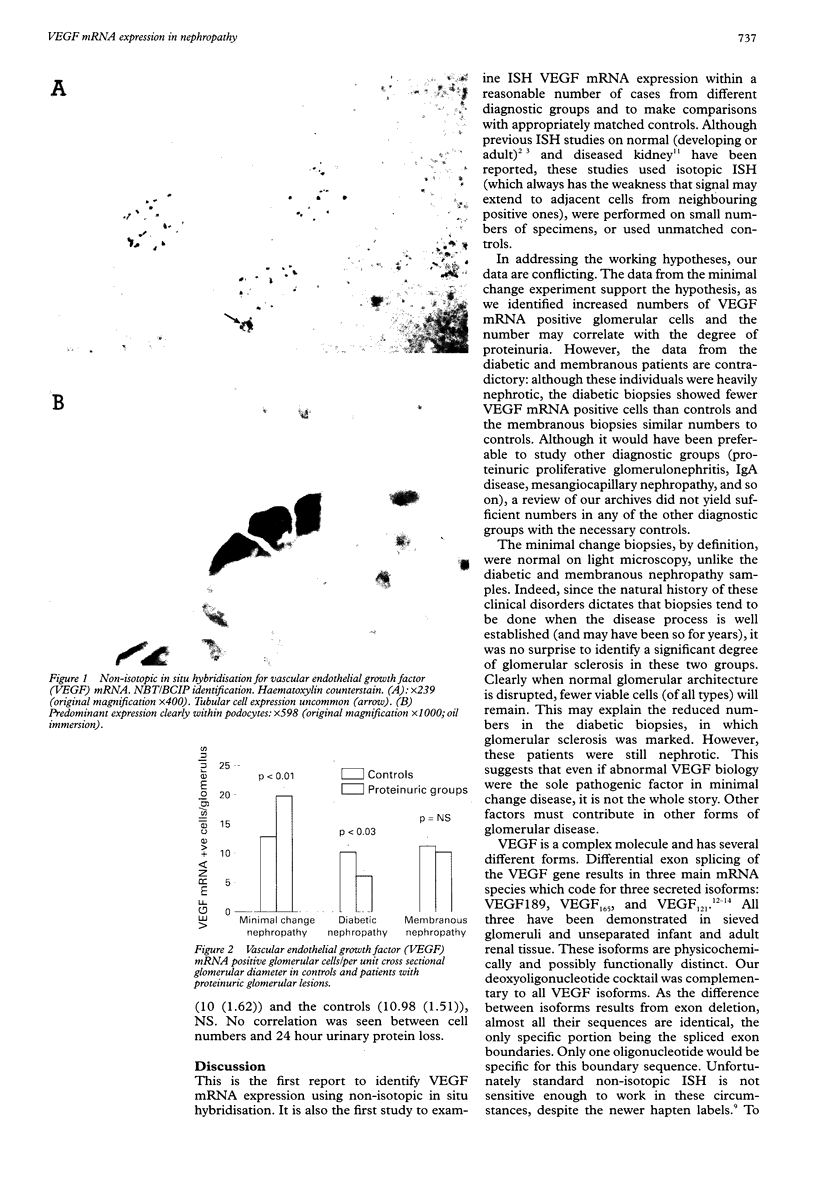
Abstract
AIM: To investigate vascular endothelial growth factor (VEGF) mRNA expression in glomerular disease in the context of heavy proteinuria. METHODS: Non-radioisotopic in situ hybridisation was performed using a cocktail of 12 deoxyoligonucleotides complementary to VEGF mRNA labelled during solid phase synthesis with 2,4-dinitrophenyl. Archival renal biopsies were studied from cases of minimal change nephropathy, membranous nephropathy, diabetic nephropathy, and controls, matched for age, sex, race, and storage time. Hybrid detection used NBT/BCIP colorimetric development. RESULTS: More VEGF mRNA positive glomerular cells per unit cross sectional diameter were seen in minimal change nephropathy (mean (SEM), 19.35 (1.5)) compared with controls (12.6 (1.73)), p < 0.01. In contrast, fewer were seen in diabetic nephropathy (5.93 (0.97)) compared with controls (9.97 (1.25)), p < 0.03. Analysis of membranous nephropathy (10 (1.62)) showed no difference from controls (10.98 (1.51)), NS. In addition, in minimal change nephropathy there was a significant correlation between 24 hour protein excretion at the time of biopsy and the number of VEGF mRNA cells per glomerulus (r = 0.08, p = 0.01). CONCLUSIONS: Using non-radioisotopic in situ hybridisation, VEGF mRNA is almost exclusively expressed by visceral glomerular epithelial cells. Abnormal numbers of cells are seen in both minimal change and diabetic nephropathy. As VEGF exists in a number of functionally distinct isoforms, further study of qualitative VEGF isoform expression in diagnostic groups is indicated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown L. F., Berse B., Tognazzi K., Manseau E. J., Van de Water L., Senger D. R., Dvorak H. F., Rosen S. Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int. 1992 Dec;42(6):1457–1461. doi: 10.1038/ki.1992.441. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997 Feb;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Gröne H. J. Angiogenesis and vascular endothelial growth factor (VEGF): is it relevant in renal patients? Nephrol Dial Transplant. 1995;10(6):761–763. [PubMed] [Google Scholar]
- Harper S. J., Bailey E., McKeen C. M., Stewart A. S., Pringle J. H., Feehally J., Brown T. A comparative study of digoxigenin, 2,4-dinitrophenyl, and alkaline phosphatase as deoxyoligonucleotide labels in non-radioisotopic in situ hybridisation. J Clin Pathol. 1997 Aug;50(8):686–690. doi: 10.1136/jcp.50.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991 Dec;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Jones P. H., Harper S., Watt F. M. Stem cell patterning and fate in human epidermis. Cell. 1995 Jan 13;80(1):83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Poltorak Z., Cohen T., Sivan R., Kandelis Y., Spira G., Vlodavsky I., Keshet E., Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997 Mar 14;272(11):7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- Pringle J. H., Ruprai A. K., Primrose L., Keyte J., Potter L., Close P., Lauder I. In situ hybridization of immunoglobulin light chain mRNA in paraffin sections using biotinylated or hapten-labelled oligonucleotide probes. J Pathol. 1990 Nov;162(3):197–207. doi: 10.1002/path.1711620305. [DOI] [PubMed] [Google Scholar]
- Roberts W. G., Palade G. E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995 Jun;108(Pt 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Connolly D. T., Van de Water L., Feder J., Dvorak H. F. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990 Mar 15;50(6):1774–1778. [PubMed] [Google Scholar]
- Shulman K., Rosen S., Tognazzi K., Manseau E. J., Brown L. F. Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol. 1996 May;7(5):661–666. doi: 10.1681/ASN.V75661. [DOI] [PubMed] [Google Scholar]
- Simon M., Röckl W., Hornig C., Gröne E. F., Theis H., Weich H. A., Fuchs E., Yayon A., Gröne H. J. Receptors of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in fetal and adult human kidney: localization and [125I]VEGF binding sites. J Am Soc Nephrol. 1998 Jun;9(6):1032–1044. doi: 10.1681/ASN.V961032. [DOI] [PubMed] [Google Scholar]
- Williams J. D., Coles G. A. Proteinuria--a direct cause of renal morbidity? Kidney Int. 1994 Feb;45(2):443–450. doi: 10.1038/ki.1994.58. [DOI] [PubMed] [Google Scholar]