Abstract
Objective
Uncontrolled HIV infection progresses to the depletion of systemic and mucosal CD4 and AIDS. Early HIV infection may be associated with increases in the concentration of MIP-3α in the blood and gut fluids. MIP-3α/CCL20 is the only chemokine known to interact with CCR6 receptors which are expressed on immature dendritic cells and both effector and memory CD8+ and CD4+ T cells. The role and prognostic value of blood levels of MIP-3α in HIV-infected individuals has yet to be described.
Methods
We determined the serum levels of MIP-3α, and IFN-γ, in 167 HIV-1-infected and 27 HIV-1-uninfected men participating in the Multicenter AIDS Cohort Study (MACS). The blood biomarkers were measured using enzyme-linked immunosorbent assays (ELISA) and the cell phenotypes using flow cytometry.
Results
Median serum levels of MIP-3α in HIV-1-infected and uninfected men was significantly different (p<0.0001) and were 21.3 pg/mL and 6.4 pg/mL respectively. The HIV-1-infected men with CD4+ T cell count <200 cells/μL showed the highest median serum MIP-3α (23.1 pg/mL). Serum levels of MIP-3α in HIV-1 infected (n=167) were negatively correlated with absolute number of CD4+ T cell (p=0.01) and were positively correlated with CD38 molecules on CD8+ T cells (p=0.0002) and with serum levels of IFN-γ (0.006).
Conclusion
Serum levels of MIP-3α concomitantly increase with plasma levels of IFN-γ, CD38 expression on CD8+ T cells, and decreased of absolute CD4+ T cells in HIV-1-infected men. A higher blood level of MIP-3α may be representation of locally high level of MIP-3α and more recruitment of immature dendritic cell at site of infection. Involvement of CCR6/CCL20 axis and epithelial cells at the recto-colonel level may enhance sexual transmission of HIV-1 in MSM and may be useful as a prognostic marker in HIV-1-infection and AIDS.
Keywords: CD4, Chemokine, HIV infection, MIP-3α/CCL20
Introduction
Chemokines are small polypeptides (90-130 amino acids) that regulate cellular activation, chemotaxis, leukocyte trafficking, and cellular adhesion. There are approximately 40-50 currently known human chemokines that are divided into two subfamilies (CXC and CC) with more than 20 receptors [1-4]. The CXC subfamily is further divided into ELR-CXC and non-ELR-CXC chemokines depending on the presence of a tripeptide (Glu-Leu-Arg) ELR-motif preceding the first cysteine residue [5].
One of the novel members of the CC chemokines is known as liver activation regulated chemokine (LARC) [6], Exodus-1 [7], macrophage inflammatory protein-3α [8], and has been classified as a CCL20 chemokine [9]. MIP-3α is the only chemokine that has high specificity for the chemokine receptor, CCR6 [10-13], which is expressed on several human cell types implicated in HIV infection, including immature dendritic cells (iDCs) [14], effector/memory CD8+ and CD4+ T cells [15,16], and interleukin-17 (IL-17) producing T cells (TH17).
CCR6 is highly expressed on peripheral blood CD4+ CCR5+ memory T cells and two populations of CD4+ T cells within the gut (α4β7+ T and TH17 cells) that are highly relevant to HIV-1-infection [17,18]. CCR6 is an independent HIV/SIV co-receptor may promote HIV transmission and disease progression in part, when other major co-receptors are affected [19]. CCL20/MIP-3α is responsible for the chemo-attraction of iDCs, effector/memory B cells and T cells. CCL20/MIP-3α has also been shown to be chemotactic for CD11b+ myeloid DCs in the Peyer's patch, and is involved in the regulation of humoral immunity and lymphocyte homeostasis.
Human β-defensin 2 and CCL20/MIP-3α both exhibit antimicrobial and anti-HIV activity that is mediated by induction of the host restriction factor apolipoprotein B mRNA-editing enzyme-catalytic ploypeptide-like 3G (APOBEC3G) [20]. Many chemokines, including CCL20/MIP-3α, show antimicrobial capability in vitro [21]. Studies have suggested that the production of CCL20/MIP-3α by epithelial cells is an important component of the innate immune defense in the female reproductive tract [22]. Berliner et al., demonstrated that human seminal plasma is able to stimulate human vaginal epithelial cells and cause the secretion of CCL20/MIP-3α, leading to the enhanced recruitment of Langerhans cell precursors to the area, acting as a possible path for HIV transmission in heterosexual individuals [23].
It has been recently shown that iDC (CD1a+ CD86−) and mature DC (CD1a+ CD86+) exclusively responded to CCL20/MIP-3α and CCL19/MIP-3β respectively, and that the number of CCR6 receptors for CCL/20MIP-3α decreases as iDCs mature DCs [24].
DCs are effective antigen presenting cells that are distributed throughout the skin and mucosal surfaces. Upon exposure to HIV-1, DCs process and present antigens to immune cells for further complex action. Furthermore, DCs may carry live HIV and may secondarily infect T-cells in lymph nodes. During this process the DCs and T-cells could be lost by direct lysis or by the action of specific cytotoxic T cells (CTLs) [25].
CCL20/MIP-3α is also a potent adjuvant in augmenting CTLs that provide strong protection in immunized animals against challenge with the vaccinia virus expressing the gag protein [26]. The role of CCR6 and its ligand has also been studied in a variety of diseases and may contribute to disease pathogenesis via autocrine and/or paracrine mechanisms [27-37].
Although DCs are known to play an important role in HIV-1 pathogenesis, the role of MIP-3α/CCL20 as a local and systemic player in HIV-1 infected individuals is poorly understood. Measurement of CCL20/MIP-3α in local microenvironments such as the gut is a challenging task. Thus, we examined CCL20/MIP-3α concentrations in the blood and found a correlation with markers of immune activation in HIV-1-infected men. We speculate that this may reflect the concentrations and the action of the MIP-3α/CCL20 at local sites.
Materials and Methods
Specimens
Serum samples from HIV-1-infected and HIV-1-uninfected men participating in the Multicenter AIDS Cohort Study (MACS) of the Natural History of AIDS at UCLA were selected based on the absolute CD4+ T cell counts (<200, 200-400, and >400 cells/μl) [38]. The institutional review board for human studies at UCLA approved the protocols and blood samples were obtained after informed consent. Blood was collected into 10 ml (Becton Dickinson VACUTAINER Systems, New Jersey) plain plastic tubes without anticoagulant for obtaining serum. Serum was separated and stored at −70°C until batch testing.
CCL20/MIP-3 α assay
Concentrations were measured using a sandwich enzyme immunoassay from R&D Systems (Minneapolis, MN, USA). The lower limit of detection was 9.0 pg/mL and the intra-assay coefficient of variation (CV) was determined to be 14.2% and 3.4% for control samples with mean concentrations of 57.3 pg/mL (n=12) and 279.0 pg/mL (n=12), respectively.
Interferon gamma (IFN-γ)
Concentrations were measured using a sandwich enzyme immunoassay from Beckman Coulter (Brea, CA, USA). The lower limit of detection was 10 U/L and the intra-assay CV was determined to be 16.5%, 6.4%, and 11.1% for control samples with mean concentrations of 47U/L (n=10), 249.7 U/L (n=10), and 3665 U/L (n=10) respectively.
Flow cytometry
Immunophenotyping and quantification of CD38 molecules on CD8+ T cells was performed using a FACSCalibur flow cytometer (Becton Dickinson, CA, U.S.A.) as previously described [39,40].
Statistical analysis
A four-parameter curve-fitting program (Bio-Rad Laboratories, Irvine, CA) was used to generate calibration curves for each enzyme immunoassay.
Descriptive statistics were used to illustrate the blood markers among study groups. Two-sample t-tests were applied to compare CCL20/MIP-3α concentrations between two study groups. Log10 transformations were applied when normal distribution assumption was not demonstrated. Spearman corrections were utilized to assess the association between two blood markers. Data were analyzed using SAS version 9.4 (SAS Institute, 2013). Graphs were produced by using SigmaPlot software version 11 (Jandel Scientific, San Rafael, CA 2008).
Results
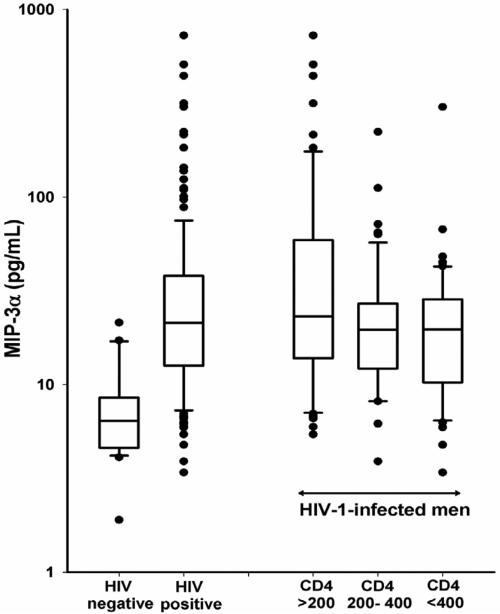
Median serum concentrations of CCL20/MIP-3α in HIV-1-uninfected and HIV-1-infected men was 21.3 pg/mL (n=167) and 6.4 pg/mL (n=27), respectively (Figure 1). CCL20/MIP-3α concentrations were significantly (p<0.0001) higher for HIV-1-infected men when compared with HIV-1-uninfected men. The median serum concentration of CCL20/MIP-3α in HIV-1-infected men with CD4+ T cell counts <200 cells/μL was 23.1 pg/mL (n=61); in HIV-1-infected men with CD4+ T cell counts of 200–400 cells/μL it was 19.6 pg/mL (n=53); and in HIV-1-infected men with CD4+ T cell counts >400 cells/μL it was 19.7 pg/mL (n=53), respectively (Figure 1). Concentrations of CCL20/MIP-3α were significantly higher in HIV-1-infected men with CD4+ T cell counts <200 cells/μL compared to the men with CD4+ T cells counts ranging from 200–400 cells/μL (p=0.0144) and >400 cells/μL (p=0.0052). There was no difference between mean concentrations of CCL20/MIP-3α when comparing HIV-1-infected men with CD4+ T cell counts of 200–400 cells/μL and with CD4+ T cell counts >400 cells/μL.
Figure 1.
log10 transformed serum concentrations of CCL20/MIP-3α in HIV-1 negative men and HIV-1 positive men with CD4+ T cell counts <200, between 200 and 400 and >400 cells/μL. The solid lines represent the median and the box represents the 25th to 75th percentiles. Individual outlying data points are presented as filled black circles.
The median, mean, standard deviation and ranges for HIV-1 RNA (viral load), white blood cell counts (WBC), lymphocyte percentage, CD38 molecules on CD8+ T cells, MIP-3α, and IFN-γ serum concentrations according to the three CD4 groups (<200, 200–400 and >400 cells /μL) are presented in Table 1.
Table 1.
Blood concentrations of markers for HIV-1 infection stratified according to CD4 cell counts.
| CD4* T-Cell Count/μL | |||
|---|---|---|---|
| Markers | <200 n=61 | 200-400 n=53 | >400 n=53 |
| $Viral load (copies/mL) | |||
| Median (range) | 4,750 (0–948,000) | 248 (0-172,000) | 98 (0-184,669) |
| Mean (SD) | 77,631 (±167,658) | 13,804 (±30,593) | 18,124(± 40,709) |
| WBC count (× 103/μL) | |||
| Median (range) | 4.1 (1.2–9.2) | 5.2 (2.2–12.6) | 5.1 (2.6–8.8) |
| Mean (SD) | 4.2 (± 1.76) | 5.3 (± 1.81) | 5.4 (± 1.36) |
| Lymphocyte (%) | |||
| Median (range) | 27.0(1–64) | 34 (12–53) | 39 (18–63) |
| Mean (SD) | 28.7 (± 13.42) | 34 (± 9.76) | 38(± 9.78) |
| *CD38 on CD8 cells | |||
| Median (Ranges) | 2,623 (143-18,543) | 1,412 (197–10,815) | 1017 (175–10,879) |
| Mean (SD) | 4,312 (±4,304) | 1,781 (± 1,781) | 1,667 (± 2,045) |
| MIP-3α (pg/mL) | |||
| Median (Ranges) | 23.1 (5.4-726) | 19.6 (3.9-222) | 19.7 (3.4–302) |
| Mean (SD) | 71.2 ( ± 128) | 27.8 (± 33) | 26.8 (± 41) |
| IFN-γ (U/L) | |||
| Median (Ranges) | 96 (0-907) | 52 (5–636) | 74(0–294) |
| Mean (SD) | 169 (± 203) | 95 (± 122) | 90 (± 74) |
Viral load=HIV RNA
expression of CD38 molecule
The correlation coefficients between serum concentrations of MIP-3α and WBC counts , percentage of lymphocytes, CD38 molecules on CD8+ T cells, IFN-γ, and HIV viral load for HIV-1-infected according to CD4+ T cell counts listed in Table 2.
Table 2.
Correlation coefficient between serum levels of MIP-3α and markers. Significant between markers have shown as bold font.
| Groups | WBC | Lymphocytes | CD38 on CD8 | IFN-γ | HIV-1 RNA |
|---|---|---|---|---|---|
| All cases | 0.11885* | −0.17731 | 0.29289 | 0.23386 | 0.13871 |
| 0.126φ | 0.0219 | 0.0002 | 0.0063 | 0.0883 | |
| 167n | 167 | 156 | 135 | 152 | |
| CD4<200 | 0.26616 | −0.28791 | 0.49085 | 0.27244 | 0.27455 |
| 0.0381 | 0.0244 | 0.0003 | 0.0670 | 0.0512 | |
| 61 | 61 | 51 | 46 | 51 | |
| CD4=200-400 | 0.09355 | −0.11102 | 0.15913 | 0.24936 | −0.04706 |
| 0.5052 | 0.4287 | 0.2598 | 0.0718 | 0.7455 | |
| 53 | 53 | 52 | 53 | 50 | |
| CD4>400 | 0.15268 | 0.03114 | 0.05899 | 0.02401 | −0.00493 |
| 0.2751 | 0.8248 | 0.6748 | 0.8894 | 0.9726 | |
| 53 | 53 | 53 | 36 | 51 |
Spearman correlation coefficient
p value
number of men
Discussion
Dendritic cells stimulate protective immunity, play a secondary role in HIV infection, and have also been known for their function as a “Trojan horse” on the HIV infection course [41]. MIP-3α may play a crucial role in the formation and function of the axis of the HIV→iDCs-CCR6←CCL20 during sexual transmission of HIV infection at the local site of the mucosal lymphoid tissues by attracting iDCs and T-cells toward epithelial cells. Song et al. have shown that intramuscular inoculation of pMIP-3α, alongside recruitment of more iDCs to the injection site, also enhances DC function [26].
It is well known that the sexual transmission of HIV-1 requires a small amount of virus at a mucosal site of inoculation to gain access to the cells that are permissive for the HIV-1 viral infection [42,43]. iDCs are constantly sampling their environment for foreign antigens, they capture theses antigens, and leave the tissue carrying the antigen to the lymph nodes and progress into mature DCs. In addition, human seminal plasma is able to stimulate the vaginal or recto-colonic epithelial (Langerhans cells) and sub epithelial cells to secrete more MIP-3α/CCL20 [23] for further recruitment of iDCs to the local site for encounters with virus. Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) at the local site can capture HIV-1 and promote infection of permissive cells in Trans-infection [44].
CCR6/MIP-3α and CCR7/MIP-3β play a major role in the constitutive trafficking of peripheral DCs along lymphatic channels to the outer surface of lymph nodes in SIV infection and this could play this role for HIV-1 infection in humans as well [45].
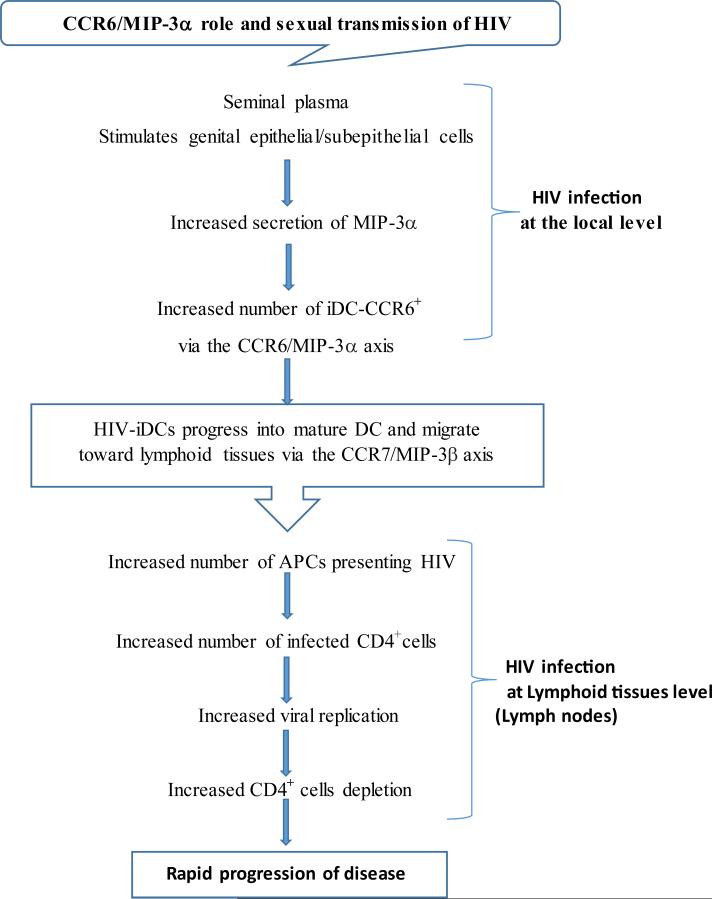
The potential mechanism of MIP-3α as part of CCR6/MIP-3α axis in the development of sexual transmission of HIV infection is summarized in a diagram (Figure 2).
Figure 2.
Role of CCR6/MIP-3α axis in sexual transmission and progression to HIV-1 infection.
The aim of our study was to investigate the relationship between the blood level of MIP-3α and local recruitments of iDC as a prognostic tool by correlating blood levels of MIP-3α with IFN-γ, viral RNA, and CD38 molecules on CD8+ T cell as an activation marker.
We found that the serum levels of MIP-3α were significantly elevated in HIV-1-infected men compared with HIV-1-uninfected men (p<0.0001). The serum levels of MIP-3α were significantly higher in HIV-1/AIDS compared to HIV-1 with absolute CD4+ T cell counts of 200-400 and >400 cells/μL. This relatively high blood concentration of MIP-3α may contributes to higher local recruitments of iDCs and poor prognoses in patients. Fontaine et al. also have shown above normal plasma levels of MIP-3α in both normal and rapid progressor of HIV-1-infected individuals [46].
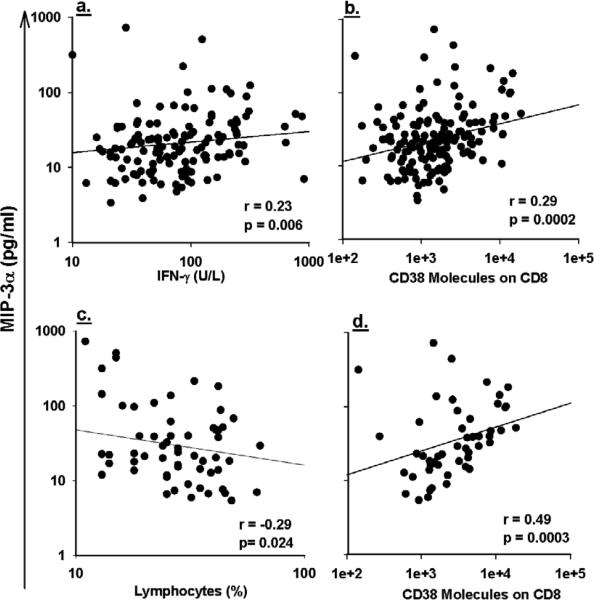
Serum levels of MIP-3α in all HIV-1-infected cases exhibited positive correlations with CD38 molecules on CD8+ T cells and IFN-γ (Figures 3a and 3b), negative correlation with lymphocyte and absolute CD4+ T cells count (0.015) but were not correlated with number of WBC, and HIV RNA (Table 2).
Figure 3.
Positive correlation between log10 transformed serum concentrations of CCL20/MIP-3α and log10 transformed of IFN-γ (a), positive correlation with log10 transformed of number of CD38 molecules on CD8+ T cells (b) shown all HIV-1-infected in the study. Negative correlation between log10 transformed serum concentrations of CCL20/MIP-3α and percentage of lymphocyte (c) and positive correlation with log10 transformed of number of CD38 molecules on CD8+ T cells(d) shown for HIV-1-infected men with CD4+ T cell counts <200 cell/μL.
Among HIV-1-infected men with CD4+ T cell counts <200 cells/μL, MIP-3α showed negative correlation with lymphocytes and positively correlation with CD38 molecules on CD8+ T-cells (Figures 3c and 3d), but was not correlated well with plasma levels of HIV RNA (p=0.051) which may be due to the effect of antiretroviral therapy on the plasma level of HIV RNA but not in the viral reservoir sites.
Studies have shown that the plasma level of HIV RNA correlates well with immune activation markers during HIV-1 infection and antiretroviral therapy, but in some cases these relationships may not be constant and strong such as we observed in our study between HIV RNA and MIP-3α for HIV-1-infected men with CD4+ T cell<200 cells/μL. In addition, high levels of MIP-3α with increased expression of CD38 molecule on CD8+ T cell may be an indication of the HIV persisting in a patient's viral reservoir tissues, and the MIP-3α in combination with other markers could be a useful prognostic test in those cases.
Since the T cell activation marker of CD38 molecules on CD8+ T cells is strongly correlated with blood levels of MIP-3α we may be able to use the blood level of MIP-3α as a surrogate marker for local levels of MIP-3α which therefore could also serve as an activation marker for iDC. Ye Ping et al. demonstrated that there were no significant differences among the four clinical stages of HIV-1 infection in the serum levels of CC-chemokines [47] and we also observed no significant differences among the two groups of patients with CD4+ T cell between 200-400 cells/μL and CD4+T cell> 400 cells/μL for MIP-3α. The weakness of our study is that the levels of MIP-3α have not been explored and measured in the fluid at the recto-colonel level.
In conclusion, the blood levels of MIP-3α in HIV-1-infected men with CD4+ T cell<200 cells/μL and all HIV-1-infected cases positively correlated with CD38 molecules expression on CD8+ T cell. The high levels of MIP-3α in HIV/AIDS may contribute to the recruitment of more iDC and other immune cells at the local site of the infection particularly the recto-colonel site of men who have sex with men (MSM) which may cause increased immune activation triggering a poor prognosis of HIV-1-infected patients. This finding could be a base for further investigations on the role and regulation of MIP-3α in the pathogenesis of HIV-1, especially at the recto-colonel region.
Acknowledgement
We thank the men who participate in the MACS, who make this and many other studies possible. We also thank John Oishi, Kevin Barrett, Denis Miles, Carlos Aquino, Ray Mercado, and Carlos Mercado for recruitment coordination; Timothy Ryner for preparation of manuscript, Patricia Hultin, Shaun Hsueh, Yegermal Asnake, and Chantal Delshad for technical assistance.
This work was supported by grants from the National Institutes of Health (U01-A1-35042); The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (U01-AI35042, 5-M01-RR00722 (GCRC), U01-A135043, U01-AI37984, U01-A135039, U01-AI35040, U01-AI37613, and U01-AI35041). This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. N01-CO56000.
References
- 1.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 2.Sallusto F, Mackay CR, Lanzavesschi A. The role of chemokine receptors in primary, effector and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 3.Rojo D, Suetomi K, Navarro J. Structural biology of chemokine receptors. Biol Res. 1999;32:263–272. doi: 10.4067/s0716-97601999000400006. [DOI] [PubMed] [Google Scholar]
- 4.Christopherson K, II, Hromas R. Chemokine regulation of normal and pathologic immune responses. Stem Cells. 2001;19:388–396. doi: 10.1634/stemcells.19-5-388. [DOI] [PubMed] [Google Scholar]
- 5.Dimberg A. Chemokines in angiogenesis. Curr Top Microbiol Immunol. 2010;341:59–80. doi: 10.1007/82_2010_21. [DOI] [PubMed] [Google Scholar]
- 6.Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- 7.Hromas R, Gray PW, Chantry D, Godiska R, Krathwohl M, et al. Cloning and characterization of exodus, a novel beta-chemokine. Blood. 1997;89:3315–3322. [PubMed] [Google Scholar]
- 8.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 9.Zlotnik A, Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 10.Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem. 1997;272:14893–14898. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- 11.Liao F, Alderson R, Su J, Ullrich SJ, Kreider BL, et al. STRL22 is a receptor for the CC chemokine MIP-3-alpha. Biochem Biophys Res Commun. 1997;236:212–217. doi: 10.1006/bbrc.1997.6936. [DOI] [PubMed] [Google Scholar]
- 12.Charbonnier AS, Kohrgruber N, Kriehuber E, Stingl G, Rot A, et al. Macrophage inflammatory protein 3-alpha is involved in the constitutive trafficking of epidermal Langerhans cells. J Exp Med. 1999;190:1755–1768. doi: 10.1084/jem.190.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 14.Farber JM. Chemokines, lymphocytes and HIV. Braz J Med Biol Res. 1998;31:11–17. doi: 10.1590/s0100-879x1998000100002. [DOI] [PubMed] [Google Scholar]
- 15.Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis B, et al. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3-alpha and is highly expressed in human dendritic cells. J Exp Med. 1997;186:837–844. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, et al. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3-alpha. J Immunol. 1999;162:186–194. [PubMed] [Google Scholar]
- 17.Ai LS, Lee SF, Chen SS, Liao F. Molecular characterization of CCR6: Involvement of multiple domains in ligand binding and receptor signaling. J Biomed Sci. 2004;11:818–828. doi: 10.1007/BF02254367. [DOI] [PubMed] [Google Scholar]
- 18.Lee AY, Eri R, Lyons AB, Grimm MC, Korner H. CC chemokine ligand 20 and its cognate receptor CCR6 in mucosal T cell immunology and inflammatory bowel disease: Odd couple or axis of evil? Front Immunol. 2013;4:194. doi: 10.3389/fimmu.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam S, Shimizu N, Hoque SA, Jinno-Oue A, Tanaka A, et al. CCR6 functions as a new co-receptor for limited primary human and simian immunodeficiency viruses. PLoS One. 2013;8:e73116. doi: 10.1371/journal.pone.0073116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafferty MK, Sun L, DeMasi L, Lu W, Garzino-Demo A. CCR6 ligands inhibit HIV by inducing APOBEC3G. Blood. 2010;115:1564–1571. doi: 10.1182/blood-2009-06-226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, et al. Many chemokines including CCL20/MIP-3-alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, et al. CCL20/MIP3-alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berlier W, Cremel M, Hamzeh H, Lévy R, Lucht F, et al. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: Involvement in the sexual transmission of HIV. Hum Reprod. 2006;21:1135–1142. doi: 10.1093/humrep/dei496. [DOI] [PubMed] [Google Scholar]
- 24.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight SC, Patterson S. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus and immunopathology. Annu Rev Immunol. 1997;15:593–615. doi: 10.1146/annurev.immunol.15.1.593. [DOI] [PubMed] [Google Scholar]
- 26.Song R, Liu S, Leong KW. Effects of MIP-1 alpha, MIP-3 alpha, and MIP-3 beta on the induction of HIV Gag-specific immune response with DNA vaccines. Mol Ther. 2007;15:1007–1015. doi: 10.1038/mt.sj.6300129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor and intrauterine infection. J Perinat Med. 2008;36:217–227. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosokawa Y, Nakanishi T, Yamaguchi D, Takahashi K, Yumoto H, et al. Macrophage inflammatory protein 3-alpha-CC chemokine receptor 6 interactions play an important role in CD4+ T-cell accumulation in periodontal diseased tissue. Clin Exp Immunol. 2002;128:548–554. doi: 10.1046/j.1365-2249.2002.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata T, Tanaka K, Inoue Y, Toiyama Y, Hiro J, et al. Macrophage inflammatory protein-3 alpha (MIP-3a) is a novel serum prognostic marker in patients with colorectal cancer. J Surg Oncol. 2013;107:160–166. doi: 10.1002/jso.23247. [DOI] [PubMed] [Google Scholar]
- 30.Kohler RE, Caon AC, Willenborg DO, Clark-Lewis I, McColl SR. A role for macrophage inflammatory protein-3 alpha/CC chemokine ligand 20 in immune priming during T cell-mediated inflammation of the central nervous system. J Immunol. 2003;170:6298–6306. doi: 10.4049/jimmunol.170.12.6298. [DOI] [PubMed] [Google Scholar]
- 31.Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3-alpha) production in normal colon and inflammatory bowel disease. Gut. 2002;51:818–826. doi: 10.1136/gut.51.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama T, Fujisawa R, Yamada H, Horikawa T, Kawasaki H, et al. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol. 2001;13:95–103. doi: 10.1093/intimm/13.1.95. [DOI] [PubMed] [Google Scholar]
- 33.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3-alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 34.Ruth JH, Shahrara S, Park CC, Morel JC, Kumar P, et al. Role of macrophage inflammatory protein-3-alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–588. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi K, Akbar SM, Horiike N, Michitaka K, Onji M. Increased serum levels of macrophage inflammatory protein-3-alpha in chronic viral hepatitis: Prognostic importance of macrophage inflammatory protein-3alpha during interferon therapy in chronic hepatitis C. J Viral Hepat. 2002;9:213–220. doi: 10.1046/j.1365-2893.2002.00354.x. [DOI] [PubMed] [Google Scholar]
- 36.Simpson KJ, Henderson NC, Bone-Larson CL, Lukacs NW, Hogaboam CM, et al. Chemokines in the pathogenesis of liver disease: So many players with poorly defined roles. Clin Sci (Lond) 2003;104:47–63. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 37.Arima Y, Harada M, Kamimura D, Park JH, Kawano F, et al. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell. 2012;148:447–457. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Detels R, Jacobson L, Margolick J, Martinez-Maza O, Muñoz A, et al. The multicenter AIDS Cohort Study, 1983 to .... Public Health. 2012;126:196–198. doi: 10.1016/j.puhe.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giorgi JV, Lyles RH, Matud JL, Yamashita TE, Mellors JW, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr. 2002;29:346–355. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 40.Hultin LE, Matud JL, Giorgi JV. Quantitation of CD38 activation antigen expression on CD8+ T cells in HIV-1 infection using CD4 expression on CD4+ T lymphocytes as a biological calibrator. Cytometry. 1998;33:123–132. doi: 10.1002/(sici)1097-0320(19981001)33:2<123::aid-cyto6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 41.Cavrois M, Neidleman J, Greene WC. The achilles heel of the trojan horse model of HIV-1 trans-infection. PLoS Pathog. 2008;4:e1000051. doi: 10.1371/journal.ppat.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sewell AK, Price DA. Dendritic cells and transmission of HIV-1. Trends Immunol. 2001;22:173–175. doi: 10.1016/s1471-4906(01)01866-x. [DOI] [PubMed] [Google Scholar]
- 43.Gosselin A, Monteiro P, Chomont N, Diaz-Griffero F, Said EA, et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol. 2010;184:1604–1616. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 45.Choi YK, Fallert BA, Murphey-Corb MA, Reinhart TA. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood. 2003;101:1684–1691. doi: 10.1182/blood-2002-08-2653. [DOI] [PubMed] [Google Scholar]
- 46.Fontaine J, Poudrier J, Roger M. Short communication: Persistence of high blood levels of the chemokines CCL2, CCL19, and CCL20 during the course of HIV infection. AIDS Res Hum Retroviruses. 2011;27:655–657. doi: 10.1089/aid.2010.0261. [DOI] [PubMed] [Google Scholar]
- 47.Ye P, Kazanjian P, Kunkel SL, Kirschner DE. Lack of good correlation of serum CC-chemokine levels with human immunodeficiency virus-1 disease stage and response to treatment. J Lab Clin Med. 2004;143:310–319. doi: 10.1016/j.lab.2004.01.012. [DOI] [PubMed] [Google Scholar]