Abstract
Objective: To report on a case of retinal pigment epithelium (RPE) toxicity apparently associated with ritonavir.
Methods: We describe a case of gradual-onset blurry vision in both eyes in a 30-year-old HIV-positive male on Highly-Active Antiretroviral Therapy (HAART) including ritonavir
Results: The patient presented with a visual acuity of 3/10 in each eye, and fundoscopy revealed paracentral pigment mottling. Computerized perimetry showed a ring-scotoma in both eyes. Fluorescein angiography revealed an anular RPE defect in both eyes, congruent with hyperautofluorescent changes on autofluorescence imaging. Full-field ERG was normal.
Conclusions: Since ritonavir has previously been linked with toxicity to the RPE, we consider this report as further evidence of this association.
Keywords: AIDS, retinal toxicity, ritonavir, retinal pigment epithelium
Zusammenfassung
Zielsetzung: Bericht über einen möglichen Zusammenhang zwischen der Toxizität des Retinalen Pigmetepithels (RPE) und Ritonavir.
Methoden: Wir beschreiben einen Fall schrittweise einsetzender Sehstörungen beider Augen eines 30-jährigen HIV-positiven Mannes auf Highly-Active Antiretroviral Therapie (HAART).
Ergebnisse: Der Patient hatte eine Sehschärfe von 3/10 in jedem Auge und eine Funduskopie zeigte eine parazentrale Pigment-Marmorierung. Eine Computerperimetrie offenbarte ein Ringskotom in beiden Augen. Eine Fluoreszenzangiographie zeigte einen ringförmigen RPE-Defekt in beiden Augen, deckungsgleich mit hyperautofluoreszenten Veränderungen im Autofluoreszenz-Imaging. Das Ganzfeld-ERG war normal.
Schlussfolgerungen: Da Ritonavir bereits mit der RPE-Toxizität in Verbindung gebracht wurde, betrachten wir diesen Bericht als weiteren Beweis dieses Zusammenhangs.
Introduction
Approximately three dozen medications have been associated with retinal toxicity [1].
Identification of the culprit is important, especially if a reasonable alternative is available. We present a case of bull’s eye maculopathy in the context of AIDS-related drugs.
Case presentation
A 30-year-old HIV-positive white male presented with complaints of blurry vision in both eyes which he had begun to notice 3 months prior. Cell count was 263 cells/µl, viral load was undetectable, and there was no previous history of AIDS-defining illness.
He had been placed on protease inhibitors ritonavir (Norvir®, Abbott Laboratories, Berkshire, UK) and atazanavir (Reyataz®, Bristol-Myers Squibb, Uxbridge, UK), and on the association of nucleoside reverse transcriptase inhibitors tenofovir/emtricitabine 245/200 mg (Truvada®, Gilead Sciences International Limited, Cambridge, UK) starting 3 years prior. He interrupted this regimen in 2008, out of his own initiative, only to have it restarted in 2010 after a symptomatic increase in the viral HIV load and also in hepatitis B viral load. Both these titers subsequently turned negative.
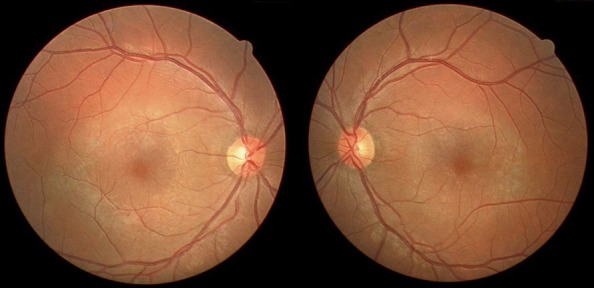
His physical examination revealed a best corrected visual acuity of 3/10 in both eyes. Fundoscopy showed a discrete perimacular ring of pigment mottling OU (Figure 1 (Fig. 1)), with 2–3 clumps of pigment in the adjacent periphery of each eye. Optic disc and vessels had a normal appearance.
Figure 1. Colour fundus photograph showing annular RPE attenuation in the macula of both eyes.
Computerized perimetry (Humphrey 60-2) showed a central scotoma in both eyes, with a foveal sensitivity of 32 and 30 dB in the right and left eyes, respectively.
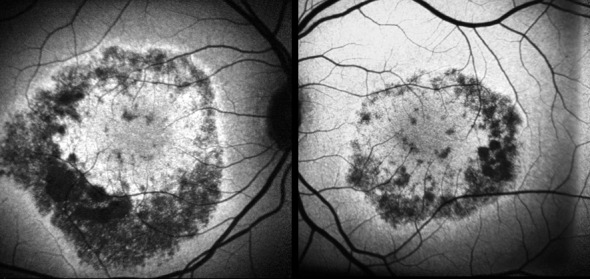
Fluorescein Angiography (FA) showed early annular hyperfluorescence consistent with bull’s eye maculopathy in both eyes (Figure 2 (Fig. 2)). SD-OCT of the macula showed retinal thinning (central foveal thickness 160 µm and 159 µm in the right and left eyes, respectively). Autofluorescence imaging showed an area of annular hypoautofluorescence congruate with the epithelial defects seen on FA (Figure 3 (Fig. 3)). The full-field ERG was normal for both cone and rod responses.
Figure 2. Fluorescein angiogram showing annular window defect in both eyes.
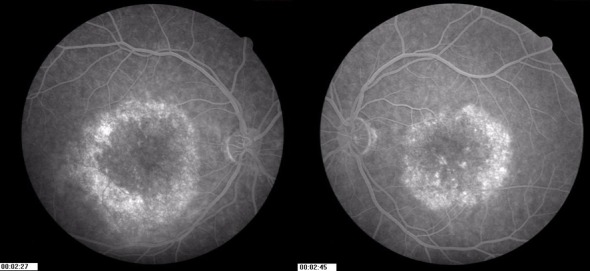
Figure 3. Fundus autofluorescence showing areas of marked hypoautofluorescence congruent with RPE changes.
There was no record of previous didanosine use, nor of anti-tuberculous drugs. Chest X-ray and VDRL were normal. Liver function tests were normal (aminotransferases, GGT and alkaline phophatase). The patient refers no family history of retinal disorders.
Discussion
Bull’s eye maculopathy occurring outside the setting of 4-aminoquinoline use, although reported, is unusual [2]. Three drugs used in the treatment of AIDS-related disorders have previously been linked with retinal epitheliopathy: the protease inhibitor ritonavir [3] (Norvir®); clofazimine (Lamprene®, Ciba Pharmaceuticals, Summit, NJ) [4], used in the management of atypical mycobacterial infections, and the reverse transcriptase inhibitor didanosine (Videx®, Bristol-Myers Squibb, Princeton, NJ) [5], [6].
The only agent common to our patient and previous cases is ritonavir.
More precisely, besides being associated with degenerative retinal changes in rodents [7], this drug has been associated with retinal pigment epithelium (RPE) changes in 3 patients, who had been on ritonavir for a minimum of 19 months [3]. Both hypertrophic and atrophic changes were seen; and these did not involve the fovea. Intraretinal crystalline deposits and other features reminiscent of idiopathic parafoveal telangiectasis were also seen, in all 3 patients [3]. Even though the latter findings were not part of the present clinical picture, there was a striking resemblance between the pattern of pigment epitheliopathy seen in our patient and that seen in the previous reports. Given the common setting of a history of treatment with ritonavir, the latter must be considered as the most likely culprit. This case seems to represent a different part of the spectrum of retinal changes potentially associated with ritonavir.
Liver dysfunction was another characteristic common to the 3 patients in Roe’s report [3]; whereas our patient had normal liver serum enzymes.
Based on rodent studies, the proposed mechanism of ritonavir toxicity has been phopholipidosis [7]. Besides the retina, liver, thyroid and kidney have also been described as targets for dose-related ritonavir toxicity [7].
Conclusions
Our case report may represent further evidence toward the retinal toxicity of the protease inhibitor ritonavir (Norvir®).
Notes
Competing interests
The authors declare that they have no competing interests. No financial support was received for this submission.
Informed consent
The patient gave his informed consent prior to inclusion of his data in this report.
References
- 1.Schwartz SG, Mieler WF. Medications and retinal toxicity. Ophthalmol Clin North Am. 2002 Dec;15(4):517–528. doi: 10.1016/S0896-1549(02)00051-2. Available from: http://dx.doi.org/10.1016/S0896-1549(02)00051-2. [DOI] [PubMed] [Google Scholar]
- 2.Weise EE, Yannuzzi LA. Ring maculopathies mimicking chloroquine retinopathy. Am J Ophthalmol. 1974 Aug;78(2):204–210. doi: 10.1016/0002-9394(74)90077-4. [DOI] [PubMed] [Google Scholar]
- 3.Roe RH, Jumper JM, Gualino V, Wender J, McDonald HR, Johnson RN, Fu AD, Cunningham ET. Retinal pigment epitheliopathy, macular telangiectasis, and intraretinal crystal deposits in HIV-positive patients receiving ritonavir. Retina (Philadelphia, Pa) 2011 Mar;31(3):559–565. doi: 10.1097/IAE.0b013e3181f0d2c4. Available from: http://dx.doi.org/10.1097/IAE.0b013e3181f0d2c4. [DOI] [PubMed] [Google Scholar]
- 4.Forster DJ, Causey DM, Rao NA. Bull's eye retinopathy and clofazimine. Ann Intern Med. 1992 May;116(10):876–877. doi: 10.7326/0003-4819-116-10-876_2. [DOI] [PubMed] [Google Scholar]
- 5.Muralha A, Reisner ML, Curi AL. Retinopatia associada ao uso de didanosina. [Retinal toxicity associated with didanosine]. Arq Bras Oftalmol. 2001;64(5):465–467. doi: 10.1590/S0004-27492001000500017. Available from: http://dx.doi.org/10.1590/S0004-27492001000500017. [DOI] [Google Scholar]
- 6.Whitcup SM, Butler KM, Caruso R, de Smet MD, Rubin B, Husson RN, Lopez JS, Belfort R, Jr, Pizzo PA, Nussenblatt RB. Retinal toxicity in human immunodeficiency virus-infected children treated with 2',3'-dideoxyinosine. Am J Ophthalmol. 1992 Jan;113(1):1–7. doi: 10.1016/s0002-9394(14)75744-7. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Scientific discussion for the approval of Norvir. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000127/WC500028725.pdf.


