Abstract
Neuromyelitis optica (NMO) and spectrum disorder (NMO/SD) represent a vexing process and its clinical variants appear to have at their pathogenic core the loss of immune tolerance to the aquaporin-4 water channel protein. This process results in a characteristic pattern of astrocyte dysfunction, loss, and demyelination that predominantly affects the spinal cord and optic nerves. Although several empirical therapies are currently used in the treatment of NMO/SD, none has been proven effective in prospective, adequately powered, randomized trials. Furthermore, most of the current therapies subject patients to long-term immunologic suppression that can cause serious infections and development of cancers. The following is the first of a 2-part description of several key immune mechanisms in NMO/SD that might be amenable to therapeutic restoration of immune tolerance. It is intended to provide a roadmap for how potential immune tolerance restorative techniques might be applied to patients with NMO/SD. This initial installment provides a background rationale underlying attempts at immune tolerization. It provides specific examples of innovative approaches that have emerged recently as a consequence of technical advances. In several autoimmune diseases, these strategies have been reduced to practice. Therefore, in theory, the identification of aquaporin-4 as the dominant autoantigen makes NMO/SD an ideal candidate for the development of tolerizing therapies or cures for this increasingly recognized disease.
RATIONALE FOR TOLERIZATION IN NMO
Neuromyelitis optica (NMO) or NMO spectrum disorder (NMO/SD) represents a potentially devastating neuroinflammatory disease in which the spinal cord and optic nerve(s) preferentially undergo astrocyte damage and demyelination.1 Recent investigations into its pathogenesis have demonstrated NMO/SD to be distinct from multiple sclerosis (MS) (figure 1).2 Identification of pathogenic anti-aquaporin 4 (anti-AQP4) antibody (NMO–immunoglobulin G [IgG])3 in context of other clinical characteristics now secures a diagnosis in seropositive disease.
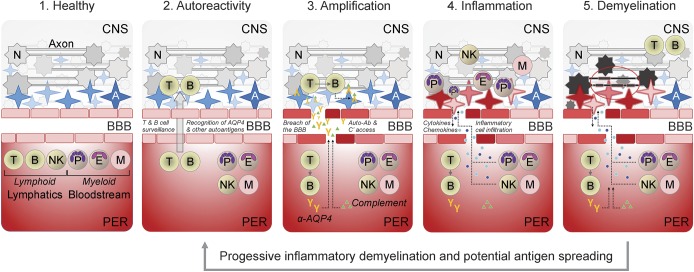
Figure 1. Integrative model of NMO/SD pathogenesis.
Distinct stages of disease are illustrated as relative temporal and spatial comparators in NMO/SD progression. Note that over the course of disease progression and demyelination, additional autoantigens may be revealed and/or recognized, potentially resulting in antigen spreading and expansion/compounding of autoreactivity. A = astrocyte (AQP4-expressing cells that support neuronal viability and function); AQP4 = aquaporin 4; B = B lymphocyte; BBB = blood-brain barrier; E = eosinophil; M = monocyte/macrophage; N = neuron; NK = natural killer cell; NMO/SD = neuromyelitis optica/spectrum disorder; P = polymorphonuclear leukocyte (neutrophil); PER = periphery; T = T lymphocyte.
Despite substantial advances in understanding NMO/SD, no existing regimen has been unambiguously proven to modify disease course, induce permanent remission, or restore lost neurologic function in adequately powered, prospective clinical trials. Continuing reliance on nonspecific agents, including corticosteroids, azathioprine, mycophenolate, and B cell depletion4,5 exposes patients to potentially serious adverse effects. Absence of animal models with complete fidelity to human NMO/SD has been a historical impediment to novel therapies.
Improved diagnosis has facilitated greater focus on developing specific therapies. Antigen-specific approaches could restore immune tolerance to AQP4 and minimize epitope spreading. In NMO/SD, dysfunctions in checkpoints governing immune cell development contribute to autoreactive immunity (figure 2). Considerable progress has been made in tolerizing strategies used to treat allergies,6 transplant and graft rejection,7 and drug hypersensitivity.8 In theory, antigen-targeted approaches spare normal immune functions. To date, similar strategies have yielded only limited success in other autoimmune diseases, such as type 1 diabetes (T1D),9 relapsing-remitting MS,10 and inflammatory bowel disease.11 Results thus far support the general concept that inhibition of antigen-specific T cell and/or B cell responses is achievable and may be durable. However, in some experimental models, tolerization strategies have worsened disease,12 perhaps because of uncertain disease etiology. Hence, in the specific context of NMO/SD, tolerization might have distinct advantages.
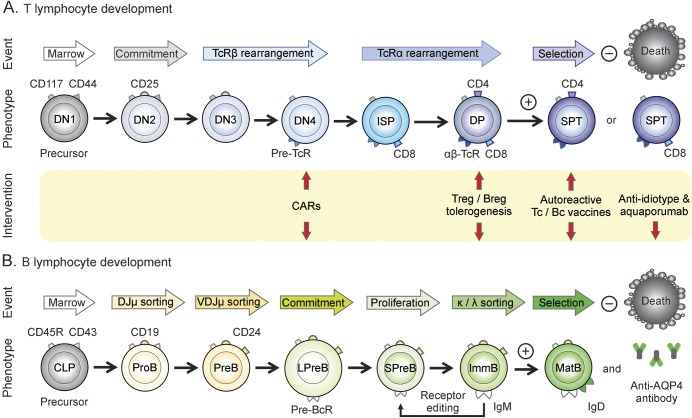
Figure 2. Comparative milestones in T and B lymphocyte maturation.
(A) T cell development. (B) B cell development. Each lineage is represented with distinguishing markers for corresponding stages, and where cell commitment occurs. Arrows indicate potential points along the development pathway where immune tolerizing interventions might be targeted in neuromyelitis optica/spectrum disorder. CAR = chimeric antigen receptor; CD = class designation marker; CLP = common lymphoid precursor; DN = double-negative thymocyte; DP = double-positive CD4+/CD8+ thymocyte; Ig = immunoglobulin; ImmB = immature B cell; ISP = immature single-positive CD8+ thymocyte; LPreB = large PreB; MatB = mature B cell; PreB = pre-B cell receptor B cell; ProB = progenitor B cell; SP = single positive CD4+ or CD8+ thymocyte; SPreB = small PreB; TcR = T cell receptor; Treg = regulatory T cell.
The Guthy-Jackson Charitable Foundation convened a meeting of experts in development of antigen-specific therapy. Key goals of this meeting were to ascertain the state-of-the-art in immune tolerization as it might be applied to NMO/SD and to catalyze innovative thinking. The following discussion considers specific opportunities and challenges to apply tolerization therapy to solve NMO/SD.
POTENTIAL STRATEGIES FOR TOLERIZATION IN NMO
Inverse DNA vaccination.
Dolgin13 and Steinman14 have built on principles of classic desensitization originating with Jenner. While traditional vaccines induce immune responses to specific antigens, inverse vaccines promote immune tolerance. Diseases in which a known antigen underlies the pathophysiology would in theory represent the most promising candidates for tolerizing therapies. Thus, seropositive NMO/SD should be ideal for antigen-specific therapy, as autoimmune responses to AQP4 are believed to be fundamental to the disease.15–17 In clinical trials, pathogenic vs tolerogenic immune responses of T and B cells would necessarily be monitored. Other diseases with similarly implicated autoantigens include myasthenia gravis (acetylcholine receptor), Graves disease (thyrotropin receptor and thyroglobulin), and T1D (multiple insulin granule antigens).18
Inverse vaccination with DNA encoding proinsulin to reinforce tolerogenic immune checkpoints has been evaluated in patients in an early-phase clinical trial in T1D.18,19 The results suggest that immune tolerance to proinsulin can be induced (p < 0.026). The study revealed an inverse relationship between antigen-restricted CD8+ T cells and serum proinsulin levels.18 A similar strategy has enabled proof-of-principle of altered responses to myelin basic protein in patients with MS.20,21 Although such approaches are attractive, relevant antigenic targets likely differ among patients and may change over time. For example, myelin oligodendrocyte glycoprotein may drive unique pathogenesis in NMO/SD and require specific tolerization strategies. Moreover, epitopes may differ in peptides as compared to parent proteins, and lipid or carbohydrate modification may influence presentation.
Inverse DNA vaccination targeting tolerization against AQP4 immunoreactivity has also been examined in experimental models of NMO/SD. A plasmid encoding AQP4 attenuated T and B cell responses specifically to this antigen, attesting to antigen specificity of this strategy (unpublished observations). In NMO/SD, clinical trials may be designed based on one or more diagnostic biomarker(s) of NMO-IgG seropositivity. In this approach, a diagnostic biomarker(s) is used as a surrogate of therapeutic efficacy and durability and serves as an outcome measure. This scenario approaches an idealized situation in which biomarkers are both the principal diagnostic gating targets and the major pathogenic factor(s).
Antiautoreactive T cell vaccines.
Roles played by autoreactive T cells in mediating autoimmunity have been established in several animal models, and complemented observations made in human disease.22 In turn, therapeutic modulation of autoreactive T cell frequency and/or function in autoimmune diseases appears achievable.23 However, regulation of all immune responses results from the “checks and balances” imposed by both effector and regulatory T cell networks (figure 3). Thus, nonspecific depletion of effector T cells could diminish immune surveillance, leading to infections or cancer.24 Similarly, indiscriminate activation or depletion of regulatory T cells (Tregs) may also result in immune dysfunction. In particular, Tregs are capable of limiting the frequency and/or activity of individual autoreactive T cell clones through recognition of their unique T cell receptor (TcR) idiotypes.25 When T cells aberrantly recognize self-antigens in an absence of adequate regulatory counterbalance, autoimmune disease might result.26 In NMO/SD, the pathogenic antibody response is dominated by class-switched IgG, indicating an underlying AQP4-reactive T cell activity.27,28 In addition, Th17 polarization of naive T cells leads to the secretion of proinflammatory cytokines in relation to target lesions, including interleukin (IL)-17A and granulocyte macrophage–colony stimulating factor.29 Such a proinflammatory cytokine profile may promote tissue destruction through recruitment of granulocytes or other cells capable of antibody-dependent cell cytotoxicity.
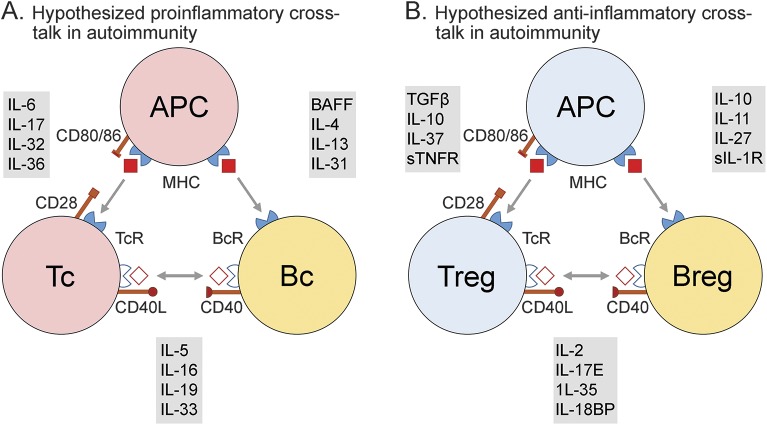
Figure 3. Hypothesized cellular and molecular crosstalk influencing NMO/SD.
(A) Theoretical proinflammatory interactions that arise from loss of immune tolerance, which may promote the onset or progression of neuromyelitis optica/spectrum disorder (NMO/SD). (B) Theoretical anti-inflammatory interactions that arise and could be amenable to therapeutic intervention in restoring immune tolerance in NMO/SD. (Cellular) APC = antigen-presenting cell; Bc = B cell; Tc = T cell; (cell surface determinants) CD = class designation; MHC = major histocompatibility complex; (soluble) BAFF = B cell activating factor; IL = interleukin; IL-18BP = IL-18 binding protein; sIL-1R = soluble IL-1 receptor; sTNFR = soluble tumor necrosis factor α receptor.
The observation that anti-idiotypic Tregs can arise spontaneously and be associated with the resolution of autoimmunity led to the concept of T cell vaccination.30,31 This strategy invokes multiple immune functions that may mitigate autoimmune disease. For example, induction of idiotype-restricted CD4+/CD25+/FoxP3+ Tregs, IL-10–secreting CD4+ Tr1 cells, and CD8+ cytotoxic T cells can result, each capable of selectively targeting autoreactive T cells that express the idiotype represented by the immunizing T cells.32
T cell–based vaccines typically use attenuated autologous autoreactive effector T cells as immunogens32 in an amplification strategy designed to target and counteract autoreactive T cells. Theoretically, this process should selectively deplete and/or functionally attenuate pathogenic T cells carrying the corresponding idiotype. Restoring an adequate Treg influence theoretically should mitigate tissue dysfunction. Clinical trials have suggested a therapeutic benefit from T cell vaccination, with minimal side effects.33 In this regard, proof-of-principle has been demonstrated in several animal models.31 In addition, therapeutic trials applying this strategy to MS, rheumatoid arthritis, and systemic lupus erythematosus–associated nephritis have been undertaken. In some of these conditions, a T cell–based vaccine approach has demonstrated considerable promise.34–36
The manufacture of autologous T cell vaccines is dependent first on identifying autoantigen-specific T cells within the peripheral blood of that patient. This goal is achieved by performing an epitope profiling assay, using overlapping peptides comprising the amino acid sequence of the target antigen. The assay readout is based on the detection of proinflammatory cytokines induced by immunodominant peptides within the target antigen. This strategy thereby identifies peptides and the associated T cell responses most relevant to the autoimmune disease in an affected individual. Next, bulk T cell lines are expanded in vitro from an autologous peripheral blood sample against the selected peptides to provide sufficient numbers of autoreactive T cells to meet dosing requirements. These particular T cells are aliquoted and cryopreserved. On demand, a dose-equivalent is thawed, formulated, and attenuated by irradiation before subcutaneous administration. Methods currently in use allow a single-cycle manufacturing process from one blood collection to be used to generate sufficient drug for at least 1 year of treatment.
Considering the aggregate evidence, a compelling case can be made for the critical role of T cells in the pathogenesis of NMO/SD.29 It follows that reestablishment of immune tolerance to AQP4 through vaccination with autologous, attenuated, and idiotype-restricted T cells represents a promising new approach to immunotherapy in this disease.
Dendritic cell vaccines.
Dendritic cells (DCs) present antigens to immune cells, including those with regulatory and autoreactive function, through major histocompatibility complex (MHC) class I and II restriction. Normally, DCs present self-antigens but do not drive autoreactive immune responses. Thus, autoreactive T cells are inactivated or anergized because of low MHC expression, inadequate costimulation (e.g., inducing anergy), or tolerogenic costimulation (e.g., PD-L1 [programed death ligand 1], IL-10, etc). In addition, DCs have important roles in maintaining tolerance by promoting regulatory cells, such as CD4+ and CD8+ Tregs, and IL-10–producing regulatory B cells.37 Immature human DCs are readily generated in the laboratory from blood monocytes and can be engineered to maintain tolerogenic phenotypes. Indeed, DC-based therapies have now entered clinical trials focusing on NMO/SD (ClinicalTrials.gov identifier: NCT02283671).
As a strategy for inducing immune tolerance in T1D, DC administration has proven relatively safe.38–42 Here, granulocyte macrophage–colony stimulating factor/IL-4 was used to generate immature CD11c+ DCs from monocytes in vitro. Transition to a proinflammatory DC phenotype is concerning when DCs are administered to human patients. To mitigate this concern, immature DCs can be treated in vitro with immunosuppressive agents such as corticosteroids, vitamin D3, and/or IL-1043 before their use as tolerizing vaccines. The resulting DCs exhibit durable tolerogenic phenotypes that prolong organ allograft survival in mice. Notably, this conditioning enhances the tolerogenic properties of DCs44 and induces high-level IL-10 production, which inhibit activated Th1 and Th17 cells, induce Tregs, and suppress autoreactive B cell functions. A related strategy involves loading of immature DCs with antisense oligonucleotides targeting key costimulatory molecules such as CD40, CD80, or CD86. Alternatively, viral vectors encoding immunoregulatory factors may potentiate tolerogenic DCs. Of note, the maintenance of DC immaturity also allows these cells to migrate into lymph nodes following antigen exposure, enhancing their tolerogenic potential.
Loading immature DCs with either native or synthetic peptides or whole antigens may also promote antigen-specific tolerance through induction of regulatory lymphocytes. This strategy addresses multiple autoantigens and/or epitopes within a target antigen(s). Thus, the goal is conditional presentation of self-antigenic epitopes by DCs to yield Treg induction. Using the entire antigen affords presentation of a broader repertoire of peptides selected by relevant MHC alleles, and potentially avoids artificial epitope(s) selection. In this respect, preloading microparticles containing antigen might facilitate DC uptake and favor tolerogenic phenotypes. Of note, early studies in which rodents were vaccinated with autoantigen-loaded DCs demonstrated antigen-specific, durable, and effective tolerance. These principles have been applied in human trials of T1D.42 Advantages may also emerge from strategies in which DCs are allowed to antigen-load in situ.
Small phase I clinical trials exploring DC vaccines in autoimmune disease have largely focused on T1D or rheumatoid arthritis. Evidence to date suggests that tolerizing DC therapies are safe. However, larger trials will be required to more thoroughly assess safety and efficacy. Despite the great interest in further assessing DC vaccines in the prevention, modulation, and curing of autoimmune diseases, key questions remain. These include optimization of DC dosing, vaccination frequency (e.g., boosting), route of administration, and identification or validation of biomarkers for assessing therapeutic efficacy.
NMO/SD appears to represent an ideal disease for DC therapy because a dominant and T cell–restricted autoantigen (AQP4) has been identified. If successful, this approach should be used as early in the disease course as is possible. The rationale for this strategy is to prevent or ameliorate the expansion of inflammatory AQP4-restricted or other antigen-restricted T lymphocytes, thereby minimizing broader tissue injury. In addition, such early intervention could in concept minimize the potential for antigen spreading or reversal of antigenic ignorance.
Antigen-coupled presentation.
Antigen-specific tolerization in animal models of disease has been pursued by a number of laboratory groups. Among these models are those for experimental autoimmune encephalomyelitis (EAE), Theiler virus–induced encephalitis, tolerance to organ transplantation, and therapy for severe allergy.6,45–50 Use of peptide-coupled or chemically fixed antigen-presenting cells (APCs) by Miller et al.6 has been one strategy. Here, unmodified splenocytes are pulsed in vitro with a disease-relevant autoantigen(s), together with a coupling agent. The host is immunized using these cells, with the goal of inducing tolerance to a target autoantigen(s). This approach was effective in reducing disease activity in a model of relapsing-remitting EAE in SJL mice.45,47 The strategy may also be attractive as prophylaxis for allotransplantation and the attenuation of alloreactive immune responses.51 Key advantages of this approach may include: (1) extensive experience in EAE and other autoimmune disease models; (2) realistic potential for targeting multiple autoantigens; (3) acceptable safety profiles in preclinical studies; (4) an apparent absence of epitope spreading; and (5) positive preliminary evidence for efficacy.45 These attributes provide a strong rationale for applying this approach to individuals with antigenically characterized autoimmune diseases such as NMO/SD.
This strategy has been translated to human trials using unaltered autologous peripheral blood mononuclear cells (PBMCs) as APCs following their isolation by therapeutic leukapheresis.52 In MS, isolated cells are first pulsed in vitro with a mixture of immunodominant myelin peptides that are identified as targets of the high-avidity myelin-specific T cell response.53 A phase I trial was conducted in which patients received graded dosages of peptide-coupled and fixed autologous PBMCs.52 Results suggested this approach was well tolerated, and induced neither immune reactivity to myelin peptides nor triggered disease relapses. Rather, the data demonstrated that the abundance of myelin-reactive T cells was substantially reduced.52 Several putative mechanisms of action have been attributed to this strategy. First, APC presentation of cross-linked peptide(s) can lead to anergy and/or functional silencing of T cells resulting from inadequate second-signaling.49,54 Here, the dominant mechanisms are most likely related to T cell recognition of peptide-coupled antigen complexes. In this scenario, T cells and/or APCs undergo apoptosis and are phagocytosed by a special subset of immature DCs or macrophages in the splenic marginal zone.45,49,50 These immature DCs/macrophages can present an autoantigen peptide(s) in a tolerogenic manner (e.g., IL-10) to pathogenic, autoreactive T cells. Each of these pathways might help dampen autoimmunity. Induction of Tregs is also likely critical in suppressing autoreactivity.45 Tolerization with peptide-coupled, fixed PBMCs undergoing apoptosis in vivo does not lead to the induction of anti-DNA autoantibodies.55 The relevant mechanism(s) involved in this immune tolerizing strategy and how it might be applied to human diseases require further study.
Despite promising results in animals, human studies have been limited to demonstrating that the abundance of myelin-specific T cells is substantially reduced52; however, this phase I study was not designed to elucidate specific mechanisms of action. The above tolerization approach with AQP4-coupled fixed APCs could, in theory, be readily adapted to NMO/SD. Moreover, epitope spreading can occur in this disease56 and may be attenuated by this strategy (reviewed in reference 57). Thus, tolerization with the entire AQP4 holoprotein or derivative immunodominant peptides may dampen autoreactivity against AQP4 and secondary autoantigen targets, providing clinical benefit. Naturally, technical challenges are likely to arise in these therapeutic strategies and their approach to the clinic. Moreover, specific biomarkers for determining efficacy will be needed to unambiguously inform the therapeutic benefit. Nonetheless, this approach holds promise for restoring immune tolerance in NMO/SD and other autoimmune conditions.
T cell receptor engineering.
The αβ TcR is the critical antigen recognition determinant on CD4+ and CD8+ T cells, conferring both antigen specificity and sensitivity. TcR diversity results from rearrangement of multiple genes (Figure 2). A key difference from antibodies is the requirement that αβ TcRs bind to peptide ligands displayed through the APC MHC. This MHC restriction focuses T cell activity against cells that bear the respective antigen. This mechanism facilitates targeting to antigen-enriched anatomical sites such as lymph nodes and infected tissues while effector T cells promote immune responses against foreign or endogenous autoantigens. CD4+ Tregs suppress these responses (part II).
Improved understanding of TcR-driven immune recognition should yield strategies to harness these cells in treating autoimmune diseases. For example, autoreactive TcR functions may be redirected to modulate their disease-propagating phenotypes. By comparison, ongoing efforts to generate therapeutic autologous T cells targeting cancer involve engineering TcRs specific to cancer peptide/class I MHC antigens.58,e1 These cells mature into effector T cells ex vivo that are then administered to the patient. Initial attempts involved CD8+ T cell clones exhibiting relatively low affinities (KD range 10–100 μM).e1 However, subsequent engineering has yielded higher affinity TcRs with KD ranges from 0.1 to 1 μM. Moreover, TcRs targeting class I antigens do not require the CD8 coreceptor and are thus capable of recruiting effector CD4+ T cells against cancer cells expressing their cognate target antigen.e2 Conceivably, this approach might also prove useful in targeting autoreactive cells. To date, TcR engineering has been accomplished using several methodologies, including yeast and phage display.e3
Engineered TcRs can be delivered using a variety of strategies. These include administration as soluble agents, via gene transfer, or as adoptive T cell therapies. Soluble TcRs will likely require affinities comparable to those of antibodies (KD in nM or pM range), and formatting as immunoglobulin fusions, bispecific agents with anti-CD3,e4 or as fusions with immunoregulatory molecules such as cytokines.e5 A high-affinity TcR against a class II MHC fused to IL-10 has been developed as a means for downregulating autoimmune responses.e6 Optimizing the tissue targeting of these constructs remains challenging.
Adoptive T cell transfer of enriched Tregs has also been used as a method of antigen-specific tolerization.e7,e8 Yet, optimal delivery of antigen-specific, stable Tregs remains to be determined. Moreover, TcR affinity and specificity of Tregse9 is less well characterized than those of effector T cells. Several studies have demonstrated that introducing class I–restricted TcR into Tregs can redirect their specific activities,e10 and that TcRs with KDs of approximately 1 μM may be of sufficient avidity to drive Treg activity.e11 TcRs directed against class I targets required lower affinities than those in effector CD4+ T cells that promote Treg activity.
Opportunities to generate mouse models and therapeutic T cells targeting AQP4 would appear to be numerous.e12 For example, in a mouse model, an immunogenic response can be elicited with effector T cells (CD4+ and/or CD8+) expressing an AQP4-specific antibody (scFv)e13 fused to a chimeric antigen receptor (CAR). Redirection of Tregs to suppress effector cells could provide greater therapeutic potential and utilize CARs directed against AQP4. Alternatively, conventional TcRs targeting AQP4 peptide/MHC class I complexes such as human leukocyte antigen (HLA)-A2 (which are expressed by 40% of the population) might prove effective. Possible advantages of CARs include their potential use of scFv from existing monoclonal antibodies.e13 Furthermore, the same scFv used in effector T cells to initiate pathogenic responses could instead be engineered to a Treg phenotype. Here, scFv affinity could be optimized using single-site complementarity determining regions mutations. Treg signaling and activity would depend on the CAR signaling domain specificity and affinity, as well as density of AQP4 displayed on target cells. Animal models that more precisely recapitulate human disease would represent significant advances toward these goals.
The TcR approach offers potential advantage over scFv/CAR, including direct targeting of Treg activity to class I–bearing tissues and regional lymph nodes, where AQP4 might be cross-presented by MHC. Ostensibly, this strategy might arrest or reverse cross-presentation of AQP4 antigens by class II MHC, which may occur early in the development of NMO/SD.e14 If so, this approach may also mitigate epitope spreading. The generation of TcRs against AQP4 peptides that bind to HLA-A2 would be relatively straightforward, given the extensive knowledge of its binding motifs and the likelihood that AQP4 contains cognate epitopes.e15 The TcR affinity in this scenario may prove inadequate, but such limitations might be overcome through the generation of CAR mutants.
AQP4 antigen–specific approaches must address issues well beyond TcR and CAR affinity. These include untoward conversion of Tregs to pathogenic phenotypes such as those exhibiting either Th1 or Th17 paradigms,e16 accurate tissue targeting, and promotion of memory and stability.e17,e18 It is possible that the same tolerance checkpoints currently being considered for rapid deletion of undesirable T cells transferred in cancer could be applied to NMO/SD.
SUMMARY AND PROSPECTUS: PART I
Immune tolerization attempts to restore central or peripheral tolerance, thereby suppressing autoimmune diseases. There is reason for optimism that this approach might offer substantial clinical benefit to individuals with NMO/SD. DNA vaccination, T cell vaccination, tolerogenic DCs, and peptide-coupling strategies have advanced to clinic trials. Other promising approaches (e.g., CAR-based or Treg-based therapeutics) are in earlier developmental stages in autoimmune diseases. Yet, defining target cell lineages to yield durable efficacy, and determining stage of disease development optimal for intervention remain crucial ongoing challenges. Past failures using similar strategies have provided critical insights that, despite the complexities of existing and emerging technologies for promoting tolerance, should illuminate the pathway forward. If so, such advances in treatment could benefit individuals with NMO/SD and other autoimmune diseases, including arresting disease progression, prolonging intervals to remission, enabling tissue repair, and ultimately avoiding chronic immunosuppressive therapy.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Gerald Nepom, Dr. Philip Bernstein, and Dr. William St. Clair for their helpful comments.
GLOSSARY
- APC
antigen-presenting cell
- AQP4
aquaporin 4
- CAR
chimeric antigen receptor
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- HLA
human leukocyte antigen
- IgG
immunoglobulin G
- IL
interleukin
- MHC
major histocompatibility complex
- NMO
neuromyelitis optica
- PBMC
peripheral blood mononuclear cell
- scFv
single chain variable fragment
- SD
spectrum disorder
- T1D
type 1 diabetes
- TcR
T cell receptor
- Treg
regulatory T cell
Footnotes
Supplemental data at Neurology.org/nn
Contributor Information
Collaborators: Guthy-Jackson Charitable Foundation International Clinical Consortium, Orhan Aktas, Lilyana Amezcua, Metha Appiwatanakul, Nasrin Asgari, Brenda Banwell, Jeffrey Bennett, James Bowen, Philippe Cabre, Tanuja Chitnis, Jeffrey Cohen, Jerome De Seze, Kazuo Fujihara, May Han, Kerstin Hellwig, Rogier Hintzen, D. Craig Hooper, Raffaele Iorio, Anu Jacob, Sven Jarius, Ho Jin Kim, Najib Kissani, Eric C. Klawiter, Ingo Kleiter, Marco Lana-Peixoto, Maria Isabel Leite, Michael Levy, Fred Lublin, Yang Mao Draayer, Romain Marignier, Marcelo Matiello, Ichiro Nakashima, Kevin C. O’Connor, Jacqueline Palace, Lekha Pandit, Friedemann Paul, Naraporn Prayoonwiwat, Claire Riley, Klemens Ruprecht, Albert Saiz, Sasitorn Siritho, Silvia Tenembaum, Brian Weinshenker, Dean Wingerchuk, and Jens Würfel
AUTHOR CONTRIBUTIONS
Larry Steinman: drafting/revising the manuscript. Amit Bar-Or: drafting/revising the manuscript. Jacinta M. Behne: revising the manuscript. Daniel Benitez-Ribas: drafting/revising the manuscript. Peter S. Chin: drafting/revising the manuscript. Michael Clare-Salzler: drafting/revising the manuscript. Donald Healey: drafting/revising the manuscript. James I. Kim: drafting/revising the manuscript. David M. Kranz: drafting/revising the manuscript. Andreas Lutterotti: drafting/revising the manuscript. Roland Martin: drafting/revising the manuscript. Sven Schippling: drafting/revising the manuscript. Pablo Villoslada: drafting/revising the manuscript. Cheng-Hong Wei: drafting/revising the manuscript. Howard L. Weiner: drafting/revising the manuscript. Scott S. Zamvil: drafting/revising the manuscript. Michael R. Yeaman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Terry J. Smith: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval.
STUDY FUNDING
This work was supported in part by the Guthy-Jackson Charitable Foundation.
DISCLOSURE
L. Steinman served on the scientific advisory board for Novartis, Receptos, Atreca, Tolerion, Teva, received travel funding and/or speaker honoraria from Biogen, Bayhill, Bayer, Celgene, Receptos, is on the editorial board for Multiple Sclerosis Journal, Proceedings of the National Academy of Science, holds a patents for antigen-specific tolerance, has a patent pending for cytokines and type 1 interferons, is on the speakers bureau for EMD Serono, received research support from NIH, has stock options and board membership in Tolerion, is on the board of directors for BioAtla. A. Bar-Or is on the scientific advisory board for Dionix, Receptos-Celgene, Roche/Genentech, Novartis, GSK, Guthy-Jackson Greater Good Foundation, Immune Tolerance Network, received travel funding and/or speaker honoraria from Receptos-Celgene, Roche/Genentech, Novartis, Sanofi-Genzyme, GSK, served on the editorial board for Neurology®, Clinical and Experimental Neuroimmunology, consulted for DioGenix, Receptos-Celgene, Roche/Genentech, Novartis, Sanofi-Genzyme, GSK, received research support from Novartis, Sanofi-Genzyme. J.M. Behne and D. Benitez-Ribas report no disclosures. P.S. Chin served as the medical director for Genentech and Novartis Pharmaceuticals Corp. M. Clare-Salzler received research support from NIH, NIAID. D. Healy has a patent pending for the treatment of B cell–mediated autoimmunities with T cell vaccination, has been CSO for Opexa Therapeutics, received research support from and holds stock/stock options in Opexa Therapeutics. J.I. Kim is an associate editor for the Journal of Negative Results in Biomedicine, is employed by Unum Therapeutics. D.M. Kranz has patents and patents pending in the areas of yeast display and T cell receptor engineering and receives license fee payments from them, consulted for AbbVie, received research support from NIH, Melanoma Research Alliance. A. Lutterotti served on the scientific advisory board for Bayer, Biogen, Novartis, Genzyme, received travel support from European Charcot Foundation, Fundacio GAEM, holds a patent with the University of Zurich, received research support from Wyss Translational Center, Austrian MS Society. R. Martin served on the scientific advisory board for Biogen, Merck Serono, Teva, Genzyme, Sanofi-Aventis, CellProtect, Neuway, received speaker honoraria from Biogen, Merck Serono, Novartis, Roche, Genzyme, holds a patent for the therapeutic efficacy of anto-CD25 monoclonal antibody treatment in combination with IFN-β in MS, consulted for Myelin Repair Foundation, The Weatherall Institute of Molecular Studies, University of Oxford, the Hertie Foundation, is a member of the Kuratorium of the Jung Foundation for Science, received research support from Novartis, Biogen, Swiss National Science Foundation, European Union Seventh Framework Program, European Research Council. S. Schippling served on the scientific advisory board for Bayer Healthcare, Biogen, Merck Serono, Novartis, Sanofi-Genzyme, TEVA, received travel funding and/or speaker honoraria from Bayer Healthcare, Biogen, Merck Serono, Novartis, Sanofi-Aventis, TEVA, is an associate editor for Frontiers in Neurology, holds a patent for therapeutic vaccination in PML using VP1 and Il7, received research support from Sanofi-Genzyme, Novartis, University of Zurich, Betty and David Koetser Foundation for Brain Research, Swiss Multiple Sclerosis Society. P. Villoslada received travel funding and/or speaker honoraria from Novartis, Roche, Genzyme, served as an academic editor for PLoS One, served on the editorial board for Neurology and Therapy, Current Treatment Options in Neurology, Multiple Sclerosis and Demyelinating Disorders, holds a patent for methylthioadenosine for the treatment of MS, Agnostic neurotrophic compounds for the treatment of brain diseases, Gene signature pattern as a biomarker for MS, Algorithm for quantifying fractal dimension in brain MRI, received research support from Novartis, Roche, Genzyme, Instituto de Salud Carlos III, European Commission, National MS Society, Fundacion Maraton TV3, holds stock or stock options in Bionure Inc., Spire Bioventures, Mint-Labs. C.-H. Wei reports no disclosures. H.L. Weiner served on the scientific advisory board for the Guthy-Jackson Charitable Foundation, Teva Pharmaceutical Industries, Biogen Idec, Novartis, Sanofi-Aventis, consulted for Therapix, Biogen, Novartis, Serono, Teva, Sanofi, received research support from National Multiple Sclerosis Society. S. Zamvil served on the scientific advisory board for BioMS, Teva Pharmaceuticals, Eli Lilly and Co., Myelin Repair Foundation, is deputy editor for Neurology®: Neuroimmunology & Neuroinflammation, consulted for Biogen Idec, Teva Neuroscience, EMD Serono, Genzyme, Novartis, Roche, is on the speakers bureau for Advanced Health Media, Biogen, received research support from NIH, NMSS, Alexander M. and June L. Maisin Foundation. M.R. Yeaman is on the scientific advisory board for Guthy-Jackson Charitable Foundation, served as an associate editor for PLoS Pathogens, holds patents for Vaccines targeting drug-resistant pathogens, immunotherapies targeting drug-resistant pathogens, novel anti-infective biological therapeutics, novel anti-infective small molecules, novel biological regulating programmed cell death, consulted for Guthy-Jackson Charitable Foundation, received research support from NovaDigm Therapeutics, Inc., Metacin Inc., US Department of Defense, NIH, holds stock or stock options for NovaDigm Therapeutics, Inc., Metacin, Inc., receives license fee and royalty payments from NovaDigm Therapeutics. T.J. Smith received research support from NIH, University of South Denmark, Bell Charitable Foundation, RPB Foundation, is a member of the Guthy-Jackson Charitable Foundation scientific advisory board, and holds patents covering the blockade of insulin-like growth factor receptor-l in autoimmune diseases. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Levy M, Wildemann B, Jarius S, et al. Immunopathogenesis of neuromyelitis optica. Adv Immunol 2014;121:213–242. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elsone L, Kitley J, Luppe S, et al. Long-term efficacy, tolerability, and retention rate of azathioprine in 103 aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder patients: a multicenter retrospective observational study from the UK. Mult Scler 2014;20:1533–1540. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura M, Nakazawa T, Doi H, et al. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol 2010;248:1777–1785. [DOI] [PubMed] [Google Scholar]
- 6.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med 1979;149:758–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh S, Schwartz RH, Singh NJ. Development and tolerization of hyperacute rejection in a transgenic mouse graft versus host model. Transplantation 2012;94:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castells M. Rapid desensitization of hypersensitivity reactions to chemotherapy agents. Curr Drug Saf 2006;1:243–251. [DOI] [PubMed] [Google Scholar]
- 9.Fousteri G, Bresson D, von Herrath M. Rational development of antigen-specific therapies for type 1 diabetes. Adv Exp Med Biol 2007;601:313–319. [DOI] [PubMed] [Google Scholar]
- 10.Turley DM, Miller SD. Prospects for antigen-specific tolerance based therapies for the treatment of multiple sclerosis. Results Probl Cell Differ 2010;51:217–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori K, Yamanishi H, Ikeda Y, et al. Oral administration of carbonic anhydrase I ameliorates murine experimental colitis induced by Foxp3-CD4+CD25− T cells. J Leukoc Biol 2013;93:963–972. [DOI] [PubMed] [Google Scholar]
- 12.Genain CP, Abel K, Belmar N, et al. Late complications of immune deviation therapy in a nonhuman primate. Science 1996;274:2054–2057. [DOI] [PubMed] [Google Scholar]
- 13.Dolgin E. The inverse of immunity. Nat Med 2010;16:740–743. [DOI] [PubMed] [Google Scholar]
- 14.Steinman L. Inverse vaccination, the opposite of Jenner's concept, for therapy of autoimmunity. J Intern Med 2010;267:441–451. [DOI] [PubMed] [Google Scholar]
- 15.Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuroscience 2010;168:1009–1018. [DOI] [PubMed] [Google Scholar]
- 16.Sato DK, Callegaro D, de Haidar Jorge FM, et al. Cerebrospinal fluid aquaporin-4 antibody levels in neuromyelitis optica attacks. Ann Neurol 2014;76:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinman L. The re-emergence of antigen-specific tolerance as a potential therapy for MS. Mult Scler 2015;21:1223–1238. [DOI] [PubMed] [Google Scholar]
- 18.Roep BO, Solvason N, Gottlieb PA, et al. Plasmid encoded proinsulin preserves C-peptide while specifically reducing proinsulin specific CD8 T cells in type 1 diabetes. Sci Transl Med 2013;5:191ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature 2005;435:612–619. [DOI] [PubMed] [Google Scholar]
- 20.Garren H, Robinson WH, Krasulová E, et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann Neurol 2008;63:611–620. [DOI] [PubMed] [Google Scholar]
- 21.Bar-Or A, Vollmer T, Antel J, et al. Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch Neurol 2007;64:1407–1415. [DOI] [PubMed] [Google Scholar]
- 22.Selmi C. Autoimmunity in 2012. Clin Rev Allergy Immunol 2013;45:290–301. [DOI] [PubMed] [Google Scholar]
- 23.Martin A, Tisch RM, Getts DR. Manipulating T cell-mediated pathology: targets and functions of monoclonal antibody immunotherapy. Clin Immunol 2013;148:136–147. [DOI] [PubMed] [Google Scholar]
- 24.Salvana EM, Salata RA. Infectious complications associated with monoclonal antibodies and related small molecules. Clin Microbiol Rev 2009;22:274–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madakamutil LT, Maricic I, Sercarz EE, Kumar V. Immunodominance in the TCR repertoire of a TCR peptide-specific CD4+ Treg population that controls experimental autoimmune encephalomyelitis. J Immunol 2008;180:4577–4585. [DOI] [PubMed] [Google Scholar]
- 26.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 2010;10:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linhares UC, Schiavoni PB, Barros PO, et al. The ex vivo production of IL-6 and IL-21 by CD4+ T cells is directly associated with neurological disability in neuromyelitis optica patients. J Clin Immunol 2013;33:179–189. [DOI] [PubMed] [Google Scholar]
- 28.Pohl M, Fisher MT, Mader S, et al. Pathogenic T cells responses against aquaporin 4. Acta Neuropathol 2011;122:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize clostridium ABC transporter. Ann Neurol 2012;72:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V, Sercarz EE. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. J Exp Med 1993;178:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J. T cell vaccination as an immunotherapy for autoimmune diseases. Cell Mol Immunol 2004;1:321–327. [PubMed] [Google Scholar]
- 32.Huang X, Wu H, Lu Q. The mechanisms and applications of T cell vaccination for autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol 2014;47:219–233. [DOI] [PubMed] [Google Scholar]
- 33.Fontana R, Bregni M, Cipponi A, et al. Peripheral blood lymphocytes genetically modified to express the self/tumor antigen MAGE-A3 induce antitumor immune responses in cancer patients. Blood 2009;113:1651–1660. [DOI] [PubMed] [Google Scholar]
- 34.Fox E, Wynn D, Cohan S, Rill D, McGuire D, Markowitz C. A randomized clinical trial of autologous T-cell therapy in multiple sclerosis: subset analysis and implications for trial design. Mult Scler 2012;18:843–852. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Li N, Zang YC, et al. Vaccination with selected synovial T cells in rheumatoid arthritis. Arthritis Rheum 2007;56:453–463. [DOI] [PubMed] [Google Scholar]
- 36.Li ZG, Mu R, Dai ZP, Gao XM. T cell vaccination in systemic lupus erythematosus with autologous activated T cells. Lupus 2005;14:884–889. [DOI] [PubMed] [Google Scholar]
- 37.Leavy O. Dendritic cells: tracing the origins of cDCs. Nat Rev Immunol 2013;13:703. [DOI] [PubMed] [Google Scholar]
- 38.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 2005;5:296–306. [DOI] [PubMed] [Google Scholar]
- 39.Vacchelli E, Vitale I, Eggermont A, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology 2012;1:1111–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantia-Smaldone GM, Chu CS. A review of dendritic cell therapy for cancer: progress and challenges. BioDrugs 2013;27:453–468. [DOI] [PubMed] [Google Scholar]
- 41.Segovia-Gamboa N, Rodriguez-Arellano ME, Rangel-Cruz R, et al. Tolerogenic dendritic cells induce antigen-specific hyporesponsiveness in insulin- and glutamic acid decarboxylase 65-autoreactive T lymphocytes from type 1 diabetic patients. Clin Immunol 2014;154:72–83. [DOI] [PubMed] [Google Scholar]
- 42.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011;34:2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boks MA, Kager-Groenland J, Haasjes MSP, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction- a comparative study on human clinical-applicable DC. Clin Immunol 2012;142:332–342. [DOI] [PubMed] [Google Scholar]
- 44.Saito M, Nagasaw M, Takada H, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med 2011;208:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol 2007;7:665–677. [DOI] [PubMed] [Google Scholar]
- 46.Karpus WJ, Pope JG, Peterson JD, Dal Canto MC, Miller SD. Inhibition of Theiler's virus-mediated demyelination by peripheral immune tolerance induction. J Immunol 1995;155:947–957. [PubMed] [Google Scholar]
- 47.Kennedy KJ, Smith WS, Miller SD, Karpus WJ. Induction of antigen-specific tolerance for the treatment of ongoing, relapsing autoimmune encephalomyelitis: a comparison between oral and peripheral tolerance. J Immunol 1997;159:1036–1044. [PubMed] [Google Scholar]
- 48.Kennedy MK, Tan LJ, Dal Canto MC, et al. Inhibition of murine relapsing experimental autoimmune encephalomyelitis by immune tolerance to proteolipid protein and its encephalitogenic peptides. J Immunol 1990;144:909–915. [PubMed] [Google Scholar]
- 49.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol 2007;178:2212–2220. [DOI] [PubMed] [Google Scholar]
- 50.Getts DR, Turley DM, Smith CE, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol 2011;187:2405–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo X, Pothoven KL, McCarthy D, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci USA 2008;105:14527–14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lutterotti A, Yousef S, Sputtek A, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med 2013;5:188ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bielekova B, Sung MH, Kadom N, Simon R, McFarland HF, Martin R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol 2004;172:3893–3904. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins MK, Mueller D, Schwartz RH, et al. Induction and maintenance of anergy in mature T cells. Adv Exp Med Biol 1991;292:167–176. [DOI] [PubMed] [Google Scholar]
- 55.Peschl P, Reindl M, Schanda K, Sospedra M, Martin R, Lutterotti A. Anti-myelin antibody responses following induction of antigen-specific tolerance with antigen-coupled cells. J Neuroimmunol 2014;275:12. [DOI] [PubMed] [Google Scholar]
- 56.Tuohy VK, Yu M, Yin L, Kawczak JA, Kinkel PR. Regression and spreading of self-recognition during the development of autoimmune demyelinating disease. J Autoimmun 1999;13:11–20. [DOI] [PubMed] [Google Scholar]
- 57.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol 2002;2:85–95. [DOI] [PubMed] [Google Scholar]
- 58.Stromnes IM, Schmitt TM, Chapuis AG, Hingorani SR, Greenberg PD. Re-adapting T cells for cancer therapy: from mouse models to clinical trials. Immunol Rev 2014;257:145–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.