rapidly than costs in other medical sectors. These costs have severe financial implications for patients with cancer and survivors and may place care out of the financial reach for many patients, despite gains in insurance coverage. Although drug costs represent only a small portion of overall cancer care costs in the United States, they receive outsized attention because of their alarming price tags and substantial price increases in recent years.
Congress should work with stakeholders to pursue solutions that will curb unsustainable costs for patients, providers, and the health care system.
Payers should design payment systems that incentivize patient-centered, high-value care and invest in infrastructure that supports a viable care delivery system.
Professional organizations should develop and disseminate clinical guidelines, tools, and resources such as Choosing Wisely to optimize patient care, reduce waste, and avoid inappropriate treatment.
Professional organizations should promote shared decision making between patients and physicians and the development of high-value treatment plans consistent with patients’ needs, values, and preferences.
Because of the myriad issues and types of health care services needed for high-quality cancer care delivery, the cancer care continuum touches on nearly all aspects of health care policy and reflects the array of challenges and opportunities facing the US health care system. ASCO will continue to: (1) track and evaluate the ever-shifting landscape in cancer care over the coming year, (2) support cancer care providers as they negotiate these growing pressures, and (3) work with policymakers to ensure that changes in the system support all patients’ access to high-quality, high-value care. Making progress in all of these areas will require a sustained, long-term effort by the multiple stakeholders involved in cancer care delivery.
Supplementary Material
Appendix
ASCO Workforce Information System
ASCO created the Workforce Information System (WIS) to assemble current data on the US oncologist supply and compare those data with the latest cancer epidemiology. For purposes of the WIS, oncologists include those who report a primary specialty of medical oncology, hematology, or hematology/oncology.
The WIS provides a data collection and analysis process that is composed of three sections: workforce supply, new entrants, and cancer incidence and prevalence. Tabulations of the number of oncologists in the United States are derived from the American Medical Association Physician Masterfile and the Centers for Medicare & Medicaid Services Physician Compare data set.84,85 Demographic data on practicing oncologists come from the Masterfile. Geographic analyses of oncologists’ practice locations are conducted using Physician Compare and US Census data (https://www.census.gov/geo/maps-data/data/tiger-line.html).
Information on fellows and residents in the oncology workforce pipeline come from published sources such as JAMA. The WIS compares the characteristics of these oncologists with those of all physicians and tracks emerging trends in the physician training pipeline. Incidence and prevalence estimates are published by the American Cancer Society and National Cancer Institute.2,3
In March 2016, ASCO released the fifth edition of WIS. To download the full report, visit www.asco.org/wis.
ASCO National Oncology Census
ASCO established the National Oncology Census to capture comprehensive, timely data that help characterize oncology practice in the United States. Begun in 2012, the census collects information about oncology services and specialties, practice settings, staffing and mergers, payer mix, patient volume, and practice pressures. ASCO is using these data to understand practice demographics and needs so that ASCO may adapt to the changing environment and be supportive of oncologists’ interests.
Launched in March 2015 and closed in July 2015, the latest census gathered 678 responses from across the country. Although this is fewer than the 974 responses cited in 2014, ASCO used new techniques in 2015 to consolidate and clean responses throughout the data collection process. Following this approach, the 2015 census covered 2,189 sites employing 13,493 oncologists, an increase from the 1,811 sites and 9,509 oncologists represented in the 2014 census.
Outreach for the 2015 census was initiated by mailing 2,600 postcards to verified and unverified practice addresses identified through the ASCO membership database, state affiliate membership lists, and responses to the 2014 census. These postcards were followed up with e-mail campaigns and telephone calls as needed.
Of those who completed the census, 611 individuals provided information about their position within the practice; 231 responders were MDs, DOs, or other PhDs providing care directly, acting as professors at academic practices, or acting as directors of medical divisions or departments; 22 responders included a mix of advanced practice nurses, registered nurses, nurse managers, and other clinical support positions; 358 were practice administrators, office managers, or executive directors or had similar occupations.
Practices were asked to select the most appropriate ownership type for their practice: academic, physician owned, or hospital or health system owned. Responders included 243 physician-owned practices, 284 hospital or health system–owned practices, and 112 academic practices. On the basis of this response rate, it is possible that ASCO does not yet have information from a significant portion of smaller practices or a number of large academic institutions.
The 2015 ASCO Trends Survey sought to expand on data collected through the census. It was launched in late September 2015 and closed in late October 2015. Whereas the census focused mainly on numeric data concerning practice type, provider population, and other quantitative data, the ASCO Trends Survey focused mainly on practice operations, asking for details on a variety of topics such as electronic health records, alternative payment models, and employee retention.
The ASCO Trends Survey was sent to a simple random sample of 394 census respondents from academic, physician-owned, and hospital or health system–owned practice settings. The sample size was chosen to accommodate a 95 confidence level and 0.05 margin of error, assuming a 60% response rate (Lohr SL: Sampling: Design and Analysis [ed 2]. Boston, MA, Brooks/Cole, 2010). The final count of complete responses for the ASCO Trends Survey was 171, resulting in an error of 0.07. The respondents had characteristics similar to those of the overarching census population according to practice setting and number of oncologists.
AUTHOR’S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The State of Cancer Care in America, 2016: A Report by the American Society of Clinical Oncology
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
M. Kelsey Kirkwood
No relationship to disclose
References
- 1.US Food and Drug Administration Hematology/Oncology (Cancer) Approvals & Safety Notifications. http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm.
- 2.American Cancer Society Cancer Facts and Figures 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf.
- 3.Howlader N NA, Krapcho M, Garshell J, et al. SEER Cancer Statistics Review, 1975-2012. http://seer.cancer.gov/csr/1975_2012/
- 4.DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2015;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 5.Sommers BD. Health care reform’s unfinished work: Remaining barriers to coverage and access. N Engl J Med. 2015;373:2395–2397. doi: 10.1056/NEJMp1509462. [DOI] [PubMed] [Google Scholar]
- 6.Sommers BD, Gunja MZ, Finegold K, et al. Changes in self-reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314:366–374. doi: 10.1001/jama.2015.8421. [DOI] [PubMed] [Google Scholar]
- 7.Congressional Budget Office Insurance Coverage Provisions of the Affordable Care Act: CBO’s March 2015 Baseline. https://www.cbo.gov/sites/default/files/cbofiles/attachments/43900-2015-03-ACAtables.pdf.
- 8.Collins SR, Rasmussen PW, Beutel S, et al. The Problem of Underinsurance and How Rising Deductibles Will Make It Worse—Findings from the Commonwealth Fund Biennial Health Insurance Survey. Washington, DC: The Commonwealth Fund; 2015. [PubMed] [Google Scholar]
- 9.Demko P. Health care spending again accelerating. http://www.politico.com/story/2015/07/health-care-spending-hike-prediction-120740. [Google Scholar]
- 10.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiJulio B, Firth J, Brodie M. Kaiser Health Tracking Poll: August 2015. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 2015. [Google Scholar]
- 12.American Cancer Society Cancer Treatment & Survivorship Facts & Figures 2014-2015. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf.
- 13.Institute of Medicine . Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 14.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Efficacy and safety results from a phase III trial of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067); J Clin Oncol; 33, 2015 (suppl; abstr LBA1) [Google Scholar]
- 15.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loftus P, Winslow R. FDA approves Bristol-Myers’s Yervoy, Opdivo for treatment of melanoma. http://www.wsj.com/articles/fda-approves-bristol-myers-yervoy-opdivo-for-treatment-of-melanoma-1443711746.
- 17.Dennis B, Harlan C. Good news from Jimmy Carter: He’s cancer free. https://www.washingtonpost.com/politics/good-news-from-jimmy-carter-hes-cancer-free/2015/12/06/ea56a120-9c65-11e5-a3c5-c77f2cc5a43c_story.html.
- 18.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration Biosimilars. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/
- 21.US Food and Drug Administration Nonproprietary Naming of Biological Products: Guidance for Industry. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm459987.pdf.
- 22.American Society of Clinical Oncology Re: FDA-2013-D-1543. http://www.asco.org/sites/www.asco.org/files/fda_biosimilars_comments.pdf.
- 23.Friends of Cancer Research Breakthrough Therapies. http://www.focr.org/breakthrough-therapies.
- 24.Genome MC. Overview of Targeted Therapies for Cancer. http://www.mycancergenome.org/content/other/molecular-medicine/overview-of-targeted-therapies-for-cancer/
- 25.US Food and Drug Administration 2015 Device Approvals. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm430692.htm.
- 26.The White House The Precision Medicine Initiative. https://www.whitehouse.gov/precision-medicine.
- 27.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 30.Philip S, Rosenberg KAB, Anderson WF. Estrogen receptor status and the future burden of invasive and in-situ breast cancers in the United States. Presented at the American Association for Cancer Research Annual Meeting, Philadelphia, PA, April 18-22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morton LM, Onel K, Curtis RE, et al. The rising incidence of second cancers: Patterns of occurrence and identification of risk factors for children and adults; Am Soc Clin Oncol; Ed Book e57-e67, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Jemal A, Siegel RL, Ma J, et al. Inequalities in premature death from colorectal cancer by state. J Clin Oncol. 2015;33:829–835. doi: 10.1200/JCO.2014.58.7519. [DOI] [PubMed] [Google Scholar]
- 33.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. doi: 10.1136/bmj.g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Preventative Services Task Force Draft recommendation statement: Breast cancer—Screening. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening.
- 35.US Preventative Services Task Force Published recommendations. http://www.uspreventiveservicestaskforce.org/BrowseRec/Index.
- 36.Wasserman Schultz D. Insurance coverage for mammograms jeopardized by new guidelines, Congresswoman and breast cancer survivor says. https://www.washingtonpost.com/news/to-your-health/wp/2015/04/23/insurance-coverage-for-mammograms-jeopardized-by-new-guidelines-congresswoman-and-breast-cancer-survivor-says/
- 37.Sabatino S, White M, Thompson T, et al. Cancer screening test use: United States, 2013. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6417a4.htm. [PMC free article] [PubMed] [Google Scholar]
- 38.Gray SW, Hicks-Courant K, Cronin A, et al. Physicians’ attitudes about multiplex tumor genomic testing. J Clin Oncol. 2014;32:1317–1323. doi: 10.1200/JCO.2013.52.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker BA, Schwaederlé M, Scur MD, et al. Breast cancer experience of the molecular tumor board at the University of California, San Diego Moores Cancer Center. J Oncol Pract. 2015;11:442–449. doi: 10.1200/JOP.2015.004127. [DOI] [PubMed] [Google Scholar]
- 40.National Cancer Institute SEER Stat Fact Sheets: All Cancer Sites. http://seer.cancer.gov/statfacts/html/all.html.
- 41.Centers for Medicare & Medicaid Services Medicare Chronic Conditions Dashboard: State Level. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Dashboard/Chronic-Conditions-State/CC_State_Dashboard.html.
- 42.Collender S. Clinical trials need cancer patients. http://www.nytimes.com/2015/06/19/opinion/clinical-trials-need-cancer-patients.html.
- 43.Hurria A, Levit LA, Dale W, et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33:3826–3833. doi: 10.1200/JCO.2015.63.0319. [DOI] [PubMed] [Google Scholar]
- 44.Dennis B. “Exceptional responders” to cancer drugs draw scrutiny from scientists. https://www.washingtonpost.com/national/health-science/looking-for-answers-in-cancers-exceptional-responders/2015/06/13/9cf0ec78-0571-11e5-a428-c984eb077d4e_story.html.
- 45.Altman D, Frist WH. Medicare and Medicaid at 50 years: Perspectives of beneficiaries, health care professionals and institutions, and policy makers. JAMA. 2015;314:384–395. doi: 10.1001/jama.2015.7811. [DOI] [PubMed] [Google Scholar]
- 46.Medicare Payment Advisory Commission . The Next Generation of Medicare Beneficiaries. 2015. Chapter 2. Washington, DC, MedPac, 2015. [Google Scholar]
- 47.Polite BN, Griggs JJ, Moy B, et al. American Society of Clinical Oncology policy statement on Medicaid reform. J Clin Oncol. 2014;32:4162–4167. doi: 10.1200/JCO.2014.56.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins SR, Rasmussen PW, Doty MM, et al. Americans’ experiences with marketplace and Medicaid coverage: Findings from the Commonwealth Fund Affordable Care Act Tracking Survey, March-May 2015. Issue Brief (Commonw Fund) 2015;16:1–17. [PubMed] [Google Scholar]
- 49.Robbins AS, Han X, Ward EM, et al. Association between the Affordable Care Act dependent coverage expansion and cervical cancer stage and treatment in young women. JAMA. 2015;314:2189–2191. doi: 10.1001/jama.2015.10546. [DOI] [PubMed] [Google Scholar]
- 50.Garfield R, Damico A, Cox C, et al. New Estimates of Eligibility for ACA Coverage Among the Uninsured. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 2015. [Google Scholar]
- 51.Hancock J. Employers shift more health costs to workers, survey finds. http://khn.org/news/employers-shift-more-health-costs-to-workers-survey-finds/ [Google Scholar]
- 52.Smith JK, Ng SC, Zhou Z, et al. Does increasing insurance improve outcomes for US cancer patients? J Surg Res. 2013;185:15–20. doi: 10.1016/j.jss.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 53.The Henry J. Kaiser Family Foundation Current status of state Medicaid expansion decisions. http://kff.org/health-reform/slide/current-status-of-the-medicaid-expansion-decision/
- 54.McMorrow S, Long SK, Fogel A. Primary care providers ordered fewer preventive services for women with Medicaid than for women with private coverage. Health Aff (Millwood) 2015;34:1001–1009. doi: 10.1377/hlthaff.2014.0907. [DOI] [PubMed] [Google Scholar]
- 55.Barnes R. Affordable Care Act survives Supreme Court challenge. https://www.washingtonpost.com/politics/courts_law/obamacare-survives-supreme-court-challenge/2015/06/25/af87608e-188a-11e5-93b7-5eddc056ad8a_story.html.
- 56.Howell T. Obamacare co-op flops have many running for coverage. http://www.washingtontimes.com/news/2015/nov/15/obamacare-co-op-flops-have-many-scrambling-for-hea/?page=all.
- 57.Mackay C, Antonelli K, Bruinooge SS, et al. Cancer center and community research program experience with insurance denials for clinical trial participation after ACA mandate; Presented at the Association of American Cancer Institutes Cancer Research Initiative Annual Meeting, Chicago, IL, July 8-9, 2015. [Google Scholar]
- 58.Polite BN. States as the laboratory for democracy: Is anybody paying attention, and does anybody care? J Clin Oncol. 2015;33:815–816. doi: 10.1200/JCO.2014.59.4135. [DOI] [PubMed] [Google Scholar]
- 59.Polite BN, Griggs JJ, Moy B, et al. American Society of Clinical Oncology policy statement on Medicaid reform. J Clin Oncol. 2014;32:4162–4167. doi: 10.1200/JCO.2014.56.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber JS, Levit LA, Adamson PC, et al. American Society of Clinical Oncology policy statement update: The critical role of phase I trials in cancer research and treatment. J Clin Oncol. 2015;33:278–284. doi: 10.1200/JCO.2014.58.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The Henry J. Kaiser Family Foundation Health Spending Trends Slideshow. http://kff.org/slideshow/health-spending-trends-slideshow/
- 62.Davis K, Stremikis K, Schoen C, et al. Mirror, mirror on the wall, 2014 update: How the U.S. health care system compares internationally. http://www.commonwealthfund.org/publications/fund-reports/2014/jun/mirror-mirror.
- 63.Tefferi A, Kantarjian H, Rajkumar SV, et al. In support of a patient-driven initiative and petition to lower the high price of cancer drugs. Mayo Clin Proc. 2015;90:996–1000. doi: 10.1016/j.mayocp.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moran Company Analysis of 2014 physician/supplier procedure summary file. http://www.themorancompany.com/services/analysis/
- 65.Walker J, Mathews AW. Small number of drugs drives big Medicare bill, spending data show. http://www.wsj.com/articles/medicare-releases-detailed-look-at-prescription-drug-spending-1430426438.
- 66.Saltz L. Perspectives on value; http://meetinglibrary.asco.org/content/115302. [Google Scholar]
- 67.Helwick C. Cost of immunotherapy projected to top $1 million per patient per year. http://www.ascopost.com/issues/july-10-2015/cost-of-immunotherapy-projected-to-top-1-million-per-patient-per-year/ [Google Scholar]
- 68.Hirschler B. Exclusive: Americans overpaying hugely for cancer drugs. http://www.reuters.com/article/us-health-pharmaceuticals-cancer-usa-idUSKCN0RM1EC20150922. [Google Scholar]
- 69.Pembroke Consulting Retail generic drug inflation reaches new heights. http://www.drugchannels.net/2014/08/retail-generic-drug-inflation-reaches.html.
- 70.Pollack A. Drug goes from $13.50 a tablet to $750, overnight. http://www.nytimes.com/2015/09/21/business/a-huge-overnight-increase-in-a-drugs-price-raises-protests.html?_r=0.
- 71.Rockoff JD. Rising drug costs to be in focus at Congressional hearing. http://www.wsj.com/articles/rising-drug-costs-to-be-in-focus-at-congressional-hearing-1449311407.
- 72.Lorenzetti L. This company is offering a $1 alternative to Turing’s $750 drug. http://fortune.com/2015/10/23/turing-daraprim-cheap-alternative/ [Google Scholar]
- 73.Mulcahy AW, Predmore Z, Mattke S. The cost savings potential of biosimilar drugs in the United States. http://www.rand.org/pubs/perspectives/PE127.html. [Google Scholar]
- 74.Johnson CY. The first in a new generation of cheaper drugs isn’t much cheaper. https://www.washingtonpost.com/news/wonk/wp/2015/09/03/the-first-in-a-new-generation-of-cheaper-drugs-isnt-much-cheaper/
- 75.Kantarjian HM, Fojo T, Mathisen M, et al. Cancer drugs in the United States: Justum pretium—The just price. J Clin Oncol. 2013;31:3600–3604. doi: 10.1200/JCO.2013.49.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.The Henry J. Kaiser Family Foundation 2015 Employer Health Benefits Survey. http://kff.org/health-costs/report/2015-employer-health-benefits-survey/
- 78.PricewaterhouseCoopers Medical cost trend: Behind the numbers 2016. https://www.pwc.com/us/en/health-industries/behind-the-numbers/assets/pwc-hri-medical-cost-trend-chart-pack-2016.pdf.
- 79.Abelson R. Health insurance deductibles outpacing wage increases, study finds. http://www.nytimes.com/2015/09/23/business/health-insurance-deductibles-outpacing-wage-increases-study-finds.html.
- 80.Radnofsky L, Armour S. Insurers win big health-rate increases. http://www.wsj.com/articles/rising-rates-pose-challenge-to-health-law-1447894371.
- 81.State Patients Equal Access Coalition Oral chemo access map. http://speac.myeloma.org/oral-chemo-access-map/
- 82.Avalere Health More than 2 million exchange enrollees forgo cost-sharing assistance. http://avalere.com/expertise/managed-care/insights/more-than-2-million-exchange-enrollees-forgo-cost-sharing-assistance.
- 83.Polsky D, Weiner J. The skinny on narrow networks in health insurance marketplace plans. http://www.rwjf.org/en/library/research/2015/06/the-skinny-on-narrow-networks-in-health-insurance-marketplace-pl.html. [Google Scholar]
- 84.American Medical Association AMA Physician Masterfile. http://www.ama-assn.org/ama/pub/about-ama/physician-data-resources/physician-masterfile.page.
- 85.Centers for Medicare & Medicaid Services Official Physician Compare Data. https://data.medicare.gov/data/physician-compare.
- 86.Brotherton SE, Etzel SI. Graduate medical education, 2014-2015. JAMA. 2015;314:2436–2454. doi: 10.1001/jama.2015.10473. [DOI] [PubMed] [Google Scholar]
- 87.US Census Bureau State & County QuickFacts. http://quickfacts.census.gov/qfd/states/00000.html.
- 88.Association of American Medical Colleges Diversity in the Physician Workforce: Facts & Figures 2014. http://aamcdiversityfactsandfigures.org/
- 89.Hamel LM, Chapman R, Malloy M, et al. Critical shortage of African American medical oncologists in the United States. J Clin Oncol. 2015;33:3697–3700. doi: 10.1200/JCO.2014.59.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.US Census Bureau . Census Urban Area Facts 2010. https://www.census.gov/geo/reference/ua/uafacts.html. [Google Scholar]
- 91.Siegel RL, Sahar L, Robbins A, et al. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev. 2015;24:1151–1156. doi: 10.1158/1055-9965.EPI-15-0082. [DOI] [PubMed] [Google Scholar]
- 92.Lin CC, Bruinooge SS, Kirkwood MK, et al. Association between geographic access to cancer care, insurance, and receipt of chemotherapy: Geographic distribution of oncologists and travel distance. J Clin Oncol. 2015;33:3177–3185. doi: 10.1200/JCO.2015.61.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin CC, Bruinooge S, Kirkwood K, et al. Association between geographic access and receipt of radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93:E367. doi: 10.1016/j.ijrobp.2015.12.012. (abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salami AC, Barden GM, Castillo DL, et al. Establishment of a regional virtual tumor board program to improve the process of care for patients with hepatocellular carcinoma. J Oncol Pract. 2014;11:e66–e74. doi: 10.1200/JOP.2014.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.American Board of Genetic Counseling ABGC 2014 Annual Business Meeting. http://www.abgc.net/ABGC/documents/BusinessMeetingABGC2014Slidesfinal.pdf.
- 96.Eichmeyer JN. Improving access to oncology genetic counseling. https://www.accc-cancer.org/oncology_issues/articles/JA14/JA14-Improving-Access-to-Oncology-Genetic-Counseling.pdf.
- 97.McDonald E, Lamb A, Grillo B, et al. Acceptability of telemedicine and other cancer genetic counseling models of service delivery in geographically remote settings. J Genet Couns. 2014;23:221–228. doi: 10.1007/s10897-013-9652-9. [DOI] [PubMed] [Google Scholar]
- 98.Buchanan AH, Datta SK, Skinner CS, et al. Randomized trial of telegenetics vs. in-person cancer genetic counseling: Cost, patient satisfaction and attendance. J Genet Couns. 2015;24:961–970. doi: 10.1007/s10897-015-9836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dawes AJ, Hemmelgarn M, Nguyen DK, et al. Are primary care providers prepared to care for survivors of breast cancer in the safety net? Cancer. 2015;121:1249–1256. doi: 10.1002/cncr.29201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.US Department of Health and Human Services National Center for Health Workforce analysis: Projecting the supply and demand for primary care practitioners through 2020. http://bhpr.hrsa.gov/healthworkforce/supplydemand/usworkforce/primarycare/
- 101.Institute of Medicine . Measuring the Impact of Interprofessional Education (IPE) on Collaborative Practice and Patient Outcomes. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 102.Accreditation Council for Graduate Medical Education ACGME Program Requirements for Graduate Medical Education in Hematology and Medical Oncology (Internal Medicine) https://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013-PR-FAQ-PIF/155_hematology_oncology_int_med_07132013.pdf.
- 103.Eid A, Hughes C, Karuturi M, et al. An interprofessionally developed geriatric oncology curriculum for hematology-oncology fellows. J Geriatr Oncol. 2015;6:165–173. doi: 10.1016/j.jgo.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harper JL, De Costa AM, Garrett-Mayer E, et al. Incorporating patient satisfaction metrics in assessing multidisciplinary breast cancer care quality. South Med J. 2015;108:372–376. doi: 10.14423/SMJ.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nolte E, Pitchforth E. What is the evidence on the economic impacts of integrated care? http://www.euro.who.int/__data/assets/pdf_file/0019/251434/What-is-the-evidence-on-the-economic-impacts-of-integrated-care.pdf. [Google Scholar]
- 106.Porter ME. Value based healthcare delivery; Presented at the annual meeting of the American Society for Clinical Oncology, Chicago, IL, May 29-June 2, 2015. [Google Scholar]
- 107.Harvard Business School Integrated Practice Units. http://www.isc.hbs.edu/health-care/vbhcd/Pages/integrated-practice-units.aspx.
- 108.Schattner E. Logistic toxicity, an unmeasured burden of healthcare. http://www.forbes.com/sites/elaineschattner/2015/08/14/logistic-toxicity-an-unmeasured-burden-of-health-care-for-people-with-cancer-and-other-illness/#6d8678b626a3. [Google Scholar]
- 109.The Advisory Board Company AMA survey: Physician satisfaction with EHR systems has plummeted. https://www.advisory.com/daily-briefing/2015/08/13/ama-survey-physician-satisfaction-with-ehr-systems-has-plummeted.
- 110.House Energy and Commerce and Ways and Means Committees H.R. 2 Medicare Access and CHIP Reauthorization Act (MACRA), 2015.
- 111.14th Congress Medicare Access and CHIP Reauthorization Act of 2015, H.R.2, 2015.
- 112.Burwell SM. Setting value-based payment goals: HHS efforts to improve U.S. health care. N Engl J Med. 2015;372:897–899. doi: 10.1056/NEJMp1500445. [DOI] [PubMed] [Google Scholar]
- 113.Centers for Medicare & Medicaid Services Bundled Payments for Care Improvement Initiative Request for Application. https://innovation.cms.gov/Files/x/Bundled-Payments-for-Care-Improvement-Request-for-Applications.pdf.
- 114.Centers for Medicare & Medicaid Services Oncology Care Model. https://innovation.cms.gov/initiatives/oncology-care/
- 115.American Society of Clinical Oncology Re: CMMI preliminary design for an oncology-focused model. http://www.asco.org/sites/www.asco.org/files/comments_on_ocm_91814.pdf.
- 116.American Society of Clinical Oncology . Physician payment reform: ASCO’s payment reform model support higher quality, more affordable cancer care. http://www.asco.org/advocacy/physician-payment-reform. [Google Scholar]
- 117.National Committee for Quality Assurance Patient-centered specialty practice recognition. http://www.ncqa.org/Programs/Recognition/Practices/PatientCenteredSpecialtyPracticePCSP.aspx. [DOI] [PubMed]
- 118.Tirodkar MA, Acciavatti N, Roth LM, et al. Lessons from early implementation of a patient-centered care model in oncology. J Oncol Pract. 2015;11:456–461. doi: 10.1200/JOP.2015.006072. [DOI] [PubMed] [Google Scholar]
- 119.NORC at the University of Chicago Annual Report 1: HCIA Disease-Specific Evaluation. https://innovation.cms.gov/files/reports/hcia-ds-firstevalrpt.pdf.
- 120.Waters TM, Webster JA, Stevens LA, et al. Community oncology medical homes: Physician-driven change to improve patient care and reduce costs. J Oncol Pract. 2015;11:462–467. doi: 10.1200/JOP.2015.005256. [DOI] [PubMed] [Google Scholar]
- 121.Community Oncology Alliance Patient Advocacy Network Oncology medical home: Improved quality and cost of care. http://coaadvocacy.org/2014/09/oncology-medical-home-improved-quality-and-cost-of-care/
- 122.Association for Value-Based Cancer Care Oncology medical home accreditation by community oncology alliance making progress. http://www.valuebasedcancer.com/vbcc-issues/2015/september-2015-vol-6-no-8/26339-oncology-medical-home-accreditation-by-community-oncology-alliance-making-progress.
- 123.Falit BP, Chernew ME, Mantz CA. Design and implementation of bundled payment systems for cancer care and radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:950–953. doi: 10.1016/j.ijrobp.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 124.Kaiser Permanente Kaiser Permanente joins Health and Human Services announcement regarding Medicare. http://share.kaiserpermanente.org/article/kaiser-permanente-joins-health-and-human-services-announcement-regarding-medicare/
- 125.UnitedHealthcare MD Anderson, UnitedHealthcare launch new cancer care payment model. http://www.uhc.com/news-room/2014-news-release-archive/md-anderson-unitedhealthcare-cancer-care-payment-model1.
- 126.Newcomer LN, Gould B, Page RD, et al. Changing physician incentives for affordable, quality cancer care: Results of an episode payment model. J Oncol Pract. 2014;10:322–326. doi: 10.1200/JOP.2014.001488. [DOI] [PubMed] [Google Scholar]
- 127.Horizon Blue Cross Blue Shield of New Jersey Horizon Blue Cross Blue Shield of New Jersey creates ground-breaking patient-centered program for cancer treatment. http://www.horizonblue.com/about-us/news-overview/company-news/horizon-bcbsnj-patient-centered-program-cancer-treatment.
- 128.Hawes K. Your guide to oncology payment reform pilots. https://www.advisory.com/research/oncology-roundtable/oncology-rounds/2015/05/your-guide-to-onc-payment-reform-pilots.
- 129.Business Wire Aetna, Moffitt Cancer Center form oncology medical home: The patient-focused model supports high-value care. http://www.businesswire.com/news/home/20150416006095/en/Aetna-Moffitt-Cancer-Center-Form-Oncology-Medical#.VgRYRN9Vikp.
- 130.Zon RT, Frame JN, Neuss MN, et al. American Society of Clinical Oncology policy statement on clinical pathways in oncology. J Oncol Pract. doi: 10.1200/JOP.2015.009134. [epub ahead of print on January 12, 2016] [DOI] [PubMed] [Google Scholar]
- 131.Plevin R. Medical schools teach students to talk with patients about care costs. http://www.npr.org/sections/health-shots/2015/09/10/438496145/medical-schools-teach-students-to-talk-with-patients-about-care-costs.
- 132.Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) with NCCN Evidence Blocks. http://www.nccn.org/evidenceblocks/
- 134.Memorial Sloan Kettering Cancer Center Drug Abacus, an interactive exploration of drug pricing: FAQs. http://www.drugabacus.org/faqs/
- 135.Miller S. We have to change how we pay for cancer drug. http://lab.express-scripts.com/insights/drug-options/we-have-to-change-how-we-pay-for-cancer-drugs.
- 136.Ollove M. States limiting patient costs for high-priced drugs. http://khn.org/news/states-limiting-patient-costs-for-high-priced-drugs/ [Google Scholar]
- 137.Hoadley J, Cubanski J, Neuman T. Medicare Part D at ten years: The 2015 marketplace and key trends, 2006-2015. http://kff.org/medicare/report/medicare-part-d-at-ten-years-the-2015-marketplace-and-key-trends-2006-2015/ [Google Scholar]
- 138.Gagnon MA, Wolfe S. Mirror, mirror on the wall: Medicare Part D pays needlessly high brand-name drug prices compared with other OECD countries and with U.S. government programs. http://carleton.ca/sppa/wp-content/uploads/Mirror-Mirror-Medicare-Part-D-Released.pdf.
- 139.Steinbrook R. The repeal of Medicare’s sustainable growth rate for physician payment. JAMA. 2015;313:2025–2026. doi: 10.1001/jama.2015.4550. [DOI] [PubMed] [Google Scholar]
- 140.Centers for Medicare & Medicaid Services Proposed policy, payment, and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2016. https://www.cms.gov/newsroom/mediareleasedatabase/fact-sheets/2015-fact-sheets-items/2015-07-08.html.
- 141.US Department of Health & Human Services What is “meaningful use”? http://www.hrsa.gov/healthit/meaningfuluse/MU%20Stage1%20CQM/whatis.html.
- 142.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 143.Schnipper LE, Lyman GH, Blayney DW, et al. American Society of Clinical Oncology 2013 top five list in oncology. J Clin Oncol. 2013;31:4362–4370. doi: 10.1200/JCO.2013.53.3943. [DOI] [PubMed] [Google Scholar]
- 144.American Society of Clinical Oncology QOPI reporting pathways: Fall 2015 round. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI%20Fall%202015%20Reporting%20Pathways-%20Full%20Measures.pdf.
- 145.Institute of Medicine . Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 146.American Society of Clinical Oncology American Society of Clinical Oncology: Barriers to Interoperability and Information Blocking. http://www.asco.org/sites/www.asco.org/files/position_paper_for_clq_briefing_09142015.pdf.
- 147.American Society of Clinical Oncology CancerLinQ takes a big leap forward. https://am.asco.org/cancerlinq™-takes-big-leap-forward.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.