Abstract
Background
Family members share genes, environment and microbial communities. If there is a strong effect of family on the salivary microbiota, controlling for family will enhance identification of microbial communities associated with cariogenesis. The current study was designed to assess the similarity of the salivary microbiome among families and the association between the salivary microbiome and dental decay taking age into account.
Methods
We selected families (n= 49) participating in the cohort study of oral health conducted by the Center for Oral Health Research in Appalachia (COHRA). All families where at least two children and at least one parent gave a saliva sample (n=173) were included. Saliva samples were collected at least one hour after eating or drinking. Following DNA extraction, the V6 region of the 16s rRNA gene was sequenced. Paired ends were joined using FLASH, sequences were de-multiplexed and filtered using QIIME 1.9.0, and taxonomy was assigned using the RDP Classifier and sequences aligned with the CORE database using PyNAST.
Results
The salivary microbiome changed with age and was more similar within families than between families. There was no difference in the diversity of the salivary microbiome by dental decay. After taking into account age and family, signals of dental decay were weak in the saliva, whether examined at the phyla, genus or operational taxonomic level.
Conclusions
The salivary microbiome does not appear to be a good indicator of dental caries.
INTRODUCTION
Every minute, the structures of the human oral cavity, the teeth, gingival sulci, tongue, hard and soft palates, buccal mucosa and tonsils are bathed by 0.3 to 0.7 milliliters of saliva, which sweeps up some of the microbes present on these structures.1 Perhaps because it is continuously secreted and swallowed, the microbial community found in saliva is the most stable over time of the microbiota in the human body.2 Saliva also averages more taxa than the gut, and the diversity – a measure of the number of taxa and their relative abundance – is the highest for all body sites measured in the Human Microbiome Project.3 However, there is little geographic structure: in a comparison of the salivary microbiome from 120 individuals from 12 different countries, sequences from different individuals in the same location were more variable than sequences from the same individual, but individuals from different locations showed no additional variation.4 Moreover, the composition of the salivary microbiome could not correctly classify into groups 161 healthy individuals aged 18-55 who followed an omnivore or ovo-lacto-vegetarian or vegan diet for at least a year. However, the participants were correctly grouped by dietary habits using results from a metabolomics screen.5
Although these descriptive studies in healthy populations find the salivary microbiome to be invariant to diet and location, some studies suggest that the salivary microbiome varies with dental decay.6–8 However, there is no consensus as to whether salivary microbial communities from caries are more or less diverse, or which taxa are associated with decay. The lack of consensus may have arisen because some of the studies were conducted in adults and others in children, ranged in sample size, and used various detection methods, including next generation sequencing, 16S rDNA microarray, and 16S rRNA-based reverse-capture oligonucleotide probes. Alternatively, there may be multiple microbial community structures associated with decay. These different structures might be more easily detected if other determinants of microbial community structure are taken into account.
Dental caries is a major public health problem worldwide; in the United States, 20% of children have untreated dental decay. Untreated dental decay leads to a variety of immediate health problems, including serious infection. Among children, untreated dental decay is the most common reason for hospitalization.9 There are several hypotheses regarding the causes of dental caries, but the proximal cause of the carious lesion, a localized dissolution of tooth structure, is the acid produced by the dental biofilm in response to free sugars.10 However, many factors can alter ones’ susceptibility to dental decay, several of which are shared among family members. Human genes influence tooth shape and enamel, salivary flow, immune response and taste.11 These phenotypes are hypothesized to directly influence the structure of the microbial communities present in plaque, the tongue and saliva, which, in turn, influence risk of dental caries. Known risk factors for dental decay, diet, hygiene, and exposure to cigarette smoke,6,12 also are shared by households. Family members share microbiota.13,14 If there is a strong effect of family on the oral microbiota, taking into account the effects of family, and thus host genetics and shared environmental factors, will enhance identification of microbial communities associated with shared environments and genetics to those associated with cariogenesis.
The current study was designed to assess the similarity of the salivary microbiome among families and the association between the salivary microbiome and dental decay taking age into account. We used samples collected as part of the first cohort study of oral health conducted by the Center for Oral Health Research in Appalachia (COHRA). Participants in COHRA are at very high risk of dental decay: among the 837 children less than 14 years of age participating in COHRA I, the median time to first dental cavity was age 10. By age 14, virtually all the children had at least one decayed tooth.15
METHODS
Study Population and Sample Collection
We selected COHRA I families (n= 49) in which at least two children and at least one parent gave a saliva sample (n=173). Samples were collected at least one hour after eating or drinking, using the Oragene Self-Collection Kit (DNA Genotek) or using a cheek swab and then preserved in an Oragene DNA kit (DNA Genotek). Collected samples were stored frozen until thawed for testing. Qualified dentists or dental hygienists evaluated participants for dental decay assigning scores for the number of decayed, missing and filled teeth. Further details about the protocol, including calibration and reliability of examiners, are published elsewhere.16 Written informed consent was obtained from all adult participants and parents of participating children. The study protocol was approved by the Institutional Review Boards at West Virginia University and the University of Pittsburgh.
DNA Extraction and Sequencing
DNA was extracted using DNeasy® Blood & Tissue kit in concert with the QIAcube with both the kit and equipment manufactured by QIAGEN (Venlo, Netherlands). The QIAcube is an automated bench top apparatus that runs a number of QIAGEN protocols reducing variability introduced by manual extraction. 100 μL of the samples were first incubated at 37° C with 80 uL enzyme cocktail for 30 minutes. The enzyme cocktail is comprised of Promega (Madison, WI, USA) cell lysis solution, lysozyme, mutanolysin, RNase A, and lysostaphin (Sigma Aldrich) in 22.5:4.5:1.125: 1.125:1 parts, respectively. After recovery, DNA was measured using a Nanodrop 2000C spectrophotometer (Thermo Scientific, Waltham, MA, USA) and stored at −80° C. We re-extracted all samples whose post-PCR concentration averaged less than 5 ng/μL on more than two measurements.
Extracted DNA was stored at −20C until thermal amplification of the 16S rRNA gene fragment using 8 base pair error-correcting barcodes with conserved primers flanking the V6 region using previously published protocols.17 The forward and reverse primer sequences mapped to the 967–985 (CAACGCGARGAACCTTACC) and 1078–1061 (ACAACACGAGCTGACGAC) on the Escherichia coli 16S rRNA segment. 3 ng of barcoded DNA from each visually confirmed, purified, and re-quantified sample was pooled into a single 1.5 mL Eppendorf tube. 140 ng of a barcoded mock community was added to the pool for quality control of sequencing. The mock community consisted of 57 species with an overrepresentation of genera typically and atypically found in abundance in a healthy human mouth. Species included Lactobacillus spp., Peptoniphilus spp., and Propionibacterium spp. Sequence data mapped to the mock community detected these over-representations (data not shown). Pooledamplicons were sequenced using the Illumina HiSeq Platform (100-cycle paired end reads) by the University of Michigan Sequencing Core.
Data Analysis and Taxonomic Assignment
The paired ends were joined using FLASH,18 which is designed to join paired ends of small fragments less than twice the length of a read. After joining the paired ends, the sequences were demultiplexed and filtered using QIIME 1.9.0.19 Following mate pair joining, 178,682,752 joined reads were demultiplexed and passed quality filtering. The number of reads per sample ranged from 20,745 to 1,726,317; all but one sample had over 200,000 reads.
We used as a reference database, CORE, a specialized database for the identification of bacteria within the oral microbiome.20 Singleton OTUs were filtered out as part of the default QIIME parameters. In addition, we excluded all OTUs below 0.05% relative abundance. Sequences were binned and aligned using the QIIME set of microbiome analysis tools (at 97% sequence identity threshold for species level) to generate a phylogenetic tree that allowed classification at species level and higher (OTU). After the OTU-table was generated, taxonomy was assigned using the RDP Classifier21 in QIIME. The sequences were then aligned using PyNast22 with the CORE-aligned sequences as template. Sequences that failed to align were omitted from the subsequent tree and OTU-table construction.
We compared the structure of bacterial communities using the UniFrac metric calculated using QIIME. All other analyses were conducted using R.
RESULTS
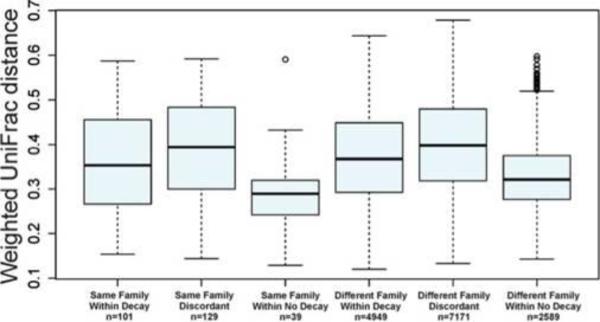
Among the 49 families, there were 91 children aged 1 to 6, and 29 aged 7 to 17, and 53 adults whose mean age was 30.3 (SD: 5.9). The number of decayed, missing and filled teeth increased with age, as did the number of teeth with active decay (Table 1). Diversity of OTUs, however, did not differ with age, nor with decay or smoking status within age groups (Table 1). Similarly, the average degree of dissimilarity among individuals within an age group was the same whether comparisons were limited to individuals with decay, individuals without decay, or individuals with different decay status; this was also true for household smoking (Table 1). Results in Table 1 are for Bray-Curtis dissimilarity; results for Euclidean distance are similar (data not shown). However, differences within families were similar to or less than between families, both within each age class ignoring decay status (Table 1) and by decay status, across age classes (Figure 1).
Table 1.
Characteristics of 173 participants from 49 families selected from the Center for Oral health Research in Appalachia Study I
| Age 1 to 6 (n=91) | Age 7 to 17 (n=29) | Adult (n=53) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Mean | (SD) | Mean | (SD) | Mean | (SD) |
| Age | 3.40 | (1.75) | 10.03 | (3.33) | 30.32 | (5.89) |
| Decayed, missing and filled teeth (DMFT) | 1.66 | (3.27) | 3.31 | (3.31) | 11.96 | (7.22) |
| Active decay (D2T> 0) | 0.91 | (2.37) | 1.52 | (2.28) | 3.96 | (3.88) |
| Shannon Diversity Index | 6.56 | (0.44) | 6.53 | (0.34) | 6.61 | (0.43) |
| Active decay | 6.52 | (0.35) | 6.65 | (0.22) | 6.60 | (0.42) |
| No active decay | 6.57 | (0.46) | 6.44 | (0.39) | 6.63 | (0.49) |
| Smokers* | 6.61 | (0.43) | 6.65 | (0.17) | 6.60 | (0.48) |
| Non-smokers | 6.49 | (0.44) | 6.47 | (0.39) | 6.61 | (0.36) |
| Bray-Curtis Dissimilarity | 0.52 | (0.11) | 0.52 | (0.09) | 0.47 | (0.12) |
| Active decay | 0.52 | (0.09) | 0.54 | (0.10) | 0.48 | (0.12) |
| No active decay | 0.52 | (0.11) | 0.49 | (0.09) | 0.43 | (0.08) |
| Between decay status (Active decay vs. No active decay) | 0.52 | (0.10) | 0.52 | (0.09) | 0.46 | (0.11) |
| Smokers* | 0.52 | (0.09) | 0.53 | (0.10) | 0.47 | (0.10) |
| Non-smokers | 0.53 | (0.12) | 0.51 | (0.09) | 0.46 | (0.13) |
| Between smoking status (Smokers vs. Non-smokers) | 0.52 | (0.11) | 0.51 | (0.09) | 0.48 | (0.12) |
| Within families | 0.44 | (0.11) | 0.44 | (0.09) | 0.37 | (0.11) |
| Between families | 0.52 | (0.11) | 0.52 | (0.09) | 0.47 | (0.12) |
For children, smoking status was assigned by whether there was a cigarette smoker in the household
Figure 1.
Weighted Unifrac distance within and between families by presence of dental decay (DMFT>0) (n=the number of comparisons). 173 participants from 49 families selected from the Center for Oral Health Research in Appalachia Study I.
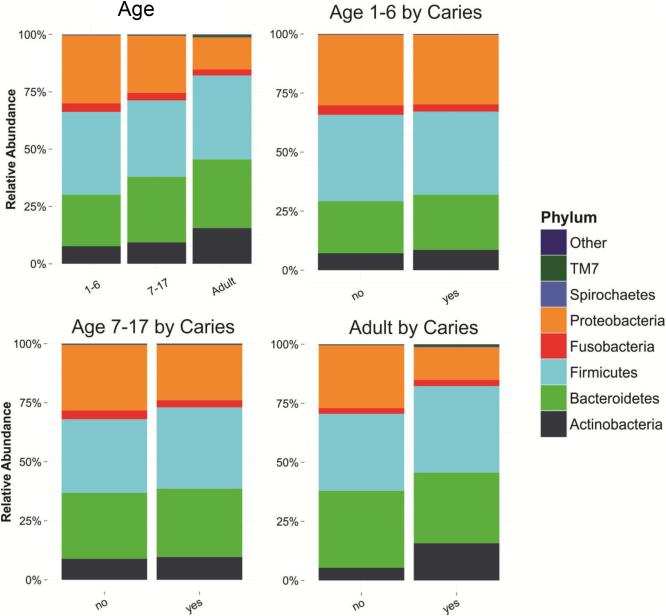
A visual comparison of the relative abundance of the most common phyla by dental decay suggested there might be differences (Figure 2). To further explore these trends, we fit a series of mixed models. Each model predicts a change in the log transformed relative abundance of one of the 8 most common phyla, using age, household smoking and presence of active decay as a predictor; family was included as a random effect. Only age was an important predictor (Table 2).
Figure 2.
Relative abundance of most common phyla by presence of active dental decay. 173 participants from 49 families selected from the Center for Oral Health Research in Appalachia Study I
Table 2.
Mixed Effect Models Predicting Change in Log Base 10 Relative Abundance of Each Phylum with D2T Score, Dentition, Smoking Household, and Urban Water.
| Phyla Mixed Effects Models (n=173 saliva specimens) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Firmicutes | Bacteroidetes | Proteobacteria | Actinobacteria | Fusobacteria | TM7 | Other | Spirochaetes | |
| Phyla relative abundance (%) | 36% | 26% | 24% | 11% | 3% | 0.5% | 0.2% | 0.1% |
| Random Effects | Variance | |||||||
| Family | 0.007 | 0.015 | 0.025 | 0.010 | 0.007 | 0.029 | 0.022 | 0.005 |
| Residual | 0.048 | 0.074 | 0.086 | 0.084 | 0.116 | 0.157 | 0.072 | 0.164 |
| Fixed Effects | Estimate(p-value) | |||||||
| Age 7-17 | −.053 (0.27) | 0.085 (0.17) | −0.119 (.007) | 0.050 (.43) | −0.079 (0.29) | 0.236 (0.008) | 0.125 (.04) | 0.089 (0.31) |
| Adult | .074(0.08) | 0.193 (0.000) | −0.331 (<<0.001) | 0.353 (<<0.001) | −0.057 (0.376) | 0.6518 (0.000) | 0.377 (<<.001) | 0.729 (0.000) |
| Household Smoking | .0008(0.96) | 0.0530 (0.24) | 0.095(.05) | −0.015 (.73) | 0.103 (0.06) | 0.015 (0.80) | −0.043 (.34) | 0.153 (0.017) |
| Active decay (D2T>0) | .000006 (0.98) | 0.005 (0.57) | −0.001 (.88) | 0.001 (.86) | 0.002 (0.84) | 0.010 (0.34) | −0.010 (.21) | 0.013 (0.25) |
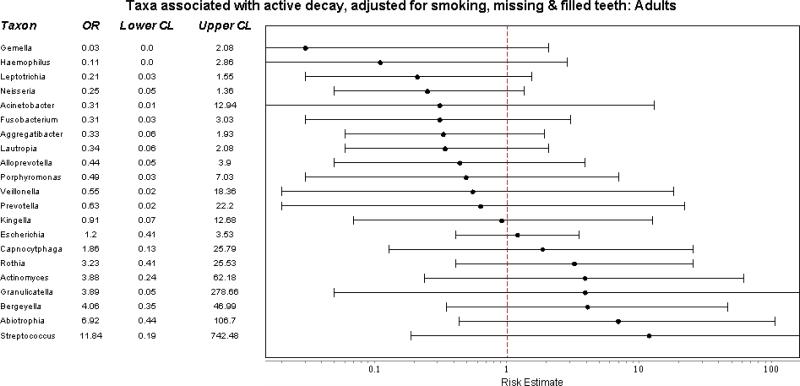
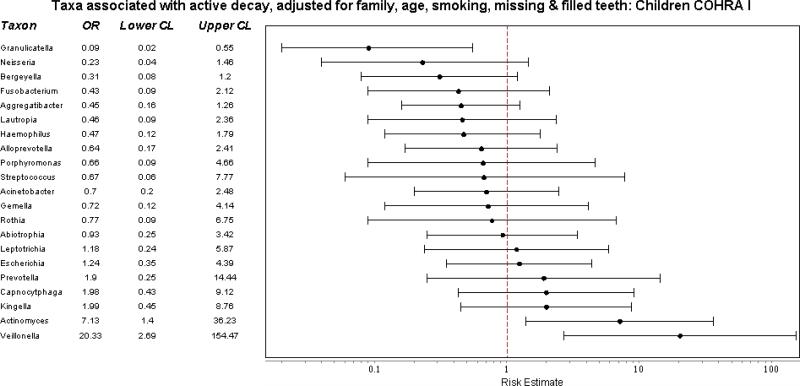
When classified at the generic level, relative abundance differed only slightly by decay status and age. Among adults, relative abundance of genera differed slightly between smokers and nonsmokers. To identify if the relative abundance of specific genera were associated with active decay after adjusting for age, smoking, and the presence of missing and filled teeth, we fit a series of logistic models, fitting separate models for each taxon. Among adults, we saw no significant associations with the relative abundance of any taxon (Figure 3a). Among children aged 1-6, a similar analysis – which also included an adjustment for family effects to account for presence of siblings, found 3 strong associations: Granulicatella was associated with a 10 fold decreased risk of active decay and Actinomyces and Veillonella a 7-fold and 20-fold increase in active decay, respectively (Figure 3b). [There was insufficient sample in the 7 to 17 age group to conduct this analysis.]
Figure 3.
Predicted associations of relative abundance of most common taxa with active dental decay, after adjusting for non-independence of family members, age, and exposure to cigarette smoke. A) Adults. B) Children aged 1-6. 173 participants from 49 families selected from the Center for Oral Health Research in Appalachia Study I
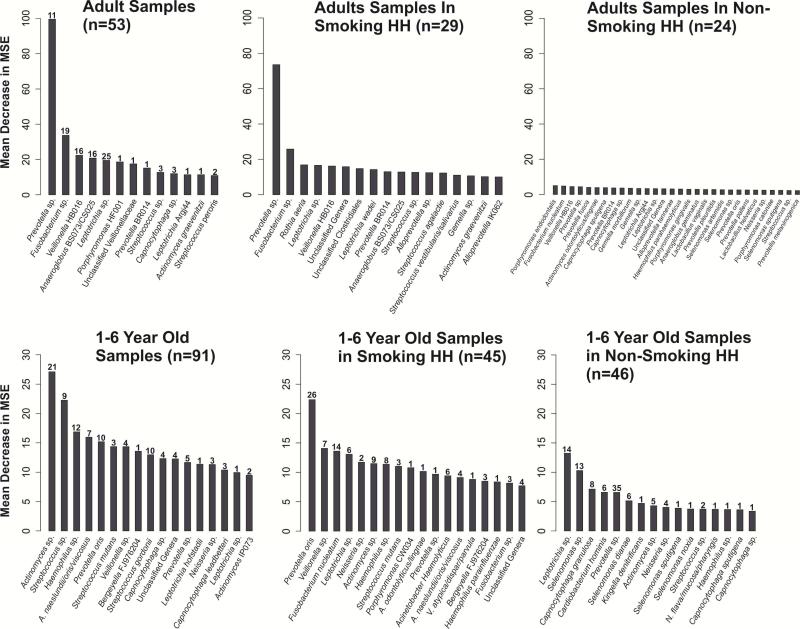
Finally, we used Random Forest to see if we could identify which genera best discriminated between those with and without active decay [https://cran.r-project.org/web/packages/randomForest/randomForest.pdf]. We did 10 runs, starting each with a different seed; Figure 4 displays the strongest predictors by the frequency they occurred for adults and 1-6 years olds by smoking status. Among adults, Prevotella – which has been previously identified with dental decay – was a strong predictor of active decay, but this was entirely driven by those who smoked cigarettes. There was no single taxon that distinguished between those with and without decay among adults who did not smoke. Among children, predictors differed between those exposed and not exposed to cigarette smoke. Different taxa were the most important predictors of decay in each group, but Actinomyces and Prevotella were best at discriminating between those with and without decay overall and within each group.
Figure 4.
Results of Random Forest Analysis: Top 10 Most Important OTUs in predicting active decay in adults (aged 18 and over) and children (aged 1-6), stratified by smoking households, and Bootstrapped 10 Times. Participants from the Center for Oral health Research in Appalachia Study I.
DISCUSSION
The major findings from this study of the salivary microbiome of 173 adults and children from 49 families are as follows. The salivary microbiome changed with age, (a marker of change from primary to permanent teeth), and the salivary microbiome was more similar within families than between families. The salivary microbiome of family members with dental decay were more similar to each other, but no more similar to non-family members with decay than to non-family members without decay. This was also true for family members without decay. This finding suggests that there are multiple salivary communities associated with health and decay. There was no difference in the diversity of the salivary microbiome by dental decay. When age and family were taken into account, signals of dental decay were weak in the saliva, whether examined at the phyla, genus or OTU level, and there was no strong signal for Streptococci, which are known to be acid producers and associated with dental decay.
Our finding of a weak signal for dental decay in saliva is consistent with previous untargeted bacterial community screens of saliva.7,23 In a study of children aged 3 to 6 with caries (n=32) and without caries (n=28), Ling et al.23 reported no significant differences in diversity in saliva samples from those with and without caries, or an association with any one genus – although some significant associations were found for dental plaque. A Danish study using the HOMIN array compared bacterial communities in saliva from 174 adults with manifest caries lesions on 3 or more surfaces to 447 randomly selected controls regardless of caries experience,6 and with a principal components analysis explained 26.3% of the variation in the set. Even so, there were no bacterial communities that uniquely identified bacterial communities in individuals with caries. In a study of saliva samples from 110 children aged 12 with and without dental decay, Gomer-Vercher et al.24 reported increasing relative abundance of Prevotella with caries, but did not find a clear clustering of bacterial communities by decay, and concluded that salivary bacterial composition was not a good biomarker for dental decay.
The lack of a strong signal for Streptococcus species may seem surprising in light of a large dental literature targeting particularly S. mutans as a cause of dental decay. This may be because we were not able to resolve Streptococcus genera to particular species: some Streptococcus species are associated with dental health and others with decay.25 Moreover, Streptococcus species are very common in the oral cavity of those with and without decay (for example, they were found in 100% of children age 3 in one study).26 The Danish study did identify associations between S. salivarus and S. parasanguinis and dental decay, but not S. mutans. However, other untargeted 16S rRNA screens in both saliva23-24 and dental plaque also found little or no signal with Streptococcus species.23
That the salivary microbiome differs between adults and children has also been noted previously.27–29 Among our study participants aged 1-6, Actinomyces and Veillonella were positively associated with dental decay and Granulicatella negatively associated. We had no strong signal for these genera among adults. In a Spanish study of unstimulated saliva from 110 12-year olds, who presumably have mixed dentition, Veillonella, but not Actinomyces, was positively associated with dental decay.24 Veillonella was also associated with dental decay in an Ohioan study of dental plaque from children aged 1 to 3 years old,28 and in a Boston study of dental plaque from 2-6 years olds with and without severe caries,30 but not in a Chinese study of dental plaque from 60 children aged 3-7.25 In the Chinese study, Actinomyces occurred significantly more often in white spots than healthy plaque, but not in cavitated lesions. By contrast, a Swedish study of 155 3 year olds reported a negative association between Actinomyces and dental decay.26 Two studies have also reported a negative association between Granulicatella and dental decay, the Swedish study26 and a Chinese study of 60 children aged 3 to 6.23 However, Granulicatella elegans was positively associated with dental decay in the Boston study30 and a Chinese study of 40 children aged 3-6 with and without severe dental decay.31 Differences in study population, sample size and variable region of the 16S rRNA sequenced may account for some of the variability. Conducting large scale screens using targeted methods for specific species that take into account known confounders are needed to clarify these disparate findings.
While the bacterial community structure found in saliva may not be a good indicator of dental caries, there is some evidence that it may be a good indicator of ongoing functions associated with dental caries. Yang et al.7 was able to distinguish between saliva from 10 adults with caries and saliva from 10 adults without caries based on functional gene structure. However, we had previously conducted an untargeted metabolomics screen using saliva from 25 sibling pairs concordant and discordant for dental decay and health.32 Although the biochemical profiles placed siblings with decay close together, sibship was more important in determining biochemical profile than the presence of decay. It appears that at least for the salivary microbiome, and current technologies, the signal of dental decay is relatively weak, and if our focus is on the etiology of caries, we will get a stronger signal by turning our attention to dental plaque.
Acknowledgements
The authors thank Jyothi Manohar, and Rachel Gicquelais for assistance in the data analysis, and Kathy Neiswanger, Jennifer Mauer for excellent data coordination.
Funding: This work was supported by R01 NIDCR R01-DE 014899.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DTW. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013;26(4):781–91. doi: 10.1128/CMR.00021-13. doi:10.1128/CMR.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarevic V, Whiteson K, Hernandez D, François P, Schrenzel J. Study of inter- and intra-individual variations in the salivary microbiota. BMC Genomics. 2010;11:523. doi: 10.1186/1471-2164-11-523. doi:10.1186/1471-2164-11-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Gao H, Mihindukulasuriya KA, et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013;14(1):R1. doi: 10.1186/gb-2013-14-1-r1. doi:10.1186/gb-2013-14-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasidze I, Li J, Quinque D, Tang K, Stoneking M. Global diversity in the human salivary microbiome. Genome Res. 2009;19(4):636–43. doi: 10.1101/gr.084616.108. doi:10.1101/gr.084616.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Filippis F, Vannini L, La Storia A, et al. Berg G, editor. The Same Microbiota and a Potentially Discriminant Metabolome in the Saliva of Omnivore, Ovo-Lacto-Vegetarian and Vegan Individuals. PLoS One. 2014;9(11):e112373. doi: 10.1371/journal.pone.0112373. doi:10.1371/journal.pone.0112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belstrøm D, Fiehn N-E, Nielsen CH, et al. Altered bacterial profiles in saliva from adults with caries lesions: a case-cohort study. Caries Res. 2014;48(5):368–75. doi: 10.1159/000357502. doi:10.1159/000357502. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Zeng X, Ning K, et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2012;6(1):1–10. doi: 10.1038/ismej.2011.71. doi:10.1038/ismej.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo AH, Yang DQ, Xin BC, Paster BJ, Qin J. Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Dis. 2012;18(6):595–601. doi: 10.1111/j.1601-0825.2012.01915.x. doi:10.1111/j.1601-0825.2012.01915.x. [DOI] [PubMed] [Google Scholar]
- 9.Nalliah RP, Allareddy V, Elangovan S, Karimbux N, Allareddy V. Hospital based emergency department visits attributed to dental caries in the United States in 2006. J Evid Based Dent Pract. 2010;10(4):212–22. doi: 10.1016/j.jebdp.2010.09.013. doi:10.1016/j.jebdp.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Sheiham A, James WPT. Diet and Dental Caries: The Pivotal Role of Free Sugars Reemphasized. J Dent Res. 2015;94(10):1341–7. doi: 10.1177/0022034515590377. doi:10.1177/0022034515590377. [DOI] [PubMed] [Google Scholar]
- 11.Shaffer JR, Wang X, McNeil DW, Weyant RJ, Crout R, Marazita ML. Genetic susceptibility to dental caries differs between the sexes: a family-based study. Caries Res. 2015;49(2):133–40. doi: 10.1159/000369103. doi:10.1159/000369103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belstrøm D, Holmstrup P, Nielsen CH, et al. Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J Oral Microbiol. 2014:6. doi: 10.3402/jom.v6.23609. doi:10.3402/jom.v6.23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. doi: 10.7554/eLife.00458. doi:10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahringer SS, Clemente JC, Corley RP, et al. Nurture trumps nature in a longitudinal survey of salivary bacterial communities in twins from early adolescence to early adulthood. Genome Res. 2012;22(11):2146–52. doi: 10.1101/gr.140608.112. doi:10.1101/gr.140608.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen A, Weyant R, McNeil D, Crout R, Marazita M, Foxman B. Family-level education and smoking habits affect siblings’ dental caries. Submitted. [DOI] [PMC free article] [PubMed]
- 16.Polk DE, Weyant RJ, Crout RJ, et al. Study protocol of the Center for Oral Health Research in Appalachia (COHRA) etiology study. BMC Oral Health. 2008;8:18. doi: 10.1186/1472-6831-8-18. doi:10.1186/1472-6831-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gloor GB, Hummelen R, Macklaim JM, et al. Microbiome profiling by illumina sequencing of combinatorial sequence-tagged PCR products. PLoS One. 2010;5(10):e15406. doi: 10.1371/journal.pone.0015406. doi:10.1371/journal.pone.0015406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–63. doi: 10.1093/bioinformatics/btr507. doi:10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303. doi:10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffen AL, Beall CJ, Firestone ND, et al. CORE: a phylogenetically-curated 16S rDNA database of the core oral microbiome. PLoS One. 2011;6(4):e19051. doi: 10.1371/journal.pone.0019051. doi:10.1371/journal.pone.0019051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. doi: 10.1128/AEM.00062-07. doi:10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26(2):266–7. doi: 10.1093/bioinformatics/btp636. doi:10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling Z, Kong J, Jia P, et al. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb Ecol. 2010;60(3):677–90. doi: 10.1007/s00248-010-9712-8. doi:10.1007/s00248-010-9712-8. [DOI] [PubMed] [Google Scholar]
- 24.Gomar-Vercher S, Cabrera-Rubio R, Mira A, Montiel-Company JM, Almerich-Silla JM. Relationship of children's salivary microbiota with their caries status: a pyrosequencing study. Clin Oral Investig. 2014;18(9):2087–94. doi: 10.1007/s00784-014-1200-y. doi:10.1007/s00784-014-1200-y. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W, Ling Z, Lin X, et al. Pyrosequencing analysis of oral microbiota shifting in various caries states in childhood. Microb Ecol. 2014;67(4):962–9. doi: 10.1007/s00248-014-0372-y. doi:10.1007/s00248-014-0372-y. [DOI] [PubMed] [Google Scholar]
- 26.Lif Holgerson P, Öhman C, Rönnlund A, Johansson I. Maturation of Oral Microbiota in Children with or without Dental Caries. PLoS One. 2015;10(5):e0128534. doi: 10.1371/journal.pone.0128534. doi:10.1371/journal.pone.0128534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, Keijser BJF. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics. 2011;4:22. doi: 10.1186/1755-8794-4-22. doi:10.1186/1755-8794-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aas JA, Griffen AL, Dardis SR, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46(4):1407–17. doi: 10.1128/JCM.01410-07. doi:10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling Z, Liu X, Wang Y, Li L, Xiang C. Pyrosequencing analysis of the salivary microbiota of healthy Chinese children and adults. Microb Ecol. 2013;65(2):487–95. doi: 10.1007/s00248-012-0123-x. doi:10.1007/s00248-012-0123-x. [DOI] [PubMed] [Google Scholar]
- 30.Kanasi E, Dewhirst FE, Chalmers NI, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44(5):485–97. doi: 10.1159/000320158. doi:10.1159/000320158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang W, Zhang J, Chen H. Pyrosequencing analysis of oral microbiota in children with severe early childhood dental caries. Curr Microbiol. 2013;67(5):537–42. doi: 10.1007/s00284-013-0393-7. doi:10.1007/s00284-013-0393-7. [DOI] [PubMed] [Google Scholar]
- 32.Foxman B, Srinivasan U, Wen A, et al. Exploring the effect of dentition, dental decay and familiality on oral health using metabolomics. Infect Genet Evol. 2014;22:201–7. doi: 10.1016/j.meegid.2013.09.020. doi:10.1016/j.meegid.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]