Summary
Adrenalectomized rats or intact rats receiving peripheral administration of β-adrenergic receptor antagonists do not develop pain following sustained COMT inhibition, suggesting a peripheral adrenergic site of action for COMT-dependent pain.
Background
Patients with chronic pain disorders exhibit increased levels of catecholamines alongside diminished activity of catechol-O-methyltransferase (COMT), an enzyme that metabolizes catecholamines. Consistent with clinical observations, our lab found that acute pharmacologic inhibition of COMT in rodents produces pain. Furthermore, we found that the development of acute COMT-dependent pain is mediated by β2-and β3-adrenergic receptors (ARs). However, the contribution of distinct populations of β2- and β3ARs to the development of persistent pain linked to abnormalities in catecholamine signaling requires further investigation.
Methods
Here, we sought to determine the contribution of peripheral, spinal, and central β2- and β3ARs to persistent COMT-dependent pain. Specifically, we implanted osmotic pumps to achieve sustained systemic delivery of the COMT inhibitor OR486 over 2-weeks. Behavioral responses to mechanical and thermal stimuli were evaluated prior to and every other day following pump implantation. Site of action was evaluated in adrenalectomized rats receiving sustained OR486 or in intact rats receiving sustained βAR antagonists peripherally, spinally or centrally alongside sustained OR486.
Results
We found that male and female rats receiving sustained OR486 exhibited mechanical allodynia, mechanical hyperalgesia and thermal hyperalgesia that lasted throughout the 2-week period. In contrast, adrenalectomized rats failed to develop COMT-dependent pain. Furthermore, peripheral, but not spinal or central, administration of the non-selective βAR antagonist propranolol, β2AR antagonist ICI-118,511 or β3AR antagonist SR59230A blocked the development of COMT-dependent pain.
Conclusions
These results demonstrate that peripheral adrenergic input is necessary for the development of persistent COMT-dependent pain, and that peripherally-acting βAR antagonists may benefit patients with chronic pain disorders.
Introduction
Chronic pain disorders including fibromyalgia (FM), headache, temporomandibular disorder (TMD) and vestibulodynia (VBD) constitute a significant healthcare problem, affecting over 100 million Americans.1-7 These disorders occur more frequently in females than males8 and are persistent in nature, characterized by pain that occurs daily and spans years. While the mechanisms underlying chronic pain are poorly understood, emerging evidence indicates a role for adrenergic pathways. Patients exhibit increased levels of catecholamines9-11 alongside diminished activity of catechol-O-methyltransferase (COMT),12,13 a ubiquitously expressed enzyme that metabolizes catecholamines to their inactive derivatives.14 Furthermore, functional variants in the COMT gene that reduce COMT activity13,15,16 are associated with increased susceptibility to FM,17-21 TMD22 and experimental pain22,23 as well as impaired response to treatment.24,25 It is estimated, based on the frequency of allele variation, that nearly two-thirds of patients with chronic pain disorders possess the low-activity COMT variants.26,27
Consistent with clinical disorders, our lab found that administration of the COMT inhibitor OR486 in rodents produces increased pain at multiple body sites and altered cognitive-affective behaviors linked to pain (e.g. avoidance of painful heat and bright light).28-30 Pharmacologic studies further revealed that COMT-dependent pain is blocked by administration of the non-selective βAR antagonist propranolol or by combined administration of selective β2- and β3AR antagonists.28-30 These results are in line with those from clinical studies, showing that propranolol alleviates pain among FM and TMD patients.31,32 Collectively, these studies suggest that increased catecholamine levels, resulting from reduced COMT activity, drive pain via β2- and β3ARs.
β2- and β3ARs are G protein-coupled receptors (GPCRs) expressed in peripheral, spinal and central regions where they could drive pain. β2ARs are located on peripheral terminals33-37 and cell bodies38-40 of primary afferent nociceptors; keratinocytes;41-43 immune cells;44-47 and adipocytes48 in the periphery and neurons49,50 and glial cells51 in the central nervous system. β3ARs are located on primary afferent nociceptors,52 adipocytes48 and immune cells45,46 in the periphery and noradrenergic neurons in brain.53 Thus, we hypothesized that peripheral, spinal, and/or central β2- and β3ARs contribute to persistent COMT-dependent pain.
To test this hypothesis, we employed a clinically-relevant model of persistent COMT-dependent pain and evaluated responses to mechanical and thermal stimuli in adrenalectomized rats, lacking peripheral epinephrine, and in intact rats receiving continuous delivery of βAR antagonists via intraplantar (i.pl.), intrathecal (i.t.), or intracerebroventricular (i.cv.) routes. Potential sexual dimorphism in the contribution of adrenergic systems to persistent COMT-dependent pain was also assessed.
Results demonstrated that male and female rats receiving sustained OR486 exhibited COMT-dependent mechanical and thermal pain, persisting for 2-weeks. In contrast, adrenalectomized rats failed to develop COMT-dependent pain. Furthermore, i.pl., but not i.t. or i.c.v., administration of the non-selective βAR antagonist propranolol, β2AR antagonist ICI118,551, or β3AR antagonist SR59230A blocked COMT-dependent pain. These findings demonstrate the importance of peripheral β2- and β3ARs in mediating persistent pain, and suggest that peripherally-acting βAR antagonists may provide an effective treatment option for patients with chronic pain disorders.
Materials and Methods
Subjects
Adult male and female Sprague-Dawley rats (N=24 intact, N=24 adrenalectomized and N= 23 sham) were purchased (Charles River Laboratories, Raleigh, NC) for the first set of experiments. For subsequent βAR antagonist experiments, adult male Sprague-Dawley rats (N=111) were bred in-house. Rats weighed between 200 and 400g for all experimental studies. Rats had ad libitum access to standard laboratory chow and water. Adrenalectomized rats were provided with saline water (0.9%) to compensate for the loss of sodium in urine due to the absence of aldosterone. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of North Carolina at Chapel Hill (UNC).
General Experimental Conditions
First, the effects of sustained COMT inhibition on pain were evaluated in intact rats receiving the COMT inhibitor OR486 or vehicle systemically for 14 days via a 2002 Alzet osmotic pump (Durect Corporation, Cupertino, CA). Next, the contribution of peripheral adrenergic systems to persistent COMT-dependent pain was evaluated in adrenalectomized rats, lacking peripheral epinephrine, or sham rats receiving OR486 or vehicle systemically for 14 days via an osmotic pump. Finally, the contribution of peripheral, spinal and central βARs to persistent COMT-dependent pain was evaluated in separate groups of intact rats receiving i.pl., i.t. or i.c.v.. βAR antagonists alongside systemic delivery of OR486 or vehicle for 14 days via an osmotic pump. The βAR antagonists were delivered via a catheter attached to a separate 2002 Alzet osmotic pump.
Animals were handled and habituated to the experimenter and environment for 4 days prior to testing. Responses to punctuate mechanical and thermal stimuli were assessed in intact and adrenalectomized animals 1 day prior to and on days 1, 3, 5, 7, 9, 11 and 13 following pump implantation. For βAR antagonist experiments, pain behaviors were assessed 1 day prior to and on days 2, 4, 6, 8, 10, 12 and 14 following pump implantation. The rest day between surgery and testing allowed animals to fully recover from catheter implantation. On baseline and testing days, rats were habituated to the mechanical and thermal testing environments for 10-15 minutes.
Drug Preparation
OR486 (Tocris, Ellisville, MO) was dissolved in a 5:3:2 ratio of dimethylsulfoxide (DMSO), 0.9% saline and ethanol.30 For peripheral experiments βAR antagonists propranolol hydrochloride (Tocris, Ellisville, MO), ICI-118,511 (Tocris, Ellisville, MO) and SR59230A (Tocris, Ellisville, MO) were each dissolved in 5:3:2 ratios of DMSO, 0.9% saline and ethanol. For i.t. and i.c.v. experiments, βAR antagonists were dissolved in 0.9% saline. Drug solutions were injected into pumps, which were placed in 15mL conical tubes containing sterile 0.9% saline and primed overnight in a dry heat bath (Lab Armor, Cornelius, OR) at 37°C. All pumps (other than those for i.t. delivery) were attached to corresponding catheters prior to priming. Subcutaneous delivery of OR486 was at a constant rate of 15mg/kg/day for a 2-week period. Peripheral delivery of propranolol hydrochloride was at 9mg/kg/day, ICI-118,511 was at 1.5mg/kg/day and SR59230A was at 1.67mg/kg/day. I.t. delivery of propranolol hydrochloride was at 50ug/day for the low dose experiments and 100ug/day for the high dose experiments; ICI-118,511 was at 30ug/day; and SR59230A was at 20ug/day. I.c.v. delivery of propranolol hydrochloride was at 50ug/day for the low dose experiments and 100ug/day for the high dose experiments; ICI-118,511 was at 30ug/day; and SR59230A was at 20ug/day.
Surgical Procedures
For all surgical procedures, rats were anesthetized by isoflurane inhalation (5% induction, 1.5-5% maintenance). Incision sites were shaved and disinfected with ethanol and betadine. Sterile technique was employed throughout the duration of all procedures according to IACUC requirements. Stainless steel wound clips (Braintree Scientific, Braintree, MA) were used to close the wounds.
For systemic delivery of OR486, a small incision was made over the left shoulder blade of the rat. Hemostats were used to create a small subcutaneous pocket, in which the pump was placed.
For i.pl. delivery of βAR antagonists, a modified version of the protocol published by Haddad et al54 was used. Pumps were attached to a Y-shaped, bifurcated 3F silicone catheter (SAI Infusion Technologies, Libertyville, IL). The pump was implanted subcutaneously over the right shoulder blade and a stainless steel 10G × 20cm semi-blunt tip trocar (SAI Infusion Technologies, Libertyville, IL) was used to subcutaneously route the catheter ends to incisions made at either hind paw. The catheter ends were attached to the plantar fascia using 4-0 silk sutures (Oasis Medical, Mettawa, IL).
For i.t. delivery55 of βAR antagonists, a small incision was made on the nape of the neck, and scissors and hemostats were used in order to lift muscle and expose the atlanta-occipital membrane. The membrane was carefully incised using the tip of scissors, causing the escape of cerebral spinal fluid (CSF). A polyurethane Alzet Short Rat IT Catheter (Durect Corporation, Cupertino, CA) was inserted into the intathecal space, dorsal to the spinal cord. The other end of the catheter was sutured to surrounding tissue and attached to the osmotic pump, which was subcutaneously implanted over the right shoulder blade.
For i.c.v. delivery56 of βAR antagonists, pumps were attached to a 38-gauge stainless steel cannula via a short vinyl catheter (Alzet Brain Infusion Kit 2, Durect Corporation, Cupertino, CA). The cannula was implanted into the right lateral ventricle (from the bregma: -0.8mm anteroposterior, -1.6mm mediolateral, -5mm dorsoventral) and was cemented to two anchoring screws on the skull. The attached pump was subcutaneously implanted over the right shoulder blade.
Assessment of Behavioral Responses to Mechanical and Thermal Stimuli
Paw withdrawal threshold was assessed using the von Frey up-down method.57 Nine calibrated and logarithmically spaced von Frey monofilaments (bending forces: 0.40, 0.68, 1.1, 2.1, 3.4, 5.7, 8.4, 13.2 and 15.0g; Stoelting, Wood Dale, IL) were applied to the plantar hind paw. First, the middle filament (3.4g) was applied to the hind paw for 3 seconds. If the rat responded with a withdrawal, an incrementally lower filament was applied. In the absence of a withdrawal, an incrementally higher filament was applied. A series of 6 total responses were recorded for each paw. Results were entered into the Paw Flick module within the National Instruments LabVIEW 2.0 software (LabVIEW, Austin, TX), which uses a logarithmic algorithm in order to determine the gram force value that would elicit paw withdrawal in 50% of trials (10(Xf+kδ)/10,000, where Xf = value [in log units] of the final von Frey hair used; k = tabular value of positive and negative responses, and δ = mean difference [in log units] between stimuli). Mechanical allodynia was defined as a heightened response to a normally innocuous stimulus, as determined by a decrease in paw withdrawal threshold.
Mechanical hyperalgesia was assessed using a normally noxious mechanical stimulus (15.0g) applied to the hind paw 10 times for a duration of 1 second, with an interstimulus interval of 1 second.30 The number of paw withdrawals was recorded for each hind paw. Mechanical hyperalgesia was defined as an increase in the number of paw withdrawals in response to a normally noxious mechanical stimulus.
Thermal hyperalgesia was assessed using the Hargreaves method.58 Animals were placed in Plexiglass chambers and a radiant beam of light was applied to the hind paw through a glass floor heated to 30°C. Paw withdrawal latencies were recorded in duplicate per paw. If the second latency recorded was not within ±4 seconds of the first, a third measure was recorded. The 2 latencies closest in value were averaged in order to determine overall latency to withdrawal. Thermal behavioral data is reported in text and figures as the difference in paw withdrawal latency from baseline (Day 0). Thermal hyperalgesia was defined as a decrease in paw withdrawal latency in response to a noxious thermal stimulus.
Statistical Analyses
Mechanical allodynia, mechanical hyperalgesia and thermal hyperalgesia data were analyzed by 2-way analysis of variance (ANOVA). Post-hoc comparisons were performed using the Bonferroni test, which corrected for multiple comparisons. Statistical significance was defined as P<0.05. All statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA).
Results
Sustained COMT inhibition produces persistent pain
Genetic and pharmacologic alterations resulting in reduced COMT activity are associated with increased experimental pain and likelihood of developing chronic pain disorders. Acute administration of the COMT inhibitor OR486 results in enhanced mechanical and thermal pain in rats.30 To evaluate the effects of sustained COMT inhibition on pain, responses to mechanical and thermal stimuli were measured in separate groups of rats receiving systemic OR486 (15mg/kg/day) or vehicle over a 2-week period. Compared to rats receiving vehicle, those receiving OR486 exhibited mechanical allodynia (F7,368=3.020, p=0.0043; Fig 1A), mechanical hyperalgesia (F7,368=2.653, p=0.0109; Fig 1B) and thermal hyperalgesia (F7,272=4.891, p<0.0001; Fig 1C) beginning on day 1 and lasting throughout the duration of the experiment. Sexual dimorphism was not observed, as both male and female rats developed mechanical allodynia (Male F1,80=101.6, p<0.0001 and Female F1,80=135.4, p<0.0001; Fig 1A, Supplemental Digital Content), mechanical hyperalgesia (Male F1,80=76.01, p<0.0001 and Female F1,80=78.18, p<0.0001; Fig 1B, Supplemental Digital Content) and thermal hyperalgesia (Male F1,56=88.98, p<0.0001 and Female F1,56=97.66, p<0.0001; Fig 1C, Supplemental Digital Content).
Figure 1.
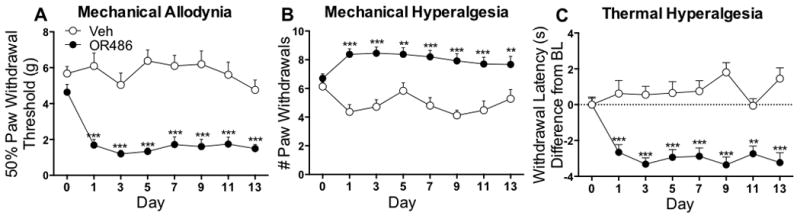
Adrenalectomized rats fail to develop persistent COMT-dependent pain
Previous work has demonstrated that acute COMT-dependent pain is mediated via β2- and β3ARs, which are located in peripheral, spinal and central regions where they could potentially drive pain transmission. To evaluate the potential contribution of peripheral adrenergic systems to COMT-dependent pain, separate groups of adrenalectomized rats (lacking peripheral epinephrine) or sham surgery rats received systemic OR486 (15mg/kg/day) or vehicle over a 2-week period and responses to mechanical and thermal stimuli were measured. Compared to sham rats receiving vehicle, those receiving OR486 developed mechanical allodynia (F3,720=114.5, p<0.0001; Fig 2A), mechanical hyperalgesia (F3,736=36.52, p<0.0001; Fig 2B) and thermal hyperalgesia (F3,720=73.75, p<0.0001; Fig 2C). In contrast, adrenalectomized rats did not develop mechanical allodynia, mechanical hyperalgesia or thermal hyperalgesia.
Figure 2.
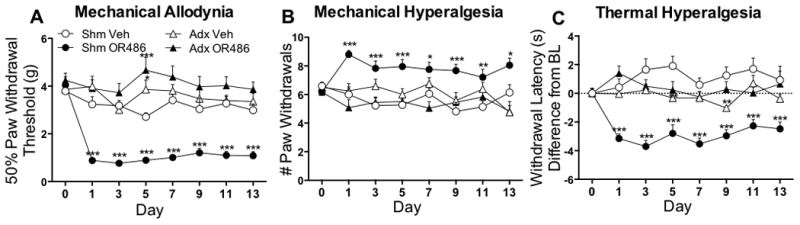
Sexual dimorphism was not observed, as both male and female sham rats developed mechanical allodynia (Male F1,160=72.50, p<0.0001 and Female F1,176=223.70, p<0.0001; Fig 2A, Supplemental Digital Content), mechanical hyperalgesia (Male F1,160=8.009, p=0.0053 and Female F1,176=118.7, p<0.0001; Fig 2B, Supplemental Digital Content) and thermal hyperalgesia (Male F1,160=87.99, p<0.0001 and Female F1,176=134.20, p<0.0001; Fig 2C, Supplemental Digital Content). Both male and female adrenalectomized rats failed to develop mechanical allodynia (Fig 2D, Supplemental Digital Content), mechanical hyperalgesia (Fig 2E, Supplemental Digital Content) and thermal hyperalgesia (Fig 2F, Supplemental Digital Content).
Peripheral βAR antagonist administration prevents the development of persistent COMT-dependent pain
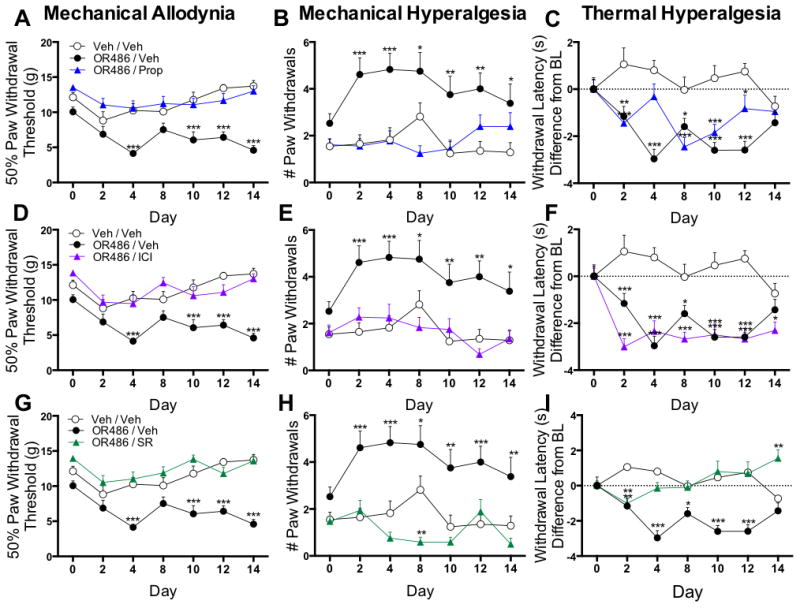
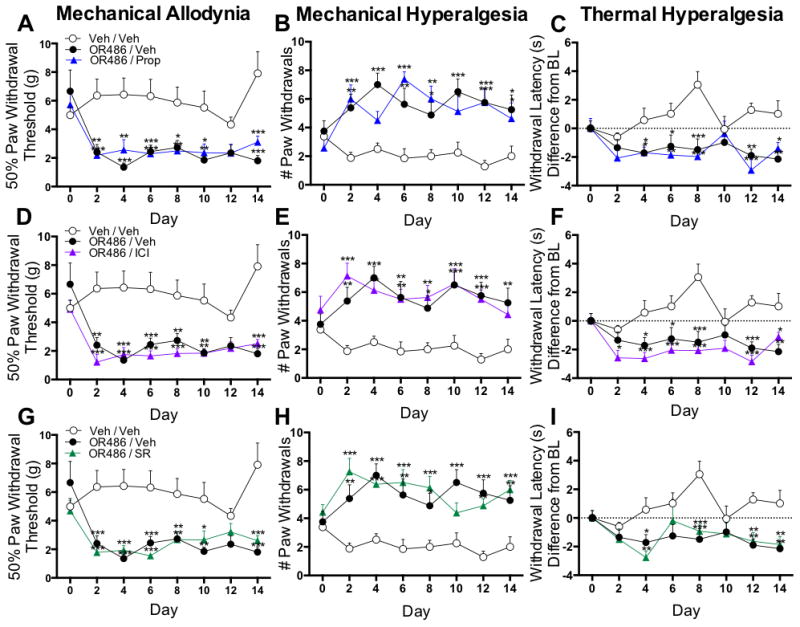
Adrenalectomized rats fail to develop persistent COMT-dependent pain, suggesting a peripheral adrenergic site of action. In order to further investigate this hypothesis, pharmacological methods were used to determine the contribution of peripheral, spinal and central βARs to persistent COMT-dependent pain. First, the contribution of peripheral βARs to mechanical and thermal pain was evaluated in separate groups of rats receiving sustained i.pl. administration of propranolol (9mg/kg/day), ICI-118,551 (1.5mg/kg/day), SR59230A (1.67mg/kg/day), or vehicle alongside sustained systemic administration of OR486 (15mg/kg/day) or vehicle over a 2-week period. Peripheral antagonist doses were selected based on the results from a preliminary study that evaluated the ability of three different doses per antagonist to reduce or block COMT-dependent pain.
Compared to rats receiving vehicle, those receiving sustained i.pl. administration of the non-selective βAR antagonist propranolol, the β2AR antagonist ICI-118,511, or the β3AR antagonist SR59230A alongside systemic OR486 did not develop mechanical allodynia (Fig 3A, F2,164=72.87, p<0.0001; Fig. 3D, F2,164=65.43, p<0.0001; Fig 3G, F2,164=90.51, p<0.0001) or mechanical hyperalgesia (Fig 3B, F2,162=41.76, p<0.0001; Fig 3E, F2,162=43.15, p<0.0001; Fig 3H, F2,162=61.97, p<0.0001). Rats receiving sustained i.pl. administration of the β3AR antagonist SR59230A also did not develop OR486-induced thermal hyperalgesia (Fig 3I, F2,163=46.24, P<0.0001). In contrast, rats receiving propranolol (Fig 3C) or ICI-118,551 (Fig 3F) alongside OR486 exhibited a 15% decrease in paw withdrawal latency from baseline, similar to rats receiving vehicle.
Figure 3.
Intrathecal βAR antagonist administration does not alter persistent COMT-dependent pain
Next, the contribution of spinal βARs to mechanical and thermal pain was evaluated in separate groups of rats receiving sustained i.t. administration of propranolol (50ug/day), ICI-118,551 (30ug/day), SR59230A (20ug/day), or vehicle alongside sustained systemic administration of OR486 (15mg/kg/day) or vehicle over a 2-week period. Intrathecal delivered antagonist doses were selected based on their ability to block pain or related behaviors in other rat models when administered i.t..59-61 Similar to animals receiving vehicle, those receiving sustained i.t. administration of the non-selective βAR antagonist propranolol, the β2AR antagonist ICI-118,511, or the β3AR antagonist SR59230A alongside systemic OR486 exhibited mechanical allodynia (Fig 4A, F2,159=16.63, p<0.0001; Fig 4D, F2,158=16.60, p<0.0001; Fig 4G, F2,173=16.13, p<0.0001), mechanical hyperalgesia (Fig 4B, F2,164=9.149, p=0.0002; Fig 4E, F2,165=13.33, p<0.0001; Fig 4H, F2,181=6.544, p=0.0018) and thermal hyperalgesia (Fig 4C, F2,164=85.26, p<0.0001; Fig 4F, F2,164=86.01, p<0.0001; Fig 4I, F2,178=81.55, p<0.0001).
Figure 4.
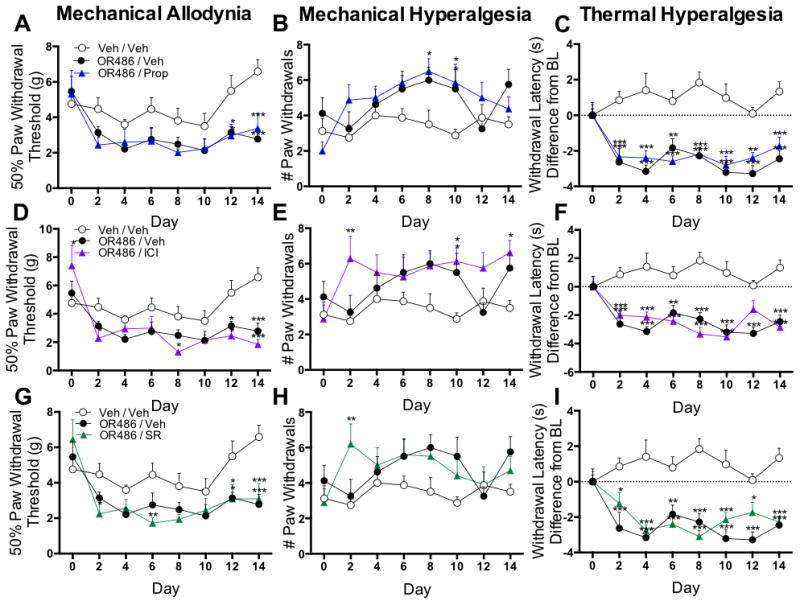
To confirm that i.t. βAR antagonists were unable to block OR486-induced pain, we performed a duplicate set of experiments using a higher dose of the non-selective βAR antagonist propranolol (100ug/day). Similar to the original dose, i.t. administration of the higher dose did not block OR486-induced mechanical allodynia (F3,182=17.42, p<0.0001; Fig 3A, Supplemental Digital Content), mechanical hyperalgesia (F3,188=5.552, p=0.0011; Fig 3B, Supplemental Digital Content) or thermal hyperalgesia (F3,187=50.96, p<0.0001; Fig 3C, Supplemental Digital Content).
Intracerebroventricular βAR antagonist administration does not alter persistent COMT-dependent pain
Finally, the contribution of central βARs to mechanical and thermal pain was evaluated in separate groups of rats receiving sustained i.c.v. administration of propranolol (50ug/day), ICI-118,551 (30ug/day), SR59230A (20ug/day), or vehicle alongside sustained systemic administration of OR486 (15mg/kg/day) or vehicle over a 2-week period. I.c.v. antagonist doses were selected based on their ability to block pain or related behaviors in other rat models.59-61 Similar to animals receiving vehicle, those receiving sustained i.c.v. administration of the non-selective βAR antagonist propranolol, the β2AR antagonist ICI-118,511, or the β3AR antagonist SR59230A alongside systemic OR486 exhibited mechanical allodynia (Fig 5A, F2,155=42.51, p<0.0001; Fig 5D, F2,157=57.15, p<0.0001; Fig 5G, F2,157=48.66, p<0.0001), mechanical hyperalgesia (Fig 5B, F2,165=41.6, p<0.0001; Fig 5E, F2,164=51.30, p<0.0001; Fig 5H, F2,164=55.35, p<0.0001) and thermal hyperalgesia (Fig 5C, F2,164=31.36, p<0.0001; Fig 5F, F2,163=43.52, p<0.0001; Fig 5I, F2,165=22.98, p<0.0001).
Figure 5.
To confirm that i.c.v. βAR antagonists are unable to block OR486-induced pain, we performed a duplicate set of experiments using a higher dose of the non-selective βAR antagonist propranolol (100ug/day). Similar to the original dose, i.c.v. administration of the higher dose did not block OR486-induced mechanical allodynia (F3,193=43.70, p<0.0001; Fig 4A, Supplemental Digital Content), mechanical hyperalgesia (F3,205=28.26, p<0.0001; Fig 4B, Supplemental Digital Content) or thermal hyperalgesia (F3,203=61.76, p<0.0001; Fig 4C, Supplemental Digital Content).
Discussion
Though the mechanisms underlying chronic pain disorders are not well described, emerging evidence suggests a role for adrenergic pathways. Employing a rodent model of sustained COMT inhibition that mimics abnormalities in catecholamine signaling observed in patients with these disorders, we demonstrate that COMT-dependent pain behaviors are mediated via peripherally, but not spinally or centrally, located β2- and β3ARs.
In previous studies, we established a causal link between low COMT and pain. Specifically, we demonstrated that a single injection of the COMT inhibitor OR486 produces pronounced increases in behavioral responses to mechanical and thermal stimuli, similar to that produced by intraplantar carrageenan. Subsequent pharmacological studies further demonstrated that the development of acute COMT-dependent pain requires the activation of β2- and β3ARs.28,30 Within hours, administration of OR486 results in increased circulating levels of nitric oxide (NO) and the pro-inflammatory cytokines tumor necrosis factor-α (TNFα), interleukin 1β (IL-1β), interleukin 6 (IL-6), and chemokine (C-C motif) ligand 2 (CCL2),28 which are nociceptive transmitters implicated in chronic pain disorders. Individuals with FM, headache and TMD exhibit increased levels of these molecules,62-65 which elicit pain by reducing nociceptor firing thresholds.66-76 NO and pro-inflammatory cytokines also elicit pain by working synergistically to potentiate one another's biosynthesis, as we observed in the OR486 model.28
Here, we utilized a more clinically-relevant model of sustained COMT inhibition, characterized by enhanced sensitivity to noxious stimuli and altered pain-relevant cognitive-affective behaviors that persist over a 2-week period, to determine the site-of-action whereby βARs mediate persistent COMT-dependent pain. The contribution of peripheral adrenergic systems was first examined in adrenalectomized rats. We found that, compared to sham surgery rats, adrenalectomized rats that lack peripheral epinephrine fail to develop OR486-induced mechanical and thermal pain. This finding is in line with those from previous studies showing that adrenalectomized rats have blunted pain responses following formalin administration77 or chronic constriction injury.78 Together, these results suggest that peripherally circulating catecholamines contribute to the transmission of pain in models of inflammatory and neuropathic pain as well as chronic pain disorders. This conclusion is further supported by studies that have demonstrated increased levels of urinary catecholamines in patients with myofacial pain10 and increased circulating plasma catecholamines in women with FM.9
As previous preclinical and clinical studies have reported sex-specific differences in COMT-related phenotypes79-83 and as males and females exhibit different COMT expression patterns,84,85 we examined the contribution of peripheral adrenergic systems to COMT-dependent pain in both males and females. Counter to our expectation, male and female rats exhibited a comparable increase in mechanical and thermal pain following sustained systemic OR486 administration, which was blocked by suppressing peripheral adrenergic tone. Our findings emphasize the importance of examining chronic pain disorders in the context of both males and females, while continuing to consider possible gender-specific effects of COMT-dependent pain in future studies and clinical applications.
The independent contribution of peripheral, spinal and central βARs to persistent COMT-dependent pain were next examined in separate groups of intact rats receiving targeted delivery of the non-selective βAR antagonist propranolol, the selective β2AR antagonist ICI-118,551, or the selective β3AR antagonist SR59230A alongside systemic OR486. We found that peripheral, but not spinal or central, administration of propranolol, ICI-118,511 or SR59230A blocked the development of COMT-dependent pain throughout the duration of the 2-week testing period. While all three antagonists blocked the development of mechanical pain, only SR59230A blocked the development of thermal heat pain. These findings significantly extend those from earlier acute COMT inhibition studies,28,30 demonstrating that peripheral β2- and β3ARs both contribute to the development of persistent mechanical pain, while peripheral β3ARs independently contribute to the development of persistent thermal pain following sustained COMT inhibition.
The peripheral contribution of β2ARs to pain is in line with results from previous studies demonstrating that epinephrine activates β2ARs located on the peripheral terminals of primary afferent nociceptors, increasing their excitability and producing a hyperalgesic state.33-37 Also, elevations in plasma levels of norepinephrine activate β2ARs to promote visceral hypersensitivity.36 In humans, variants of the β2AR gene known to influence receptor expression are associated with increased risk of TMD.86
The contribution of peripheral β3ARs to persistent pain is more novel. Peripherally expressed β3ARs are known for their ability to regulate norepinephrine-induced changes in metabolism and thermoregulation.87 In 2010, it was discovered that β3ARs are expressed on primary afferent nociceptors, where they drive norepinephrine-induced ATP release and contribute to neuropathic pain behavior.88 Recently, β3ARs have also been shown to mediate formalin-induced TMJ pain.89 In contrast to acute COMT-dependent thermal pain, which requires coincident activation of both β2- and β3ARs,30 persistent COMT-dependent thermal pain requires independent activation of peripheral β3ARs. Unlike most GPCRs, including β2ARs, β3ARs do not undergo desensitization after agonist stimulation.90,91 Thus, β3ARs are uniquely positioned to stimulate downstream effectors for prolonged periods of time.
In addition to their location on primary afferent nociceptors, β2- and β3ARs are expressed on numerous peripheral cell types where they could potentially mediate pain, including: immune cells involved in adaptive responses (T-cells, mast cells, and macrophages), adipocytes, keratinocytes, and satellite glia. T-cells, mast cells, and macrophages are immune cells in the periphery that express βARs and, following their activation by epinephrine or norepinephrine, orchestrate inflammatory responses. Increased catecholamine levels following stress or pharmacologic manipulation lead to activation of T-cells, increased expression of β2- and β3ARs,47 and production of IL-1, IL-6, and CCL2.92 T-cell infiltration in the spinal dorsal horn of adult rats has been shown to contribute to pain following nerve injury.93,94 In line with these findings, patients with FM have more activated T-cells circulating in blood compared to healthy controls.95 Epinephrine activates mast cells and stimulates release of IL-1β, IL-6 and other pro-inflammatory cytokines in a β2AR-dependent manner.44 Increased activation of mast cells has been observed in numerous chronic pain disorders, including FM, headache, vestibulodynia, and irritable bowel syndrome.96-101 Agonist activation of β2ARs expressed on macrophages in vitro results in activation of intracellular kinases and subsequent release of IL-6. In line with these findings, sustained systemic administration of epinephrine in mice results in β2AR-mediated increases in the activation of macrophages and production of IL-6.45,46
Adipocytes are cells in the periphery that express both β2- and β3ARs and specialize in storing energy as fat.48 In addition, they interface with immune cells to regulate inflammatory responses.102 Notably, adipocytes produce 30% of the IL-6 circulating in the body103 and studies have shown that activation of β3ARs on adipocytes produces a robust increase in IL-6 levels in plasma104 as well as in TNFα,105 CCL2,106 and NO107 levels in vitro.
Keratinocytes and satellite glial cells reside near the peripheral terminals and cell bodies, respectively, of primary afferent nociceptors. While a direct link between βAR activation on these cell types and pain has yet to be established, catecholamine-induced activation of β2ARs expressed on keratinocytes results in increased activation of intracellular kinases and release of IL-6.41-43 Similarly, activation of satellite glia by catecholamines results in βAR-mediated increases in intracellular cyclic nucleotides that facilitate neuronal-glial communication.108
Collectively, these findings demonstrate the importance of β2- and β3ARs located on immunoregulatory cells in the periphery to persistent COMT-dependent pain, accounting for clinical observations that βAR antagonists provide pain relief for patients with FM and TMD.31,32,109 While these findings seem inconsistent with the ability of antidepressants to alleviate persistent pain by increasing synaptic levels of catecholamines, it is important to note that the analgesic effect of antidepressants is associated with descending inhibition of pain via actions at α2ARs or D2DARs in the spinal dorsal horn.110,111 Thus, catecholamines can exert divergent influences on nociception as a function of localization and net influence on neuronal excitability. By determining where, when, and how β2- and β3ARs and their downstream effectors mediate COMT-dependent pain, the field will better understand the diverse nature of catecholamine signaling so that patients suffering from disorders resulting from reduced COMT and/or elevated catecholamines receive the most relevant treatments.
While the studies herein utilized a clinically-relevant rodent model of sustained COMT inhibition, additional mechanistic studies will implement a COMT-/- mouse model in order to more accurately represent the endogenously low levels of COMT activity observed in pain patients. Future studies are also necessary to elucidate the specific cell signaling pathways responsible for the initiation and maintenance of β2- and β3AR-mediated pain. Finally, clinical studies are required to evaluate the efficacy of peripheral β2- and β3AR antagonist therapy in patients with chronic pain disorders and related conditions.
In conclusion, we utilized a clinically-relevant animal model that portrays characteristics of patients with chronic pain disorders to demonstrate that both male and female rats are susceptible to the development of persistent COMT-dependent pain, which is mediated via peripherally located β2- and β3ARs. These findings suggest that peripheral β2- and β3AR antagonist therapy may be an effective option for the treatment of chronic pain disorders as well as those with overlapping peripheral β-adrenergic mechanisms (e.g. complex regional pain syndrome112).
Supplementary Material
What we already know about this topic.
Decreased catecholamine-O-methyltransferase (COMT) activity is associated with increased clinical and experimental pain in humans and inhibition of COMT in animals results in hypersensitivity
Although β-adrenoceptors appear important to these observations, the site(s) of receptor activation are unknown
What this article tells us that is new.
In rats, sustained administration of a COMT inhibitor produces hypersensitivity to mechanical and thermal stimuli which is prevented by peripheral, but not spinal or supraspinal administration of β-adrenoceptor antagonists, suggesting a peripheral site of action
Acknowledgments
Funding: This work was funded by NIH/NINDS R01 NS072205 to A.N. and P01 NS045685 to A.N.
The authors thank SAI-Infusion Technologies for their assistance with catheter design.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- 1.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547–55. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Pizzo PA. Relieving pain in America: insights from an Institute of Medicine committee. JAMA. 2014;312:1507–8. doi: 10.1001/jama.2014.12986. [DOI] [PubMed] [Google Scholar]
- 3.Medicine Io. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. 2014 doi: 10.7205/MILMED-D-16-00012. [DOI] [PubMed] [Google Scholar]
- 4.Rey E, Talley NJ. Irritable bowel syndrome: novel views on the epidemiology and potential risk factors. Dig Liver Dis. 2009;41:772–80. doi: 10.1016/j.dld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 2013;14:T20–32. doi: 10.1016/j.jpain.2013.07.014. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–91. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Wesselmann U, Bonham A, Foster D. Vulvodynia: Current state of the biological science. Pain. 2014;155:1696–701. doi: 10.1016/j.pain.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SB, Maixner DW, Greenspan JD, Dubner R, Fillingim RB, Ohrbach R, Knott C, Slade GD, Bair E, Gibson DG, Zaykin DV, Weir BS, Maixner W, Diatchenko L. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain. 2011;12:T92–101. doi: 10.1016/j.jpain.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: a pilot study in fibromyalgia. Arthritis Rheum. 2000;43:872–80. doi: 10.1002/1529-0131(200004)43:4<872::AID-ANR19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Evaskus DS, Laskin DM. A biochemical measure of stress in patients with myofascial pain-dysfunction syndrome. J Dent Res. 1972;51:1464–6. doi: 10.1177/00220345720510053501. [DOI] [PubMed] [Google Scholar]
- 11.Perry F, Heller PH, Kamiya J, Levine JD. Altered autonomic function in patients with arthritis or with chronic myofascial pain. Pain. 1989;39:77–84. doi: 10.1016/0304-3959(89)90177-2. [DOI] [PubMed] [Google Scholar]
- 12.Marbach JJ, Levitt M. Erythrocyte catechol-O-methyltransferase activity in facial pain patients. J Dent Res. 1976;55:711. doi: 10.1177/00220345760550043801. [DOI] [PubMed] [Google Scholar]
- 13.Smith SB, Reenila I, Mannisto PT, Slade GD, Maixner W, Diatchenko L, Nackley AG. Epistasis Between Polymorphisms in COMT, ESR1, and GCH1 Influences COMT Enzyme Activity and Pain. Pain. 2014 doi: 10.1016/j.pain.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 15.Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–10. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 16.Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–3. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 17.Barbosa FR, Matsuda JB, Mazucato M, de Castro Franca S, Zingaretti SM, da Silva LM, Martinez-Rossi NM, Junior MF, Marins M, Fachin AL. Influence of catechol-O-methyltransferase (COMT) gene polymorphisms in pain sensibility of Brazilian fibromialgia patients. Rheumatol Int. 2012;32:427–30. doi: 10.1007/s00296-010-1659-z. [DOI] [PubMed] [Google Scholar]
- 18.Cohen H, Neumann L, Glazer Y, Ebstein RP, Buskila D. The relationship between a common catechol-O-methyltransferase (COMT) polymorphism val(158) met and fibromyalgia. Clin Exp Rheumatol. 2009;27:S51–6. [PubMed] [Google Scholar]
- 19.Gursoy S, Erdal E, Herken H, Madenci E, Alasehirli B, Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int. 2003;23:104–7. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda JB, Barbosa FR, Morel LJ, Franca Sde C, Zingaretti SM, da Silva LM, Pereira AM, Marins M, Fachin AL. Serotonin receptor (5-HT 2A) and catechol-O-methyltransferase (COMT) gene polymorphisms: triggers of fibromyalgia? Rev Bras Reumatol. 2010;50:141–9. [PubMed] [Google Scholar]
- 21.Vargas-Alarcon G, Fragoso JM, Cruz-Robles D, Vargas A, Vargas A, Lao-Villadoniga JI, Garcia-Fructuoso F, Ramos-Kuri M, Hernandez F, Springall R, Bojalil R, Vallejo M, Martinez-Lavin M. Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish patients with fibromyalgia. Arthritis Res Ther. 2007;9:R110. doi: 10.1186/ar2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 23.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 24.Mobascher A, Brinkmeyer J, Thiele H, Toliat MR, Steffens M, Warbrick T, Musso F, Wittsack HJ, Saleh A, Schnitzler A, Winterer G. The val158met polymorphism of human catechol-O-methyltransferase (COMT) affects anterior cingulate cortex activation in response to painful laser stimulation. Mol Pain. 2010;6:32. doi: 10.1186/1744-8069-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakvag TT, Klepstad P, Baar C, Kvam TM, Dale O, Kaasa S, Krokan HE, Skorpen F. The Val158Met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain. 2005;116:73–8. doi: 10.1016/j.pain.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Barbosa F, Matsuda J, Mazucato M, de Castro França S, Zingaretti S, da Silva L, Martinez-Rossi N, Júnior M, Marins M, Fachin A. Influence of catechol-O-methyltransferase (COMT) gene polymorphisms in pain sensibility of Brazilian fibromialgia patients. Rheumatology International. 2012;32:427–430. doi: 10.1007/s00296-010-1659-z. [DOI] [PubMed] [Google Scholar]
- 27.Desmeules J, Chabert J, Rebsamen M, Rapiti E, Piguet V, Besson M, Dayer P, Cedraschi C. Central pain sensitization, COMT Val158Met polymorphism, and emotional factors in fibromyalgia. J Pain. 2014;15:129–35. doi: 10.1016/j.jpain.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Hartung JE, Ciszek BP, Nackley AG. beta2- and beta3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines. Pain. 2014;155:1346–55. doi: 10.1016/j.pain.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kline RHt, Exposto FG, O'Buckley SC, Westlund KN, Nackley AG. Catechol-O-methyltransferase inhibition alters pain and anxiety-related volitional behaviors through activation of beta-adrenergic receptors in the rat. Neuroscience. 2015;290:561–9. doi: 10.1016/j.neuroscience.2015.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain. 2009;10:542–52. doi: 10.1016/j.jpain.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics. 2010;20:239–48. doi: 10.1097/FPC.0b013e328337f9ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–9. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–60. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PubMed] [Google Scholar]
- 35.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–12. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Rui YY, Zhou YY, Ju Z, Zhang HH, Hu CY, Xiao Y, Xu GY. Adrenergic beta2-receptors mediates visceral hypersensitivity induced by heterotypic intermittent stress in rats. PLoS One. 2014;9:e94726. doi: 10.1371/journal.pone.0094726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khasar SG, Green PG, Miao FJ, Levine JD. Vagal modulation of nociception is mediated by adrenomedullary epinephrine in the rat. Eur J Neurosci. 2003;17:909–15. doi: 10.1046/j.1460-9568.2003.02503.x. [DOI] [PubMed] [Google Scholar]
- 38.Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci. 2006;24:527–34. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- 39.Ochoa-Cortes F, Guerrero-Alba R, Valdez-Morales EE, Spreadbury I, Barajas-Lopez C, Castro M, Bertrand J, Cenac N, Vergnolle N, Vanner SJ. Chronic stress mediators act synergistically on colonic nociceptive mouse dorsal root ganglia neurons to increase excitability. Neurogastroenterol Motil. 2014;26:334–45. doi: 10.1111/nmo.12268. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Zhu HY, Jin Y, Zhou Y, Hu S, Liu T, Jiang X, Xu GY. Adrenergic signaling mediates mechanical hyperalgesia through activation of P2X3 receptors in primary sensory neurons of rats with chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G710–9. doi: 10.1152/ajpgi.00395.2014. [DOI] [PubMed] [Google Scholar]
- 41.Dasu MR, Ramirez SR, La TD, Gorouhi F, Nguyen C, Lin BR, Mashburn C, Stewart H, Peavy TR, Nolta JA, Isseroff RR. Crosstalk between adrenergic and toll-like receptors in human mesenchymal stem cells and keratinocytes: a recipe for impaired wound healing. Stem Cells Transl Med. 2014;3:745–59. doi: 10.5966/sctm.2013-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koizumi H, Tanaka H, Ohkawara A. beta-Adrenergic stimulation induces activation of protein kinase C and inositol 1,4,5-trisphosphate increase in epidermis. Exp Dermatol. 1997;6:128–32. doi: 10.1111/j.1600-0625.1997.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 43.Pullar CE, Isseroff RR. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci. 2006;119:592–602. doi: 10.1242/jcs.02772. [DOI] [PubMed] [Google Scholar]
- 44.Chi DS, Fitzgerald SM, Pitts S, Cantor K, King E, Lee SA, Huang SK, Krishnaswamy G. MAPK-dependent regulation of IL-1- and beta-adrenoreceptor-induced inflammatory cytokine production from mast cells: implications for the stress response. BMC Immunol. 2004;5:22. doi: 10.1186/1471-2172-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiarella SE, Soberanes S, Urich D, Morales-Nebreda L, Nigdelioglu R, Green D, Young JB, Gonzalez A, Rosario C, Misharin AV, Ghio AJ, Wunderink RG, Donnelly HK, Radigan KA, Perlman H, Chandel NS, Budinger GR, Mutlu GM. beta(2)-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. J Clin Invest. 2014;124:2935–46. doi: 10.1172/JCI75157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim MH, Gorouhi F, Ramirez S, Granick JL, Byrne BA, Soulika AM, Simon SI, Isseroff RR. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6. J Invest Dermatol. 2014;134:809–17. doi: 10.1038/jid.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laukova M, Vargovic P, Csaderova L, Chovanova L, Vlcek M, Imrich R, Krizanova O, Kvetnansky R. Acute stress differently modulates beta1, beta2 and beta3 adrenoceptors in T cells, but not in B cells, from the rat spleen. Neuroimmunomodulation. 2012;19:69–78. doi: 10.1159/000329002. [DOI] [PubMed] [Google Scholar]
- 48.Collins S, Surwit RS. The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res. 2001;56:309–28. doi: 10.1210/rp.56.1.309. [DOI] [PubMed] [Google Scholar]
- 49.Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–9. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholson R, Dixon AK, Spanswick D, Lee K. Noradrenergic receptor mRNA expression in adult rat superficial dorsal horn and dorsal root ganglion neurons. Neurosci Lett. 2005;380:316–21. doi: 10.1016/j.neulet.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 51.Stone EA, Ariano MA. Are glial cells targets of the central noradrenergic system? A review of the evidence Brain Res Brain Res Rev. 1989;14:297–309. doi: 10.1016/0165-0173(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 52.Kanno T, Yaguchi T, Nishizaki T. Noradrenaline stimulates ATP release from DRG neurons by targeting beta(3) adrenoceptors as a factor of neuropathic pain. J Cell Physiol. 2010;224:345–51. doi: 10.1002/jcp.22114. [DOI] [PubMed] [Google Scholar]
- 53.Claustre Y, Leonetti M, Santucci V, Bougault I, Desvignes C, Rouquier L, Aubin N, Keane P, Busch S, Chen Y, Palejwala V, Tocci M, Yamdagni P, Didier M, Avenet P, Le Fur G, Oury-Donat F, Scatton B, Steinberg R. Effects of the beta3-adrenoceptor (Adrb3) agonist SR58611A (amibegron) on serotonergic and noradrenergic transmission in the rodent: relevance to its antidepressant/anxiolytic-like profile. Neuroscience. 2008;156:353–64. doi: 10.1016/j.neuroscience.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Haddad F, Adams GR. Inhibition of MAP/ERK kinase prevents IGF-I-induced hypertrophy in rat muscles. J Appl Physiol (1985) 2004;96:203–10. doi: 10.1152/japplphysiol.00856.2003. [DOI] [PubMed] [Google Scholar]
- 55.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–6. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 56.DeVos SL, Miller TM. Direct intraventricular delivery of drugs to the rodent central nervous system. J Vis Exp. 2013:e50326. doi: 10.3791/50326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 58.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 59.Dumka VK, Tandan SK, Raviprakash V, Tripathi HC. Central noradrenergic and cholinergic modulation of formaldehyde-induced pedal inflammation and nociception in rats. Indian J Physiol Pharmacol. 1996;40:41–6. [PubMed] [Google Scholar]
- 60.Fukui M, Nakagawa T, Minami M, Satoh M. Involvement of beta2-adrenergic and mu-opioid receptors in antinociception produced by intracerebroventricular administration of alpha,beta-methylene-ATP. Jpn J Pharmacol. 2001;86:423–8. doi: 10.1254/jjp.86.423. [DOI] [PubMed] [Google Scholar]
- 61.Kanzler SA, Januario AC, Paschoalini MA. Involvement of beta3-adrenergic receptors in the control of food intake in rats. Braz J Med Biol Res. 2011;44:1141–7. doi: 10.1590/s0100-879x2011007500127. [DOI] [PubMed] [Google Scholar]
- 62.Kubota E, Kubota T, Matsumoto J, Shibata T, Murakami KI. Synovial fluid cytokines and proteinases as markers of temporomandibular joint disease. J Oral Maxillofac Surg. 1998;56:192–198. doi: 10.1016/s0278-2391(98)90868-0. [DOI] [PubMed] [Google Scholar]
- 63.Uzar E, Evliyaoglu O, Yucel Y, Ugur Cevik M, Acar A, Guzel I, Islamoglu Y, Colpan L, Tasdemir N. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in pateints with migraine. Eur Rev Med Pharmacol Sci. 2011;10:1111–1116. [PubMed] [Google Scholar]
- 64.Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, Floridi A, Calabresi P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46:200–7. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 65.Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W, Nackley AG. Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 2011;152:2802–12. doi: 10.1016/j.pain.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Omote K, Hazama K, Kawamata T, Kawamata M, Nakayaka Y, Toriyabe M, Namiki A. Peripheral nitric oxide in carrageenan-induced inflammation. Brain Res. 2001:171–175. doi: 10.1016/s0006-8993(01)02733-0. [DOI] [PubMed] [Google Scholar]
- 67.Holguin A, O'Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Bravmann C, Martin D, Fleshner M, Maier SF, Watkins LR. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-I (nNOS) Pain. 2004;110:517–30. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Kuboyama K, Tsuda M, Tsutsui M, Toyohara Y, Tozaki-Saitoh H, Shimokawa H, Yanagihara N, Inoue K. Reduced spinal microglial activation and neuropathic pain after nerve injury in mice lacking all three nitric oxide synthases. Mol Pain. 2011;7:50. doi: 10.1186/1744-8069-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–31. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 70.Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci. 1998;18:7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 2008;434:293–8. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 72.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1β sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obreja O, Biasio W, Andratsch M, Lips KS, Rathee PK, Ludwig A, Rose-John S, Kress M. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain. 2005;128:1634–41. doi: 10.1093/brain/awh490. [DOI] [PubMed] [Google Scholar]
- 74.Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1B potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 75.Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–99. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- 77.Vissers KC, De Jongh RF, Crul BJ, Vinken P, Meert TF. Adrenalectomy affects pain behavior of rats after formalin injection. Life Sci. 2004;74:1243–51. doi: 10.1016/j.lfs.2003.07.040. [DOI] [PubMed] [Google Scholar]
- 78.Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci. 2004;24:8595–605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–6. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–23. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–45. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- 82.White TP, Loth E, Rubia K, Krabbendam L, Whelan R, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Paillere ML, Nees F, Paus T, Pausova Z, Rietschel M, Robbins T, Smolka MN, Shergill SS, Schumann G, Consortium I. Sex differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence. Neuropsychopharmacology. 2014;39:2560–9. doi: 10.1038/npp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belfer I, Segall SK, Lariviere WR, Smith SB, Dai F, Slade GD, Rashid NU, Mogil JS, Campbell CM, Edwards RR, Liu Q, Bair E, Maixner W, Diatchenko L. Pain modality- and sex-specific effects of COMT genetic functional variants. Pain. 2013;154:1368–76. doi: 10.1016/j.pain.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381–9. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- 85.Chen LX, Fang Q, Chen Q, Guo J, Wang ZZ, Chen Y, Wang R. Study in vitro and in vivo of nociceptin/orphanin FQ(1-13)NH2 analogues substituting N-Me-Gly for Gly2 or Gly3. Peptides. 2004;25:1349–54. doi: 10.1016/j.peptides.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 86.Diatchenko L, Anderson AD, Slade GD, Fillingim RB, Shabalina SA, Higgins TJ, Sama S, Belfer I, Goldman D, Max MB, Weir BS, Maixner W. Three major haplotypes of the beta2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:449–62. doi: 10.1002/ajmg.b.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strosberg AD. Structure and function of the beta 3-adrenergic receptor. Annu Rev Pharmacol Toxicol. 1997;37:421–50. doi: 10.1146/annurev.pharmtox.37.1.421. [DOI] [PubMed] [Google Scholar]
- 88.Kanno T, Yaguchi T, Nishizaki T. Noradrenaline stimulates ATP release from DRG neurons by targeting beta(3) adrenoceptors as a factor of neuropathic pain. J Cell Physiol. 2010;224:345–51. doi: 10.1002/jcp.22114. [DOI] [PubMed] [Google Scholar]
- 89.Favaro-Moreira NC, Parada CA, Tambeli CH. Blockade of beta(1)-, beta(2)- and beta(3)-adrenoceptors in the temporomandibular joint induces antinociception especially in female rats. Eur J Pain. 2012;16:1302–10. doi: 10.1002/j.1532-2149.2012.00132.x. [DOI] [PubMed] [Google Scholar]
- 90.Liggett SB, Freedman NJ, Schwinn DA, Lefkowitz RJ. Structural basis for receptor subtype-specific regulation revealed by a chimeric beta 3/beta 2-adrenergic receptor. Proc Natl Acad Sci U S A. 1993;90:3665–9. doi: 10.1073/pnas.90.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao W, Luttrell LM, Medvedev AV, Pierce KL, Daniel KW, Dixon TM, Lefkowitz RJ, Collins S. Direct binding of activated c-Src to the beta 3-adrenergic receptor is required for MAP kinase activation. J Biol Chem. 2000;275:38131–4. doi: 10.1074/jbc.C000592200. [DOI] [PubMed] [Google Scholar]
- 92.Slota C, Shi A, Chen G, Bevans M, Weng NP. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun. 2015;46:168–79. doi: 10.1016/j.bbi.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–22. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vicuna L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, Prokosch S, Riva P, Griffin RS, Njoo C, Gehrig S, Mall MA, Arnold B, Devor M, Woolf CJ, Liberles SD, Costigan M, Kuner R. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med. 2015;21:518–23. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Russell IJ, Vipraio GA, Michalek JE, Craig FE, Kang YK, Richards AB. Lymphocyte markers and natural killer cell activity in fibromyalgia syndrome: effects of low-dose, sublingual use of human interferon-alpha. J Interferon Cytokine Res. 1999;19:969–78. doi: 10.1089/107999099313523. [DOI] [PubMed] [Google Scholar]
- 96.Feng B, La JH, Schwartz ES, Gebhart GF. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome Am J Physiol Gastrointest Liver Physiol. 2012;302:G1085–98. doi: 10.1152/ajpgi.00542.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Graziottin A, Skaper SD, Fusco M. Mast cells in chronic inflammation, pelvic pain and depression in women. Gynecol Endocrinol. 2014;30:472–7. doi: 10.3109/09513590.2014.911280. [DOI] [PubMed] [Google Scholar]
- 98.Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13:237–40. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 99.Petra AI, Panagiotidou S, Stewart JM, Conti P, Theoharides TC. Spectrum of mast cell activation disorders. Expert Rev Clin Immunol. 2014;10:729–39. doi: 10.1586/1744666X.2014.906302. [DOI] [PubMed] [Google Scholar]
- 100.Regauer S, Eberz B, Beham-Schmid C. Mast cell infiltrates in vulvodynia represent secondary and idiopathic mast cell hyperplasias. APMIS. 2015;123:452–6. doi: 10.1111/apm.12372. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Z, Cherryholmes G, Mao A, Marek C, Longmate J, Kalos M, Amand RP, Shively JE. High plasma levels of MCP-1 and eotaxin provide evidence for an immunological basis of fibromyalgia. Exp Biol Med (Maywood) 2008;233:1171–80. doi: 10.3181/0712-RM-328. [DOI] [PubMed] [Google Scholar]
- 102.Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. 2014;16:1484–92. doi: 10.1111/cmi.12336. [DOI] [PubMed] [Google Scholar]
- 103.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 104.Mohamed-Ali V, Flower L, Sethi J, Hotamisligil G, Gray R, Humphries SE, York DA, Pinkney J. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab. 2001;86:5864–9. doi: 10.1210/jcem.86.12.8104. [DOI] [PubMed] [Google Scholar]
- 105.Fu L, Isobe K, Zeng Q, Suzukawa K, Takekoshi K, Kawakami Y. beta-adrenoceptor agonists downregulate adiponectin, but upregulate adiponectin receptor 2 and tumor necrosis factor-alpha expression in adipocytes. Eur J Pharmacol. 2007;569:155–62. doi: 10.1016/j.ejphar.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 106.Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Isoproterenol stimulates monocyte chemoattractant protein-1 expression and secretion in 3T3-L1 adipocytes. Regul Pept. 2006;135:12–6. doi: 10.1016/j.regpep.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Canova NK, Lincova D, Kmonickova E, Kamenikova L, Farghali H. Nitric oxide production from rat adipocytes is modulated by beta3-adrenergic receptor agonists and is involved in a cyclic AMP-dependent lipolysis in adipocytes. Nitric Oxide. 2006;14:200–11. doi: 10.1016/j.niox.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Gilman AG, Nirenberg M. Effect of catecholamines on the adenosine 3′:5′-cyclic monophosphate concentrations of clonal satellite cells of neurons. Proc Natl Acad Sci U S A. 1971;68:2165–8. doi: 10.1073/pnas.68.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wood PB, Kablinger AS, Caldito GS. Open trial of pindolol in the treatment of fibromyalgia. Ann Pharmacother. 2005;39:1812–6. doi: 10.1345/aph.1G014. [DOI] [PubMed] [Google Scholar]
- 110.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 111.Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol. 1999;19:467–89. doi: 10.1023/a:1006986824213. [DOI] [PubMed] [Google Scholar]
- 112.Li W, Shi X, Wang L, Guo T, Wei T, Cheng K, Rice KC, Kingery WS, Clark JD. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain. 2013;154:1224–36. doi: 10.1016/j.pain.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


