Abstract
Background
Sarcoidosis is a granulomatous inflammatory disease of unknown cause. Its prevalence in Germany is approximately 46 per 100 000 persons.
Methods
This article is based on pertinent publications retrieved by a selective search in PubMed.
Results
A presumptive diagnosis of sarcoidosis is made in any patient with a granulomatous inflammation that is not explained by any other identifiable cause, such as an infection or foreign body. Non-caseating granulomas containing epithelioid cells are its histological hallmark. Recently developed diagnostic techniques, including positron emission tomography and magnetic resonance imaging, have made it easier to detect organ involvement and to assess the activity of the disease. The pattern of organ involvement varies from patient to patient. Many patients have a systemic inflammatory reaction with subfebrile or febrile temperatures, night sweats, weight loss, diminished physical reserve, and fatigue. Sarcoidosis often resolves spontaneously. Detection of organ involvement is not necessarily an indication for treatment, but treatment is clearly needed if there is symptomatic cardiac involvement or any involvement of the central nervous system. Oral corticosteroids are the first line of treatment. Their long-term use can cause serious complications.
Conclusion
The treatment of patients with sarcoidosis, particularly those with complicated disease courses, requires close collaboration of the primary care physician with a specialized interdisciplinary center.
Sarcoidosis is a relatively rare disease that affects patients all over the world (1). Estimates of its prevalence in Europe have ranged from 1 to 64 per 100 000 persons (1). It is more common in Scandinavia than in southern Europe. Two studies from Germany yielded the same prevalence of approximately 46 per 100 000 persons (2, 3). Sarcoidosis can arise at any age; it has two incidence peaks, one in young adulthood and another, lower one around age 60 (4). It more commonly affects women (5). Seasonal clustering of the initial manifestation of sarcoidosis has been noted, with onset most commonly in the spring and somewhat less commonly in the fall, particularly for the acute-onset form of the disease (4, e1).
Methods
The author systematically searched PubMed for pertinent publications from 1960 onward on the basis of her scientific and clinical experience. The publications considered for this article included reviews; reports of randomized and controlled trials, registry studies, and case-control studies; guidelines; and case reports.
Learning goals
This article is intended to inform the reader about
how sarcoidosis is diagnosed,
how organ manifestations are treated,
what tests are used to assess the disease course,
and the role of these tests in treatment monitoring.
Epidemiology.
Estimates of the prevalence of sarcoidosis in Europe range from 1 to 64 per 100 000 persons. It has two incidence peaks, one in young adulthood and another around age 60.
Definition and differential diagnosis
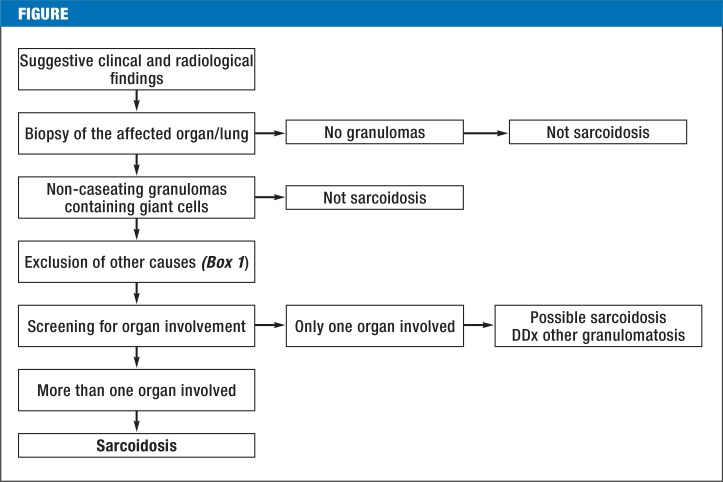
Sarcoidosis is a granulomatous inflammation that has no identifiable cause, such as an infection or a foreign body. It is thus a diagnosis of exclusion (Figure 1, Box 1) (6). Non-caseating granulomas with epithelioid cells are its histological hallmark and generally enable the pathologist to distinguish it from other systemic granulomatoses (6, 7). Sarcoidosis can nonetheless be difficult to tell apart from mycobacterial and, in particular, atypical mycobacterial disease, which often produces non-caseating granulomas, and from certain fungal diseases (rare in Germany) such as coccidiodomycosis and histoplasmosis (7). Cat scratch disease and toxoplasmosis are important differential diagnoses of sarcoidosis that mainly affect the lymphatic system. Chronic berylliosis (usually an occupational disease) and granulomatous changes due to immunodeficient states and cancer may be impossible to distinguish from sarcoidosis on histological grounds alone (6). The same holds for certain very rare hereditary diseases, such as Blau syndrome (e2). A granulomatous inflammation resembling sarcoidosis can also be induced by certain drugs, including recombinant interferon- α or - β (8). According to case reports, antibody therapy directed against tumor necrosis factor α (TNF-α) can induce such a reaction as well (9). This is paradoxical, because infliximab has been used successfully to treat sarcoidosis and the absence of TNF-α has actually been found to impede the development of granulomas.
Figure.
Algorithm for the diagnosis of sarcoidosis, modified from Baughman et al. (26).
DD, differential diagnosis
Box 1. The differential diagnosis of systemic diseases with granulomas that contain epithelioid and giant cells.
Tuberculosis (infection with M. tuberculosis)
Atypical mycobacterial disease
Fungal infection, histoplasmosis, coccidioidomycosis
Bartonellosis, toxoplasmosis, brucellosis
Sarcoid-like lesions due to cancer
Sarcoid-like lesions due to an immunodeficiency
Sarcoid-like lesions due to medications
Heavy-metal-associated granulomatosis
Chronic berylliosis
Systemic vasculitis
Isolated pulmonary disease: exogenous allergic alveolitis, silicosis
Definition.
Sarcoidosis is a granulomatous inflammation that has no identifiable cause, such as an infection or a foreign body. It is thus a diagnosis of exclusion.
Pathogenesis
The cause of sarcoidosis is not yet known (10). In view of the heterogeneity of the disease, it is assumed to have a variety of triggers. 4% of cases are familial, implying a genetic predisposition. Black Americans are more commonly and more severely affected than whites, while Japanese patients have a different pattern of prevalence of individual organ manifestations than Europeans (e3). Environmental influences are known as well. In Europe and Asia, there is a marked north-south prevalence gradient for sarcoidosis (e4).
Clinical features
The clinical features are determined by the pattern of organ involvement. Many patients have a systemic inflammatory reaction with subfebrile or febrile temperatures, night sweats, weight loss, diminished physical reserve, and fatigue (11).
Pathogenesis.
The cause of sarcoidosis is unknown. In view of the heterogeneity of the disease, it is assumed to have a variety of triggers.
The reported rates of organ involvement vary widely across studies, both because of ethnic variation and because the rate of detection of organ involvement (often asymptomatic) depends on the diagnostic method used (Table) (12, 13). The lung is the most commonly involved organ (4, e5): many studies have shown that the the pulmonary parenchyma and mediastinal lymph nodes are involved in over 90% of patients. Other lymph nodes, the liver, and the eyes are involved in 15–20% of all patients (e6). About 15% of patients of European ancestry have cutaneous involvement (4, 14). Cardiac and musculoskeletal involvement are now detected at much higher rates than before (10–20% each) since the introduction of magnetic resonance imaging (MRI) and 18-fluorodeoxyglucose positron emission tomography [18F-FDG-PET] for this purpose (15). The previous rate of detection of cardiac involvement was 2–7%, despite a prevalence of more than 20% in autopsy studies (16). The central nervous system is involved in 5–10% of patients (17). About 5% have chronic sinusitis.
Table. Various manifestations of sarcoidosis classified by organ.
| Organ* | Manifestations |
|---|---|
| Lung (> 90%) |
Mediastinal lymphadenopathy. parenchymal involvement (alveoli/interstitium). pulmonary fibrosis. bronchial involvement (app. 10%. often obstructive). pleural involvement (rare). pulmonary hypertension (rare in patients of European descent) |
| Liver (15–20%) |
Nodular parenchymal involvement. cirrhosis. involvement of the biliary pathways. cholestasis. biliary cirrhosis. vascular involvement/stenosis due to lymphadenopathy (rare). portal hypertension |
| Skin (app. 15%) |
Localized involvement. usually papular; lupus pernio (involvement of the face). sarcoidosis in a scar (e.g.. a tattoo). erythema nodosum (histologically not a granuloma. nonspecific finding). erythema anulare |
| Heart (2–5%) |
Conducting system (common; bundle branch block. AV block). myocardial involvement (danger of ventricular tachycardia). pericardial involvement (rare) |
| Kidney (app. 5%) |
Nephrolithiasis. renal failure of postrenal cause (hypercalcemia). interstitial nephritis |
| Central nervous system (app. 5%) | Nodular lesions. pituitary involvement (often with diabetes insipidus). encephalopathy. meningeal involvement |
| Peripheral nervous system (app. 5%) | Facial nerve palsy. other cranial nerve deficits. mononeuritis multiplex. polyradiculitis. polyneuropathy |
| Eye (15–20%) |
Lacrimal gland involvement. granulomatous conjunctivitis. anterior uveitis (iridocyclitis. trabeculitis with glaucoma). intermediate uveitis. posterior uveitis (choroiditis. periphlebitis retinae). optic neuritis. orbital granuloma |
| Bone (app. 1%) |
Nodular lesions. cystic lesions involving the joints (Jüngling’s disease). central necrotizing foci in the skull (very rare in patients of European descent). bone marrow involvement |
*The percentages for the involvement of various organs are derived from an American case-control study (26).
Special constellations of organ involvement
The triad of ankle arthritis, mediastinal lymphadenopathy, and erythema nodosum is called Löfgren syndrome. The combination of parotitis, uveitis, mediastinal lymphadenopathy, and (sometimes) facial nerve palsy is called Heerfordt syndrome (e7). Sarcoidosis affecting the skin of the face is called lupus pernio.
Pulmonary involvement.
Many studies have shown that the the pulmonary parenchyma and mediastinal lymph nodes are involved in over 90% of patients. Sarcoidosis that does not involve the lungs is rare.
Diagnostic evaluation
Imaging studies
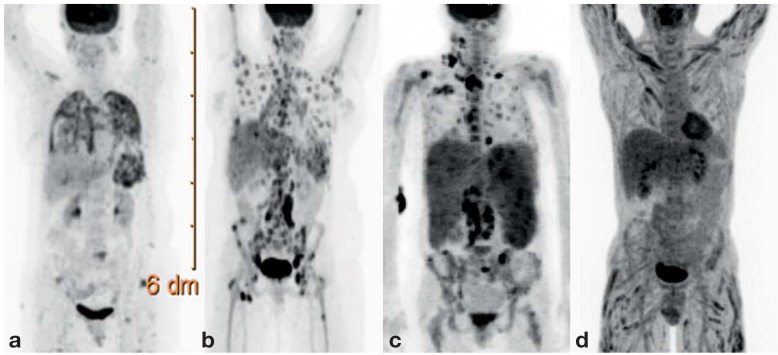
Recent developments in diagnostic imaging, particularly PET and MRI, have made organ involvement in sarcoidosis easier to detect (15, 18). On the other hand, new problems arise since evidence of organ involvement does not directly indicate the need for treatment. Moreover, patients are often troubled by the detection of clinically insignificant organ manifestations. Assessing the clinical relevance of the findings of imaging studies has become the key issue and is an increasingly complex task for the treating physicians. Many different diagnostic imaging techniques are available. Pulmonary involvement is well seen in a conventional chest x-ray. The abdominal organs and superficial lymph nodes can be visualized with ultrasonography. High-resolution computerized tomography (HRCT) is an optimal means of demonstrating lung involvement and mediastinal lymphadenopathy, but at the cost of exposure to ionizing radiation (e8). Often, there is a discrepancy between imaging and functional impairment. Although HRCT may show diffuse pulmonary involvement (particularly of the fine nodular type), pulmonary function is often no more than mildly impaired (19, e9). MRI is suitable for the detection and follow-up of cardiac and central nervous system (CNS) involvement (20, e10). Whole-body MRI can also be used to search for organ involvement (21) but is only available in a few centers. A further option is 18F-FDG-PET (Figure 2) (e11– e13), which reveals active inflammation in all organs where it is present. Special PET techniques and relevant experience are needed to detect cardiac and CNS involvement, as these two organ systems have high rates of metabolic activity.
Figure 2.
18F-FDG-PET findings in multi-organ involvement. a) Lung and spleen involvement; b) diffuse bone and lymph node involvement;c) marked involvement of the lymph nodes and spleen, as well as less marked involvement of the bone marrow and liver. d) Marked, diffuse muscle involvement with relatively mild involvement of the lymph nodes and liver.
Diagnostic imaging.
Recent developments in diagnostic imaging have markedly improved the detection rates for organ involvement by sarcoidosis.
Confirming the diagnosis by biopsy
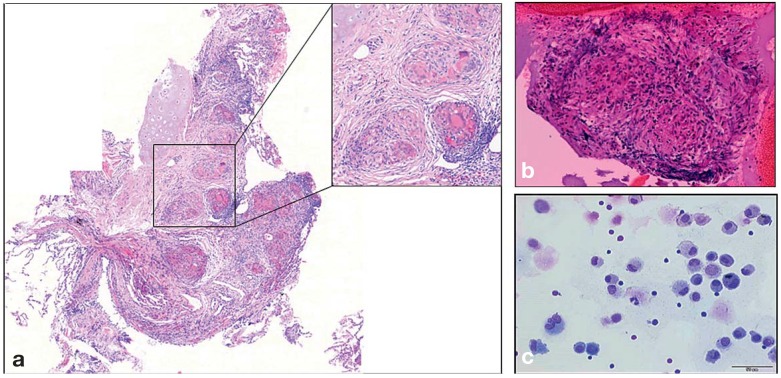
There is a consensus that an initial diagnosis of sarcoidosis should be confirmed by biopsy, because later confirmation is often made more difficult by treatment, and the potential indications for treatment in the patient’s further course may then be impossible to assess (22, 23). Angiotensin converting enzyme (ACE) and the soluble interleukin-2 receptor (sIL-2R), two commonly used biomarkers for sarcoidosis, are nonspecific and therefore unsuitable for confirming the diagnosis. The lung is the most common site of a confirmatory biopsy, because pulmonary involvement is very common and because bronchoscopy affords easy access. The mediastinal lymph nodes are easily reached for sampling with an ultrasonically guided fine-needle biopsy (EBUS), the lung parenchyma with a transbronchial biopsy (TBB) (Figure 3) (e14– e16). These two methods in combination have the highest diagnostic yield (more than 90%) (e16). As bronchoscopy with EBUS is a highly safe method enabling very good assessment of the mediastinal lymph nodes, it is recommended for confirming the diagnosis of Löfgren syndrome (24, 25, e15– e17). Moreover, bronchoalveolar lavage (BAL) should be performed during bronchoscopy (25). Differential and immune cytology of the BAL sample typically reveal CD4 lymphocytosis (25), and part of the sample should be sent for microbiological studies to rule out an infectious cause. When organs other than the lungs and mediastinal lymph nodes are biopsied, native tissue should also be sent to the microbiological laboratory, because, despite the use of PCR-based techniques, formalin-fixed tissue only rarely suffices for the exclusion of florid infection (22). Culture is the gold standard for the detection of any pathogen in the differential dignosis of sarcoidosis (e18). The tine test and more recent tests based on interferon production (IGRA) cannot rule out florid tuberculosis with absolute certainty, nor can they detect atypical mycobacterial disease (5).
Figure 3.
Cytological and histological findings in sarcoidosis
a) transbronchial lung biopsy specimen, with non-caseating granulomas containing epthelioid and giant cells
b) a granuloma obtained by ultrasound-guided needle biopsy of a mediastinal lymph node
c) cell smear of a bronchoalveolar lavage (BAL) specimen, showing the typical abundance of CD4+ T lymphocytes (small, round cells with a large nucleus and little cytoplasm). Most of the other cells are alveolar macrophages with a large amount of cytoplasm.
Confirmation of the diagnosis by biopsy.
The lung is the most common site of a confirmatory biopsy, because pulmonary involvement is very common and because bronchoscopy affords easy access.
Organ screening
Any clinical evidence that suggests organ involvement calls for meticulous further investigation (Box 2). Whenever an initial diagnosis of sarcoidosis is made, the lungs, heart, and eyes should always be examined by the corresponding specialists.
Box 2. Screening for organ involvement.
-
Standard
physical examination including eye exam
chest x-ray
pulmonary function tests, diffusion capacity
abdominal ultrasonography
echocardiography
ECG, holter ECG
urinary and serum calcium levels
serum creatinine and urea levels
AST, ALT, AP, gamma-GT
-
Optional
MRI if CNS involvement is suspected
MRI if muscle involvement is suspected
18F-FDG-PET if diffuse bone involvement is suspected, or to search for foci of involvement
AP, alkaline phosphatase; 18F-FDG-PET; 18-fluordeoxyglucose positron emission tomography; gamma-GT, gamma-glutamy ltransferase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; MRI, magnetic resonance imaging; ECG, electrocardiography
Most types of organ involvement arise within two years of the onset of disease. Thus, annual organ screening is indicated, particularly in the first few years after diagnosis.
Disease course
Sarcoidosis often takes a favorable course. The rate of spontaneous resolution, particularly in Löfgren syndrome and in patients with asymptomatic organ involvement, is very high (85%). Spontaneous resolution generally occurs within six months of onset (13, 26, 27). Follow-up appointments are recommended every three months in the first year after diagnosis, and then annually for the next 3–5 years, in patients who have no indication for treatment and no further problems (5, 26, 28). Some 10% have a progressive course despite immunosuppressive treatment (5, 26, 27). The complications of long-term corticosteroid treatment are a major problem; many patients become obese and develop a complicated metabolic syndrome (5, 29, e19).
Disease course.
Sarcoidosis often takes a favorable course. The rate of spontaneous resolution, particularly in Löfgren syndrome and in patients with asymptomatic organ involvement, is very high (85%).
Sarcoidosis mildly elevates mortality (1), with death due mainly to cardiac and CNS involvement (1). In Europe and the USA, sarcoidosis accounts for approximately 3% of all lung transplantations (30, e20), the main indications being sarcoidosis-induced pulmonary fibrosis, cystic changes with mycetomes, and pulmonary hypertension (30, e21, e22). Sudden cardiac death in young adults may often be due to sarcoidosis (16, e23, e24).The CNS can be affected by sarcoidosis not just directly, but also by a (very rare) sarcoidosis-associated leukencephalopathy, which is usually fatal (31, e25).
Treatment
The physician deciding whether and how to treat sarcoidosis should keep in mind the high rate of spontaneous resolution, the high prevalence of asymptomatic organ involvement, and the major complications of long-term corticosteroid use (5, 26). There is no doubt that serious cardiac arrhythmias, myocardial involvement, and CNS involvement are clear indications for treatment. There is an international consensus that any other type of organ involvement should be treated only if symptomatic, and only if there is a risk of permanent damage to the organ (5, 12, 22, 28). Thus, for example, an extensive diagnostic evaluation of the lungs with pulmonary function tests and (usually ergometric) stress testing is required before any treatment for pulmonary sarcoidosis is initiated (5, 12). Treatment is indicated only if the pulmonary function and diffusion capacity are markedly restricted, with resulting diminution of physical reserve. An analogous evaluation of any involved organ system is required before the initiation of treatment. For the liver, treatment is considered indicated when the liver enzyme concentrations are at least three times the upper limit of normal (28). The biliary system is very rarely affected in isolation; in such cases, treatment decisions are based on the alkaline phosphatase (AP) level. Hypercalcemia arises commonly in sarcoidosis because of increased production of active vitamin D3 (calcitriol) through the activity of the enzyme 1-α-hydroxylase, which is produced by macrophages within granulomas (32). Hypercalcemia that does not respond to dietary measures and avoidance of sunlight is a clear indication for the treatment with prednisone. Because of their predisposition to hypercalcemia, patients with sarcoidosis should not be given calcium or vitamin D supplements to prevent osteoporosis. If they have osteoporosis, biphosphonates are recommended as the primary treatment (32). Ocular involvement that does not respond to treatment with local immune suppression is another common indication for treatment (e6, e26).
Mortality.
Sarcoidosis mildly elevates mortality, with death due mainly to cardiac and CNS involvement. In Europe and the USA, sarcoidosis accounts for app. 3% of all lung transplantations.
No treatment for sarcoidosis has yet been approved by the European regulatory authority. Oral corticosteroid administration is the standard treatment (29), even though the scientific evidence underlying it is inadequate by current standards. There has been only a single randomized, placebo-controlled, multicenter trial from Finland, in which patients with sarcoidosis (radiological class I and II) were given oral corticosteroids for three months, followed by inhaled corticosteroids for a further 15 months. The treated patients had a significantly higher forced vital capacity and better radiological findings 5 years after the start of the trial (33). All of the relevant specialty societies currently consider the performance of any further multicenter, placebo-controlled trials regarding the use of corticosteroids in sarcoidosis to be unethical.
Indications for treatment.
high-grade arrhythmia, myocardial involvement, CNS involvement
symptomatic organ involvement that might lead to permanent organ damage
Most experts recommend that the initial treatment of sarcoidosis should be with prednisone at a dose of 0.5 mg per kilogram of body weight (kgBW) (28, 29). Neurosarcoidosis is the sole exception, as higher doses must be used to overcome the blood-brain barrier (17). According to current guidelines, systemic corticosteroids (if indicated) should be given for at least 6 months, with a gradual reduction of the dose over the ensuing 6 months (28). If sarcoidosis is reactivated while the dose is still above the maintenance level of 7.5 mg per day (27), immunosuppressive combination therapy with methotrexate or azathioprine should be considered (22, 27). These two drugs are mainly used as second-line treatment, with the goal of limiting the corticosteroid dose; in a recently published study, it was shown that the immunosuppressive effects of the two drugs are roughly equivalent (34). This study, however, was neither blinded nor controlled and involved merely a comparison of retrospectively acquired data from two different centers that used different treatment strategies. The use of azathioprine or methotrexate in younger patients is problematic, particularly in those wishing to have children. In the latter case, the patient should be extensively informed and counseled before the treatment is begun. Both of these immunosuppressive drugs are teratogenic and potentially toxic to the bone marrow and liver, especially with long-term use. For this reason, regular monitoring of the differential bloodcount and liver enzyme levels is required. Moreover, both drugs produce gastrointestinal side effcts, whch may appear immediately after the start of treatment and take a fulminant course. If unacceptable side effects of methotrexate or aza-thioprine arise, or if these drugs are contraindicated, then combination therapy with leflunomide or myco-phenolate mofetil can be considered as well (29, e19, e27). The evidence for this combination is very weak.
Standard treatment.
Oral corticosteroid administration is the standard treatment, even though the scientific evidence underlying it is inadequate by current standards.
Second-line treatment.
immunosuppressive combination therapy with methotrexate or azathioprine is used as second-line treatment. These two drugs are mainly given in order to limit the corticosteroid dose.
In case sarcoidosis progresses despite immunosuppressive combination therapy and the progression is found to be due to increased inflammatory activity, an international consensus holds that anti-TNF-α antibody treatment with infliximab or adalimumab should be considered (5, 26, e19). Studies have shown that anti-TNF-α antibodies are effective only if they also block the membrane-bound form of TNF-α. A double-blind, placebo-controlled, multicenter clinical trial of add-on treatment with infliximab showed a significant improvement of forced vital capacity by 2.5% in 24 weeks compared to placebo, but no improvement of the quality of life (35). The same trial also revealed a significant improvement of cutaneous involvement (if present) under treatment with infliximab (e28). Case series, and the author’s own clinical experience, also indicate very good efficacy against neurosarcoidosis (35). Infliximab can be given at a dose of 3 mg/kgBW per day combined with prednisone + azathioprine/ methotrexate, or else 5 mg/kgBW combined with a corticosteroid alone. This treatment is usually maintained for 1 year (36, 37). Infliximab has not been approved in Germany for the treatment of sarcoidosis and must therefore be given off label. A request for reimbursement for the cost of infliximab treatment must be submitted to the patient’s statutory health carrier on the basis of the scientific evidence presented above.
Treatment monitoring and biomarkers
Before any treatment is begun, a biomarker and a diagnostic technique for involvement of the organ in question should be chosen and documented, so that the response to treatment can be assessed. Many different biomarkers have been used successfully to assess organ function. The inflammatory activity of sarcoidosis can be assessed very well with the soluble interleukin-2 receptor α (sIL-2R or sCD25) or neopterin; these two biomarkers can thus be used for treatment monitoring (38). Their blood levels vary considerably, however, depending on the organ involved. The sIL-2R level is misleadingly elevated in patients with renal failure (e29).
Anti-TNF-alpha antibody treatment.
If sarcoidosis progresses despite immunosuppressive combination therapy and the progression is found to be due to increased inflammatory activity, anti-TNF-alpha antibody treatment with infliximab or adalimumab should be considered.
Overview
Sarcoidosis is a rare disease with heterogeneous clinical features. Whenever the presumptive diagnosis of sarcoidosis is made, it should be confirmed by biopsy, and a florid infection or other granulomatous process should be excluded. The diagnosis of sarcoidosis does not, in itself, imply a need for treatment. Rather, the indication for treatment should be carefully evaluated in the light of a functional diagnostic assessment of all involved organs, which should also be used for treatment monitoring and guidance. Sarcoidosis generally responds to corticosteroid monotherapy, but about 10% of patients have steroid-resistant progressive disease.
Biomarkers.
The inflammatory activity of sarcoidosis can be assessed very well with the soluble interleukin-2 receptor alpha (sIL-2R or sCD25) or neopterin. These two biomarkers are suitabe for treatment monitoring.
Further information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their
15-digit“uniform CME number” (einheitliche Fortbildungsnummer, EFN).
The EFN must be entered in the appropriate field in the cme.aerzteblatt.de
website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 13 November 2016, and earlier CME units until the dates indicated:
“The Diagnosis and Treatment of Nail Diseases” (Issue 29–30/2016) until
16 October 2016,
“Breastfeeding and Complementary Feeding” (issue 25/2016) until
18 September 2016,
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the histological hallmark of sarcoidosis?
ulceration of the upper mucosal layers
granulocytic infiltration
necrotizing vasculitis
non-caseating granulomas containing epithelioid cells
hyperkeratosis and marked parahyperkeratosis
Question 2
Which of the following findings are compatible with sarcoidosis?
The tine test is negative and the IGRA is positive.
The polymerase chain reaction and culture for Mycobacterium tuberculosis are positive, but acid-fast bacilli cannot be demonstrated.
The Kveim test is negative.
Multiple organs are involved, histological examination reveals non-caseating granulomas, and other causes have been excluded.
Atypical mycobacteria have been demonstrated with the polymerase chain reaction.
Question 3
What clinical triad is called the Löfgren syndrome?
nystagmus, intention tremor, und dysarthria
ptosis, miosis, and enophthalmos
goiter, exophthalmos, and tachycardia
juvenile arthritis, chronic congestive heart failure, and psoriasis
ankle arthritis, mediastinal lymphadenopathy, and erythema nodosum
Question 4
Which of the findings below is obligatory for the confirmation of a diagnosis of sarcoidosis?
The diagnosis should be made entirely on clinical grounds.
An experienced radiologist can make the diagnosis with certainty on the basis of a high-resolution chest CT.
Whenever the presumptive diagnosis of sarcoidosis is made, mycobacterial cultures should be obtained.
An elevated ACE level suffices.
Genetic testing can confirm the diagnosis with certainty.
Question 5
What drugs are used for the second-line treatment of sarcoidosis?
ibuprofen and diclofenac
methotrexate and azathioprine
calcium inhibitors and antibiotics
adalimumab and infliximab
mitoxantrone and cyclophosphamide
Question 6
What is the current standard for the initial treatment of sarcoidosis?
prednisone 1mg/kgBW per day for 1 month, then 0.5 mg/kgBW per day for 1 month
prednisone 0.5 mg/kgBW per day and azathioptine 150 mg/d for 6 months
rifampicin 600 mg, isoniazid 300 mg, and pyrazinamide 2000 mg/d for 4 months
prednisone 0.5 mg/kgBW per day with calcium and vitamin D supplementation
prednisone 0.5 mg/kgBW per day for 1 month, then gradual reduction over the ensuing 6 months
Question 7
What test is part of the routine diagnostic screening for organ involvement in sarcoidosis?
pulmonary function tests
magnetic resonance imaging
cardiac catheterization
24-hour blood pressure measurement
phlebography of the pelvis and lower limbs
Question 8
You have diagnosed Löfgren syndrome in a 23-year-old man on the basis of the characteristic triad of manifestations. What should you do next?
Start corticosteroid treatment immediately.
Thoroughly inform and counsel the patient, telling him that no further tests are needed, as Löfgren syndrome generally resolves spontaneously.
Send the patient to a dermatologist at once to confirm the diagnosis.
Test for genetic polymorphism of BTNL2 and treat with corticosteroids or not depending on the result.
Inform the patient of the prognosis and recommend, as a first step, histologic confirmation of the diagnosis with bronchoscopy (EBUS), followed by symptomatic treatment with nonsteroidal anti-inflammatory drugs and local treatment for joint swelling.
Question 9
Your 68-year-old patient with newly diagnosed sarcoidosis involving the lungs and liver has the following findings: vital capacity 85%, CO diffusion capacity 73%, PaO2 at rest 78 mmHg, GOT 40 U/L, GPT 50 U/L. The gamma-interferon test is positive, and no acid-fast bacilli are found in the sputum. Mycobacterial cultures are negative. What should you do?
Start corticosteroid treatment immediately.
To confirm the diagnosis, order an 18F-FDG-PET to search for foci of disease.
Change the diagnosis to active tuberculosis and start treatment with four antimycobacterial drugs.
Recommend regular follow-up for the time being, as neither the pulmonary involvement nor the hepatic involvement in this patient is currently an indication for prednisone treatment.
Start combination therapy with infliximab, as azathioprine and methotrexate are contraindicated.
Question 10
A patient with known sarcoidosis comes to see you for the first time, having recently suffered an episode of acute renal failure due to obstructive nephrolithiasis. What should you do?
Before initiating prednisone treatment, send him to the hospital for a renal biopsy, as you suspect renal involvement with sarcoidosis.
Measure urinary and serum calcium levels and advise him, for the time being, to avoid dairy products and keep out of direct sunlight.
Obtain endocrinological consultation for a suspected abnormality of parathyroid metabolism.
Recommend the initiation of prednisone treatment and vitamin D supplementation to prevent osteoporosis.
Send him to a urologist, as the kidney stones are not related to sarcoidosis.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest of statement
Prof. Prasse has received payment from Novartis for conducting research projects on their behalf.
References
- 1.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scharkoff T. Epidemiologie der Sarkoidose. Pneumologie. 1993;47:588–592. [PubMed] [Google Scholar]
- 3.Pabst S, Skowasch D, Grohe C. Sarkoidose. Pneumologie. 2012;66:96–109. doi: 10.1055/s-0030-1257126. quiz 10. [DOI] [PubMed] [Google Scholar]
- 4.Loddenkemper R, Kloppenborg A, Schoenfeld N, Grosser H, Costabel U. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis—results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheiten. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178–182. [PubMed] [Google Scholar]
- 5.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–1167. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 6.Myers JL, Tazelaar HD. Challenges in pulmonary fibrosis: 6-Problematic granulomatous lung disease. Thorax. 2008;63:78–84. doi: 10.1136/thx.2004.031047. [DOI] [PubMed] [Google Scholar]
- 7.Rosen Y. Pathology of sarcoidosis. Semin Respir Crit Care Med. 2007;28:36–52. doi: 10.1055/s-2007-970332. [DOI] [PubMed] [Google Scholar]
- 8.Buss G, Cattin V, Spring P, Malinverni R, Gilliet M. Two cases of interferon-alpha-induced sarcoidosis Koebnerized along venous drainage lines: new pathogenic insights and review of the literature of interferon-induced sarcoidosis. Dermatology. 2013;226:289–297. doi: 10.1159/000346244. [DOI] [PubMed] [Google Scholar]
- 9.Shin JI, Kim DS. Development of sarcoidosis during anti-TNF-alpha treatment: what is the mechanism? Clin Exp Rheumatol. 2009;27 author reply 5. [PubMed] [Google Scholar]
- 10.Moller DR. Potential etiologic agents in sarcoidosis. Proc Am Thorac Soc. 2007;4:465–468. doi: 10.1513/pats.200608-155MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gvozdenovic BS, Mihailovic-Vucinic V, Ilic-Dudvarski A, Zugic V, Judson MA. Differences in symptom severity and health status impairment between patients with pulmonary and pulmonary plus extrapulmonary sarcoidosis. Respir Med. 2008;102:1636–1642. doi: 10.1016/j.rmed.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Kirsten D. Lungensarkoidose: aktuelle Diagnostik und Therapie. Dtsch Med Wochenschr. 2013;138:537–541. doi: 10.1055/s-0032-1332898. [DOI] [PubMed] [Google Scholar]
- 13.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 14.Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685–702. doi: 10.1016/j.ccm.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Lynch JP, 3rd, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. 2014;35:372–390. doi: 10.1055/s-0034-1376889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandolin R, Lehtonen J, Airaksinen J, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 17.Segal BM. Neurosarcoidosis: diagnostic approaches and therapeutic strategies. Curr Opin Neurol. 2013;26:307–313. doi: 10.1097/WCO.0b013e3283608459. [DOI] [PubMed] [Google Scholar]
- 18.Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007;132:1949–1953. doi: 10.1378/chest.07-1178. [DOI] [PubMed] [Google Scholar]
- 19.Koyama T, Ueda H, Togashi K, Umeoka S, Kataoka M, Nagai S. Radiologic manifestations of sarcoidosis in various organs. Radiographics. 2004;24:87–104. doi: 10.1148/rg.241035076. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P. Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2015;16:949–958. doi: 10.1093/ehjci/jev142. [DOI] [PubMed] [Google Scholar]
- 21.Hostettler KE, Bratu VA, Fischmann A, Tamm M, Studler U. Whole-body magnetic resonance imaging in extrathoracic sarcoidosis. Eur Respir J. 2014;43:1812–1815. doi: 10.1183/09031936.00008914. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 23.Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406–412. doi: 10.1378/chest.123.2.406. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med. 2012;106:883–892. doi: 10.1016/j.rmed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Costabel U, Bonella F, Ohshimo S, Guzman J. Diagnostic modalities in sarcoidosis: BAL, EBUS, and PET. Semin Respir Crit Care Med. 2010;31:404–408. doi: 10.1055/s-0030-1262207. [DOI] [PubMed] [Google Scholar]
- 26.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183:573–581. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baughman RP, Judson MA. Relapses of sarcoidosis: what are they and can we predict who will get them? Eur Respir J. 2014;43:337–339. doi: 10.1183/09031936.00138913. [DOI] [PubMed] [Google Scholar]
- 28.Judson MA. The treatment of pulmonary sarcoidosis. Respir Med. 2012;106:1351–1361. doi: 10.1016/j.rmed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Grutters JC, van den Bosch JM. Corticosteroid treatment in sarcoidosis. Eur Respir J. 2006;28:627–636. doi: 10.1183/09031936.06.00105805. [DOI] [PubMed] [Google Scholar]
- 30.Taimeh Z, Hertz MI, Shumway S, Pritzker M. Lung transplantation for pulmonary sarcoidosis. Twenty-five years of experience in the USA. Thorax. 2016;71:378–379. doi: 10.1136/thoraxjnl-2015-207497. [DOI] [PubMed] [Google Scholar]
- 31.Jamilloux Y, Néel A, Lecouffe-Desprets M, et al. Progressive multifocal leukoencephalopathy in patients with sarcoidosis. Neurology. 2014;82:1307–1313. doi: 10.1212/WNL.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 32.Baughman RP, Janovcik J, Ray M, et al. Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:113–120. [PubMed] [Google Scholar]
- 33.Pietinalho A, Tukiainen P, Haahtela T, Persson T, Selroos O. Finnish Pulmonary Sarcoidosis Study Group: Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24–31. doi: 10.1378/chest.121.1.24. [DOI] [PubMed] [Google Scholar]
- 34.Vorselaars AD, Wuyts WA, Vorselaars VM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805–812. doi: 10.1378/chest.12-1728. [DOI] [PubMed] [Google Scholar]
- 35.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 36.Russell E, Luk F, Manocha S, Ho T, O’Connor C, Hussain H. Long term follow-up of infliximab efficacy in pulmonary and extra-pulmonary sarcoidosis refractory to conventional therapy. Semin Arthritis Rheum. 2013;43:119–124. doi: 10.1016/j.semarthrit.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Hostettler KE, Studler U, Tamm M, Brutsche MH. Long-term treatment with infliximab in patients with sarcoidosis. Respiration. 2012;83:218–224. doi: 10.1159/000328738. [DOI] [PubMed] [Google Scholar]
- 38.Prasse A, Katic C, Germann M, Buchwald A, Zissel G, Müller-Quernheim J. Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med. 2008;177:330–336. doi: 10.1164/rccm.200705-742OC. [DOI] [PubMed] [Google Scholar]
- e1.Sipahi Demirkok S, Basaranoglu M, Dervis E, Bal M, Karayel T. Analysis of 87 patients with Lofgren’s syndrome and the pattern of seasonality of subacute sarcoidosis. Respirology. 2006;11:456–461. doi: 10.1111/j.1440-1843.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- e2.Caso F, Costa L, Rigante D, et al. Caveats and truths in genetic, clinical, autoimmune and autoinflammatory issues in Blau syndrome and early onset sarcoidosis. Autoimmun Rev. 2014;13:1220–1229. doi: 10.1016/j.autrev.2014.08.010. [DOI] [PubMed] [Google Scholar]
- e3.Mirsaeidi M, Machado RF, Schraufnagel D, Sweiss NJ, Baughman RP. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438–449. doi: 10.1378/chest.14-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Hosoda Y, Yamaguchi M, Hiraga Y. Global epidemiology of sarcoidosis. What story do prevalence and incidence tell us? Clin Chest Med. 1997;18:681–694. doi: 10.1016/s0272-5231(05)70412-3. [DOI] [PubMed] [Google Scholar]
- e5.Judson MA, Baughman RP. How many organs need to be involved to diagnose sarcoidosis?: An unanswered question that, hopefully, will become irrelevant. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:6–7. [PubMed] [Google Scholar]
- e6.Baughman RP, Lower EE, Kaufman AH. Ocular sarcoidosis. Semin Respir Crit Care Med. 2010;31:452–462. doi: 10.1055/s-0030-1262213. [DOI] [PubMed] [Google Scholar]
- e7.Judson MA. The clinical features of sarcoidosis: A comprehensive review. Clin Rev Allergy Immunol. 2015;49:63–78. doi: 10.1007/s12016-014-8450-y. [DOI] [PubMed] [Google Scholar]
- e8.Nunes H, Brillet PY, Valeyre D, Brauner MW, Wells AU. Imaging in sarcoidosis. Semin Respir Crit Care Med. 2007;28:102–120. doi: 10.1055/s-2007-970336. [DOI] [PubMed] [Google Scholar]
- e9.Criado E, Sánchez M, Ramírez J, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30:1567–1586. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- e10.Jeudy J, Burke AP, White CS, Kramer GB, Frazier AA. Cardiac sarcoidosis: the challenge of radiologic-pathologic correlation: from the radiologic pathology archives. Radiographics. 2015;35:657–679. doi: 10.1148/rg.2015140247. [DOI] [PubMed] [Google Scholar]
- e11.Mostard RL, Voo S, van Kroonenburgh MJ, et al. Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med. 2011;105:1917–1924. doi: 10.1016/j.rmed.2011.08.012. [DOI] [PubMed] [Google Scholar]
- e12.Mostard RL, van Kroonenburgh MJ, Drent M. The role of the PET scan in the management of sarcoidosis. Curr Opin Pulm Med. 2013;19:538–544. doi: 10.1097/MCP.0b013e328363ed0d. [DOI] [PubMed] [Google Scholar]
- e13.Mostard RL, Verschakelen JA, van Kroonenburgh MJ, et al. Severity of pulmonary involvement and (18)F-FDG PET activity in sarcoidosis. Respir Med. 2013;107:439–447. doi: 10.1016/j.rmed.2012.11.011. [DOI] [PubMed] [Google Scholar]
- e14.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med. 2012;106:883–892. doi: 10.1016/j.rmed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- e15.Nakajima T, Yasufuku K, Kurosu K, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis–comparisons with other bronchoscopic diagnostic modalities. Respir Med. 2009;103:1796–1800. doi: 10.1016/j.rmed.2009.07.013. [DOI] [PubMed] [Google Scholar]
- e16.Plit M, Pearson R, Havryk A, Da Costa J, Chang C, Glanville AR. Diagnostic utility of endobronchial ultrasound-guided transbronchial needle aspiration compared with transbronchial and endobronchial biopsy for suspected sarcoidosis. Intern Med J. 2012;42:434–438. doi: 10.1111/j.1445-5994.2011.02446.x. [DOI] [PubMed] [Google Scholar]
- e17.Costabel U, Bonella F, Ohshimo S, Guzman J. Diagnostic modalities in sarcoidosis: BAL, EBUS, and PET. Semin Respir Crit Care Med. 2010;31:404–408. doi: 10.1055/s-0030-1262207. [DOI] [PubMed] [Google Scholar]
- e18.Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997;156:1–25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- e19.Baughman RP, Grutters JC. New treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approaches. Lancet Respir Med. 2015;10:813–822. doi: 10.1016/S2213-2600(15)00199-X. [DOI] [PubMed] [Google Scholar]
- e20.Schultz HH, Andersen CB, Steinbruuchel D, Perch M, Carlsen J, Iversen M. Recurrence of sarcoid granulomas in lung transplant recipients is common and does not affect overall survival. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:149–153. [PubMed] [Google Scholar]
- e21.Baughman RP, Lower EE. Who dies from sarcoidosis and why? Am J Respir Crit Care Med. 2011;183:1446–1447. doi: 10.1164/rccm.201103-0409ED. [DOI] [PubMed] [Google Scholar]
- e22.Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138:1078–1085. doi: 10.1378/chest.09-2002. [DOI] [PubMed] [Google Scholar]
- e23.Bagwan IN, Hooper LV, Sheppard MN. Cardiac sarcoidosis and sudden death. The heart may look normal or mimic other cardiomyopathies. Virchows Arch. 2011;458:671–678. doi: 10.1007/s00428-010-1003-8. [DOI] [PubMed] [Google Scholar]
- e24.Sparrow PJ, Merchant N, Provost YL, Doyle DJ, Nguyen ET, Paul NS. CT and MR imaging findings in patients with acquired heart disease at risk for sudden cardiac death. Radiographics. 2009;29:805–823. doi: 10.1148/rg.293085715. [DOI] [PubMed] [Google Scholar]
- e25.Hohlfeld SK, Gunthard HF, Zeitz J, Locher P, Bachli E. Progressive multi-focal leukoencephalopathy as a rare lethal complication in untreated sarcoidosis. BMJ Case Rep. 2012 doi: 10.1136/bcr.03.2011.4036. pii: bcr0320114036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e26.Gundlach E, Hoffmann MM, Prasse A, Heinzelmann S, Ness T. Interleukin-2 receptor and angiotensin-converting enzyme as markers for ocular sarcoidosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147258. e0147258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e27.Vorselaars AD, van Moorsel CH, Deneer VH, Grutters JC. Current therapy in sarcoidosis, the role of existing drugs and future medicine. Inflamm Allergy Drug Targets. 2013;12:369–377. doi: 10.2174/18715281113126660062. [DOI] [PubMed] [Google Scholar]
- e28.Baughman RP, Judson MA, Lower EE, et al. Infliximab for chronic cutaneous sarcoidosis: a subset analysis from a double-blind randomized clinical trial. Sarcoidosis Vasc Diffuse Lung Dis. 2016;32:289–295. [PubMed] [Google Scholar]
- e29.Verwoerd A, Vorselaars AD, van Moorsel CH, Bos WJ, van Velzen-Blad H, Grutters JC. Discrepant elevation of sIL-2R levels in sarcoidosis patients with renal insufficiency. Eur Respir J. 2015;46:277–280. doi: 10.1183/09031936.00005315. [DOI] [PubMed] [Google Scholar]