Abstract
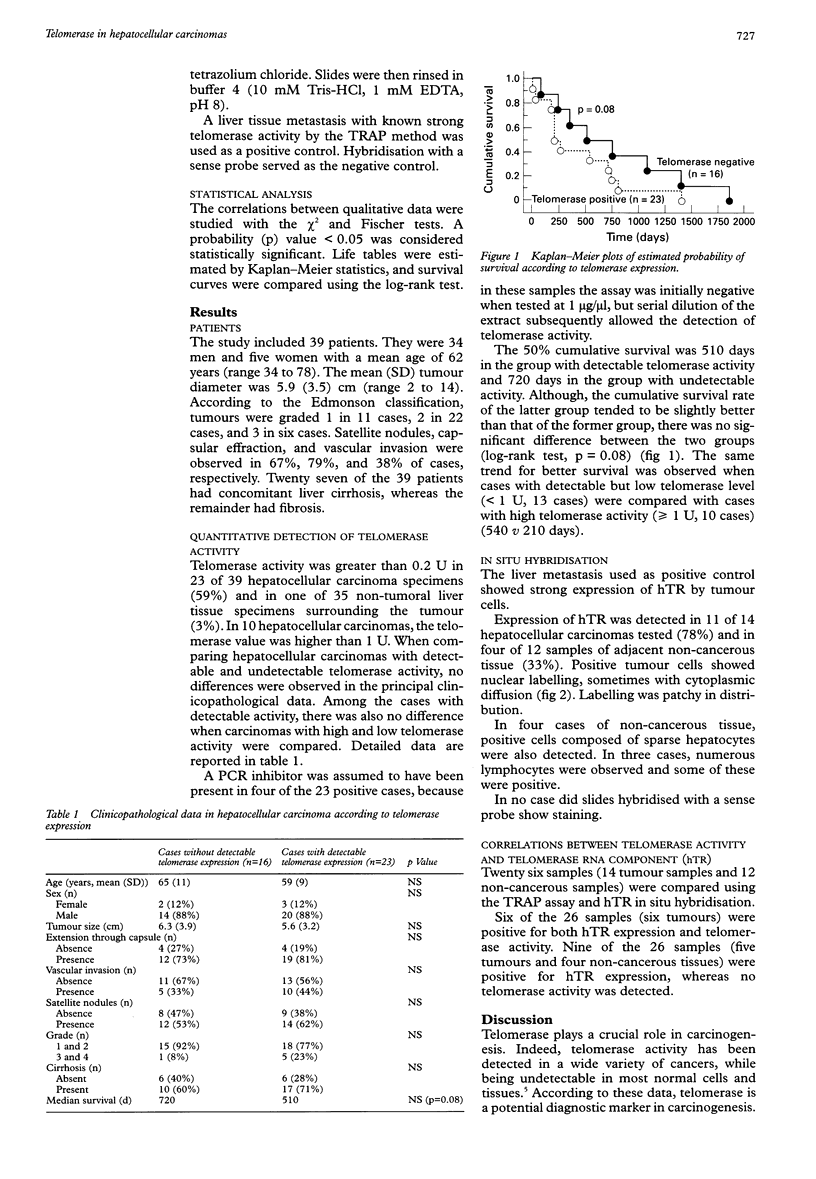
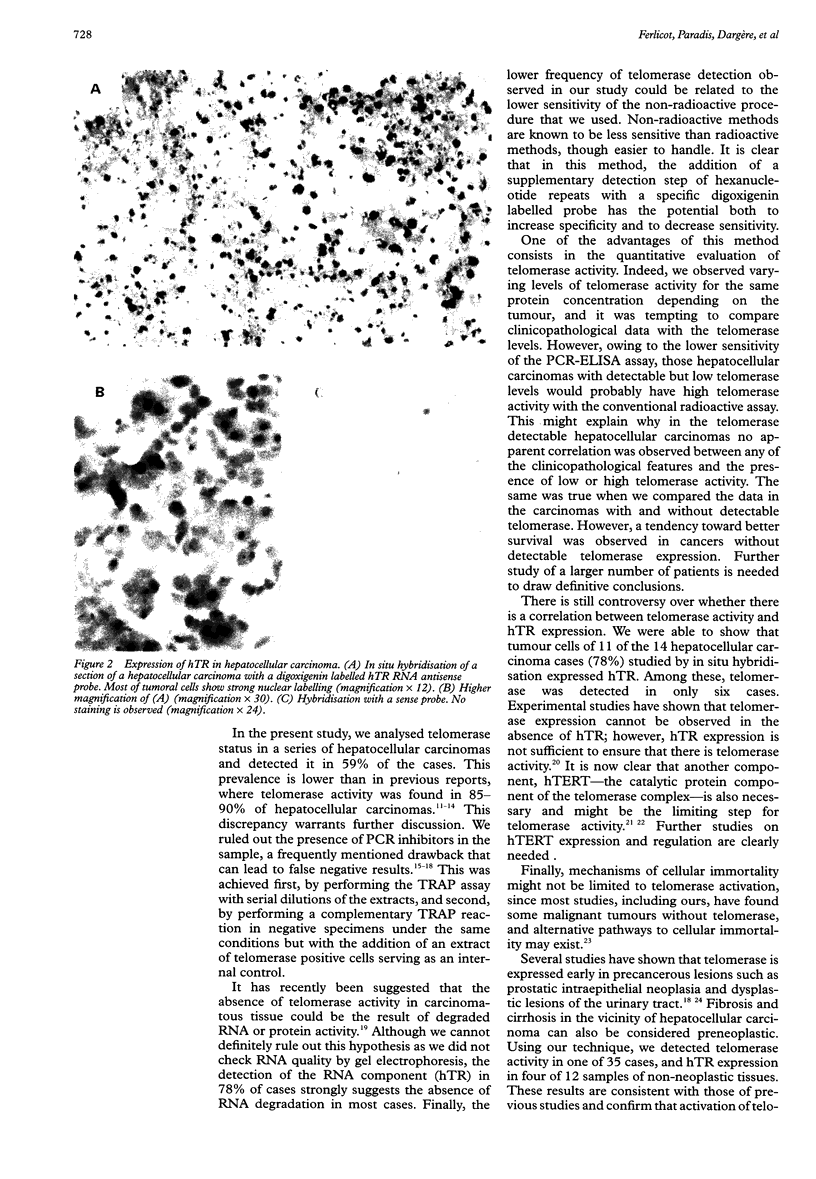
BACKGROUND: While telomerase is undetectable in most normal somatic tissues, telomerase activation has been detected in many immortal cell lines and various cancers. AIM: To investigate telomerase expression in hepatocellular carcinoma, and to assess the expression of the RNA component of telomerase, hTR. METHODS: 39 hepatocellular carcinomas were studied using a telomerase polymerase chain reaction (PCR) enzyme linked immunosorbent assay, which does not require radioactive PCR amplification and yields a semiquantitative measurement. Expression of hTR was also assessed by a non-radioactive in situ hybridisation procedure. The correlations between these two markers and the clinicopathological data were analysed. RESULTS: Telomerase activity was detected in 23 of 39 hepatocellular carcinoma specimens (59%). Comparison of hepatocellular carcinoma with and without telomerase expression, or with high and low telomerase (10 cases v 13 cases), showed no differences in the principal clinicopathological data. Although median survival was lower in the group with detectable telomerase activity than in that with undetectable activity (510 v 720 days) the difference was not significant (log-rank test, p = 0.08). hTR expression was detected in 11 of 14 cases of hepatocellular carcinoma tested (78%) and in four of 12 samples of adjacent non-cancerous tissue (33%). Five tumours and four non-cancerous tissues were positive for hTR, whereas no telomerase activity was detected in these. CONCLUSIONS: The presence of telomerase activity in hepatocellular carcinomas is confirmed. No correlation was observed between clinicopathological data and telomerase expression in hepatocellular carcinoma, but survival seemed better in the absence of telomerase expression. hTR seems to be more widely expressed than telomerase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blasco M. A., Lee H. W., Hande M. P., Samper E., Lansdorp P. M., DePinho R. A., Greider C. W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997 Oct 3;91(1):25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Collins K. Structure and function of telomerase. Curr Opin Cell Biol. 1996 Jun;8(3):374–380. doi: 10.1016/s0955-0674(96)80013-5. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Meyerson M., Eaton E. N., Ellisen L. W., Caddle S. D., Haber D. A., Weinberg R. A. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998 Mar 5;16(9):1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- Feng J., Funk W. D., Wang S. S., Weinrich S. L., Avilion A. A., Chiu C. P., Adams R. R., Chang E., Allsopp R. C., Yu J. The RNA component of human telomerase. Science. 1995 Sep 1;269(5228):1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kojima H., Yokosuka O., Imazeki F., Saisho H., Omata M. Telomerase activity and telomere length in hepatocellular carcinoma and chronic liver disease. Gastroenterology. 1997 Feb;112(2):493–500. doi: 10.1053/gast.1997.v112.pm9024303. [DOI] [PubMed] [Google Scholar]
- Kolquist K. A., Ellisen L. W., Counter C. M., Meyerson M., Tan L. K., Weinberg R. A., Haber D. A., Gerald W. L. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet. 1998 Jun;19(2):182–186. doi: 10.1038/554. [DOI] [PubMed] [Google Scholar]
- Kyo S., Kunimi K., Uchibayashi T., Namiki M., Inoue M. Telomerase activity in human urothelial tumors. Am J Clin Pathol. 1997 May;107(5):555–560. doi: 10.1093/ajcp/107.5.555. [DOI] [PubMed] [Google Scholar]
- Levy M. Z., Allsopp R. C., Futcher A. B., Greider C. W., Harley C. B. Telomere end-replication problem and cell aging. J Mol Biol. 1992 Jun 20;225(4):951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- Meyerson M., Counter C. M., Eaton E. N., Ellisen L. W., Steiner P., Caddle S. D., Ziaugra L., Beijersbergen R. L., Davidoff M. J., Liu Q. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997 Aug 22;90(4):785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Miura N., Horikawa I., Nishimoto A., Ohmura H., Ito H., Hirohashi S., Shay J. W., Oshimura M. Progressive telomere shortening and telomerase reactivation during hepatocellular carcinogenesis. Cancer Genet Cytogenet. 1997 Jan;93(1):56–62. doi: 10.1016/s0165-4608(96)00329-9. [DOI] [PubMed] [Google Scholar]
- Nakamura T. M., Morin G. B., Chapman K. B., Weinrich S. L., Andrews W. H., Lingner J., Harley C. B., Cech T. R. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997 Aug 15;277(5328):955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- Niida H., Matsumoto T., Satoh H., Shiwa M., Tokutake Y., Furuichi Y., Shinkai Y. Severe growth defect in mouse cells lacking the telomerase RNA component. Nat Genet. 1998 Jun;19(2):203–206. doi: 10.1038/580. [DOI] [PubMed] [Google Scholar]
- Tahara H., Kuniyasu H., Yokozaki H., Yasui W., Shay J. W., Ide T., Tahara E. Telomerase activity in preneoplastic and neoplastic gastric and colorectal lesions. Clin Cancer Res. 1995 Nov;1(11):1245–1251. [PubMed] [Google Scholar]
- Wellinger R. J., Ethier K., Labrecque P., Zakian V. A. Evidence for a new step in telomere maintenance. Cell. 1996 May 3;85(3):423–433. doi: 10.1016/s0092-8674(00)81120-4. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W., Piatyszek M. A. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995 Sep 25;23(18):3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P., Bosman F. T., Benhattar J. Tissue quality is an important determinant of telomerase activity as measured by TRAP assay. Biotechniques. 1998 Oct;25(4):660–662. doi: 10.2144/98254dt05. [DOI] [PubMed] [Google Scholar]
- Yashima K., Litzky L. A., Kaiser L., Rogers T., Lam S., Wistuba I. I., Milchgrub S., Srivastava S., Piatyszek M. A., Shay J. W. Telomerase expression in respiratory epithelium during the multistage pathogenesis of lung carcinomas. Cancer Res. 1997 Jun 15;57(12):2373–2377. [PubMed] [Google Scholar]
- Yashima K., Piatyszek M. A., Saboorian H. M., Virmani A. K., Brown D., Shay J. W., Gazdar A. F. Telomerase activity and in situ telomerase RNA expression in malignant and non-malignant lymph nodes. J Clin Pathol. 1997 Feb;50(2):110–117. doi: 10.1136/jcp.50.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Sugino T., Tahara H., Woodman A., Bolodeoku J., Nargund V., Fellows G., Goodison S., Tahara E., Tarin D. Telomerase activity in bladder carcinoma and its implication for noninvasive diagnosis by detection of exfoliated cancer cells in urine. Cancer. 1997 Jan 15;79(2):362–369. doi: 10.1002/(sici)1097-0142(19970115)79:2<362::aid-cncr20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Telomeres: beginning to understand the end. Science. 1995 Dec 8;270(5242):1601–1607. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]



