Abstract
Optimal therapy and prognosis of crescentic-IgA nephropathy (C-IgAN) are not known. Reported treatment options for C-IgAN include combination of corticosteroids and cyclophosphamide for 6 months. The role of mycophenolate sodium in C-IgAN is unknown. We report a case of C-IgAN that was successfully treated with combination immunosuppressive therapy.
Key words: Acute kidney injury, crescentic nephropathy, glomerular diseases, IgA nephropathy, mycophenolate sodium
Introduction
Rapidly progressive glomerulonephritis (RPGN) is a rare clinical syndrome that is characterized by signs of glomerulonephritis (dysmorphic red blood cells [RBCs], proteinuria), rapid and progressive deterioration in renal function and is associated with the extensive crescent formation surrounding ≥50% of glomeruli on kidney biopsy. RPGN accounts for only 2–4% of all glomerulonephritis. RPGN can be classified as primary (i.e., in absence of underlying preexisting glomerular disease, e.g., anti-glomerular basement membrane (GBM) antibody disease, renal limited vasculitis) or secondary to systemic disease (e.g., postinfectious, Goodpasture's syndrome, microscopic polyangiitis, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, systemic lupus erythematosus) or could be superimposed on underlying preexisting glomerular disease (e.g., crescentic-IgA nephropathy [C-IgAN]).
C-IgAN commonly present as RPGN. While acute kidney injury (AKI) may be the initial presentation in C-IgAN, it can rarely present as nephrotic syndrome mainly associated with advanced glomerulosclerosis. The optimal therapy and precise prognosis of C-IgAN are not known. The treatment of C-IgAN has not been studied in the randomized clinical trial. Observational clinical studies have showed some clinical improvement with the combination of corticosteroids, cyclophosphamide and plasma exchange for variable period of time. A recent study[1] of patients with C-IgAN found that nearly 70% of the patients progressed to end-stage renal disease (ESRD) in 5 years despite receiving immunosuppressive therapy. A randomized controlled trial of mycophenolate mofetil (MMF) treatment in severe IgA nephropathy by Chen et al.[2] showed that the use of mycophenolate is associated with decrease in proteinuria within 3 months. However, there is no study to evaluate the role of mycophenolate sodium in C-IgAN. We report a case of C-IgAN that was successfully treated with combination immunosuppressive therapy including mycophenolate with long-term follow-up.
Case Report
A 54-year-old male presented to his primary doctor with runny nose, fever, chills, and facial swelling. The patient was started on oral moxifloxacin for 10 days with no improvement. Past medical history was significant for hypertension, gout, nephrolithiasis, and hyperlipidemia. He denied any past medical history of diabetes mellitus, hepatitis, or blood transfusion. Eleven days after the initial presentation, labs revealed AKI with elevated serum creatinine of 3.8 mg/dl, microscopic hematuria, and massive proteinuria (18 g/day). Review of the system was negative for nausea, vomiting, diarrhea, dyspnea, gross hematuria, arthralgia, or rash. He denied the use of nonsteroidal anti-inflammatory drugs and herbal medication. There was no history of intravenous (IV) drug use.
On physical examination, blood pressure was elevated at 197/101 mmHg, and there was pitting edema of bilateral lower extremity. The rest of the physical examination was unremarkable. At the time of initial presentation, patient had serum creatinine of 3.0 mg/dl, serum albumin 2.6 g/dl, total protein 5.1 g/dl, total cholesterol was 271 mg/dl and low-density lipoprotein level was 198 mg/dl. Complete blood test and liver function test were unremarkable. Urinalysis showed urine protein of 500 and RBC 25-50. A 24-h urine collection revealed 18 g of proteinuria. Further laboratory investigations for secondary causes of glomerulonephritis revealed normal level of complements C3 and C4. Antinuclear antibody, cryoglobulin level, hepatitis B, hepatitis C, P-anti-neutrophil cytoplasmic antibody (ANCA), C-ANCA, and anti-GBM Ab were all negative. Sonogram of the kidneys revealed normal sized kidneys with echogenic parenchyma. The patient subsequently underwent ultrasound-guided kidney biopsy.
Kidney biopsy findings
On renal biopsy, 39 glomeruli were identified. One glomerulus was globally sclerosed. Over half showed cellular crescent and 11 glomeruli demonstrated endocapillary hypercellularity [Figure 1]. There were mild patchy interstitial fibrosis and tubular atrophy. Immunofluorescence showed granular staining in glomerular mesangial areas for IgA and C3 [Figure 2]. IF was negative for IgG, IgM, C1q, fibrinogen and albumin. Electron microscopy revealed electron-dense deposits in the mesangium and extensive foot processes effacement. The final biopsy diagnosis was consistent with C-IgAN.
Figure 1.
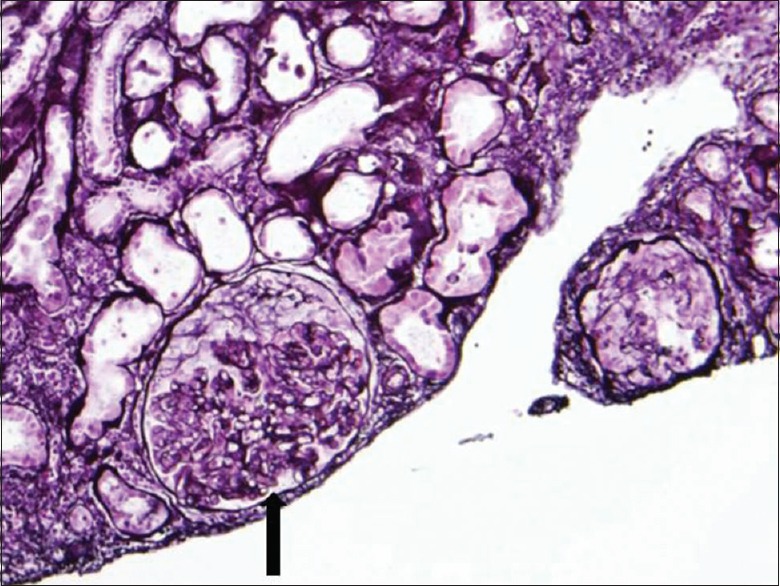
Light microscopic examination of kidney biopsy specimen revealed mesangial hypercellularity and crescent formation (arrow) in majority of glomeruli
Figure 2.
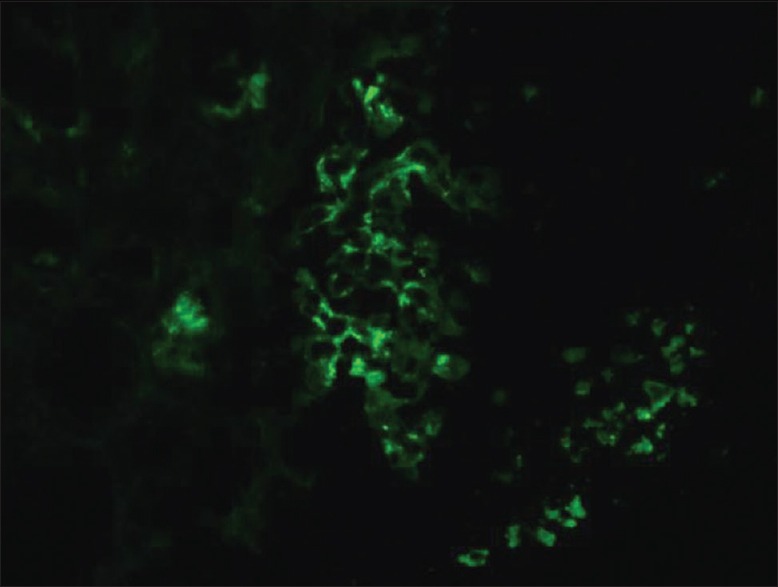
Immunofluorescence showed granular staining in the mesangium for IgA
Clinical course
The patient was initially started on IV pulse corticosteroid for 3 days, followed by oral corticosteroid therapy and monthly IV cyclophosphamide for 6 months. Serum creatinine subsequently improved to 2.2 mg/dl. In addition, the patient was initiated on an angiotensin receptor blocker. After 6 months of (the above) therapy, 24-h urine protein decreased to 2 g and serum creatinine improved to 1.6 mg/dl. The patient was subsequently started on oral mycophenolate sodium 360 mg twice daily for six additional months as maintenance therapy. He was gradually tapered off oral corticosteroid therapy. At follow-up after 4 years of initial presentation, patient maintains normal renal function and has no proteinuria.
Discussion
IgA nephropathy is one of the most common glomerular diseases worldwide. Its presentation varies from asymptomatic hematuria to progressive renal failure requiring renal replacement therapy. On histopathology, the presence of mesangial hypercellularity, endocapillary hypercellularity, glomerulosclerosis, tubular atrophy and interstitial fibrosis is associated with poor prognosis.[3] IgA nephropathy patients may have extracapillary proliferation which is characterized by noncircumferential crescent formation. Bitencourt-Dias et al. studied 144 pts with IgA nephropathy retrospectively.[4] They found that 18% of the patients had crescents on kidney biopsy. The presence of crescents was associated with hypertension, proteinuria, increased initial serum creatinine level and progression to ESRD.[4] In a multicenter study, Lv et al.[1] concluded that C-IgAN had a poor prognosis and initial serum creatinine level on presentation may predict kidney failure in patients with this disease.
C-IgAN has poor renal prognosis without therapy. The 2012 Kidney Disease Improving Global Outcomes guidelines suggested the use the steroid and cyclophosphamide in patients with IgA nephropathy and rapidly progressive C-IgAN, which is similar to the treatment of ANCA vasculitis.[5] However, there are no randomized controlled trials for the treatment of C-IgAN.
In an observational retrospective study by Roccatello et al.,[6] intensive combination therapy with pulse steroid, cyclophosphamide and plasma exchange for 2 months showed a substantial delay in the onset of dialysis. While, the intensive treatment seemed sufficient to delay the progression of disease, it was inadequate to reverse the crescent formation and chronic changes. Tumlin et al.[7] treated 12 patients with crescentic, proliferative IgA nephropathy with pulse steroid and IV cyclophosphamide for 6 months. After 6 months of treatment, they found a significant decrease in serum creatinine level and proteinuria. On repeat biopsy after the treatment, they found that the endocapillary proliferation, cellular crescents, and karyorrhexis were eliminated. One of 12 patients progressed to ESRD after 36 months of follow-up.
Chen et al.[2] conducted a randomized control trial of MMF treatment in severe IgA nephropathy. They concluded that mycophenolate was more effective in reducing proteinuria and correcting serum lipid level, especially in patients with severe IgA nephropathy and proteinuria >2 g/day. However, there is no clinical study looking at the use of mycophenolate in the initial treatment of C-IgAN or as maintenance therapy.
Our patient presented with nephrotic syndrome and AKI. Kidney biopsy was consistent with C-IgAN. A combination of corticosteroid and cyclophosphamide-based induction therapy for 6 months followed by mycophenolate-based maintenance therapy resulted in complete remission of nephrotic syndrome and AKI in our patient. Our patient continues to be in complete clinical remission with normal renal function, 4 years after completing combined immunosuppressive therapy. Based on our experience, one can consider mycophenolate sodium for maintenance therapy in C-IgAN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We like to thank Dr. Xiaotong Wang (pathologist) for providing us with the renal pathology results and pictures. This case was presented as a poster at the American Society of Nephrology Kidney Week 2013 in Atlanta, GA in November 2013.
References
- 1.Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol. 2013;24:2118–25. doi: 10.1681/ASN.2012101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang Y, et al. A randomized control trial of mycophenolate mofeil treatment in severe IgA nephropathy. Zhonghua Yi Xue Za Zhi. 2002;82:796–801. [PubMed] [Google Scholar]
- 3.Lee MJ, Kim SJ, Oh HJ, Ko KI, Koo HM, Kim CH, et al. Clinical implication of crescentic lesions in immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29:356–64. doi: 10.1093/ndt/gft398. [DOI] [PubMed] [Google Scholar]
- 4.Bitencourt-Dias C, Bahiense-Oliveira M, Saldanha LB, Barros RT, Woronik V. Comparative study of IgA nephropathy with and without crescents. Braz J Med Biol Res. 2004;37:1373–7. doi: 10.1590/s0100-879x2004000900012. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 6.Roccatello D, Ferro M, Coppo R, Giraudo G, Quattrocchio G, Piccoli G. Report on intensive treatment of extracapillary glomerulonephritis with focus on crescentic IgA nephropathy. Nephrol Dial Transplant. 1995;10:2054–9. [PubMed] [Google Scholar]
- 7.Tumlin JA, Lohavichan V, Hennigar R. Crescentic, proliferative IgA nephropathy: Clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant. 2003;18:1321–9. doi: 10.1093/ndt/gfg081. [DOI] [PubMed] [Google Scholar]


