Abstract
B cell anti-host antibody production plays a central role in chronic graft-vs-host disease (cGVHD). T follicular helper (TFH) cells drive B cell responses and are implicated in this process. Given differences in cGVHD incidence between umbilical cord blood (UCB) and adult donor transplant recipients, we evaluated TFH cell reconstitution kinetics to define graft source differences and their potential pathogenic role in cGVHD. Although we observed significantly fewer TFH cells in the blood of UCB recipients (vs. matched related donors (MRD)) early after transplantation, by 1 year the numbers of TFH cells were similar. Additionally, at both early (day 60) and late (1 year) time points, TFH cell phenotype was predominantly central memory cells in both cohorts. TFH cells were functional and able to produce multiple cytokines (INF-γ, TNF-α, IL-2, IL-17 and IL-21) following stimulation. In contrast to mouse models where an enhanced frequency of splenic TFH cells contributes to cGVHD, patients with cGVHD showed significantly depleted circulating TFH cells following both UCB and MRD transplantation. Low numbers of TFH cells early after UCB transplantation could directly contribute to less cGVHD in this cohort. Additionally, systemic therapy (including steroids and calcineurin inhibitors) may contribute to decreases in TFH cells in patients with cGVHD. These data provide further evidence supporting the importance of TFH cells in cGVHD pathogenesis.
Introduction
Blood and marrow transplantation is one of the only curative therapies for patients with hematological malignancies that are refractory to current chemotherapy regimens. Rapid lymphocyte recovery is essential for optimal protection against pathogens over the lifetime of a transplant recipient. In addition to their anti-microbial function, donor lymphocytes also mediate graft-vs-leukemia effects1. Unfortunately, donor lymphocytes are also responsible for one of the major complications of hematopoietic cell transplantation (HCT), graft-vs-host disease (GVHD). The pathophysiology of acute GVHD has been extensively studied in mice and humans2 and more recently there has been an increasing emphasis to better understand the pathophysiology of cGVHD3. For instance, several groups have established that donor B cells produce antibody directed against host antigens in both mice and humans experiencing cGHVD4-6. This is most evident in seminal studies by Miklos showing that in sex-mismatched transplants, B cells from female donors produce antibodies against male recipient antigens6,7. Accordingly, strategies targeting bulk B cells (with rituximab8) or their signaling machinery (with ibrutinib9) have been used to treat both experimental murine cGVHD and in humans with encouraging results in early human trials4,10. Current therapies including corticosteroids and calcineurin inhibitors broadly target immune cells, however, there are a lack of therapeutic interventions directed at specific T cell subsets for treatment of cGVHD.
More recently, a subset of T cells known to drive B cell responses in secondary lymphoid tissues, called T follicular helper (TFH) cells, has been increasingly characterized in mice11 and humans12,13. In humans, TFH cells can be identified in the periphery, herein referred to as pTFH cells13,14.These T cells are defined by the co-expression of CD4 and among others, the chemokine receptor CXCR5. Under normal circumstances, TFH cells provide B cell help through expression of costimulatory molecules including CD40L, PD-1, and ICOS13. Moreover, they produce key cytokines (e.g., IL-21) in germinal centers which activate B cells to undergo class switching and induce antibody production11. In murine experimental cGVHD models, we have previously shown that TFH cells drive germinal center B cells and the production of antibodies causing injury to host tissues within the lung, liver, thymus, spleen, and colon5. In this model, blocking several effector molecules, including ICOS and IL-21 from donor TFH cells prevents or reverses germinal center formation and cGVHD5.
Although immune recovery and function following HCT has been studied for years, a more in depth look at the cell subsets directly involved in complications, such as cGVHD, has lagged. Additionally, as our availability of donor pools grows through the use of related, unrelated, or umbilical cord blood (UCB) sources15-17, there may be considerable differences in the transplanted lymphocytes (i.e., graft composition) and lymphocyte subset recovery post-transplant. This in turn, may be associated with differences in clinical outcome. Notably, recipients of UCB transplantation experience less cGVHD than bone marrow (BM) and/or peripheral blood stem cell (PBSC) sources18, including those from matched related donors (MRDs) which have traditionally been the stem cell source of choice.
Given the role of TFH cells in murine models of cGHVD, we asked whether or not there were differences in human TFH cells between donor sources that could explain differences in cGVHD.
Methods
Transplant protocols and GVHD prophylaxis
Patients were treated using a variety of different conditioning regimens and cell sources described below. For myeloablative transplantation patients received cyclophosphamide (60 mg/kg × 2 days), +/−fludarabine (25 mg/m2 × 3 days) and total body irradiation (13.2 Gy over 4 days)19. Non myeloablative intensity conditioning (NMA) consisted of cyclophosphamide (50 mg/kg × 1), fludarabine (30 mg/m2 × 5) and total body irradiation (2 Gy). In patients without prior intensive chemotherapy equine ATG was added at (15 mg/kg × 6 doses). GVHD prophylaxis consisted of mainly MMF (2-3 gm/day divided BID from days −3 to 30) and cyclosporine A (CSA, to keep trough levels 200-400) or sirolimus (to keep trough levels 8-12, from days −3 to 100 and then a taper thereafter) or methotrexate and CSA.
Patient Samples
Kinetic and phenotypic studies were completed using cryopreserved PBMCs from patients undergoing UCB (n=15) or matched sibling PBSC/BM transplantation (n=15; n=14 PBSC and n=1 BM) at days +60, 100, 180 and 365. For the analysis of pTFH cells and cGVHD, an additional 10 patients with cGVHD from each stem cell source were used. cGVHD was diagnosed using recent NIH consensus conference guidelines20. All patients consented to donating peripheral blood lymphocytes at various times following transplantation using Institutional Review Board approved clinical trials and all of the above clinical research has been conducted according to the declaration of Helsinki.
Antibodies and flow cytometry
The following antibodies were used: CD8 BV650, CD4 BV605, and CD57 PacBlue all obtained from Biolegend. CXCR5-APC and CD3-PercpCy5.5 were obtained from Becton Dickinson. CD27 APC-eFLuor 780, CD45RO PeCy7, and Fixable Viability Dye eFluor 506 were all obtained from eBiosciences. Flow cytometry was done on a BD LSRII and data analyzed using FlowJo (Treestar). To identify TFH cells, samples were gated on live cells within the lymphocyte gate after doublet discrimination. TFH cells were then defined by gating on CD3+CD8−CD4+CXCR5+ cells.
Assessment of TCR-induced Cytokine Production of pTFH Cells Post-transplant
Patient samples were thawed overnight in basal media containing 10% FBS with no additional cytokine support. The following day, cells were washed, counted, and resuspended at 5-10×106/mL. To evaluate for TCR induced intracellular CD40 ligand (CD154) expression and cytokine production, unstimulated PMBCs were compared to SEB (staphylcoccal enterotoxin B) stimulated cells in the presence of brefeldin A and monesin (added after 1 hour incubation). Briefly, cells were incubated for in the presence or absence of SEB stimulation for 6 hours at 37 degrees Celsius. At the end of the incubation period, the reaction was stopped by washing the cells three times followed by staining for the following cell surface and intracellular cytokines using previously published methods 21. The following antibodies were used: CD154 APC Vio-770 (Miltenyi Biotechnology), CD3 FITC, IL-21 PE, CXCR5 PerCP-Cy5.5, IL-17A Alexa Fluor 647 (Becton Dickinson), CD8 AF700, CD4 BV605, IFNy PacBlue, TNFα BV650, IL-2 PeCy7 (Biolegend), and Fixable Viability Dye eFluor 506 (eBiosciences). Flow cytometry was done on a BD LSRII and data analyzed using FlowJo (Treestar).
Statistical analysis
Where results of multiple samples are summarized, data are expressed as the mean ± standard deviation (SD) for the indicated number (n=). For studies with two groups, differences between groups were analyzed by Student T test or the Mann-Whitney U test, unless otherwise indicated. For comparison of multiple groups, analysis of variance (ANOVA) was utilized. If significant then an unpaired T test was used and p values were corrected using Bonferroni correction for multiple comparisons. Statistical significance was indicated as NS, p> 0.05, *p≤ 0.05, **p< 0.01, and ***p< 0.001. Results were considered significant at p values of 0.05 or less (GraphPad Software, San Diego, CA).
Results
Reconstitution of peripheral T follicular helper cells following HCT
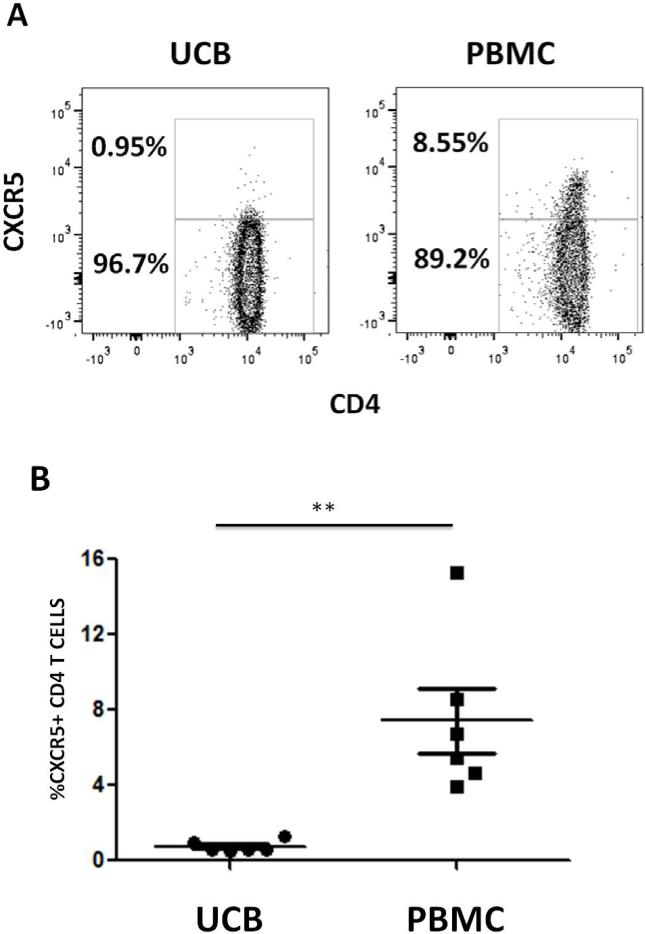
Although reconstitution of several T lymphocyte subsets following HCT has been described16,22, there are currently no published data regarding the recovery of pTFH cells in humans following HCT. Given the role of TFH cells in driving B cell responses and the association of host-reactive antibodies in cGVHD6, our initial study hypothesis was that higher levels of circulating TFH cells would correlate with cGVHD. Before addressing this, we first investigated the content of pTFH cells (defined by CD3+CD4+CXCR5+) in UCB and adult PB. As shown in Figure 1, UCB is essentially devoid of pTFH cells, while they are readily detectable in healthy individuals (UCB vs. adult blood 0.7 ± 0.3% vs 7.4 ± 4.2%, p=0.002, n=6, Figure 1). Therefore, we sought to compare this specific subset in recipients of UCB (n=15) and MRD (n=15) at 60 days to 1 year after transplantation. Patient characteristics are shown in table 1. Briefly, all patients received reduced intensity transplantation and the two groups did not differ significantly with respect to ATG use in the conditioning regimen, age, portion with aGVHD or severity of aGVHD. There were differences in baseline diseases, with a higher proportion of UCB patients having AML or MDS. The majority of patients showed donor engraftment at D100 in the T cell compartment.
Figure 1. PBMCs from adult donors contain more pTFH cells than UCB.
Mononuclear cell fractions were isolated from normal UCB and normal PB samples and stained with CD3, CD4, and CXCR5. pTFH CD4+CXCR5+ populations were gated from the Live CD3+ population in each sample. A) Example gating for quantification of pTFH CD4+CXCR5+ populations in an UCB mononuclear cell fraction (left) and a PBMC (right). B) Percentage of pTFH CD4+CXCR5+ cells found in UCB (n=6) versus PBMC (n=6). **p=0.002, Mann-Whitney t-test.
Table 1.
Patient Demographics
| PBSC/BM | UCB | p value | |
|---|---|---|---|
| n=15 | n=15 | ||
| Sex | 0.69 | ||
| Male | 10(66.7%) | 11(73.3%) | |
| Age | 0.25 | ||
| Median(Min-Max) | 63.2(23.4-72.8) | 56.7(44.8-69.5) | |
| Diagnosis | 0.35 | ||
| ALL | 1(6.7%) | 2(13.3%) | |
| AML | 5(33.3%) | 7(46.7%) | |
| Other Leukemia | 0 | 1(6.7%) | |
| Myelodysplasia | 2(13.3%) | 3(20.0%) | |
| Non-Hodgkin's | 4(26.7%) | 2(13.3%) | |
| Lymphoma | |||
| Hodgkin's Lymphoma | 3(20.0%) | 0 | |
| Conditioning | 1.00 | ||
| RIC | 15(100.0%) | 15(100.0%) | |
| ATG in Conditioning | 1.00 | ||
| Yes | 3(20.0%) | 3(20.0%) | |
| GVHD prophylaxis | 1.00 | ||
| CSA +/− MMF +/− MTX | 13(86.7%) | 13(86.7%) | |
| Siro +/− Tacro +/−MMF | 2(13.3%) | 2(13.3%) | |
| aGVHD II-IV | 0.23 | ||
| Yes | 3(20.0%) | 6(40.0%) | |
| No | 12(80.0%) | 9(60.0%) | |
| Time to aGVHD II-IV | 0.79 | ||
| Median days (Min-Max) | 37.0(30.0-38.0) | 39.0(21.0-115.0) | |
| D+100 Donor Engraftment in CD3+ Fraction | 0.14 | ||
| n | 15 | 11 | |
| Median (Min-Max) | 99.0 (56.0-100.0) | 100.0 (75.0-100.0) | |
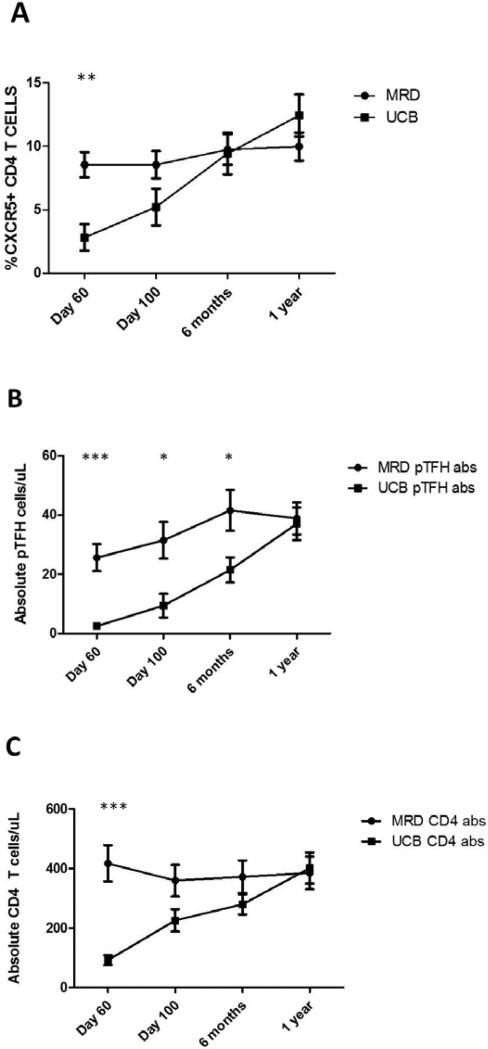
Based on our initial findings showing differences in CD4+CXCR5+ T cells between UCB and adult peripheral blood (above, Figure 1), we predicted differences in their reconstitution kinetics based on stem cell source. In MRD recipients, the proportion of pTFH cells was stable over time, whereas UCB recipients (Figure 2A) had significantly lower percentages of pTFH cells at day 60 compared to MRD transplant recipients (8.5 ± 1.0% vs 2.8 ± 1.0%, p=0.001, Figure 2A) with similar levels at 1 year post transplant (10 ± 1.1% vs 12.4 ± 1.7%, p=0.23, Figure 2A). To ensure that these differences in frequency were not related to disproportionate recovery of the CD4 T cell compartment, we also evaluated absolute cell counts of both pTFH and CD4 T cells from each group. As shown in Figure 2B, the absolute count of pTFH cells increases over 1 year in each group, with significantly more pTFH cells in MRD recipients at day 60, day 100, and 6 months. At day 60, UCB recipients also had significantly fewer total CD4 T cells, however, the numbers of CD4 T cells converge at later time points (Figure 2C), similar to other reports16,22.
Figure 2. The pTFH population expands in UCB recipients while the pTFH population remains stable in MRD recipients.
A) Quantification of pTFH cells (CD3+CD4+CXCR5+) in patients undergoing UCB (n=15) or matched sibling PBSC/BM transplantation (n=15) at day 60, day 100, 6 months, and 1 year post-transplantation. At day 60 post-transplant, UCB recipients had significantly lower percentages of pTFH cells compared to MRD transplant recipients (8.5% ± 1.0 vs 2.8% ± 1.0. ***p value 0.001, Mann-Whitney t-test). B-C) Absolute numbers of pTFH (B) and CD4 T cells (C) in MRD and UCB recipients displayed in panel A. There are significantly more pTFH cells in MRD recipients at day 60 (25.6 ± 4.5 vs 2.5 ± 0.7. ***p value 0.001), day 100 (31.5 ± 6.2 vs 9.4 ± 4.0. *p value 0.02), and 6 months (41.6 ± 6.9 vs 21.5 ± 4.2. *p value 0.02) compared to UCB recipients. There are significantly more CD4 T cells in MRD recipients at D60 following transplant (417.5 ± 60.6 vs 92.2 ± 18.8. ***p value 0.001) compared to UCB recipients.
We conclude that differences between donor stem cell sources affect reconstitution kinetics of pTFH cells in the recipient, likely due to donor graft content. However, it is not known whether there are differences in the characteristics of the reconstituting pTFH cells between the two groups, as this could influence differences in cGVHD between the two stem cell sources.
Recipients of MRD and UCB transplants have phenotypically and functionally similar pTFH cells
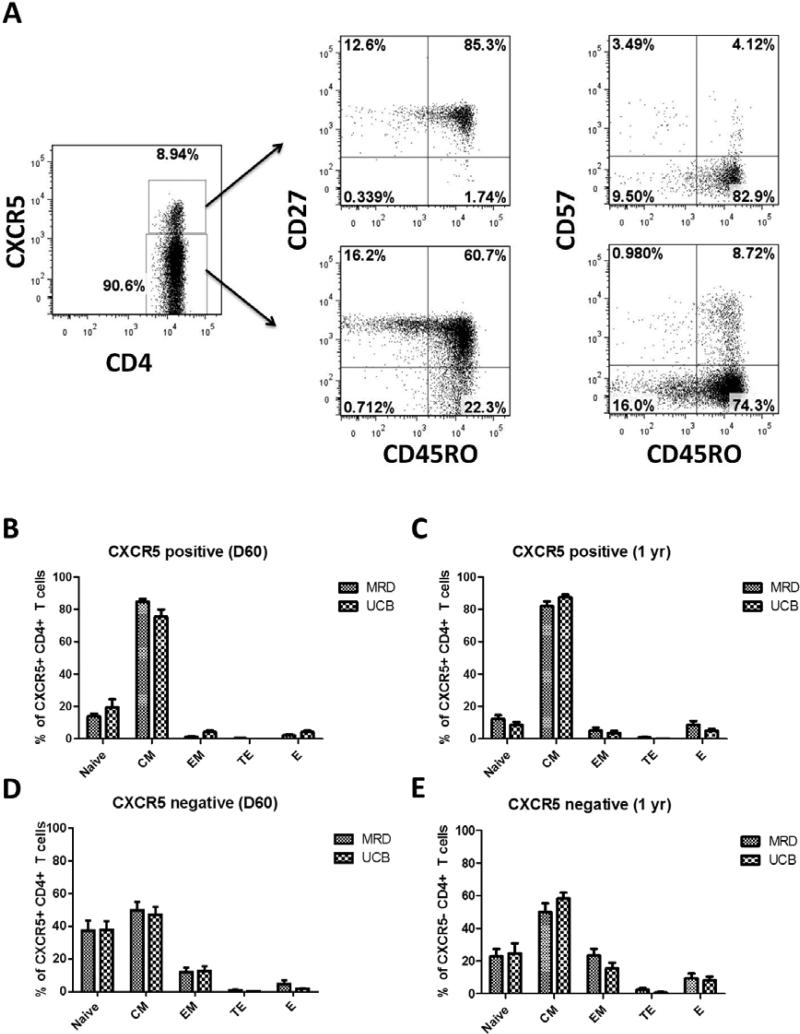
Previous studies have shown that pTFH cells have a surface phenotype of central memory cells (Tcm)23. Given that UCB grafts contain essentially only naive CD4 T cells, their maturation to TFH cells could be altered or delayed as they require further differentiation in the secondary lymphoid tissues24. In contrast, since a proportion of the T cells transferred with MRD grafts contain CXCR5 expressing cells previously exposed to antigen in the donor, they would not be expected to require these initial steps of differentiation. Instead, they could undergo homeostatic proliferation and perhaps recover faster than UCB recipients. To assess whether or not there were cellular differences in the reconstituting pTFH cells between graft sources, we evaluated them for phenotypic differences in CD4+CXCR5+ T cells.
Helper CD4 T cells can be subdivided into different subsets based on established surface markers. These include naïve (CD45RO−CD27+), central memory (CD45RO+CD27+), effector memory (CD45RO+CD27−), effector (CD45RO+CD57+), and terminal effector (CD45RO−CD57+) CD4 T cells. As shown in Figure 3A, each of these subsets are present in both the CXCR5+ and CXCR5− populations of CD4 T cells in recipients of HCT (representative example, MRD at day 60). When evaluating the CXCR5+ cells in UCB and MRD recipients, we found that the majority of them are predominantly central memory cells at both 60 days (84.7 ± 1.6% vs 75.4 ± 4.6%, p value=0.17, Figure 3B) and 1 year (83.1 ± 2.6% vs 88.2 ± 1.4%, p value=0.4, Figure 3C). Of note, there were no detectable terminal effector cells in the CXCR5+ compartment of UCB recipients at day 60 (Figure 3B) and they were significantly reduced at 1 year (0.9 ± 0.2% vs 0.2 ± 0.2%, p value=0.03) (Figure 3C). When examining the CXCR5− cells, we found no significant differences between the two donor sources. The majority were predominantly naïve, central memory, and effector memory CD4 T cells at both 60 days (37.4 ± 6.1% vs 37.6 ± 5.5%, 49.7 ± 5.4% vs 46.9 ± 5.0%, and 11.8 ± 3.0% vs 12.7 ± 2.8%, respectively, p values >0.05, Figure 3D) and 1 year (22.9 ± 4.6% vs 25.0 ± 4.2%, 50.0 ± 5.3% vs 53.6 ± 2.7%, and 23.6 ± 4.0% vs 17.6 ± 2.3%, respectively, p values >0.05, Figure 3E). These data demonstrate that, even in UCB recipients, circulating pTFH cells following HCT have a predominant CM phenotype and there are no major differences between donor sources.
Figure 3. MRD and UCB pTFH cells possess a central memory cell phenotype.
(A) Representative gating path and characterization of pTFH cells following MRD HCT at D60. (B-E) At D60 and 1 year post transplant, CD4 T cells from peripheral blood were gated from the Live CD3+ population. Subsequently, CXCR5+ (2B, 2D) and CXCR5− (2C, 2E) populations within the CD4+ subset were analyzed for CD45RO, CD27, and CD57 expression. Populations identified: naïve (CD45RO−CD27+), central memory (CD45RO+CD27+), effector memory (CD45RO+CD27−), effector (CD45RO+CD57+), and terminal effector (CD45RO−CD57+). In both MRD and UCB recipients, the majority of CXCR5+ cells are central memory cells at day 60 (3B) and 1 year (3C). CXCR5− cells were predominantly naïve, central memory and effector memory CD4 T cells at both 60 days (3D) and 1 year (3E).
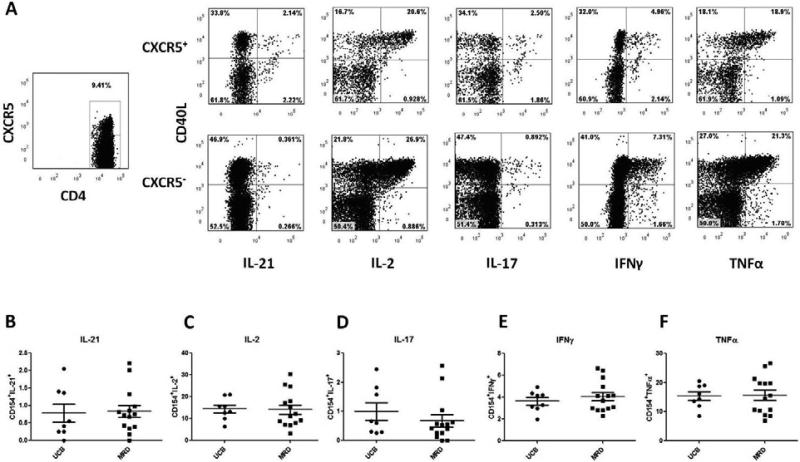
We next set out to determine the function of pTFH cells in transplant recipients. pTFH cells from both UCB and MRD recipients at D+360 produced multiple cytokines (INF-γ, TNF-α, IL-2, IL-17 and IL-21) following direct TCR stimulation with SEB. Prior studies have shown that TFH cells use CD40L to provide B cell help, thus we focused on the cytokine production in this population11,14. The percentage of CXCR5+CD40L+ cells producing cytokines did not differ based on stem cell source (Figure 4A-F). Because we found no CD4 T cell specific differences between the two donor sources, and because murine studies show increases in splenic TFH cell frequency in the setting of a cGHVD, we next asked whether or not TFH cell numbers and function were affected by cGVHD.
Figure 4. pTFH cells from both UCB and MRD recipients produce multiple cytokines in response to T cell receptor stimulation.
Representative gating path and characterization of pTFH cells stimulated with SEB following MRD HCT at 1 year. CD4 T cells from peripheral blood were gated from the live CD3+ population. Subsequently, CXCR5+ and CXCR5− populations within the CD4+ subset were analyzed for expression of CD40L and multiple cytokines including IL-21, IL-2, IL-17, IFNγ, and TNFα. Graphs B-F show compiled data for CXCR5+ cells that coexpress CD40L and B) IL-21, C) IL-2, D) IL-17, E) IFNy, or F) TNFα. Differences between donors (B-F) were non-significant.
pTFH cells are lower in patients with cGVHD
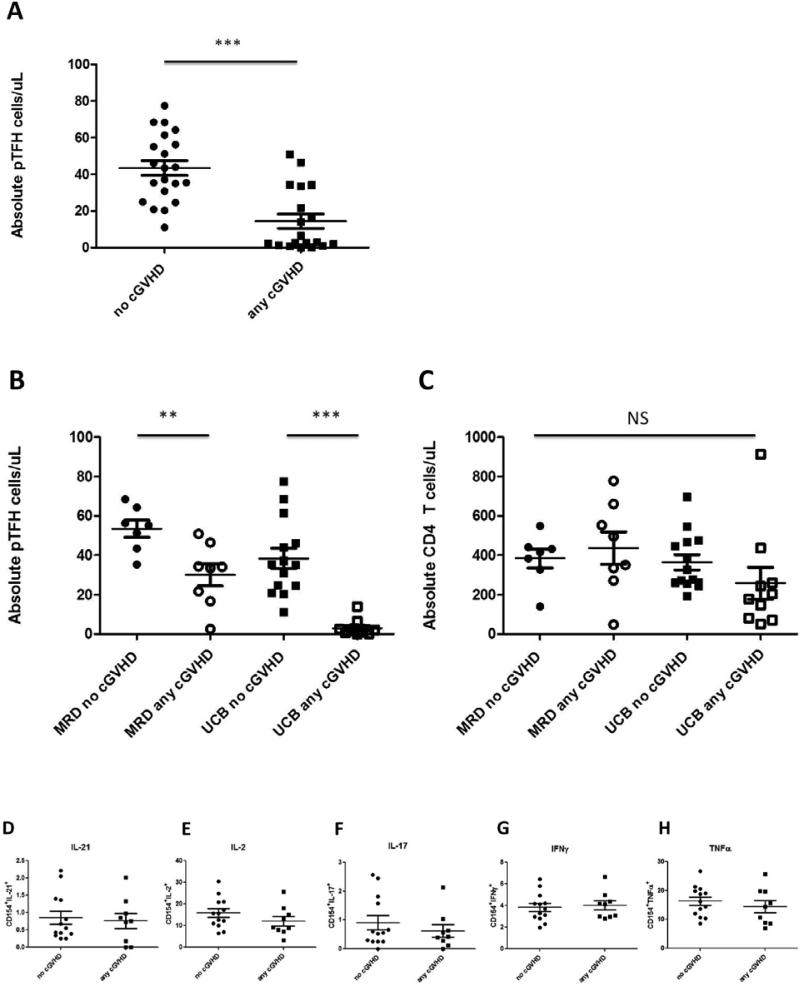
Some models of murine cGVHD are characterized by pathogenic B cell activity and chronic antibody deposition that is dependent on TFH cells5,9. However, these studies evaluated splenocytes and not the peripheral blood. As routine tissue sampling is difficult in any human study, we evaluated TFH cells circulating in the peripheral blood to determine if the absolute number of pTFH cells varied in patients with cGVHD (n=19) to those without cGVHD (n=21) at 1 year after transplantation, irrespective of disease severity (limited or extensive). Interestingly, when patients with ongoing cGVHD were analyzed at 1 year, there were significantly lower absolute counts of pTFH cells compared to those without cGVHD (14.4 ± 3.9 vs 43.4 ± 4.0, p=<0.0001) (Figure 5A). This effect was still present when broken down by donor source based on the presence or absence of cGVHD (MRD, 53.5 ± 4.3 vs 30.1 ± 5.6 per uL, p=0.007; UCB 38.4 ± 5.2 vs 3.1 ± 1.2 per uL, p=< 0.0001) (Figure 5B). Both groups were treated with similar average doses of prednisone, either with or without additional immunosuppressive therapy (CSA, sirolimus, or MMF; supplemental Table 1). Conversely, there were no differences in absolute numbers of CD4 T cells in those with ongoing cGVHD versus those without (MRD, 384.3 ± 47.9 vs 436.6 ± 82.2 per uL, p=0.61, UCB 364.9 ± 38.4 vs 258.5 ± 81.0 per uL, p=0.21) (Figure 5C), perhaps suggesting that current GVHD therapies may decrease the number of pTFH cells. Thus, in contrast to our initial hypothesis, pTFH cells are lower in patients with cGVHD. As in figure 4, we also evaluated available samples for differences in cytokine production based on the presence or absence of cGHVD. Similar to there being no functional differences based on stem cell source, we found no differences in cytokine production based on presence or absence of cGHVD (Figure 5D-H).
Figure 5. pTFH cells are significantly depleted in patients with ongoing cGVHD.
(A) pTFH counts in patients without cGVHD (n=21) compared to patients with cGVHD (limited or extensive) (n= 19) at 1 year post-transplantation (43.4 ± 4.0 vs 14.4 ± 3.9 cells/ul, *** p <0.0001. Mann-Whitney t-Test.). (B) Quantification of pTFH cells in patients without versus with any cGVHD grouped by donor source (MRD, 53.5 ± 4.3 vs 30.1 ± 5.6, ** p=0.007; UCB 38.4 ± 5.2 vs 3.1 ± 1.2, ***p < 0.0001). (C) Absolute CD4 counts in MRD and UCB recipients in patients with or without cGVHD (MRD, 384.3 ± 47.9 vs 436.6 ± 82.2, NS, p=0.61; UCB 364.9 ± 38.4 vs 258.5 ± 81.0, NS, p=0.21). (D-H) CD4 T cells from peripheral blood were gated from the Live CD3+ population following stimulation with SEB. Subsequently, CXCR5+ and CXCR5− populations within the CD4+ subset were analyzed for expression of CD40L and multiple cytokines including IL-21, IL-2, IL-17, IFNγ, and TNFα. Graphs B-F show compiled data for CXCR5+ cells that coexpress CD40L and B) IL-21, C) IL-2, D) IL-17, E) IFNy, or F) TNFα. Differences between donors (B-F) were non-significant.
Discussion
Characterization of immune reconstitution following HCT remains an important goal. There are significant differences in the lymphocyte composition and recovery following UCB and PBSC/BM transplant procedures 22. In some studies these differences, particularly in T cells, may contribute to higher risks of viral infections. With regard to TFH cells, mouse models demonstrate that diminished TFH cell activity in neonates results in reduced serologic vaccine responses25. There is strong evidence in humans to support the role of circulating TFH cells in response to pathogens or vaccines14,23. They are also expanded in the periphery of patients with existing autoimmune disorders13,26,27,28. We have previously shown that TFH cells play an important role in driving cGVHD pathogenesis in mice5 and were interested if similar mechanisms could explain human cGVHD.
Despite significant HLA disparity between the donor and recipient, UCB transplantation results in equivalent frequencies of aGVHD and a lower frequency of cGVHD compared to MRD sources18. As the balance of T and B cell immunity drives both protection against pathogens as well as GVHD post-HCT, we hypothesized that there would be donor-dependent differences in pTFH after allo-HCT that could contribute to differences in cGVHD. We characterized the recovery of pTFH cells based on stem cell source and found slower recovery of the pTFH cells (absolute numbers and frequency) in UCB recipients compared to MRDs. This is likely explained by UCB grafts containing essentially no pTFH cells and all naïve T cells, which require additional differentiation steps to become TFH cells in the recipient. Interestingly, we found significantly lower absolute numbers of pTFH in UCB recipients up to 6 months after transplant, which could partially contribute to lower levels of cGVHD in these patients. By 1 year post transplant there were similar numbers of pTFH cells in both groups. This was unexpected as TFH cells in MRD grafts have a significant starting advantage and are presumably exposed to similar growth stimuli (e.g. IL-2, IL-7 and IL-15) in a lymphopenic environment. The cytokines driving TFH differentiation in humans are still ill-defined29, but similar to other lineages, are likely restrained by homeostatic mechanisms. Previous exposure to antigen in the donor could also contribute to higher rates of in cGVHD in MRD recipients (as UCB T cells are essentially all naive). Antigen experienced TFH cells could undergo homeostatic proliferation in the recipient and drive this pathogenic process. As in male HCT recipients of female donors, homeostatic or antigen driven proliferation of TFH cells primed against H-Y antigens could explain higher rates of cGVHD in this setting6,7. HY-specific T cells would support the development of pathologic B cells, as evidenced by the production and predictive nature of anti-HY antibodies in recipients of sex-mismatched transplants6. In our current study, we did not evaluate for TCR diversity in circulating TFH cells to see if this positively or negatively correlates with cGVHD, nor were we able to actually prove that the TFH cells were of donor origin due to the small numbers of cells in the research sample.However, engraftment analysis of the total T cell population at most time points tested was 100% donor.
Given the above differences in the graft content of naïve and memory T cells, we predicted there would also be skewing of the phenotype of reconstituting pTFH cells. As UCB grafts contain essentially no CXCR5-expressing CD4 T cells upon transfer, they must traverse the entire developmental process11,24. When looking at CXCR5+CD4+ cells in UCB and MRD recipients, we found that the majority of cells were central memory cells and there were no significant differences between the two donor types at 60 days or one year post-transplant. There were also similar levels of naïve, effector memory, effector, and terminal effector CD4 T cells. These data suggest that even in UCB recipients, circulating TFH cells are antigen experienced. Other studies show that TFH cells can be further distinguished based on CXCR3 expression14 and respond specifically to antigen inducing the co-stimulatory protein ICOS. Here, we did not evaluate whether or not these same CXCR3+ICOS+ pTFH cells are capable of driving antibody production in cGVHD (e.g. against H-Y minor histocompatibility antigens), though this could be the basis of future studies. We do show that the TFH cells in the blood of patients at 1 year after transplant are functional and able produce an array of cytokines including IL-21, IL-2, IL-17, IFN-γ and TNF-α. Our data also suggest that the phenotypic differences in TFH cells between donor sources likely cannot account for differences in levels of cGHVD, as both contain equal numbers of central memory and effector TFH cells. Because we found no CD4 T cell specific differences between the two donor sources, we next asked whether or not the presence of cGVHD affected TFH cell numbers.
Given that TFH cells are known to help support pathologic B cell responses and drive cGVHD in mice, we proposed that higher levels of donor pTFH cells could thus drive cognate B cells and chronic antibody production directed against the host. Depletion of TFH effector cytokines (e.g. IL-21) or co-stimulatory ligands (ICOS and CD40L) blocks germinal center formation and can prevent or reverse ongoing cGVHD in a model of multi-organ system disease with bronchiolitis obliterans5. These data suggest multiple pathways that could serve as potential candidates to halt cGVHD by targeting TFH cell effector functions. Thus, we hypothesized that peripheral TFH cells would be increased in cGVHD and serve as a promising target in patients affected by this transplant complication. When studying PBMCs of patients with ongoing cGVHD, we were surprised to find significantly lower percentages and absolute numbers of TFH cells. Interestingly, this appears to be independent of donor source. These findings may suggest homing out of the peripheral blood and into secondary lymphoid tissues or alternatively, a loss of pTFH cells as part of the cGVHD process. Additionally, as the patients in our study with cGVHD all receive immune suppression (e.g., prednisone, CSA, sirolimus), this likely contributes to decreased pTFH cells. It is interesting to note that CXCR5+CD4+ T cells, as opposed to the bulk CD4 T cell population, are decreased independent of cGVHD or treatment. This may suggest that current therapies are broadly targeting TFH cells and provides preliminary evidence that inhibition of human TFH cells in vivo would be a promising target for cGVHD treatment or prophylaxis. In mice, it is clear that inhibiting specific signaling pathways (e.g. BTK or ICOS) or cytokines (e.g. IL-21) in TFH cells can halt and potentially reverse the adverse effects of cGVHD at least in some rodent models10. Based on the above and our data showing a very low frequency of pTFH cells in UCB grafts and a low rate of cGVHD in this setting, it is tempting to speculate that selective depletion of pTFH cells might be a strategy to reduce cGVHD in adult donor transplantation.
In summary, there are significant differences in the TFH cells between donor sources. UCB grafts are essentially absent of TFH cells but make up approximately 5-15% of PB CD4+ cells. pTFH cells in UCB and MRD recipients were predominantly central memory cells with similar percentages at early and late time points suggesting phenotypic differences are unlikely to explain differences in cGVHD. Interestingly, pTFH cells are lower in UCB recipients early on after transplant which could help explain lower levels of cGVHD in these patients. While TFH cells in UCB recipients are significantly lower early after transplant, they reconstitute to similar numbers as PBSC/BM recipients by 1 year post-transplantation. Lastly, we show that TFH cells are markedly depleted in patients with ongoing cGVHD, likely as a result of homing, cGVHD itself or the treatment with immunosuppressive therapy. Collectively, these findings show that TFH cells are associated with cGHVD and that slower recovery of pTFH cells may contribute to protection of cGVHD after UCB transplantation.
Supplementary Material
Highlights.
There are significant differences in peripheral TFH cell recovery following HCT depending on donor source
TFH cell phenotypes following HCT are similar between donor sources and do not differ in patients with or without cGVHD
Peripheral blood TFH cells are significantly decreased in patients with cGVHD
Acknowledgements
Authorship statement: D.A.K. performed experiments, analyzed data, and wrote the paper. H.W. performed and interpreted experiments. M.A., R. B., M.L.M, S.G.H., D.J.W. and S.C., J.S.M., J.E.W., and B.R.B all provided samples, clinical input and edited the paper. M.R.V. analyzed data, wrote and edited the paper. Research reported in this publication was supported by the NIH MSTP Grant T32 GM008244 (DAK) and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 (DAK, MRV, JSM, BRB). This work was also supported by NIH R01 AI100879 (MRV and JSM), 5R01AI100879 (MRV, DJW, SC, JSM), P01 CA142106 (BRB), and P01 CA065493-21 (MRV, SC, JSM, BRB and JEW).
Footnotes
Financial disclosure: The authors have nothing to disclose. Conflict of interest statement: There are not conflicts of interest to report.
References
- 1.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 2.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socie G, Ritz J, Martin PJ. Current Challenges in Chronic Graft-versus-Host Disease. Biology of Blood and Marrow Transplant. 2010;16:S146–S151. doi: 10.1016/j.bbmt.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Sarantopoulos S, Ritz J. Aberrant B-cell homeostasis in chronic GVHD. Blood. 2015;125:1703–1707. doi: 10.1182/blood-2014-12-567834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123:3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakasone H, Tian L, Sahaf B, et al. Allogeneic HY antibodies detected 3 months after female-to- male HCT predict chronic GVHD and nonrelapse mortality in humans. Blood. 2015;125:3193–3201. doi: 10.1182/blood-2014-11-613323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubovsky JA, Flynn R, Du J, et al. Ibrutinib treatment ameliorates murine chronic graft-versus- host disease. The Journal of Clinical Investigation. 2014;124:4867–4876. doi: 10.1172/JCI75328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarantopoulos S, Blazar BR, Cutler C, Ritz J. B cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:16–23. doi: 10.1016/j.bbmt.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotty S. Follicular Helper CD4 T Cells (TFH). Annual Review of Immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 12.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. Cxc Chemokine Receptor 5 Expression Defines Follicular Homing T Cells with B Cell Helper Function. The Journal of Experimental Medicine. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013:5. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115:3861–3868. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 16.Szabolcs P, Cairo MS. Unrelated umbilical cord blood transplantation and immune reconstitution. Seminars in Hematology. 2010;47:22–36. doi: 10.1053/j.seminhematol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund TC, Boitano AE, Delaney CS, Shpall EJ, Wagner JE. Advances in umbilical cord blood manipulation[mdash]from niche to bedside. Nat Rev Clin Oncol. 2015;12:163–174. doi: 10.1038/nrclinonc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutman JA, Myint H, Lee CK, Smith C, Nguyen V, Pollyea DA. Chronic graft versus host disease and immunosuppression burden is significantly lower following adult cord blood transplantation versus matched unrelated donor transplantation.. BMT Tandem Meeting; Grapevine, TX. ASBMT; 2014. [DOI] [PubMed] [Google Scholar]
- 19.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagasia M, Greinix H, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adelman CA, Chattopadhyay S, Bieker JJ. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free media. Development. 2002;129:539–549. doi: 10.1242/dev.129.2.539. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallikkuth S, Parmigiani A, Silva SY, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–993. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014 Oct 16;41(4):529–42. doi: 10.1016/j.immuni.2014.10.004. 2014 doi: 101016/jimmuni201410004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debock I, Jaworski K, Chadlaoui H, et al. Neonatal Follicular Th Cell Responses Are Impaired and Modulated by IL-4. The Journal of Immunology. 2013;191:1231–1239. doi: 10.4049/jimmunol.1203288. [DOI] [PubMed] [Google Scholar]
- 26.Luo C, Li Y, Liu W, et al. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. J Neuroimmunol. 2013;256:55–61. doi: 10.1016/j.jneuroim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Li XY, Wu ZB, Ding J, et al. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjogren's syndrome. Biochem Biophys Res Commun. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 28.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt N, Liu Y, Bentebibel S-E, et al. The cytokine TGF-[beta] co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.