Abstract
Division of labor in insect societies relies on simple behavioral rules, whereby individual colony members respond to dynamic signals indicating the need for certain tasks to be performed. This in turn gives rise to colony-level phenotypes. However, empirical studies quantifying colony-level signal-response dynamics are lacking. Here, we make use of the unusual biology and experimental amenability of the queenless clonal raider ant Cerapachys biroi, to jointly quantify the behavioral and physiological responses of workers to a social signal emitted by larvae. Using automated behavioral quantification and oocyte size measurements in colonies of different sizes and with different worker to larvae ratios, we show that the workers in a colony respond to larvae by increasing foraging activity and inhibiting ovarian activation in a progressive manner, and that these responses are stronger in smaller colonies. This work adds to our knowledge of the processes that link plastic individual behavioral/physiological responses to colony-level phenotypes in social insect colonies.
Keywords: division of labor, ovarian development, automated behavioral analysis, larvae, social behavior, social communication
Introduction
Insect societies are striking examples of highly integrated “superorganisms” that can homeostatically respond to changes in the social and physical environment (Hölldobler and Wilson 2008). Several models exist that attempt to explain how social groups consisting of cognitively simple individuals can display seemingly complex behavior by following simple behavioral rules (Duarte et al. 2011). The majority of these models assume that individual colony members respond to dynamic signals that indicate the need within the colony for certain tasks to be performed. Variation in individual response thresholds and/or variation in exposure to these signals are in turn implied to give rise to a division of labor. However, experimental work on signal-response dynamics in social insect colonies remains scarce. It includes work on the fanning behavior of bumblebees in response to nest temperature (Weidenmuller 2004), in which nestmates were shown to consistently differ in response thresholds. Other examples include trophallaxis in response to the larvae:worker ratio in ants (Cassill and Tschinkel 1999), departure of new foragers in response to the return of successful foragers to the nest (Schafer et al. 2006) and the onset of foraging in response to brood, worker and queen pheromones in honeybees (Pankiw et al. 1998a; Pankiw et al. 1998b; Leoncini et al. 2004).
In social Hymenoptera, larvae are generally the ‘end-users’ of resources and the only brood developmental stage requiring food, and they are able to signal their hunger level to workers (Cassill and Tschinkel 1995; Creemers et al. 2003; den Boer and Duchateau 2006; Kawatsu 2013). The number, size and hunger level of larvae can thus be expected to be major drivers of worker foraging activity.
Larvae also have a physiological effect on workers in the honeybee (Arnold et al. 1994; Mohammedi et al. 1998; Oldroyd et al. 2001; Traynor et al. 2014) and three species of ants (Heinze et al. 1996; Teseo et al. 2013; Villalta et al. 2015). In these species, larvae inhibit worker egg-laying and this effect appears to necessitate direct physical contact between workers and larvae (Arnold et al. 1994; Villalta et al. 2015), possibly mediated via short-range brood pheromones, which have been characterized in honeybees (Arnold et al. 1994; Le Conte et al. 2001) but are currently unknown in ants.
Here we investigate the effects of larvae on worker physiology (ovarian development) and behavior (foraging activity) in the clonal raider ant Cerapachys biroi. In this ant species, queens are absent and all the workers can reproduce parthenogenetically (Tsuji and Yamauchi 1995). All colony members switch between reproduction and colony maintenance/brood care tasks, giving rise to a stereotypical colony cycle (Ravary 2002; Ravary et al. 2006; Oxley et al. 2014). Each colony alternates between two phases: a ca. 3-week long reproductive phase, in which the workers synchronously lay parthenogenetic, diploid eggs and do not forage, and a ca. 2-week long brood care phase during which a subset of the workers forage while the others tend to the brood, and no eggs are laid. Colonies in the reproductive phase typically contain eggs and pupae, while colonies in the brood care phase contain larvae and newly emerged callow workers. The colony cycle is controlled by the brood: the presence of larvae in a colony inhibits ovary activation in workers (Teseo et al. 2013) and maintains the colony in the brood care phase (Ravary et al. 2006). As in other ant species, larvae of C. biroi are reared communally, but trophallaxis from workers to larvae hasn’t been reported in this species. Instead, workers place larvae onto prey items on which they feed directly. The clonal and phasic reproduction mode of C. biroi provides optimal control over factors that are known to affect individual behavior and physiology, such as genotype and age, and that would otherwise introduce ‘noise’ in the measured responses, i.e. confounding sources of inter-individual variation.
Here, we aim to measure ‘response curves’ for the plastic changes in behavior and physiology occurring in groups of C. biroi workers in response to varying intensities of the larval signal. Depending on how it is emitted and received, the purported larval signal could in principle elicit several types of individual and colony responses. For example, in a very simple scenario, the response of each worker could be turned ‘on’ in the presence of any number of larvae and ‘off’ in the absence of larvae. If so, the colony would show no response in the absence of larvae, but a similar response in the presence of any number of larvae. Alternatively, each worker’s response might be proportional to the signal intensity it perceives. Perceived signal intensity might in turn be directly proportional to the number of larvae present in the colony, or might depend on the contact rate between larvae and workers, in which case the larvae:worker ratio can also be expected to affect the colony response.
We also aim to quantify variability in the workers’ individual physiological response to the putative larval signal. Variation in ovarian activation among nestmates is of particular interest in social insects, as it is indicative of reproductive skew, a ‘precursor’ of reproductive division of labor. Most ant species have complete and fixed reproductive division of labor between fertile queens and sterile workers, with little or no flexibility in individual reproductive strategies. Species with totipotent workers provide valuable systems to study if and how reproduction is regulated by the physical and social environments that individuals experience (Heinze 2008; Field et al. 2010).
Methods
Experimental Design
56 experimental colonies were set up with callow (1–3 days old) workers and young (3–5 days old) larvae in varying number, so as to represent five larvae:worker ratios ranging from 0 to 1 across two different colony sizes (8 and 16 workers) (Table 1). A ratio of 1 corresponds to the estimated ratio found in a typical (i.e. large, healthy) laboratory stock colony in the brood care phase, while the absence of larvae corresponds to the reproductive phase and triggers egg-laying within 4–7 days in most worker groups. Group sizes (8 and 16) were chosen on the basis of previous experiments, which showed that colonies of that size are fully functional: they have high worker and brood survival, and display the full spectrum of worker behavior (foraging, nursing, synchronized egg-laying), as well as the stereotypical colony cycle of the species (Ulrich, unpublished). All workers and all larvae were derived from the same stock colony, ensuring they were precisely age-matched and genotype-matched (clonal line A; MLL1 in Kronauer et al., (2012)). Ovariole number varies from 2 to 6 in C. biroi and correlates with body size (Ravary and Jaisson 2004). To minimize any effect of variation in reproductive physiology, we aimed to only use 2-ovariole workers in this experiment, and size-screened the workers by eye to achieve this. Each colony was provided with a number of fire ant (Solenopsis invicta) worker pupae equal to the number of workers present in the colony at the start of the experiment. Colonies were kept in clear Petri dishes (50mm diameter × 9mm high) with a moist plaster of Paris floor. The colonies were kept at 25 °C ± 0.5 °C and 65% ± 5% humidity under constant illumination for the duration of the experiment.
Table 1.
Experimental treatments and replicate numbers.
| 0 larvae | 1 larva | 2 larvae | 4 larvae | 8 larvae | 16 larvae | |
|---|---|---|---|---|---|---|
| 8 workers | ratio = 0 n = 5 |
ratio = 1/8 n = 7 |
ratio = 1/4 n = 6 |
ratio = 1/2 n = 5 |
ratio = 1 n = 5 |
|
| 16 workers | ratio = 0 n = 5 |
ratio = 1/8 n = 7 |
ratio = 1/4 n = 6 |
ratio = 1/2 n = 5 |
ratio = 1 n = 5 |
After seven days, all workers and larvae were counted and workers were frozen (−80 °C) for later dissection. The duration of the experiment was determined by dissecting subsets of individuals from control colonies (workers without larvae; not listed in Table 1) on consecutive days. As soon as dissections revealed fully developed oocytes in the control individuals, all experimental colonies were frozen. Thus, the experiment ended before any eggs were laid, ensuring that our measurement of ovarian development was not confounded by the presence of ‘empty’ ovarioles following egg-laying. One colony (‘10E’: 16 larvae, 16 workers) was excluded from all analyses due to a mistake during experiment setup.
Ovarian Development
Five to six workers were randomly collected from each colony, dissected and their ovaries photographed using a Leica Z16APO microscope mounted with a DFC450 camera. The area of the two largest oocytes was scored manually from each image (ImageJ). The scoring was performed blindly with respect to experimental treatment. 95.6% (307 out of 321) of the dissected workers had 2 ovarioles, while the rest (4.4%) had 3 or 4 ovarioles. To check whether the presence of these individuals biased our data, we compared largest oocyte sizes between workers with 3–4 ovarioles and workers with 2 ovarioles from the same colony. In all but one colony, the size of the largest oocyte of workers with 3–4 ovarioles was within the range (i.e. not the highest or the lowest) covered by the other, 2-ovariole workers of the same colony.
Behavior
14 webcams (Logitech C910) were used to acquire images of each colony every 326 ± 0.13 seconds (mean ± SE) for the duration of the experiment, resulting in the acquisition of 1730 frames per colony. Custom-made tracking software written in MATLAB 2015a was used to measure foraging activity. In each frame, groups of adjacent ant-colored pixels (ant ‘blobs’) were identified following contrast-based image segmentation. In a colony, maximum aggregation (all workers clustered in one location) results in a single large blob, while minimal aggregation (all workers isolated) results in as many small blobs as there are ants in the colony. The larvae were always clustered in a single location (the ‘nest’) along with a varying number of workers, while the rest of the workers explored the Petri dish. This allowed us to define the proportion of foragers as (# blobs −1)/# workers. Note that we use the term ‘foraging’ loosely here, as it encompasses all tasks taking place away from the nest (e.g. scouting, food search and processing). The accuracy of the tracking algorithm was assessed by comparing it to manual tracking performed on 9 to 11 frames per colony. This comparison showed that overall, 96.9% of ants were successfully detected and 94.6% of the automatically detected ‘blobs’ were actual ants (as opposed to false positives, e.g. dark food debris). Images of 9 colonies (3A, 3D, 4A, 4C, 6B, 6C, 7E, 7G, 8F) with particularly high rates of false positives (> 9%) were inspected visually. In all cases, the increased error rate was caused by food debris that were systematically mistaken for ants by the tracking software, inflating our estimate of foraging activity. The proportion of foragers in these colonies was corrected to account for these errors. Two colonies (‘5E’: 8 larvae, 8 workers; ‘8E’: 4 larvae, 16 workers) were excluded from all behavioral analyses because Petri dish lids were not fitted correctly and images could therefore not be analyzed automatedly.
Data analysis
The proportion of foragers per frame averaged over frames 1500 to 1700 (see justification in Results section) was used as behavioral response variable for each colony. The largest oocyte size averaged over 5–6 workers per colony was used as physiological response variable. The largest oocyte size was used for consistency with previously published work (Teseo et al. 2013), but we carried out the same analysis using the average of the two largest oocytes for each individual for comparison. Similarly, because it is debatable whether it is more appropriate to sample an equal number of workers per colony or an equal proportion of workers per colony when estimating the mean and the variance of a trait, we compared the two approaches to assess whether the sampling method would affect our results. To this aim, we randomly resampled 3 (out of the 5 or 6) dissected workers per colony of 8 workers, so as to obtain ovarian development data for 37.5% of the ants from each colony in all cases. Effects of worker number, larvae number and their interaction on each response variable were investigated using ordinal logistic regressions (function clm of package ordinal in R version 2.15.1). These models are non-parametric to account for the fact that neither response variable was normally distributed. We evaluated the significance of effects and their interaction by comparing models using log-likelihood ratio tests (LRTs) following deletion of terms (starting with the interaction). Terms for which deletion did not significantly decrease model fit were omitted, until only significant terms remained in the model (α = 0.05). Pairwise comparisons of treatments were performed using non-parametric multiple pairwise comparisons following Kruskal-Wallis rank-sum tests (function kruskalmc of package pgirmess in R), as described in Siegel and Castellan (1988).
Results
Average larval survival was high (mean ± standard deviation: 0.88±0.22) and wasn’t affected by the treatment type (Kruskal-Wallis rank sum test: χ29=11.53, p=0.24). The same was true for adult survival (0.99 ± 0.04; χ29=8.63, p=0.47).
Ovarian development
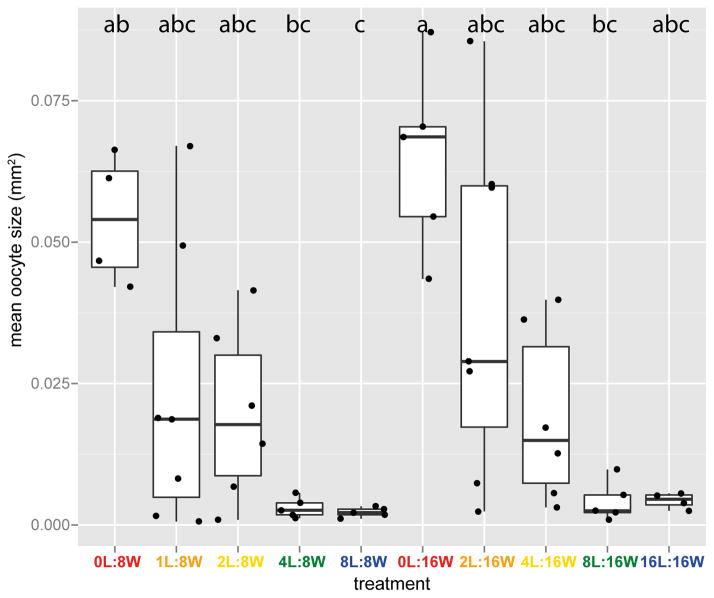
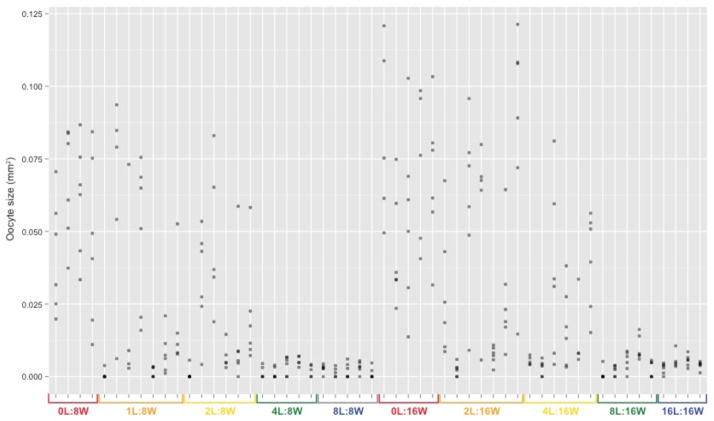
Worker ovarian development varied considerably across treatments, from ovaries containing no visible oocyte, to ovaries containing full-size eggs (Figure 1). Oocyte size was positively affected by the number of workers present in the colony (χ21=8.88, p=0.003; mean of the two largest oocytes: χ21=8.15, p=0.004; resampled data: χ21=10.47, p=0.001) and negatively affected by the number of larvae they tended (χ21=21.71, p=3.18 × 10−6; mean of the two largest oocytes: χ21=20.59, p=5.70 × 10−6; resampled data: χ21=20.58, p=5.72 × 10−6) with an interaction between the efffect of larvae and worker numbers being weak or absent (χ21=3.48, p=0.062; mean of the two largest oocytes: χ21=4.20, p=0.041; resampled data: χ21=2.68, p=0.101). Interestingly, treatments with an intermediate larvae:worker ratio (1/8 and 1/4) displayed a large amount of variation between replicate colonies. For example, the mean oocyte size for colonies in the treatment group 1L:8W covered the entire range of values observed in other treatments at the same group size, from 0L:8W to 8L:8W, and the same was true for treatment group 2L:16W, with respect to 0L:16W and 16L:16W. In other words, at intermediate larvae:worker ratios, some colonies behave like colonies with a larvae:worker ratio of 1 while others behave like colonies with no larvae. We then investigated variation in ovarian development between individuals within colonies, using standard deviation in oocyte size as a measure of reproductive skew (Figure 2). Note that in species such as C. biroi where all colony members can reproduce, standard deviation is equivalent to the index for reproductive skew of Keller and Vargo (1993). We found that reproductive skew between nestmates significantly varied across treatments: inter-individual variation in ovarian development within a colony increased with the number of workers (χ21=6.78, p=0.009) and decreased with the number of larvae (χ21=29.44, p=5.75 × 10−8) present in the colony. We found substantial inter-individual variation within colonies in all the treatments in which the larvae:worker ratio was less than 1/2. At ratios of 1/2 and 1, however, all colony members had ovary scores equal or close to 0. As a result, the distribution of individual ovary development in colonies with a larvae:worker ratio of 0 showed little to no overlap with that of colonies with a larvae:worker ratio of 1/2 or 1, whereas treatments with a ratio of 1/8 and 1/4 showed overlap with all other treatments. We asked whether inter-individual variation in ovarian development (within-colony variation) was increased at intermediate ratios, which would for example occur if a weak larval signal intensity triggers a response in some workers but not others (either because the signal intensity is close to the average worker response threshold, which it only crosses in some workers, or because only a subset of workers are exposed to the signal due to low contact rates). However, reproductive skew at intermediate larvae:worker ratios was not higher than at a ratio of 0 (relevant pairwise comparisons: 0L:8W vs. 1L:8W and 2L:8W: ns, 0L:16W vs. 2L:16W and 4L:16W: ns).
Fig. 1.
Box plot of the colony mean largest oocyte size as a function of colony composition. A full-size egg is ca. 0.1 mm2. Each box shows the median (bold horizontal line), first and third quartiles (“hinges”) and 95% confidence interval of the median (“notches”). Colors denote larvae:worker ratios. Dots represent colonies. Letters indicate significance of pairwise tests: treatments that do not share a letter differ in average oocyte size. The results of pairwise tests were qualitatively the same when using the mean size of the two largest oocytes instead of the size of the single largest oocyte
Fig. 2.
Individual oocyte size as a function of colony composition. Dots represent individuals (5–6 individuals per colony; superposed dots are indicated in black). Colors denote the larvae:worker ratio
Behavior
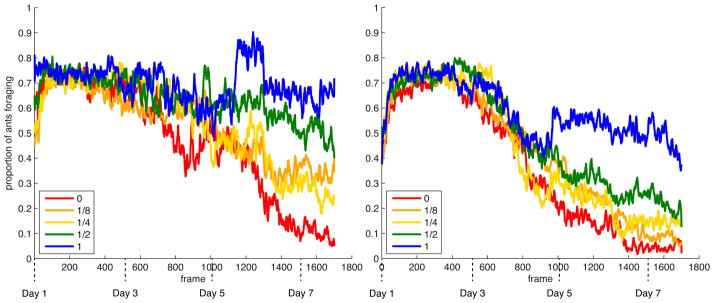
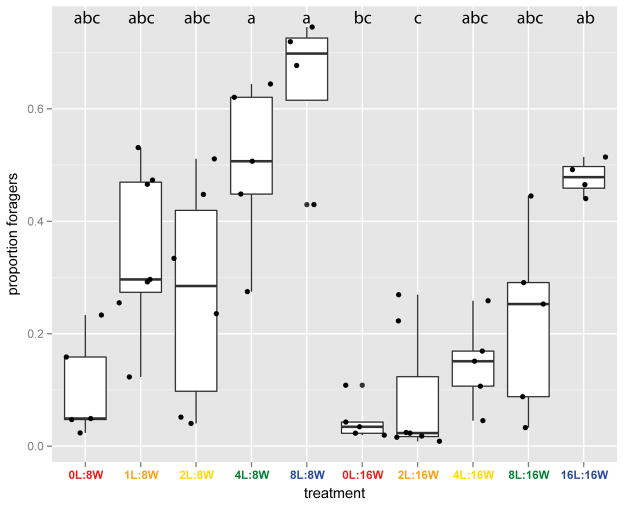
Colony activity, as measured by the proportion of workers found in the foraging arena away from the nest, was high (around 70%) across all treatments for the first half of the experiment, likely as a result of initial disturbance during experiment setup (Figure 3). We therefore restricted our analyses to the average foraging activity observed in frames 1500 to 1700, corresponding to the last 18 hours of the experiment. Restricting the analysis of the behavioral data to the end of the experiment also makes it more directly comparable to the physiological data, which were collected at the end of the experiment. Foraging activity within this timeframe was negatively affected by the number of workers (χ21=41.56, p=9.46 × 10−10), and positively affected by the number of larvae (χ21=40.99, p=1.25 × 10−9) in the colony. A significant interaction (χ21=7.99, p=0.005) between these two effects resulted from a steeper increase in foraging activity with larvae number in 8-worker colonies compared to 16-worker colonies (Figure 4). Between colony variability in foraging activity appeared to be higher at intermediate larvae:worker ratios (1/8, 1/4 and 1/2) relative to ratios of 0 or 1 (Figure 4).
Fig. 3.
Average proportion of foragers over time for colonies containing 8 workers (left panel) or 16 workers (right panel) at different larvae:worker ratios (color code in box insert). Error bars are omitted for readability
Fig. 4.
Box plot of the mean proportion of foragers per colony over frames 1500–1700 as a function of colony composition. Each box shows the median (bold horizontal line), first and third quartile (“hinges”) and 95% confidence interval of the median (“notches”). Colors denote larvae:worker ratios. Dots represent colonies. Letters indicate significance of pairwise tests: treatments that do not share a letter differ in average foraging activity
If ovarian development and foraging behavior were regulated jointly, we would expect ovarian development to correlate negatively with the intensity of foraging activity within a treatment. We tested this in the treatment groups that presented enough inter-colony variability in behavior and physiology to warrant such analysis, namely the larvae:worker ratios 1/8 and 1/4 of each group size (the other treatments, 0, 1/2 and 1 had little variation in behavior and/or ovarian development among replicate colonies; Figures 1 and 4). The hypothesis was not supported in any of these treatment groups (Spearman’s rank correlation: S= 96, p=0.088, n=7; S=60, p=0.136, n=6; S=86, p=0.236, n=7; S=38, p=0.083, n=5 for treatment groups 1L:8W, 2L:8W, 2L:16W and 4L:16W respectively), and we can therefore not exclude the possibility that the two responses are regulated by two distinct components of the larval signal.
Discussion
Making use of the unusual biology and experimental amenability of C. biroi, we jointly quantified the behavioral and physiological responses of workers to the intensity of a larval signal. We show that colonies of workers of identical age and genotype respond to the presence of larvae by increasing foraging activity and inhibiting ovarian activation in a dose-dependent way. Intermediate response intensity at intermediate signal intensities appeared to mainly result from increased colony-level response variability. This increased variability could occur because the responses of individual workers are non-independent, i.e. because workers influence each other. Alternatively, small differences in the intensity of the larval signal across replicate colonies (for example because larvae in some colonies emit a stronger signal than in others) could result in large differences in colony response at intermediate larvae:worker ratios.
Within-colony, between-individual response variability could be measured for ovarian development and was substantial in all treatments with a larvae:worker ratio less than 1/2, but did not vary between these treatments. The sources of inter-individual variation in behavior and physiology in C. biroi and other social insects are still largely unknown. In this experiment, differences in individual genotype and age can be excluded as sources of variation. Response variability could instead stem from plastic differences in response thresholds (e.g. determined during larval development) or differential exposure to the relevant signal during the experiment (e.g. variation in the contact rate between each worker and the larvae).
Reproductive synchrony between nestmates is central to the ecology of C. biroi and is enforced through policing (Teseo et al. 2013). Any form of variability in individual response to a reproduction-inhibiting signal has the potential to disrupt the colony cycle in this system. For example, if a weak signal intensity (e.g. small number of larvae) inhibited egg-laying in most but not all workers, the colony might continuously produce larvae in small quantities and be caught in a constant brood care phase with low reproductive output. The fact that we observed incomplete ovarian inhibition in colonies with a low (<1/2) larvae:worker ratio suggests that such a colony cycle breakdown might have occurred in these colonies over time. Both responses measured in this experiment were strongly affected by the number of larvae present in the colony, but also, and less expectedly, by the number of workers. Group size is thought to affect virtually any aspect of social life, including division of labor, communication and immunity (Dornhaus et al. 2012). We found that 16-worker colonies had higher ovarian development and a lower proportion of foragers than 8-worker colonies, suggesting they might be able to produce more offspring while investing less into foraging, and thus might have increased fitness, everything else (colony age and genetic structure, resource availability, brood:worker ratio) being equal. Furthermore, our results show that 16-worker colonies had increased reproductive skew. These findings are in line with theoretical predictions and/or empirical evidence that larger colonies display increased division of labor (Gautrais et al. 2002; Thomas and Elgar 2003; Holbrook et al. 2011) and are energetically more efficient (Waters et al. 2010). However, we cannot determine whether the observed effects are due to worker number per se, or to the increased density in 16-worker colonies, since the nest boxes used for all treatments were equal in size.
There is increasing behavioral and genetic evidence that foraging behavior and ovarian development are functionally anti-correlated at several levels of organization (e.g. across members of a colony or across strains of the same species) in social insects (West-Eberhard 1996; Amdam et al. 2004; Dolezal et al. 2013). In C. biroi, ovarian development and foraging are separated in time across the two phases of the colony cycle, rather than across different individuals (Oxley et al. 2014). Here, we show that the two functions are also negatively correlated across groups of workers consisting of genetically identical individuals exposed to different intensities of a signal. The fact that larvae increased foraging activity while decreasing ovarian development in workers generally supports the hypothesis that brood care and reproduction are functionally linked. However, we did not find negative correlations between the behavioral and physiological responses within treatments, and can therefore not conclude that the two responses are regulated by the same components of the larval signal, i.e. by the same pheromone(s) and/or mechanical signal(s). It is still unclear what the exact nature of the larval signal triggering the observed responses is. Contrary to honeybees (Arnold et al. 1994; Le Conte et al. 2001), a brood pheromone has not yet been characterized in ants (Morel and Vandermeer 1988). Furthermore, any ant larval signal need not be entirely chemically conveyed but may also have a mechanical component, since the larvae are in contact with the workers throughout their development and are known to actively solicit food from them (Cassill and Tschinkel 1995; Creemers et al. 2003; den Boer and Duchateau 2006; Kawatsu 2013).
By quantifying behavior and reproductive physiology in experimental colonies of known composition, this work adds to our knowledge of signal-response dynamics in social insect colonies and sheds light on the processes that link individual responses to colony-level phenotypes. It also illustrates how small differences in colony composition (larvae number, worker number) can lead to genotype- and age-independent, plastic changes in worker physiology and behavior.
Further work, in particular involving individual behavioral tracking, will be needed to understand behavioral plasticity, individual specialization and division of labor in this and other social insects.
Significance Statement.
Insect societies display seemingly complex collective behavior, although they are composed or cognitively simple individuals. Understanding the rules that underlie collective behavior and division of labor remains a major challenge in social insect biology. In this study, we quantify the behavioral and physiological response of workers to varying intensities of a larval signal in the clonal ant Cerapachys biroi. We find that the workers in a colony plastically respond to larvae by increasing foraging activity and inhibiting ovarian activation in a progressive manner, and that both responses are stronger in smaller colonies.
By showing how simple signal-response dynamics can shape important aspects of colony function, this work adds to our knowledge of the processes that link plastic individual responses to colony-level phenotypes in social insect colonies.
Acknowledgments
This work was supported by grant 1DP2GM105454-01 from the NIH, a Klingenstein-Simons Fellowship Award in the Neurosciences, and a Searle Scholar Award to D.J.C.K. Y.U. was supported by a Swiss National Science Foundation Advanced Postdoc Mobility Fellowship (P300P3-147900) and a Rockefeller University Women & Science Fellowship. D.B. was supported by the Rockefeller University Summer Undergraduate Research Fellowship Program. R.L. was supported by a Marie Curie International Outgoing Fellowship (PIOF-GA-2012-327992). Jonathan Saragosti helped design and build the setup used to acquire behavioral data.
References
- Amdam GV, Norberg K, Fondrk MK, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc Natl Acad Sci U S A. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold G, Le Conte Y, Trouiller J, Hervet H, Chappe B, Masson C. Inhibition of Worker Honeybee Ovaries Development by a Mixture of Fatty-Acid Esters from Larvae. Cr Acad Sci Iii-Vie. 1994;317:511–515. [Google Scholar]
- Cassill DL, Tschinkel WR. Allocation of Liquid Food to Larvae Via Trophallaxis in Colonies of the Fire Ant, Solenopsis invicta. Anim Behav. 1995;50:801–813. [Google Scholar]
- Cassill DL, Tschinkel WR. Effects of colony-level attributes on larval feeding in the fire ant, Solenopsis invicta. Insectes Soc. 1999;46:261–266. [Google Scholar]
- Creemers B, Billen J, Gobin B. Larval begging behaviour in the ant Myrmica rubra. Ethol Ecol Evol. 2003;15:261–272. [Google Scholar]
- den Boer S, Duchateau MJHM. A larval hunger signal in the bumblebee Bombus terrestris. Insectes Soc. 2006;53:369–373. [Google Scholar]
- Dolezal AG, Johnson J, Hoelldobler B, Amdam GV. Division of labor is associated with age-independent changes in ovarian activity in Pogonomyrmex californicus harvester ants. J Insect Physiol. 2013;59:519–524. doi: 10.1016/j.jinsphys.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Dornhaus A, Powell S, Bengston S. Group size and its effects on collective organization. Annu Rev Entomol. 2012;57:123–41. doi: 10.1146/annurev-ento-120710-100604. [DOI] [PubMed] [Google Scholar]
- Duarte A, Weissing FJ, Pen I, Keller L. An Evolutionary Perspective on Self-Organized Division of Labor in Social Insects. Annu Rev Ecol Evol S. 2011;42:91–110. [Google Scholar]
- Field J, Paxton RJ, Soro A, Bridge C. Cryptic plasticity underlies a major evolutionary transition. Curr Biol. 2010;20:2028–31. doi: 10.1016/j.cub.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Gautrais J, Theraulaz G, Deneubourg JL, Anderson C. Emergent polyethism as a consequence of increased colony size in insect societies. J Theor Biol. 2002;215:363–373. doi: 10.1006/jtbi.2001.2506. [DOI] [PubMed] [Google Scholar]
- Heinze J. The demise of the standard ant (Hymenoptera: Formicidae) Myrmecological News. 2008;11:9–20. [Google Scholar]
- Heinze J, Trunzer B, Oliveira PS, Holldobler B. Regulation of reproduction in the neotropical ponerine ant, Pachycondyla villosa. J Insect Behav. 1996;9:441–450. [Google Scholar]
- Holbrook CT, Barden PM, Fewell JH. Division of labor increases with colony size in the harvester ant Pogonomyrmex californicus. Behav Ecol. 2011;22:960–966. [Google Scholar]
- Hölldobler B, Wilson EO. The superorganism: the beauty, elegance, and strangeness of insect societies. 1. W.W. Norton; New York: 2008. [Google Scholar]
- Kawatsu K. Effect of nutritional condition on larval food requisition behavior in a subterranean termite Reticulitermes speratus (Isoptera: Rhinotermitidae) J Ethol. 2013;31:17–22. [Google Scholar]
- Keller L, Vargo EL. Reproductive structure and reproductive roles in colonies of eusocial insects. In: Keller L, editor. Queen Number and Sociality in Insects. Oxford University Press; 1993. pp. 16–44. [Google Scholar]
- Kronauer DJC, Pierce NE, Keller L. Asexual reproduction in introduced and native populations of the ant Cerapachys biroi. Mol Ecol. 2012;21:5221–5235. doi: 10.1111/mec.12041. [DOI] [PubMed] [Google Scholar]
- Le Conte Y, Mohammedi A, Robinson GE. Primer effects of a brood pheromone on honeybee behavioural development. P Roy Soc B-Biol Sci. 2001;268:163–168. doi: 10.1098/rspb.2000.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini I, Le Conte Y, Costagliola G, Plettner E, Toth AL, Wang MW, Huang Z, Becard JM, Crauser D, Slessor KN, Robinson GE. Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc Natl Acad Sci U S A. 2004;101:17559–17564. doi: 10.1073/pnas.0407652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammedi A, Paris A, Crauser D, Le Conte Y. Effect of Aliphatic Esters on Ovary Development of Queenless Bees (Apis mellifera L.) Naturwissenschaften. 1998;85:455–458. [Google Scholar]
- Morel L, Vandermeer RK. Do Ant Brood Pheromones Exist. Ann Entomol Soc Am. 1988;81:705–710. [Google Scholar]
- Oldroyd BP, Wossler TC, Ratnieks FLW. Regulation of ovary activation in worker honey-bees (Apis mellifera): larval signal production and adult response thresholds differ between anarchistic and wild-type bees. Behav Ecol Sociobiol. 2001;50:366–370. [Google Scholar]
- Oxley PR, Ji L, Fetter-Pruneda I, McKenzie SK, Li C, Hu HF, Zhang GJ, Kronauer DJC. The Genome of the Clonal Raider Ant Cerapachys biroi. Curr Biol. 2014;24:451–458. doi: 10.1016/j.cub.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiw T, Huang Z-Y, Winston ML, Robinson GE. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J Insect Physiol. 1998a;44:685–692. doi: 10.1016/s0022-1910(98)00040-7. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE, Fondrk MK. Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera) Behav Ecol Sociobiol. 1998b;44:193–198. [Google Scholar]
- Ravary F. The reproductive cycle of thelytokous colonies of Cerapachys biroi Forel (Formicidae, Cerapachyinae) Insectes Soc. 2002;49:114–119. [Google Scholar]
- Ravary F, Jahyny B, Jaisson P. Brood stimulation controls the phasic reproductive cycle of the parthenogenetic ant Cerapachys biroi. Insectes Soc. 2006;53:20–26. [Google Scholar]
- Ravary F, Jaisson P. Absence of individual sterility in thelytokous colonies of the ant Cerapachys biroi Forel (Formicidae, Cerapachyinae) Insectes Soc. 2004;51:67–73. [Google Scholar]
- Schafer RJ, Holmes S, Gordon DM. Forager activation and food availability in harvester ants. Anim Behav. 2006;71:815–822. doi: 10.1016/j.anbehav.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric statistics for the behavioural sciences. MacGraw Hill Int; New York: 1988. pp. 213–214. [Google Scholar]
- Teseo S, Kronauer DJC, Jaisson P, Chaline N. Enforcement of Reproductive Synchrony via Policing in a Clonal Ant. Curr Biol. 2013;23:328–332. doi: 10.1016/j.cub.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Elgar MA. Colony size affects division of labour in the ponerine ant Rhytidoponera metallica. Naturwissenschaften. 2003;90:88–92. doi: 10.1007/s00114-002-0396-x. [DOI] [PubMed] [Google Scholar]
- Traynor KS, Le Conte Y, Page RE. Queen and young larval pheromones impact nursing and reproductive physiology of honey bee (Apis mellifera) workers. Behav Ecol Sociobiol. 2014;68:2059–2073. doi: 10.1007/s00265-014-1811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Yamauchi K. Production of Females by Parthenogenesis in the Ant, Cerapachys Biroi. Insectes Soc. 1995;42:333–336. [Google Scholar]
- Villalta I, Angulo E, Devers S, Cerdá X, Boulay R. Regulation of worker egg laying by larvae in a fission-performing ant. Anim Behav. 2015;106:149–156. [Google Scholar]
- Waters JS, Holbrook CT, Fewell JH, Harrison JF. Allometric scaling of metabolism, growth, and activity in whole colonies of the seed-harvester ant Pogonomyrmex californicus. Am Nat. 2010;176:501–10. doi: 10.1086/656266. [DOI] [PubMed] [Google Scholar]
- Weidenmuller A. The control of nest climate in bumblebee (Bombus terrestris) colonies: interindividual variability and self reinforcement in fanning response. Behav Ecol. 2004;15:120–128. [Google Scholar]
- West-Eberhard MJ. Natural History and Evolution of Paper Wasp. In: Turillazzi S, West-Eberhard MJ, editors. Natural History and Evolution of Paper Wasp. Oxford Univ. Press; New York: 1996. pp. 290–317. [Google Scholar]