Abstract
Chronic kidney disease of unknown etiology (CKDu) is a major healthcare issue in Sri Lanka. This study included 125 consecutive patients with a diagnosis of CKDu undergoing renal biopsy at one hospital from 2008 to 2012. Associations between renal outcome parameters, epidemiological data, and histopathological findings were examined and regression models constructed based on univariate associations with outcome variables as serum creatinine >1.2 and stage of CKD >3. The mean patient age was 46.21 years (standard deviation = 11.64). A marked male predominance was noted. A positive family history of CKD was seen in 35.8%. Prominent histopathological features were glomerular sclerosis (94.8%), interstitial infiltration (76%) with lymphocytic infiltration, interstitial fibrosis (71.2%), and tubular atrophy (70.4%). Importantly, significant histological changes were seen in patients with early CKDu. For CKD stage >3 independent associations were: interstitial fibrosis [P = 0.005; odds ratio (OR) =0.153] and interstitial infiltrate (P = 0.030; OR = 0.2440. For serum creatinine >1.2, independent predictors were >50% glomerular sclerosis (P = 0.041; OR = 0.92), tubular atrophy (P = 0.034; OR = 0.171, and more than 40 residential life years (P = 0.009; OR = 9.229). Chronic tubulointerstitial nephritis (TIN) appears to be the predominant histopathological finding in patients with CKDu, with significant renal pathology established early on in the course of the disease. Interstitial infiltration appears to be an independent association of advancing CKD, CKDu, histopathology, histology, and TIN.
Key words: Chronic kidney disease of unknown etiology, histopathology, histology, tubulointerstitial nephritis
Introduction
Chronic kidney disease (CKD) encompasses a spectrum of clinical and laboratory derangements manifesting with chronic and progressive decline of renal functions. A systematic review of population-based studies worldwide reports a composite median prevalence of CKD in persons aged 30 years or older of 7.2%.[1] CKD causes considerable impact on morbidity and mortality. The impact of CKD is especially pronounced in developing countries like Sri Lanka, where resource limitations result in lack of access to essential dialysis facilities and renal transplant services.
Major etiological associations of CKD include diabetes mellitus and hypertension. The etiological profile of CKD varies with regard to geographical territories in Sri Lanka. Epidemiological studies based on patients with CKD from the Western provinces of the country report a more conventional epidemiological profile.[2,3] In contrast, a high prevalence of CKD is reported from the North Central province of the country among patients without the aforementioned traditional risk factors.[4] This province is an area of around 10,000 km2 in the dry zone of the country and comprises mostly of farming communities. The unusual characteristics of CKD in these patients led to a definition of CKD of unknown etiology (CKDu). A multifactorial etiology is proposed for this disease. Chronic agrochemical exposure,[5] Ochratoxin A,[6] fluoride (in conjunction with sodium and calcium),[7] and heavy metals such as cadmium[8] are possible etiological agents.
Histopathological studies have reported that interstitial fibrosis and tubular atrophy with or without nonspecific interstitial mononuclear cell infiltration are the dominant features in patients with CKDu. Glomerular sclerosis, glomerular collapse, and features of vascular pathology such as fibrous intimal thickening and arteriolar hyalinosis are also common.[9] It has also been determined that tubular damage is an early pathophysiological mechanism, as evidenced by the increased excretion of the tubular markers alpha1-microglobulin and N-acetyl-beta-D: Glucosaminidase in the urine of patients with CKDu.[10]
There is paucity of data on the associations of the histopathological parameters and the epidemiological, clinical, and laboratory determinants of CKDu. It is in this background that this study is positioned and aimed to create a synergistic clinico-pathological model of CKDu which could serve as the basis for prognostication and guidance of the management. Pathological correlations with laboratory data could be further utilized for the design of screening tools targeted toward an early diagnosis. In addition, this model can be utilized toward identifying potential therapeutic targets for the disease.
Subjects and Methods
This study recruited the patients presenting to the Teaching Hospital Anuradhapura with a diagnosis of CKDu, from 2008 to 2012 at the Teaching Hospital Anuradhapura, Sri Lanka. This hospital is the final Tertiary Care Referral Unit for patients with renal disease originating from the North Central Province of Sri Lanka. The majority of the reported cases of CKDu in Sri Lanka originate from this province. This sample is, therefore, more representative of the population characteristics of CKDu in Sri Lanka. CKDu was defined according to the criteria developed by the Scientific Committee of the National Research Programme for CKDu launched by World Health Organization together with the Ministry of Healthcare and Nutrition Sri Lanka.[11] The following patients were screened for inclusion: Patients with CKD with no evidence of diabetes mellitus, chronic or severe hypertension, snake bite, glomerulonephritis or urological diseases, together with normal HBA1C (<6.5%), and blood pressure <160/100 mmHg untreated or <140/90 mmHg on up to two antihypertensive medications. Patients who refused consent for biopsy or with compelling contraindications for biopsy namely, biopsy site infection, active renal or peri-renal infection, structural, and anatomical renal abnormalities of the kidney, cysts and renal masses and those with a solitary kidney, coagulopathy, and uncontrolled hypertension were excluded from the study.
Ultrasound guided renal biopsy was performed on all eligible consenting patients, by an independent operator blinded to patient details. The renal biopsy specimens were fixed in 10% formalin and embedded in paraffin. Sections were analyzed after hematoxylin and eosin, periodic acid–Schiff, periodic acid-Schiff silver methenamine, and Masson's trichrome staining. Immunofluorescence studies were performed in selected cases. Histological examinations were made independently by consultant pathologists. All biopsy specimens were assessed for adequacy and inadequate biopsies were repeated.
Concurrent investigations were also performed supplementary to clinical assessment based on the presentation and the biopsy findings to delineate etiology. These included fasting blood sugar and hemoglobin A1c to exclude diabetes, HIV serology, VDRL, urine full report, ESR, CRP, ultrasound abdomen with renal duplex computerized tomography. Hepatitis B and C serology, antinuclear factor, and autoimmune screen including complement C3 and C4 were performed where required to exclude an alternate cause for renal dysfunctionRenal functions were evaluated with serum creatinine, estimated glomerular filtration rate (eGFR) and urine microscopy, spot albumin, and 24 h protein excretion. Immunofluorescence studies were also performed to exclude immunoglobulin A (IgA) nephropathy and other glomerulonephritis in selected cases.
General sociodemographic, epidemiological, and clinical data were obtained. Concurrent laboratory data, including the serum creatinine, urine full report, and spot albumin measurement, were analyzed. Staging of patients was done using GFR following the National Kidney Foundation Kidney Disease Outcome Quality Initiative Guidelines (KDOQI).[12] The Cockcroft-Gault formula[13] was used to calculate eGFR for given values of serum creatinine.
Descriptive statistics were used for sociodemographic, epidemiological, and clinical data. The associations between pathological parameters and relevant sociodemographic and clinical data were examined using the Chi-square test. Nonparametric statistical analysis was performed when necessary. The level of significance was considered as 0.05. Logistic regression models were constructed for the major renal outcomes including serum creatinine, estimated GFR, and stage of CKD based on the standard KDOQI classification. Sociodemographic variables and histopathological data were included in the construction of the model. All variables which are noted to have a P < 0.2 on univariate analysis were considered for inclusion. Independent predictors were selected at a P < 0.05 and adjusted odds ratios (OR) extracted.
Results
Based on the aforementioned selection criteria, 125 patients were included in the study. A total of 150 patients were diagnosed with possible CKDu at this unit during the above study period. Eighteen individuals did not undergo renal biopsy as they did not consent for the procedure. Renal biopsy was abandoned due to technical constraints in four individuals and three biopsies demonstrated inadequate yield. Table 1 gives a description of the sociodemographic data of the study population. The mean age of the population was 46.21 years (standard deviation [SD] =11.64). The majority were ethnically Sinhalese. A marked male predominance was noted (73.6%), and clustering of the disease was noted in the age groups of 31–60 years.
Table 1.
Sociodemographic data of the study population
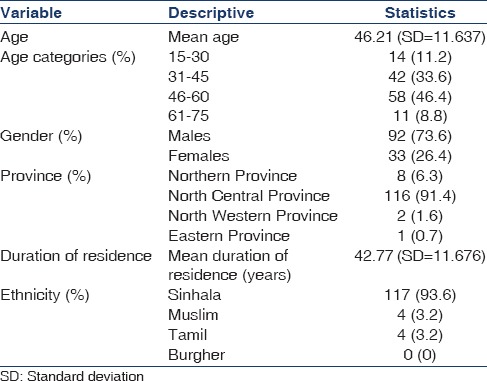
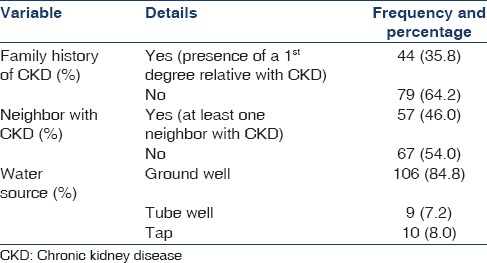
The province of origin in most cases was the North Central Province. A considerable percentage of patients (35.8%) had a positive family history of CKD (defined as presence of a 1st degree relative with CKD) and a neighbor (46%) with CKD [Table 2]. Water consumption was found to be mainly from ground wells (84.8%).
Table 2.
Epidemiological data
The renal parameters in the patients with CKDu are shown in Table 3. The mean serum creatinine was 1.9 (SD = 0.791) and mean eGFR was 43.3. The majority of the patients were classified as CKD stage 3 and 4 based on the eGFR. It is notable that, despite a diagnosis of CKDu, 25.6% of the patients had undetectable proteinuria and 28.8% of the patients had trace proteinuria as detected by a urinary dipstick. A significant amount of leukocytes were seen in urine in 19.2% of the patients, and significant red blood cells were demonstrable in 9.6% of the patients.
Table 3.
Renal parameters

Histopathological data
The renal biopsies of patients with CKDu demonstrated the following prominent abnormalities: (a) Glomerular sclerosis and glomerular collapse, (b) interstitial fibrosis, (c) tubular fibrosis, (d) tubular atrophy, (e) interstitial infiltration, (f) presence of casts, and (h) arteriolar hyalinosis. Prominent histopathological features were noted to be glomerular sclerosis (94.8%), interstitial infiltration (76%), interstitial fibrosis (71.2%), and tubular atrophy (70.4%). The presence of casts was also observed in 54.3%. Lymphocytes were noted to be the prominent infiltrating cell (97.9% of 95 cases). Low rates of periglomerular fibrosis were observed (16%). None of the patients included in the study had evidence of tubular fibrosis. Arteriolar hyalinosis characteristic of hypertensive nephropathy was also observed only in a limited number of cases. Mesangial hypercellularity was also noted in 10.4% of the biopsy specimens analyzed.
Table 4 presents the descriptive histopathology of the study cohort. Glomerular sclerosis >50%, tubular atrophy, interstitial fibrosis, and interstitial infiltrate were seen in 45.9%, 72.4%, 74.5%, and 72.4%, respectively of the patients with CKD stage <3 and in 51.3%, 75.2%, 75.2%, and 78.8%, respectively in patients with serum creatinine <1.2. This demonstrates that pathological changes are established in early stages of renal impairment and early stage CKD. In patients with dipstick proteinuria <+2, the above pathological abnormalities were seen in 31.3%, 75%, 78.1%, and 68.8% of the patients, respectively.
Table 4.
Descriptive histopathology
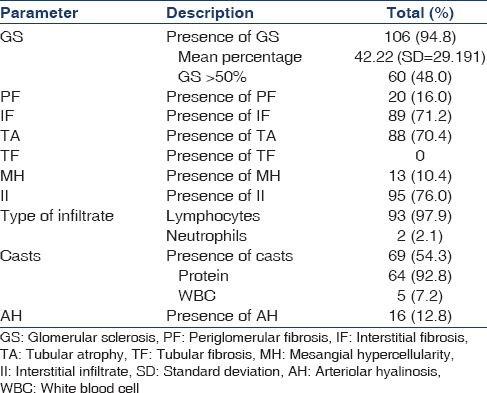
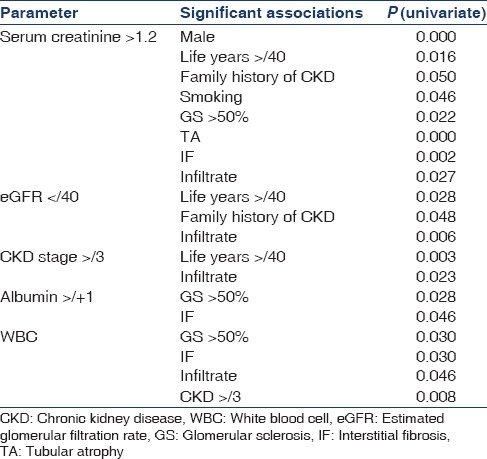
Table 5 presents the univariate associations with renal outcomes. Serum creatinine >1.2 was significantly associated with male gender, duration of residence in the North Central province >40 years, family history of CKD, and smoking. Clinicopathological association was noted with the presence of tubular atrophy, interstitial fibrosis, and interstitial infiltrate. However, upon consideration of renal function based on the eGFR, a value of <40 was significantly associated with residential life years >40, family history of CKD, and presence of interstitial infiltrate. Patients with CKD stage III or more had similar associations. Clinicopathological correlations were also noted with other renal parameters. Urine spot albumin >+2 was significantly associated with the presence of glomerular sclerosis and interstitial fibrosis while significant excretion of WBC was associated with glomerular sclerosis, interstitial infiltrate, and interstitial fibrosis. Immunofluorescence testing for IgA nephropathy was performed in 13/125 (10.4%) of all biopsy specimens. All examined specimens were negative for IgA deposition.
Table 5.
Univariate associations of renal outcomes
Logistic regression analysis for renal outcomes
Renal outcomes selected for logistic regression analysis were serum creatinine and CKD stage. Serum creatinine values were dichotomized based on the median due to the nonparametric nature of the distribution, and the cutoff was taken as 0.2. For this regression model, the independent variables selected were smoking, family history of CKD, glomerular sclerosis percentage, tubular atrophy, interstitial fibrosis, and interstitial infiltrate. For the model based on CKD stage, the independent variables selected were renal WBC excretion, tubulitis, interstitial fibrosis, interstitial infiltrate, and residential life years >40. Backward logistic regression methodology was utilized.
For CKD stage >3, the independent predictors noted were presence of interstitial fibrosis (P = 0.005; adjusted OR = 0.153 (95% CI = 0.041–0.572) and presence of interstitial infiltrate (P = 0.030; adjusted OR = 0.244 (95% CI = 0.067–0.892). The Nagelkerke R2 for the model was 0.412. Independent predictors of elevated serum creatinine (>1.2 mg/dl) were, presence of >50% glomerular sclerosis (P = 0.041; adjusted OR = 0.92 (95% CI = 0.009–0.910), tubular atrophy (P = 0.034; adjusted OR = 0.171 (95% CI = 0.0033–0.874) and more than >40 years of residence in the North Central province (P = 0.009 (OR = 9.229; 95% CI = 1.724–49.314). Nagelkerke R2 for this model was 0.563.
Discussion
The findings of this study reflect the features of CKDu as tubulointerstitial nephritis (TIN) with progressive lymphocytic infiltration with advancing stages of CKD. The selection criteria and definition of CKDu are more robust when compared to previous pathological studies, and the sample size is larger.[10,14]
CKDu in this group of patients had a male predominant distribution with the mean age noted to be 46 years. A similar male predominance has been identified in previous studies.[15] This is consistent with the postulation that CKDu occurs due to occupational exposure, since the majority of males in these areas of the country are engaged in agricultural activities with possible exposure to agrochemicals, pesticides, and contaminated soil and water exposure. However, other factors may also account for the marked gender predilection noted. A considerable percentage of patients had a positive family history of the disease. This is most likely to reflect common etiological exposure among members of the same family. However, the possibilities of a genetic basis for the disease should be investigated further. Similar genetic associations have been explored in CKD of unknown etiology within native tribes of New Mexico.[16] A clustering is noted in individuals aged 31-60 years. The relative sparing of younger age groups may reflect the exposure dependent nature of the disease.
The histopathological pattern observed among CKDu patients in this study is consistent with predominant TIN as evidenced by widespread tubular atrophy, interstitial mononuclear infiltrate, and prominent widespread interstitial fibrosis. Glomerular sclerosis as observed in the above series is also associated with chronic TIN and is probably a secondary process related to tubular atrophy and periglomerular fibrosis. This finding is consistent with previous histopathological characterizations of CKDu in Sri Lanka.[9] Several etiological agents have been associated with chronic TIN in other settings. These include pharmacological agents such as analgesics, metabolic disturbances such as hypercalcemia, autoimmune disease, exposure to toxins such as aristolochic acid as implicated in Balkan nephropathy and Chinese herbal nephropathy, heavy metal exposure (cadmium and lead) malignancies, obstructive uropathy, and vascular disease.
It is notable that significant renal pathological changes including glomerular sclerosis, tubular atrophy, interstitial fibrosis, and interstitial inflammation occur early in the disease. A similar trend is noted with dipstick proteinuria >2. Furthermore, more than 50% of glomerular sclerosis and tubular atrophy were found to be independently associated with serum creatinine at a cutoff of 1.2 by logistic regression analysis. By considering all of these findings, it is postulated that renal damage may not be associated with alteration in renal functions and other markers such as albuminuria in the initial stages. Patients in this study did not undergo microalbuminuria estimation that could have investigated lower cutoff values of proteinuria.
Early detection of CKDu is a challenge. As we found that since most renal pathology is established with mild elevation of serum creatinine and +2 albuminuria, it is imperative to explore other biomarkers of early CKDu, as conventional renal parameters may not be useful in the early detection of the disease.
Interstitial inflammation was associated with serum creatinine >1.2, CKD stage >3, eGFR <40, and significant WBC excretion in urine on univariate analysis. Furthermore, it was noted to be an independent association in patients with CKD >3 in logistic regression analysis. These findings indicate a pivotal role of interstitial inflammation in the progressive pathophysiology of CKDu. This is keeping with the findings of current research into the pathophysiology of CKD which demonstrates that the renal mass reduction resulting from the renal insult ultimately leads to infiltration and activation of immunocompetent cells and induction of mesangial proliferation and cytokine overproduction.[17] This ultimately induces scarring and fibrosis of glomeruli, tubules, and interstitium resulting in declining GFR. Therefore, in CKDu, it may be postulated that immunocompetent cells are the main driving force of the disease. The offending agents should be identified causing this inflammation. Future therapeutic approaches should target this inflammatory process.
More than 40 residential life years were found to be associated with adverse renal outcomes on univariate analysis with significance persisting on logistic regression for serum creatinine >1.2. This reflects the probable cumulative exposure effect to an environmental antigen which is possibly driving the disease. It further advocates screening and follow-up programs for these patients.
The clinicopathological correlations have implications for the early detection and prognostic modeling of CKDu. Novel markers should be investigated and the question should be raised whether high-risk individuals, that is, males between 30 and 60 with a strong family and epidemiological history with significant cumulative resident life years, should undergo renal biopsy as a screening procedure. However, such procedures should be further validated in experimental settings before being generalized to clinical practice.
A small number of biopsy specimens demonstrated mesangial expansion. Mesangial hypercellularity and increase in mesangial matrix are associated with chronic glomerulonephritides such as variants of minimal change disease,[18] focal segmental glomerulosclerosis,[19] and diabetic nephropathy.[20] A similar pattern has been observed in IgA nephropathy which occurs as a result of immune complex deposition and immune cellular infiltration.[21] The presence of immune complex was not detected with immunofluorescence. These findings may indicate a spectrum of glomerulonephritis with immune-based etiology in addition to TIN in CKDu. However, other histopathological features of chronic glomerulonephritis such as glomerular hyalinosis were not observed.
The reason for the poor correlation between eGFR and pathological features is unclear. Possible explanations are that the equation of calculation of the eGFR may be influenced by racial factors not previously assessed in the Sri Lankan population.
Correlational analysis for toxins and environmental exposures, including heavy metals and fertilizer residues in the above study cohort, is currently under evaluation as a follow-up to this study.
Conclusions
The findings of this study demonstrate that the predominant histopathological forms of CKDu are consistent with chronic TIN, and furthermore, that the disease is predominant among males, aged between 30 and 60 with a positive family history, and epidemiological history of CKDu. Conventional markers of renal function such as serum creatinine and eGFR are not sensitive for detection of the disease as most of the pathological changes are already established before a change in these markers are discernible. Interstitial infiltration may be the driving force behind the progression of renal disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijewickrama ES, Weerasinghe D, Sumathipala PS, Horadagoda C, Lanarolle RD, Sheriff RM. Epidemiology of chronic kidney disease in a Sri Lankan population: Experience of a tertiary care center. Saudi J Kidney Dis Transpl. 2011;22:1289–93. [PubMed] [Google Scholar]
- 3.Gooneratne IK, Ranaweera AK, Liyanarachchi NP, Gunawardane N, Lanerolle RD. Epidemiology of chronic kidney disease in a Sri Lankan population. Int J Diabetes Dev Ctries. 2008;28:60–4. doi: 10.4103/0973-3930.43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrajith R, Nanayakkara S, Itai K, Aturaliya TN, Dissanayake CB, Abeysekera T, et al. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environ Geochem Health. 2011;33:267–78. doi: 10.1007/s10653-010-9339-1. [DOI] [PubMed] [Google Scholar]
- 5.Peiris-John RJ, Wanigasuriya JK, Wickremasinghe AR, Dissanayake WP, Hittarage A. Exposure to acetylcholinesterase-inhibiting pesticides and chronic renal failure. Ceylon Med J. 2006;51:42–3. doi: 10.4038/cmj.v51i1.1382. [DOI] [PubMed] [Google Scholar]
- 6.Wanigasuriya KP, Peiris H, Ileperuma N, Peiris-John RJ, Wickremasinghe R. Could ochratoxin A in food commodities be the cause of chronic kidney disease in Sri Lanka? Trans R Soc Trop Med Hyg. 2008;102:726–8. doi: 10.1016/j.trstmh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Chandrajith R, Dissanayake CB, Ariyarathna T, Herath HM, Padmasiri JP. Dose-dependent Na and Ca in fluoride-rich drinking water – Another major cause of chronic renal failure in tropical arid regions. Sci Total Environ. 2011;409:671–5. doi: 10.1016/j.scitotenv.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 8.Wanigasuriya KP, Peiris-John RJ, Wickremasinghe R. Chronic kidney disease of unknown aetiology in Sri Lanka: Is cadmium a likely cause? BMC Nephrol. 2011;12:32. doi: 10.1186/1471-2369-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanayakkara S, Komiya T, Ratnatunga N, Senevirathna ST, Harada KH, Hitomi T, et al. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ Health Prev Med. 2012;17:213–21. doi: 10.1007/s12199-011-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanayakkara S, Senevirathna ST, Karunaratne U, Chandrajith R, Harada KH, Hitomi T, et al. Evidence of tubular damage in the very early stage of chronic kidney disease of uncertain etiology in the North Central Province of Sri Lanka: A cross-sectional study. Environ Health Prev Med. 2012;17:109–17. doi: 10.1007/s12199-011-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Research Programme for Chronic Kidney Disease of Unknown Aetiology in Sri Lanka. [Last accessed on 2015 May 19]. Available from: http://www.epid.gov.lk/web/pdf/wer_2009/vol_36_no_49_english.pdf .
- 12.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Athuraliya NT, Abeysekera TD, Amerasinghe PH, Kumarasiri R, Bandara P, Karunaratne U, et al. Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 2011;80:1212–21. doi: 10.1038/ki.2011.258. [DOI] [PubMed] [Google Scholar]
- 15.Senevirathna L, Abeysekera T, Nanayakkara S, Chandrajith R, Ratnatunga N, Harada KH, et al. Risk factors associated with disease progression and mortality in chronic kidney disease of uncertain etiology: A cohort study in Medawachchiya, Sri Lanka. Environ Health Prev Med. 2012;17:191–8. doi: 10.1007/s12199-011-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoy WE, Megill DM, Hughson MD. Epidemic renal disease of unknown etiology in the Zuni Indians. Am J Kidney Dis. 1987;9:485–96. doi: 10.1016/s0272-6386(87)80075-6. [DOI] [PubMed] [Google Scholar]
- 17.López-Novoa JM, Martínez-Salgado C, Rodríguez-Peña AB, López-Hernández FJ. Common pathophysiological mechanisms of chronic kidney disease: Therapeutic perspectives. Pharmacol Ther. 2010;128:61–81. doi: 10.1016/j.pharmthera.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Allen WR, Travis LB, Cavallo T, Brouhard BH, Cunningham RJ., 3rd Immune deposits and mesangial hypercellularity in minimal change nephrotic syndrome: Clinical relevance. J Pediatr. 1982;100:188–91. doi: 10.1016/s0022-3476(82)80632-x. [DOI] [PubMed] [Google Scholar]
- 19.Schoeneman MJ, Bennett B, Greifer I. The natural history of focal segmental glomerulosclerosis with and without mesangial hypercellularity in children. Clin Nephrol. 1978;9:45–54. [PubMed] [Google Scholar]
- 20.Sterzel RB, Schulze-Lohoff E, Marx M. Cytokines and mesangial cells. Kidney Int Suppl. 1993;39:S26–31. [PubMed] [Google Scholar]
- 21.Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, et al. IgA nephropathy: Molecular mechanisms of the disease. Annu Rev Pathol. 2013;8:217–40. doi: 10.1146/annurev-pathol-011110-130216. [DOI] [PubMed] [Google Scholar]


