Abstract
Sulfide mineral processing often produces large quantities of wastewaters containing acid-generating inorganic sulfur compounds. If released untreated, these wastewaters can cause catastrophic environmental damage. In this study, microbial fuel cells were inoculated with acidophilic microorganisms to investigate whether inorganic sulfur compound oxidation can generate an electrical current. Cyclic voltammetry suggested that acidophilic microorganisms mediated electron transfer to the anode, and that electricity generation was catalyzed by microorganisms. A cation exchange membrane microbial fuel cell, fed with artificial wastewater containing tetrathionate as electron donor, reached a maximum whole cell voltage of 72 ± 9 mV. Stepwise replacement of the artificial anolyte with real mining process wastewater had no adverse effect on bioelectrochemical performance and generated a maximum voltage of 105 ± 42 mV. 16S rRNA gene sequencing of the microbial consortia resulted in sequences that aligned within the genera Thermoplasma, Ferroplasma, Leptospirillum, Sulfobacillus and Acidithiobacillus. This study opens up possibilities to bioremediate mining wastewater using microbial fuel cell technology.
Keywords: Microbial fuel cell, Electricity generation, Acidophile, Mining, Wastewater
1. Introduction
The world's demand for metals requires the continued mining and processing of metal-bearing ores. A common treatment process for extracting valuable metals from metal-sulfide minerals involves crushing and subsequent flotation to create a mineral concentrate suitable for further (bio)hydrometallurgical treatment. In addition to the valuable metal-containing phases, the low economic value mineral pyrite (FeS2) is also usually present. Pyrite is oxidized during ore crushing and flotation [1], [2], during which time the inorganic sulfur compound (ISC) thiosulfate () is produced (Eq. (1)).
| (1) |
Additional sources of ISCs in sulfide mineral processing include hydrogen sulfide from flotation of molybdenum and from ammoniacal thiosulfate leaching for gold recovery. The generated ISCs are contained in the processed waters that carry waste (gangue) minerals to tailings ponds, where the solid waste metal sulfides sink to the bottom of the pond. The tailings are covered in water to impair ingress of oxidation that, in turn, retards the release of acid and metals (reviewed in [3]). In the tailings pond, acidophilic microorganisms can subsequently oxidize thiosulfate to tetrathionate (; Eq. (2)) and ultimately, the sulfur moiety will end up as sulfuric acid (Eq. (3)), lowering the pH to values typically between 1 and 3. This provides requisite conditions for the growth of extremely acidophilic microorganisms that have an optimum growth pH < 3 [4].
| (2) |
| (3) |
If released to recipient water bodies, these ISCs can pose environmental risks, including microbial oxidation to sulfuric acid and the depletion of dissolved oxygen (reviewed in [5]). The most common treatment method for ISC-containing mining wastewaters is to raise the pH with ‘lime’ (CaO) before release of the water. A drawback of this technique is the production of a metal-containing sludge that requires safe disposal. Further investigated biotechnological solutions include metal precipitation with biologically produced hydrogen sulfide at near neutral [6] or acidic [7], [8] pH, as well as biological oxidation of the ISCs to sulfuric acid prior to neutralization with lime and release to downstream water bodies [1]. However, neither of these methods have been extensively implemented at mining sites around the world.
Microbial fuel cells (MFCs) are bioelectrochemical systems capable of sustainable microbial oxidation of a substrate in the anode compartment, while reducing electron acceptors in the cathode, with the flow of electrons forming an electrical current (reviewed in [9]). The general principles of MFCs (Fig. 1) are that the electron donor is oxidized in the anode compartment, often by microorganisms attached as a biofilm to the anode surface and, in the absence of competing electron acceptors, they pass their electrons to the anode. Microorganisms able to donate electrons at the anode can be termed ‘electricigens’ and the most commonly investigated species come from the genera Geobacter and Shewanella [10], [11]. Electrons donated to the anode are chemically or biologically reduced at the cathode. Research into MFCs has intensified in the past decade, and they have been extensively tested for treatment of organic carbon containing wastewaters at neutral pH to simultaneously generate a current and remove the waste product (reviewed in [12]). Most MFCs use microbial communities from ‘non-extreme’ environments such as municipal wastewater, activated sludge or sediment [13], [14], [15], [16], while MFCs using extremophilic microorganisms are less common. Several studies have documented the use acidophiles in MFCs fed with organic carbon and energy sources [17], [18], [19], [20], [21]. However, to treat ISCs in mining wastewaters at acidic pH values, it is required to utilize microorganisms that donate electrons to the anode during growth using carbon dioxide and inorganic compounds as carbon and energy sources, respectively. However, research into the use of ISCs in MFCs is rare. A single study has shown that a mixed culture of acidophilic microorganisms is capable of generating electricity from tetrathionate, with Acidithiobacillus ferrooxidans and Ferroplasma spp. being the dominant populations present in the anolyte and on the bioanode surface [22]. The maximum current and power densities achieved during operation were 79.6 mA m−2 and 13.9 mW m−2, respectively, but the low coulombic efficiency of 5% suggested that processes other than donation of the electrons to the anode also occurred [22].
Fig. 1.
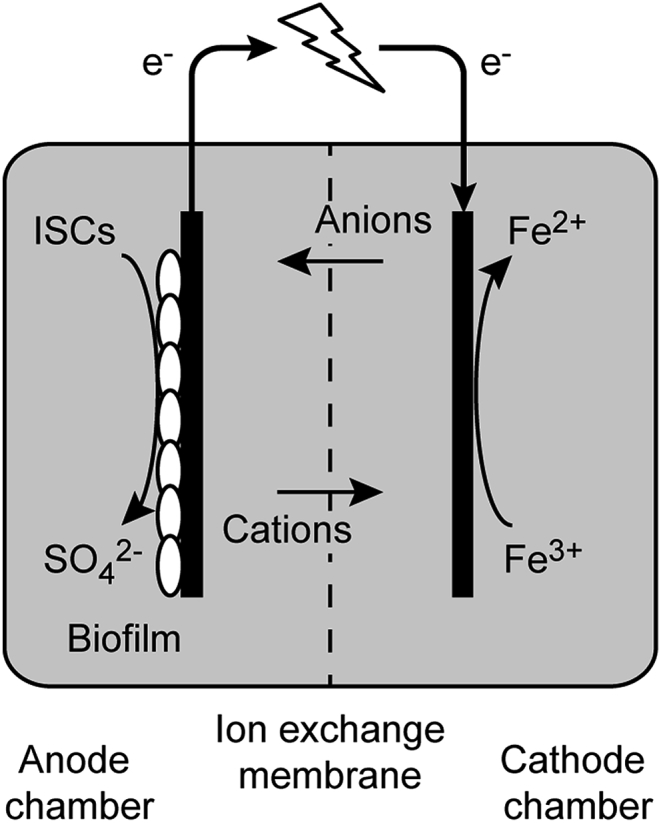
Schematic diagram of a MFC showing the ISC oxidizing biofilm on the anode, the ferric iron-reducing cathode, with the two compartments separated by an ion exchange membrane. Horizontal arrows denote the exchange of ions across the membrane.
In this study, the feasibility of using acidophilic microorganisms in MFCs fed with wastewater from an industrial sulfide mineral flotation process was investigated. If successful, the acid-generating ISCs would be removed before pH neutralization of water and release to recipient water bodies. The specific aims were to select suitable acidophilic microbes to remove the ISC compounds while simultaneously producing an electrical current in MFCs.
2. Materials and methods
2.1. Inoculum and growth conditions
Acidophilic microorganisms were enriched from a pH 2.5 to 2.7 underground acid mine stream sediment from a poly-metal sulfide mineral mine located in Kristineberg, Sweden [23] and an acid sulfate soil containing metastable iron sulfides from Vaasa, Finland [24]. These environments were chosen as they were likely to contain populations able to grow anaerobically at low pH while utilizing ISCs [24], [25], [23].
Initial selection of the microbial consortium was carried out in MFCs containing an anolyte of autoclaved mineral salts medium (adjusted to pH 2 using sulfuric acid) plus sterile filtered (0.22 μm membrane filter; Sarstedt, Nümbrecht, Germany) trace elements solution [26] and various concentrations of filter-sterilized potassium tetrathionate and/or sodium thiosulfate. Additional ISC substrate (raising the ISC concentrations to the original values) was added to the anolyte when the concentration dropped below 1 mM in the fed batch system. Inorganic carbon for autotrophic growth was provided either by flushing the anolyte with CO2 gas, or when the anolyte was changed by the addition of sterile filtered 10 mM (final concentration) sodium bicarbonate. The catholyte consisted of 35.7 mM ferric iron as Fe2(SO4)3 adjusted to pH 1.5 using sulfuric acid. All MFCs were incubated at room temperature (21 ± 2 °C) for up to a maximum of 156 days.
Based upon the stable performance and current generated, it was decided to utilize the Kristineberg culture fed with tetrathionate for further work to degrade ISCs in mining process waters. The effect of increasing fractions of process wastewater obtained from the Boliden AB sulfide mineral flotation process was evaluated by parallel MFCs containing an anolyte of mineral salts medium plus 5 mM tetrathionate, compared to an anolyte containing increasing amounts of process wastewater. Finally, the effects of anion and cation exchange membranes on the performance of MFCs, and microbial community changes in the anion exchange membrane MFC, were also addressed. During each experimental stage, the cell voltage, anode potential and cathode potential were recorded. Tetrathionate and inorganic carbon were added when the anolyte was replaced each time the process wastewater concentration was increased.
The ISC content of the wastewater was determined by ion chromatography and inductively coupled plasma optical emission spectrometer as previously described [27], [28] and found to contain 138.3 ± 0.3 mg/L thiosulfate (1.22 mM; no other ISCs or volatile sulfur compounds were detected). Modeling of the sulfur compound species after adjusting the pH to pH 2.0 in the anolyte by OLI Analyzer Studio software (Version 3.1 (2001), OLI Systems Inc., NJ) suggested the ISCs would remain chemically stable as thiosulfate during the MFC operation in the absence of microbial catalyzed oxidation.
2.2. MFC construction and operation
The MFCs used in this study were two-chamber flow-through microbial fuel cells (Supplemental File 1) constructed as described elsewhere [29]. The anode and cathode chambers had an equal volume of 33 cm3 and were separated with either a cation or anion exchange membrane (Mega a.s., Czech Republic). The outer sides of the anode and cathode chambers were covered by carbon paper (Graphite foil, Coidan Graphite Products, USA) pressed together with graphite electrodes (MR graphite, Germany). The anolyte and catholyte were continuously pumped (L/S Digital Drive, Masterflex, Sweden) from recirculation bottles at a flow rate of 50 mL/min. For each MFC, the volume of anolyte was 500 mL and the volume of catholyte was 1000 mL. The graphite electrodes were constantly connected to a resister (1000 Ω, unless otherwise stated).
The anolyte and catholyte pH was measured using a phenomenal Ag-AgCl pH meter (VWR, Sweden). Microbial consumption of tetrathionate was measured by cyanolysis [26]. Anode and cathode potentials were measured against Ag/AgCl reference electrodes (B2820, SI analytics, Germany) connected to the anolyte via glass capillaries (QiS, the Netherlands) filled with 3 M KCl. The theoretical anode potential was calculated based on the proposed tetrathionate degradation in Eq. (4) [30], [31], [32], with adjustment for pH 2, 5 mM tetrathionate addition, and a temperature of 21 °C, according to Eq. (5) [9].
| (4) |
| (5) |
where Eanode is the theoretical anode potential and E0 is the standard electrode potential. Total iron in the anolyte was measured on a flame atomic absorption spectrometer (AAnalyst 400, PerkinElmer, USA). Polarization experiments were performed by stepwise decreasing the external resistance (∞, 1000 Ω, 560 Ω, 220 Ω, 100 Ω, 56 Ω, and 10 Ω) and the process was subsequently reversed to obtain maximum power and internal resistance of the MFC systems. The power obtained and current values were then normalized to the anode surface area (22 cm2). Cyclic voltammetry tests (μStat 400, Dropsens, Spain) were performed to investigate the existence of extracellular mediators produced by microorganisms.
2.3. Microbial community profiling
Community genomic DNA samples were prepared by vacuum filtration of cells through sterile 0.2 μm membrane filters (Sartorius Stedim, Germany) followed by DNA extraction procedures as described in the PowerWater DNA Isolation Kit (MO BIO, USA). The quality and quantity of DNA samples were checked utilizing a Qubit machine (Qubit®2.0, Life technologies, Sweden) and a Nanodrop device (NANODROP 2000, Thermo SCIENTIFIC, USA), respectively. The microbial community was assessed by amplification of a portion of the 16S rRNA gene using PCR primers 341F and 805R [33] as described elsewhere [34]. Sequencing was carried out at the Science for Life Laboratory (Stockholm, Sweden; www.scilifelab.se) with Illumina MiSeq pair-end sequencing technologies. 16S rRNA gene reads were analyzed using the UPARSE pipeline (Edgar 2013), before annotation using the SINA/SILVA database (SILVA 119) [35], and final data handling in Explicet 2.10.5 (Robertson et al., 2013). 16S rRNA gene sequences were submitted to the NCBI database with the accession numbers:SRR3491402 and SRR2962706.
3. Results and discussion
3.1. Enrichment of acidophilic microorganisms in the anode compartment
Two Kristineberg enrichment MFCs were operated for 61 days: one with 5 mM tetrathionate and the second with a mixture of 3 mM tetrathionate plus 3 mM thiosulfate (Supplemental File 2). The tetrathionate-fed MFC reached a maximum whole cell voltage of 35 mV on day 61, whereas the tetrathionate-plus thiosulfate-fed MFC took until day 59 to reach a whole cell voltage of approximately zero. The Vaasa acid sulfate soil MFCs fed with 5 mM tetrathionate or 5 mM thiosulfate took a longer time to generate a positive whole cell voltage compared to the Kristineberg tetrathionate-fed MFC and peaked at voltages of 48 and 42 mV, respectively (Supplemental File 2). The time to select for a microbial consortium that generated a positive whole cell potential took longer than 15 days in all cases. As these MFCs were operated for an extended time period it was necessary to add further substrate that resulted in a dip in cell voltage that took approximately 10 days to recover. It is possible that this dip was due to a small concentration of oxygen entering the system that was consumed before electron donor addition to the anode recommenced. The anode potentials with 5 mM tetrathionate as substrate were in the range of 550–600 mV (Supplemental File 2) that was considerably higher than the calculated value of −272 mV (versus Ag/AgCl electrode at pH 2, 20 °C, and 2 g L−1 tetrathionate). Despite the variations in microbial communities present, this result was consistent with previously reported tetrathionate-fed acidic MFCs and was attributed to MFC losses and lower reaction efficiencies [22].
The whole cell voltages produced and coulombic efficiencies were lower than those achieved with acidic anolyte MFCs fed with organic carbon and energy containing wastewaters [21], [36]. The increased whole cell voltage with organic carbon was likely due to the higher energy present in organic carbon compounds. In addition, the low coulombic efficiency in this study may have been due to the energy required for extremely acidophilic microorganisms to maintain pH homeostasis, such as proton pumping out of the cytoplasm (reviewed in [37]) and reverse electron transport to generate NADH [31]. The whole cell voltages in this study were also lower than obtained with 2.0–2.5 g L−1 (7.7–9.6 mM) tetrathionate [22], potentially due to the higher substrate concentration in the previous study.
3.2. Sulfide mineral flotation process water MFC performances
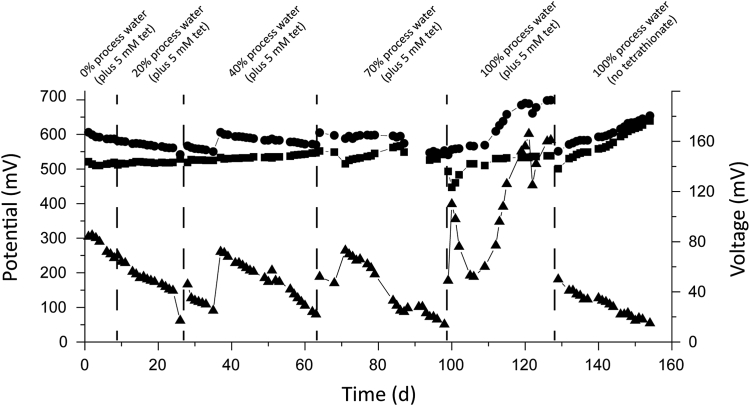
The average cation exchange membrane whole cell voltage fed with 5 mM tetrathionate (in the absence of process wastewater) was 76 ± 7 mV (Table 1 and Fig. 2). The coulombic efficiency was 3.0% and a power density of 2.5 mW m−2, values similar to previous observed tetrathionate-fed MFCs [22]. The whole cell voltage values fluctuated when new substrate was added (Fig. 2), indicating that cell voltage depended on the availability of substrate. Increasing the percentage of process wastewater slightly decreased the average and maximum whole cell voltages until 100% process wastewater plus tetrathionate was added when the whole cell voltage had an average of 105 ± 42 mV and a maximum of 166 mV. The increase in whole cell voltage may have been due to transfer of electrons to the anode from the 1.22 mM thiosulfate present in the process wastewater. The maximum whole cell voltage in the presence of 100% process water and no additional tetrathionate decreased to 29 ± 9 mV, likely due to the decreased availability of tetrathionate. A parallel control MFC with artificial mineral salts medium containing tetrathionate (but no process wastewater) also resulted in substrate utilization coupled to generation of a whole cell potential, although the achieved power densities and coulombic efficiencies were lower than in the presence of process wastewater (Table 1). This suggested that any potential contaminants in the process wastewater (e.g. residues of flotation chemicals [38]) did not have any negative effects on the MFC performance.
Table 1.
Experimental parameters and electrochemistry of the MFCs treating ISCs in sulfide mineral flotation process wastewater and tetrathionate fed control MFCs (without process wastewater).
| Process water (%)a | Tet (mM)b | Thio (mM)c | Voltage (mV)d |
EAn (mV)e |
PD (mW m−2)f | Tet used (mM day−1)g | CE (%)h | ||
|---|---|---|---|---|---|---|---|---|---|
| Ave ± SD | Max | Ave ± SD | Min | ||||||
| Cation exchange membrane test | |||||||||
| 0 | 5.00 | 0.00 | 76 ± 7 | 85 | 515 ± 5 | 510 | 2.5 | 0.28 | 3.0 |
| 20 | 5.00 | 0.25 | 51 ± 11 | 64 | 518 ± 3 | 515 | 1.1 | 0.64 | 0.89 |
| 40 | 5.00 | 0.49 | 45 ± 16 | 72 | 515 ± 4 | 519 | 0.88 | 0.52 | 1.0 |
| 70 | 5.00 | 0.86 | 41 ± 20 | 73 | 560 ± 50 | 515 | 0.73 | 0.45 | 1.0 |
| 100 | 5.00 | 1.23 | 105 ± 42 | 166 | 518 ± 27 | 447 | 4.8 | 0.10 | 11.8 |
| 100 | 0.00 | 1.23 | 29 ± 9 | 50 | 575 ± 37 | 501 | 0.37 | 0.05 | ND |
| Cation exchange membrane control | |||||||||
| 0 | 5.00 | 0.00 | 72 ± 9 | 85 | 531 ± 8 | 522 | 2.3 | NDi | NDi |
| 0 | 5.00 | 0.00 | 48 ± 9 | 58 | 530 ± 4 | 525 | 1.0 | 0.78 | 0.69 |
| 0 | 5.00 | 0.00 | 55 ± 20 | 103 | 508 ± 19 | 510 | 1.3 | 0.85 | 0.72 |
| 0 | 5.00 | 0.00 | 22 ± 33 | 50 | 571 ± 71 | 548 | 0.21 | 0.59 | 0.41 |
| 0 | 5.00 | 0.00 | 53 ± 20 | 84 | 623 ± 40 | 539 | 1.2 | 1.07 | 0.55 |
| 0 | 5.00 | 0.00 | 23 ± 9 | 42 | 645 ± 22 | 615 | 0.23 | 3.35 | ND |
| Anion exchange membrane test | |||||||||
| 0 | 5.00 | 0.00 | 43 ± 15 | 76 | 573 ± 18 | 539 | 0.80 | 0.50 | 0.96 |
| 20 | 5.00 | 0.25 | 23 ± 28 | 72 | 563 ± 34 | 488 | 0.23 | 2.00 | 0.13 |
| 40 | 5.00 | 0.49 | 42 ± 2 | 48 | 509 ± 5 | 503 | 0.77 | 1.53 | 0.31 |
| 70 | 5.00 | 0.86 | 61 ± 10 | 85 | 476 ± 5 | 468 | 1.6 | 0.83 | 0.82 |
| 100 | 5.00 | 1.23 | 53 ± 13 | 84 | 506 ± 26 | 462 | 1.2 | 0.64 | 0.93 |
| 100 | 0.00 | 1.23 | 29 ± 16 | 54 | 514 ± 26 | 473 | 0.37 | 0.06 | ND |
| Anion exchange membrane control | |||||||||
| 0 | 5.00 | 0.00 | 55 ± 17 | 92 | 562 ± 21 | 528 | 1.3 | 0.36 | 1.7 |
| 0 | 5.00 | 0.00 | 17 ± 17 | 41 | 558 ± 16 | 528 | 0.13 | 2.34 | 0.08 |
| 0 | 5.00 | 0.00 | 17 ± 16 | 45 | 562 ± 18 | 532 | 0.13 | 1.90 | 0.10 |
| 0 | 5.00 | 0.00 | 45 ± 16 | 73 | 525 ± 18 | 500 | 0.88 | 2.11 | 0.24 |
| 0 | 5.00 | 0.00 | 30 ± 12 | 50 | 519 ± 17 | 502 | 0.39 | 0.64 | 0.52 |
| 0 | 5.00 | 0.00 | 55 ± 9 | 67 | 479 ± 17 | 458 | 1.3 | 0.90 | ND |
Percentage amended sulfide mineral flotation process water.
Tetrathionate concentration in the anolyte.
Thiosulfate concentration in the sulfide mineral flotation process water amended to the anolyte.
Cell voltage presented as an average ± SD and maximum value.
Potential versus Ag/AgCl electrode presented as an average ± SD and minimum value.
Power density.
Tetrathionate consumption (note, no tetrathionate was added in the 100% process wastewater without tetrathionate).
Coulombic efficiency (note, CE not calculated as no tetrathionate was added).
ND, not determined.
Fig. 2.
Anode (■) and cathode (●) potentials and cell voltages (▴) of the cation exchange membrane MFCs inoculated with the Kristineberg acid mine drainage stream sediment and with increasing amounts of amended sulfide mineral flotation process water.
Similar whole cell voltage values and anode potentials were achieved using the anion exchange membrane MFCs (Table 1). However, the power densities and coulombic efficiencies were lower than with the cation exchange membrane. A stable whole cell voltage was observed in the first 15 days with 100% process wastewater without added tetrathionate after which additional tetrathionate (1 mM) was amended after 16 and 24 days, resulting in increased whole cell voltage (Table 1). This suggested the MFC was substrate-limited under batch mode, and it would be interesting to investigate whether higher voltage could be achieved in continuous mode. An anion exchange membrane control MFC (with no process wastewater) also generated an electrical voltage with no statistically valid difference between the data sets (Table 1). This also supported the hypothesis that the process wastewater did not inhibit the MFC.
3.3. Microbial community in the anion exchange membrane MFC
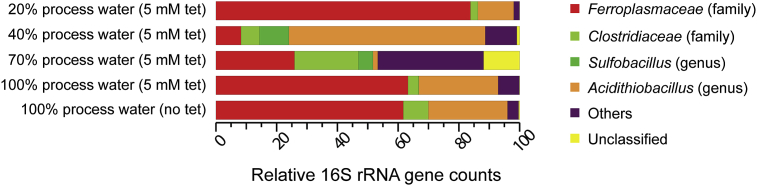
The microbial community of the tetrathionate-fed Kristineberg-inoculated MFC was dominated by strains with 16S rRNA genes that aligned (in order of greatest to least number of sequence reads) within the genera Thermoplasma, Ferroplasma, Leptospirillum, and Acidithiobacillus (Supplemental File 3). This was similar to the microbial community of the Kristineberg acid mine drainage stream (rather than the stream sediment used in this study) that is dominated by Acidithiobacillus spp., but also contains both Thermoplasma- and Ferroplasma-like species [23]. The presence of strains with 16S rRNA gene sequences similar to Acidithiobacillus was not surprising, as some species grow anaerobically utilizing elemental sulfur [39] and A. ferrooxidans is present in other acidic, tetrathionate-fed MFCs [22]. The low number of Acidithiobacillus-like 16S rRNA gene reads compared to, for instance, Ferroplasma-like species that have not previously been shown to utilize ISCs, was surprising. However, it is believed that the Ferroplasma-like population played a role in the MFCs, as it was retained during successive subculturing and re-inoculation in increasing concentrations of the mining process water (Fig. 3). In addition, the presence of 16S rRNA gene sequences similar to those from the genus Leptospirillum was also surprising, as characterized species from this genus have not previously been shown to grow anaerobically [40]. It is possible that the Leptospirillum-like population was carried over from the acid mine drainage stream sediment inoculum [23], although they potentially also play an active role in the MFCs, as similar populations were reported in tetrathionate-fed MFCs [22]. The microbial community from the anode compartment of the tetrathionate-fed acid-sulfate-soil-inoculated MFC was also investigated by 16S rRNA gene sequencing. The identified community consisted of 16S rRNA gene sequences that aligned within the Acidithiobacillus and Sulfobacillus genera (Supplemental File 3). Some cultured Sulfobacillus species are facultative anaerobes [41], although species from this genus have not previously been identified in MFCs. The 16S rRNA gene sequencing data identified some populations that for instance, have not previously been demonstrated to utilize ISCs. These results highlight the difficulty of assigning functional and metabolic traits based solely upon 16S rRNA gene similarity, and this type of data must be interpreted with caution.
Fig. 3.
Microbial community development (based upon relative 16S rRNA gene counts) with increasing amounts of amended sulfide mineral flotation process wastewater in the anion exchange membrane MFC originally inoculated from the Kristineberg culture. Abbreviation: tet, tetrathionate.
The microbial community from the tetrathionate-fed, anion exchange membrane MFC originally inoculated from the Kristineberg culture with increasing concentrations of mining process water continued to be dominated by 16S rRNA gene sequences that aligned within the Ferroplasmaceae family and Acidithiobacillus genus present in the inoculum (Fig. 3). In addition, 16S RNA gene sequences that aligned within the Sulfobacillus genus were also selected that were not part of the dominating community in the preliminary MFCs. Finally, a ‘tail’ of low abundance species was present that might also play a role in substrate utilization and electricity generation in the MFC. This mixed microbial community was similar to that identified in tetrathionate-fed MFCs inoculated with mining process waters [22], but varied from another low pH bioelectrochemical system in which organic carbon-oxidizing Acidiphilium spp. were detected on the anode of a pH 3 sediment/water interface microcosm in the Rio Tinto river [19].
3.4. Role of microorganisms in electricity generation
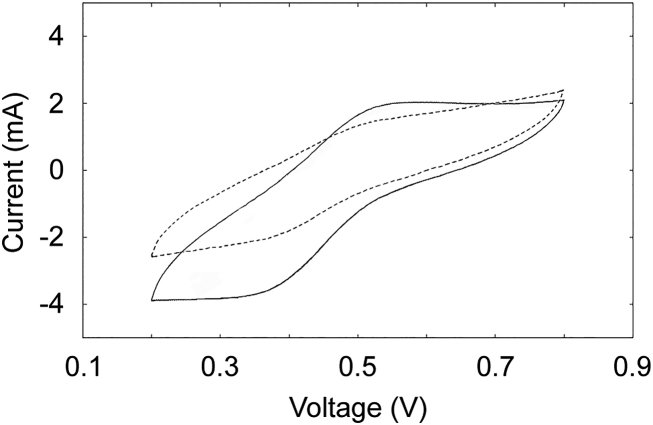
Cyclic voltammetry was applied to the anodes during electricity generation by the process wastewater test and mineral salts medium controls to investigate the role of microbial catalyzed electricity generation. The results showed that, compared to the abiotic control, cation exchange membrane MFCs inoculated with the Kristineberg acid mine drainage stream sediment produced more current during cyclic voltammetry (Fig. 4). A similar result was obtained with the other MFCs operated at the same time (Supplemental File 4). The current production indicated the acidophilic microorganisms at least partially mediated electron transfer to the anode.
Fig. 4.
Cyclic voltammetry of the cation exchange membrane MFCs inoculated with the Kristineberg acid mine drainage stream sediment in the presence of 100% process wastewater with no additional tetrathionate (solid line) and an abiotic control (dashed line).
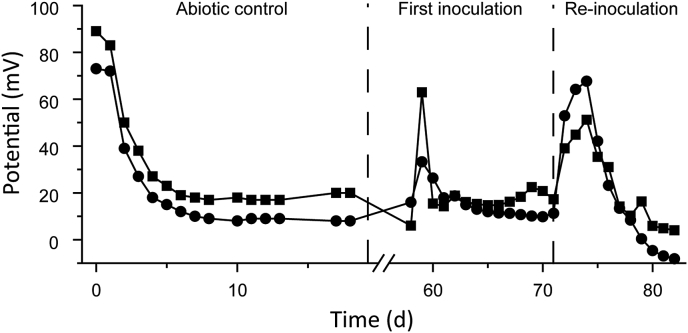
Additional abiotic controls were carried out to investigate electrical voltage generation in the absence of acidophilic microorganisms compared to data from the inoculated MFCs with 100% mining process water without additional tetrathionate (Fig. 5). The whole cell voltage values of the inoculated MFCs dropped slowly in a linear manner compared to the rapid decrease in control MFCs (Fig. 1 & Table 1). This suggested the ISC had a certain chemical energy that can be extracted as electricity in a chemical fuel cell, but the microorganisms increased the efficiency of the process by catalyzing substrate degradation and transferring electrons to the anode. This was also supported by the inoculation of acidophilic microorganisms into the anolyte that resulted in increased whole cell voltage (Fig. 5).
Fig. 5.
Development of whole cell voltage in abiotic control anion exchange (■) and cation exchange (●) chemical fuel cells before subsequent inoculation from their respective MFCs. The x-axis has been broken between days 18 and 58.
3.5. Potential substrate utilization pathway
A speculative pathway for substrate utilization in the ISC-fed MFCs is hydrolysis of tetrathionate to thiosulfate, elemental sulfur and sulfate carried out by Acidithiobacillus spp. [31]. Anaerobic growth of A. ferrooxidans with elemental sulfur as substrate has been suggested to occur, at least in part, by disproportionation to form hydrogen sulfide and sulfate [39]. This was supported by the end products from tetrathionate-fed MFCs [22] and accumulation of a yellow precipitate on the anode surface in this study (visual observation), that was confirmed as elemental sulfur by transmission electron microscopy [22]. 16S rRNA gene sequences that aligned with Ferroplasma and Thermoplasma genera were surprising since, although cultured species from this genera can grow anaerobically [42], [43], they have not been shown to grow utilizing ISCs [44]. It is possible that these populations were able to utilize organic carbon released from the autotrophic species [45]; that the Ferroplasma-like species acted as an electron shuttle in a similar manner to synergistic interactions between contaminant-degrading species and electron transfer to the anode by e.g. Geobacter spp. (reviewed in [46]); or alternatively, a novel species able to utilize ISCs had been selected. These hypotheses are under investigation via metagenomic and metatranscriptomic analyses of the anion exchange membrane MFC population.
In conclusion, this study demonstrated that electrical currents can be generated by acidophilic microorganisms from real industrial wastewater, opening up the possibility of using this technology to bioremediate mining process wasters by bioelectrochemistry. A quick response time from tetrathionate addition to increased whole cell voltage indicated that electricity generation was highly dependent on the level of substrate, and it may be possible to increase cell voltage by continuously supplying the MFC with process wastewater. The microorganisms selected in the population included microorganisms with 16S rRNA gene sequences similar to those previously identified in an acidic MFC (e.g. Acidithiobacillus-like and Ferroplasma-like species), as well as a population aligning with the Sulfobacillus genus not previously identified in MFCs.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
The research leading to these results has received funding from the European Union Seventh Framework Program (FP7/2012-2016) under grant agreement number 282970 (BioElectroMET). We thank our consortium members for comments on the manuscript. Sequencing was carried out at the National Genomics Infrastructure hosted by Science for Life Laboratory. Bioinformatic computations were performed on resources provided by SNIC through the Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under project b2013127.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.resmic.2016.04.010.
Contributor Information
Gaofeng Ni, Email: gaofeng.ni@lnu.se.
Stephan Christel, Email: stephan.christel@lnu.se.
Pawel Roman, Email: Pawel.Roman@wetsus.nl.
Zhen Lim Wong, Email: zhen.wong07@yahoo.co.uk.
Martijn F.M. Bijmans, Email: Martijn.Bijmans@wetsus.nl.
Mark Dopson, Email: mark.dopson@lnu.se.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Liljeqvist M., Sundkvist J.E., Saleh A., Dopson M. Low temperature removal of inorganic sulfur compounds from mining process waters. Biotechnol Bioeng. 2011;108:1251–1259. doi: 10.1002/bit.23057. [DOI] [PubMed] [Google Scholar]
- 2.Schippers A., Jozsa P.G., Sand W. Sulfur chemistry in bacterial leaching of pyrite. Appl Environ Microbiol. 1996;62:3424–3431. doi: 10.1128/aem.62.9.3424-3431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson D.B. Recent developments in microbiological approaches for securing mine wastes and for recovering metals from mine waters. Minerals. 2014;4:279–292. [Google Scholar]
- 4.Rzhepishevska O.I., Lindström E.B., Tuovinen O.H., Dopson M. Bioleaching of sulfidic tailing samples with a novel, vacuum-positive pressure driven bioreactor. Biotechnol Bioeng. 2005;92:559–567. doi: 10.1002/bit.20609. [DOI] [PubMed] [Google Scholar]
- 5.Dopson M., Johnson D.B. Biodiversity, metabolism and applications of acidophilic sulfur- metabolizing micro-organisms. Environ Microbiol. 2012;14:2620–2631. doi: 10.1111/j.1462-2920.2012.02749.x. [DOI] [PubMed] [Google Scholar]
- 6.Sahinkaya E., Ozkaya B., Kaksonen A.H., Puhakka J.A. Sulfidogenic fluidized-bed treatment of metal-containing wastewater at low and high temperatures. Biotechnol Bioeng. 2007;96:1064–1072. doi: 10.1002/bit.21195. [DOI] [PubMed] [Google Scholar]
- 7.Ňancucheo I., Johnson D.B. Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb Biotechnol. 2012;5:34–44. doi: 10.1111/j.1751-7915.2011.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bijmans M.F.M., De Vries E., Chun-Hui Y., Buisman C.J.N., Lens P., Dopson M. Sulfate reduction at pH 4.0 for treatment of process and wastewaters. Biotechnol Prog. 2010;29:1029–1037. doi: 10.1002/btpr.400. [DOI] [PubMed] [Google Scholar]
- 9.Logan, Bruce E., Bert Hamelers, René Rozendal, Uwe Schröder, Jürg Keller, Stefano Freguia. Microbial fuel cells: methodology and technology. Environ Sci Technol. 2006;40:5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- 10.Caccavo F., Lonergan D.J., Lovley D.R., Davis M., Stolz J.F., McInerney M.J. Geobacter sulfurreducens sp. nov., a hydrogen-oxidizing and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkateswaran K., Moser D.P., Dollhopf M.E., Lies D.P., Saffarini D.A., MacGregor B.J. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol. 1999;49:705–724. doi: 10.1099/00207713-49-2-705. [DOI] [PubMed] [Google Scholar]
- 12.He C.-S., Mu Z.-X., Yang H.-Y., Wang Y.-Z., Mu Y., Yu H.-Q. Electron acceptors for energy generation in microbial fuel cells fed with wastewaters: a mini-review. Chemosphere. 2015;140:12–17. doi: 10.1016/j.chemosphere.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Logan B.E., Murano C., Scott K., Gray N.D., Head I.M. Electricity generation from cysteine in a microbial fuel cell. Water Res. 2005;39:942–952. doi: 10.1016/j.watres.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.R., Jung S.H., Regan J.M., Logan B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour Technol. 2007;98:2568–2577. doi: 10.1016/j.biortech.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y., Wang X., Logan B.E., Lee H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol. 2008;78:873–880. doi: 10.1007/s00253-008-1360-2. [DOI] [PubMed] [Google Scholar]
- 16.Sun J., Hu Y-y, Bi Z., Cao Y-q. Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber microbial fuel cell. Bioresour Technol. 2009;100:3185–3192. doi: 10.1016/j.biortech.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Dopson M., Ni G., Sleutels T.H.J.A. Possibilities for extremophilic microorganisms in microbial electrochemical systems. FEMS Microbiol Rev. 2015 doi: 10.1093/femsre/fuv044. In-press, fuv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borole A.P., O'Neill H., Tsouris C., Cesar S. A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum. Biotechnol Lett. 2008;20:1367–1372. doi: 10.1007/s10529-008-9700-y. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Munoz J., Amils R., Fernandez V.M., De Lacey A.L., Malki M. Electricity generation by microorganisms in the sediment-water interface of an extreme acidic microcosm. Int Microbiol. 2011;14:73–81. doi: 10.2436/20.1501.01.137. [DOI] [PubMed] [Google Scholar]
- 20.Malki M., De Lacey A.L., Rodriguez N., Amils R., Fernandez V.M. Preferential use of an anode as an electron acceptor by an acidophilic bacterium in the presence of oxygen. Appl Environ Microbiol. 2008;74:4472–4476. doi: 10.1128/AEM.00209-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H., Kim B., Kim J., Lee T., Yu J. Electricity generation and microbial community in microbial fuel cell using low-pH distillery wastewater at different external resistances. J Biotechnol. 2014;186:175–180. doi: 10.1016/j.jbiotec.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Sulonen M.L., Kokko M.E., Lakaniemi A.M., Puhakka J.A. Electricity generation from tetrathionate in microbial fuel cells by acidophiles. J Hazard Mater. 2015;284c:182–189. doi: 10.1016/j.jhazmat.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 23.Liljeqvist M., Ossandon F.J., González C., Rajan S., Stell A., Valdes J. Metagenomic analysis reveals adaptations to a cold-adapted lifestyle in a low-temperature acid mine drainage stream. FEMS Microb Ecol. 2015;91 doi: 10.1093/femsec/fiv011. [DOI] [PubMed] [Google Scholar]
- 24.Wu X., Wong Z.L., Sten P., Engblom S., Österholm P., Dopson M. Microbial community potentially responsible for acid and metal release from an Ostrobothnian acid sulfate soil. FEMS Microbiol Ecol. 2013;84:555–563. doi: 10.1111/1574-6941.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X., Sten P., Engblom S., Nowak P., Osterholm P., Dopson M. Impact of mitigation strategies on acid sulfate soil chemistry and microbial community. Sci Tot Environ. 2015;526:215–221. doi: 10.1016/j.scitotenv.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 26.Dopson M., Lindström E.B. Potential role of Thiobacillus caldus in arsenopyrite bioleaching. Appl Environ Microbiol. 1999;65:36–40. doi: 10.1128/aem.65.1.36-40.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roman P., Veltman R., Bijmans M.F., Keesman K.J., Janssen A.J. Effect of methanethiol concentration on sulfur production in biological desulfurization systems under haloalkaline conditions. Environ Sci Technol. 2015;49(15):9212–9221. doi: 10.1021/acs.est.5b01758. [DOI] [PubMed] [Google Scholar]
- 28.Roman P., Bijmans M.F.M., Janssen A.J.H. Quantification of individual polysulfides in lab-scale and full-scale desulfurisation bioreactors. Environ Chem. 2014;11:702–708. [Google Scholar]
- 29.Ter Heijne A., Liu F., Weijden R., Weijma J., Buisman C.J., Hamelers H.V. Copper recovery combined with electricity production in a microbial fuel cell. Environ Sci Technol. 2010;44:4376–4381. doi: 10.1021/es100526g. [DOI] [PubMed] [Google Scholar]
- 30.Meulenberg R., Scheer E.J., Pronk J.T., Hazeu W., Bos P., Kuenen J.G. Metabolism of tetrathionate in Thiobacillus acidophilus. FEMS Microbiol Lett. 1993;112:167–172. [Google Scholar]
- 31.Quatrini R., Appia-Ayme C., Denis Y., Jedlicki E., Holmes D., Bonnefoy V. Extending the models for iron and sulfur oxidation in the extreme acidophile Acidithiobacillus ferrooxidans. BMC Genom. 2009;10:394. doi: 10.1186/1471-2164-10-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangold S., Valdes J., Holmes D.S., Dopson M. Sulfur metabolism in the extreme acidophile Acidithiobacillus caldus. Front Microbio. 2011;2:17. doi: 10.3389/fmicb.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hugerth L.W., Wefer H.A., Lundin S., Jakobsson H.E., Lindberg M., Rodin S. DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies. Appl Environ Microbiol. 2014;80:5116–5123. doi: 10.1128/AEM.01403-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindh M.V., Figueroa D., Sjöstedt J., Baltar F., Lundin D., Andersson A. Transplant experiments uncover Baltic Sea basin-specific responses in bacterioplankton community composition and metabolic activities. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X.M., Cheng K.Y., Selvam A., Wong J.W.C. Bioelectricity production from acidic food waste leachate using microbial fuel cells: effect of microbial inocula. Process Biochem. 2013;48:283–288. doi: 10.1016/j.biortech.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Slonczewski J.L., Fujisawa M., Dopson M., Krulwich T.A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol. 2009;55:1–79. doi: 10.1016/S0065-2911(09)05501-5. [DOI] [PubMed] [Google Scholar]
- 38.Dopson M., Sundkvist J.-E., Lindström E.B. Toxicity of metal extraction and flotation chemicals to Sulfolobus metallicus and chalcopyrite bioleaching. Hydrometallurgy. 2006;81:205–213. [Google Scholar]
- 39.Osorio H., Mangold S., Denis Y., Nancucheo I., Johnson D.B., Bonnefoy V. Anaerobic sulfur metabolism coupled to dissimilatory iron reduction in the extremophile Acidithiobacillus ferrooxidans. Appl Environ Microbiol. 2013;79:2172–2181. doi: 10.1128/AEM.03057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hippe H. Leptospirillium gen. nov (ex Markoysan 1972), nom. rev., including Leptospirillium ferrooxidans sp nov (ex Markoysan 1972), nom. rev. and Leptospirillium thermoferrooxidans sp nov (Golovacheva et al. 1992) Int J Syst Evol Microbiol. 2000;50:501–503. doi: 10.1099/00207713-50-2-501. [DOI] [PubMed] [Google Scholar]
- 41.Johnson D.B., Joulian C., d'Hugues P., Hallberg K.B. Sulfobacillus benefaciens sp. nov., an acidophilic facultative anaerobic Firmicute isolated from mineral bioleaching operations. Extremophiles. 2008;12:789–798. doi: 10.1007/s00792-008-0184-4. [DOI] [PubMed] [Google Scholar]
- 42.Dopson M., Baker-Austin C., Bond P. Towards determining details of anaerobic growth coupled to ferric iron reduction by the acidophilic archaeon 'Ferroplasma acidarmanus' Fer1. Extremophiles. 2007;11:159–168. doi: 10.1007/s00792-006-0029-y. [DOI] [PubMed] [Google Scholar]
- 43.Segerer A., Langworthy T.A., Stetter K.O. Thermoplasma acidophilum and Thermoplasma volcanium sp. nov. from Solfatara Fields. Syst Appl Microbiol. 1988;10:161–171. [Google Scholar]
- 44.Dopson M., Baker-Austin C., Hind A., Bowman J.P., Bond P.L. Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp. nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl Environ Microbiol. 2004;70:2079–2088. doi: 10.1128/AEM.70.4.2079-2088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ňancucheo I., Johnson D.B. Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl Environ Microbiol. 2010;76:461–467. doi: 10.1128/AEM.01832-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H., Luo H., Fallgren P.H., Jin S., Ren Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol Adv. 2015;33:317–334. doi: 10.1016/j.biotechadv.2015.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.