Abstract
Background
DBS is an effective treatment for movement disorders, but it is relatively complex, invasive, and costly. Little is known about whether stimulation mode alters pulse generator (battery) longevity in routine clinical care. The aim of this study was to compare battery longevity during monopolar versus bipolar stimulation in patients who underwent DBS for movement disorders.
Methods
We evaluated 2,902 programming adjustments and calculated the average stimulator settings for 393 batteries in 200 unique patients with Parkinson's disease (PD) and essential tremor (ET). We classified the pulse generators into different stimulation modes (monopolar, bipolar, tripolar, and double monopolar) and compared battery longevity with Kaplan–Meier's survival analyses using the log‐rank test. We exclusively implanted the Medtronic 3387 lead with adjacent electrode contacts separated by 1.5 mm.
Results
Mean pulse generator longevity was 47.6 ± 1.6 months regardless of diagnosis or stimulation mode. Bipolar stimulation mode was associated with greater longevity than monopolar stimulation (56.1 ± 3.4 vs. 44.2 ± 2.1 months; P = 0.006). This effect was most pronounced when stimulation parameters were at low‐to‐moderate intensity settings. Double monopolar configuration was associated with less pulse generator longevity than conventional stimulation modes (37.8 ± 5.6 vs. 49.7 ± 1.9; P = 0.014).
Conclusion
Implanted pulse generators initially programmed in bipolar mode provided 1 year of additional battery longevity versus monopolar mode in this large, retrospective series of patients with ET and PD. Given satisfactory efficacy for motor symptoms, bipolar stimulation mode is a feasible alternative programming strategy at the initiation of DBS therapy.
Keywords: Parkinson's disease, tremor, motor control, deep brain stimulation
DBS is a treatment option for neurological symptoms that do not respond optimally to oral medications and other conv‐entional therapies.1, 2, 3 The longevity of the implanted pulse generator (IPG) varies depending on the stimulation target, underlying disease process, electrode location, and other variables, and these factors contribute directly to surgical morbidity and cost over time.4, 5 As the indications and demand for DBS expand, there is an increasing need to understand factors that contribute to this important clinical outcome. Stimulator programming adjustments are simple, reversible, and already part of routine care for established DBS indications; therefore, identification and characterization of potential strategies to optimize battery longevity could have broad implications for clinical practice.
DBS exerts its clinical effects by generating rapid, precisely timed electrical pulses from one or more contacts on a linear electrode array in the brain. The two most common stimulation modes are monopolar (with the cathode/negative electrode contact in the brain and the anode/positive contact in the pulse generator in the chest wall) and bipolar (with the anode and cathode contacts in much closer proximity, both within the brain). Although similar in many respects, the therapeutic impedances are typically greater during bipolar versus monopolar stimulation, indicating that at a given stimulation intensity, a constant voltage DBS device will deliver less current with bipolar versus monopolar stimulation. Monopolar stimulation, however, produces a larger volume of tissue activation (VTA) than bipolar stimulation, typically yielding lower stimulation thresholds for both symptomatic efficacy and potential side effects.6 Conversely, bipolar stimulation is associated with a dense, smaller VTA, potentially requiring more intense stimulation to achieve comparable efficacy. Given these competing factors, it is not known whether bipolar or monopolar stimulation is associated with less‐frequent battery replacement surgeries in clinical practice.
A previous study found that DBS programming in configurations other than exclusively bipolar (monopolar or mixed) predicted shorter battery life in a heterogeneous sample of patients with Parkinson's disease (PD), essential tremor (ET), dystonia, and cerebellar outflow tremor.7 Additionally, we recently found significantly shorter battery longevity in patients with pallidal DBS for dystonia versus those with subthalamic or thalamic DBS for PD and ET, and patients with ET and PD experienced similar overall battery longevity.4 In the context of these observations, we evaluated the following questions: (1) Does pulse generator longevity differ between the two most commonly used stimulation modes (bipolar vs. monopolar) in a large sample of patients?; (2) Is the magnitude of this putative effect clinically significant?; (3) Does clinical efficacy differ based upon monopolar versus bipolar stimulation mode?; and (4) Does battery longevity differ between conventional stimulation modes (monopolar, bipolar) versus other modes (double monopolar, tripolar)? Strategies to optimize pulse generator longevity have the potential to decrease surgical morbidity and cost in patients who undergo DBS for refractory neurological symptoms.
Materials and Methods
We evaluated consecutive DBS patients who underwent either subthalamic or ventral intermediate thalamic DBS for PD and ET at the University of Alabama at Birmingham (UAB; Birmingham, AL) between 1998 and 2012. This study was approved by the UAB Institutional Review Board. Informed consent was not obtained individually from subjects because this retrospective study used deidentified data generated as part of routine care. We only included patients with single‐channel, constant voltage devices (Soletra; Medtronic, Inc., Minneapolis, MN), and our approach to programming is similar to published practice parameters.8
Upon initial programming, a survey of each of the four DBS electrode contacts was performed in monopolar mode to determine stimulation thresholds for side effects and clinical improvement. We routinely perform a more abbreviated bipolar mode survey, focusing on the specific contacts that showed the greatest symptomatic improvement during monopolar stimulation. The decision for monopolar or bipolar stimulation mode for chronic stimulation was based on clinical response to stimulation, regardless of the location of the electrode contact, with goals of maximizing efficacy, minimizing potential side effects, and optimizing the stimulation intensity thresholds to elicit these responses. We always adjusted stimulator settings to maximal symptomatic benefit and have published motor and nonmotor outcomes post‐DBS for PD and ET in previous studies.4, 9, 10 In a minority of patients with symptoms refractory to conventional monopolar or bipolar stimulation, alternative stimulation modes, such as double monopolar (two cathode electrode contacts in the brain and the anode in the pulse generator in the chest wall) and tripolar (two cathodes and one anode contact, all in the brain) were utilized. The vast majority of devices showed 100% usage upon routine interrogation, and we do not routinely recommend that patients deactivate their device. The decision to replace the battery was based on routine care, either when stimulator interrogation showed a battery voltage approaching end of life (typically <3.6 V) or when symptoms reemerged subacutely or acutely upon complete depletion of the battery.
We collected demographic data and recorded the indication for surgery, brain hemisphere, stimulation target, and longevity for each IPG. All of our patients underwent stereotactic placement of a DBS Medtronic lead model 3387 (Medtronic, Inc.). Every individual programming adjustment (stimulation mode with active contacts, voltage, pulse width, and stimulation frequency) was entered into a database, and we calculated the average DBS settings over time. Based upon these average settings, each device was classified as monopolar, bipolar, double monopolar, or tripolar. In a minority of cases, the IPG was not in a single stimulation mode for greater than 90% of its use, such that the stimulation mode was classified as “mixed” and not included in the primary analyses. Patients with device infection or hardware failure not related to battery depletion were excluded, and we censored patients whose IPG had not yet expired and those lost to follow‐up to their most recent clinical encounter date.
We used Kaplan–Meier's survival analysis to test the primary hypothesis that battery longevity differed between bipolar and monopolar stimulation modes in patients with PD and ET. Survival analysis is ideally suited for evaluating a time‐delimited, dichotomous outcome such as battery expiration for the following reasons: (1) It accounts for devices that have not expired over relatively long follow‐up intervals; (2) it captures devices that have been implanted relatively recently (the issue of “right censoring”); (3) it is less arbitrary in terms of determining the endpoint for the statistical test; and (4) it provides more granular time resolution of the outcome of interest. Subgroups for the survival analyses were as follows: (1) monopolar versus bipolar mode with patients divided into low‐, moderate‐, and high‐stimulation intensity groups (stimulation intensity defined as average voltage × pulse width × frequency); (2) monopolar versus bipolar mode in patients with average stimulation voltages above and below 3.6 V (a voltage threshold that has specific relevance to the energy efficiency with the Soletra device); (3) conventional stimulation modes (monopolar and bipolar) versus double monopolar and tripolar modes; and (4) PD versus ET, regardless of stimulation mode. The low‐, moderate‐, and high‐stimulation intensity groupings were arbitrarily defined as <45,000, >45,000 and <80,000, and >80,000 V·μS·Hz, respectively. Additional secondary analyses compared the electrode impedances in monopolar versus bipolar stimulation mode with a paired t test in a cross‐sectional subset of 195 IPGs.
Statistical Analysis
To compare the clinical efficacy of monopolar versus bipolar stimulation, we evaluated changes in five different movement parameters at 6 months postop in 83 consecutive PD patients who underwent unilateral subthalamic DBS. We analyzed 34 monopolar versus 34 bipolar patients, excluding 15 patients because they were adjusted alternative stimulation modes (double monopolar, tripolar), or else they were switched between two or more stimulation modes during their initial 6 months of DBS therapy. The outcomes measured included UPDRS parts 2 and 3 off medications, UPDRS part 4, the time to rise from a chair in seconds measured with a stopwatch, and the number of steps taken walking comfortably through a hallway over 1 minute. The test statistic was repeated‐measures analysis of variance evaluating an effect of time within subjects and stimulation mode (monopolar vs. bipolar) by time across. Statistical analyses were conducted utilizing the statistical packages SPSS (version 19.0; SPSS, Inc., Chicago, IL), and the one minus survival Kaplan–Meier plots were generated with OriginPro software (version 9.0; Originlab Corporation, Wellesley, MA). We used P < 0.05 as the significance threshold for all statistical tests.
Results
We evaluated 2,902 stimulator adjustments in 393 Soletra IPGs from 200 unique patients with PD and ET. There were no significant differences in baseline characteristics between patients who underwent monopolar versus bipolar stimulation, except for a slightly greater average stimulation voltage during monopolar stimulation (3.6 vs. 3.4 V; Table 1). A flow chart describes how IPGs were categorized based upon stimulation mode (Fig. 1). Mean estimate for IPG longevity was 47.6 ± 1.6 months, regardless of diagnosis and stimulation mode (all data presented as mean ± standard error). At our level of statistical power, there was no effect of diagnosis of PD or ET on battery longevity (P = 0.97).
Table 1.
Demographic data and mean stimulation settings by stimulation configuration
| Monopolar | Bipolar | P Value (Monopolar vs. Bipolar) | Double Monopolar | Tripolar | |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 63.3 ± 0.8 | 62.5 ± 1.0 | 0.502 | 64.9 ± 1.7 | 69.0 ± 2.5 |
| Sex, n (%) | |||||
| Male | 119 (74.8) | 74 (60.7) | 20 (71.4) | 16 (61.5) | |
| Female | 40 (25.2) | 48 (39.3) | 8 (28.6) | 10 (38.5) | |
| Diagnosis, n (%) | |||||
| PD | 117 (73.6) | 88 (72.1) | 13 (46.4) | 18 (69.2) | |
| ET | 42 (26.4) | 34 (27.9) | 15 (53.6) | 8 (30.8) | |
| Stimulator settings (mean ± SD) | |||||
| Voltage, V | 3.6 ± 0.1 | 3.4 ± 0.1 | 0.041 | 3.2 ± 0.1 | 3.5 ± 0.2 |
| Pulse width, ms | 100.2 ± 2.5 | 100.6 ± 3.3 | 0.927 | 126.8 ± 8.0 | 112.8 ± 7.0 |
| Frequency, H | 170.1 ± 1.3 | 173.4 ± 1.3 | 0.090 | 181.8 ± 1.8 | 177.1 ± 4.4 |
| Power (mean ± SD) | 62.3 ± 2.3 | 61.8 ± 2.7 | 0.882 | 75.0 ± 5.6 | 69.2 ± 5.4 |
| Low | 29.6 ± 1.0 | 29.8 ± 1.0 | 0.938 | ||
| Voltage | 3.0 ± 0.1 | 2.8 ± 0.1 | 0.455 | ||
| Pulse width | 65.0 ± 2.1 | 65.4 ± 2.5 | 0.896 | ||
| Frequency | 156.6 ± 2.8 | 164.9 ± 2.8 | 0.040 | ||
| Moderate | 52.6 ± 1.1 | 54.0 ± 1.4 | 0.444 | ||
| Voltage | 3.5 ± 0.1 | 3.5 ± 0.1 | 0.835 | ||
| Pulse width | 90.5 ± 2.1 | 91.0 ± 2.2 | 0.866 | ||
| Frequency | 168.4 ± 2.1 | 172.0 ± 2.1 | 0.237 | ||
| High | 92.7 ± 3.3 | 101.4 ± 3.4 | 0.076 | ||
| Voltage | 4.0 ± 0.1 | 3.8 ± 0.1 | 0.300 | ||
| Pulse width | 131.8 ± 4.3 | 145.7 ± 4.9 | 0.037 | ||
| Frequency | 179.1 ± 1.5 | 182.8 ± 1.1 | 0.069 | ||
SD, standard deviation.
Figure 1.
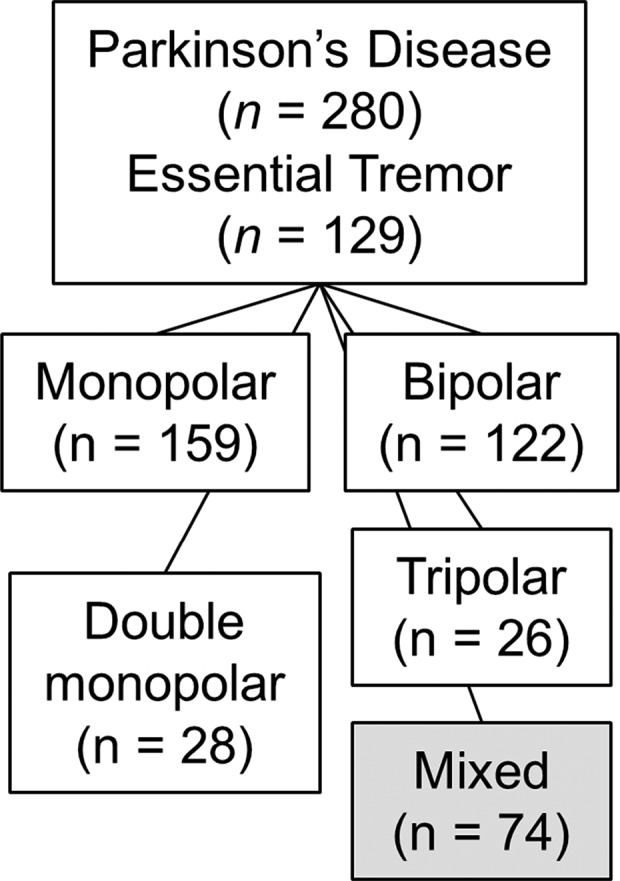
Flow chart illustrating patient classification into different groups based upon stimulation mode.
Bipolar stimulation mode was associated with greater battery longevity than monopolar stimulation (56.1 ± 3.4 vs. 44.2 ± 2.1 months; P = 0.006; Fig. 2A), and bipolar DBS had the most pronounced effects on battery longevity when other stimulation parameters (voltage, pulse width, and frequency) were in the low‐to‐moderate range (77.3 ± 4.9 vs. 61.7 ± 4.0 months, 60.6 ± 4.6 vs. 46.0 ± 2.5, and 28.3 ± 1.7 vs. 29.2 ± 2.1 at low‐, moderate‐, and high‐stimulation intensities, respectively; Fig. 2B; Table 2).
Figure 2.
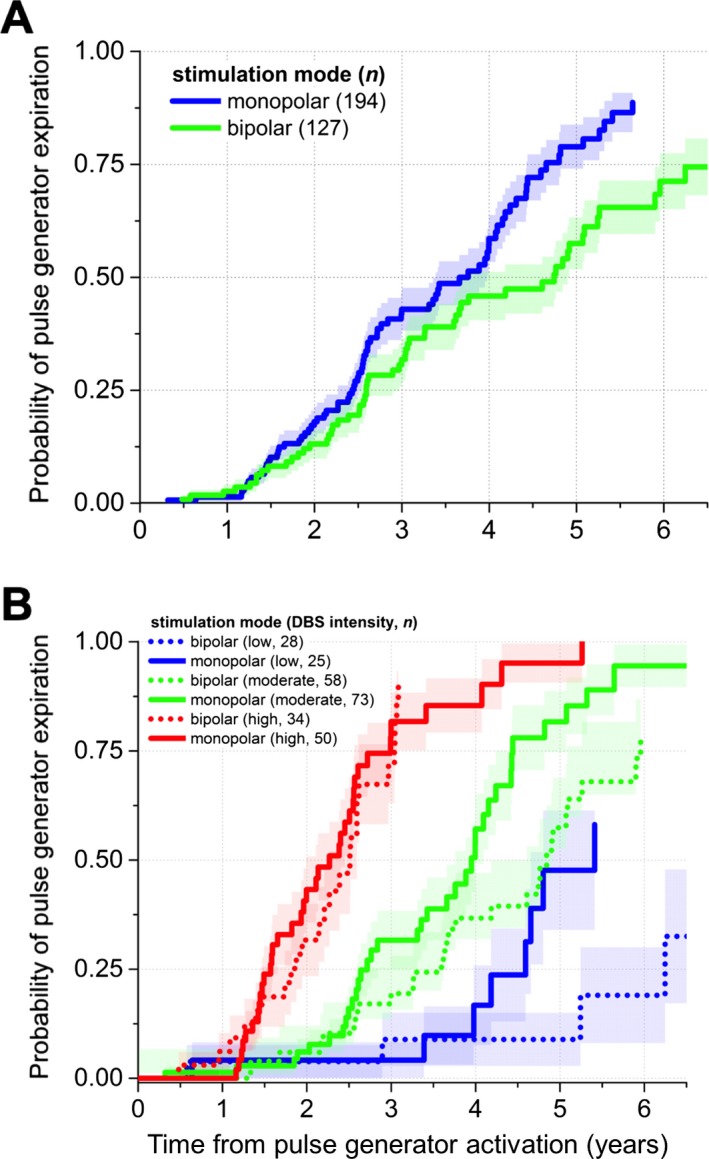
Bipolar stimulation is associated with greater pulse generator longevity than monopolar stimulation in patients with DBS for movement disorders. (A) Primary outcome comparing bipolar to monopolar stimulation in patients with subthalamic DBS for PD and thalamic stimulation for ET. Bipolar DBS is associated with approximately 1 year of additional battery longevity versus monopolar stimulation. (B) Subgroup analyses across different average stimulation intensities show that the most substantial effects of bipolar mode on Soletra (Medtronic, Inc., Minneapolis, MN) battery longevity occur at low‐to‐moderate overall stimulation intensities.
Table 2.
Longevity in months by intensity of stimulation
| Intensity (×1000)a | Longevity, Months (Mean ± SD) | P Value | |
|---|---|---|---|
| Monopolar | Bipolar | ||
| <45 | 41.7 ± 2.5 | 49.1 ± 4.2 | 0.123 |
| 45–80 | 32.8 ± 1.5 | 37.7 ± 2.5 | 0.072 |
| >80 | 21.4 ± 1.2 | 24.8 ± 1.7 | 0.100 |
Intensity calculated multiplying voltage × pulse width ×frequency.
SD, standard deviation.
We further investigated whether the linear distance between the anode and cathode contacts during bipolar stimulation related to battery longevity (i.e., bipolar electrode contacts separated by 3, 6, or 9 mm). We found that the median IPG longevities, based upon distance between anode and cathode contacts, were 44.1 [31.1, 62.6], 58.1 [43.4, 60.9], and 86.6 [70.8, 104.6] months for distances of 3, 6, and 9 mm, respectively (median and 95% confidence interval; P = 0.02, log‐rank test; Fig. 3). Double monopolar stimulation was associated with markedly shorter battery longevity versus both conventional DBS settings (bipolar and monopolar) and tripolar stimulation (37.8 ± 5.6 vs. 49.7 ± 1.9 and 51.2 ± 6.0 months, respectively; P = 0.014; Fig. 4).
Figure 3.
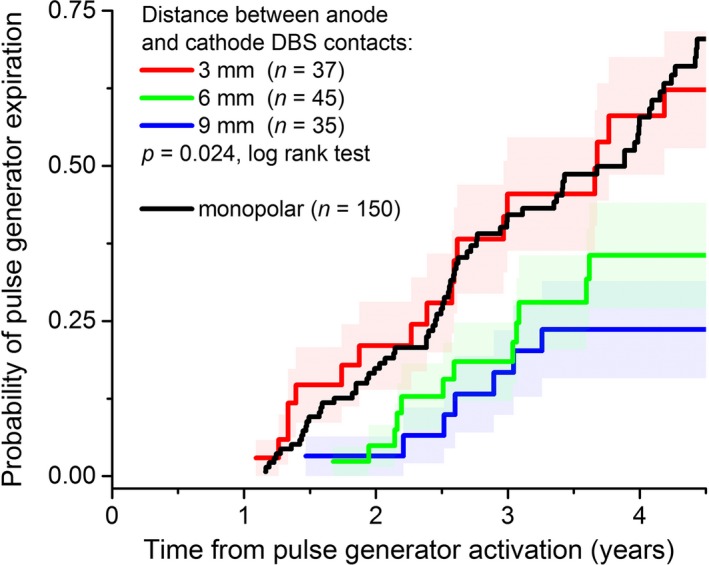
The distance between the anode and cathode contacts during bipolar stimulation alters pulse generator longevity. More widely spaced anode and cathode contacts during bipolar stimulation are associated with greater battery longevity versus either more narrowly spaced contacts or monopolar DBS. Importantly, these results arise from the use of only the Medtronic 3387 lead (Medtronic, Inc., Minneapolis, MN), with adjacent contacts separated by 1.5 mm.
Figure 4.
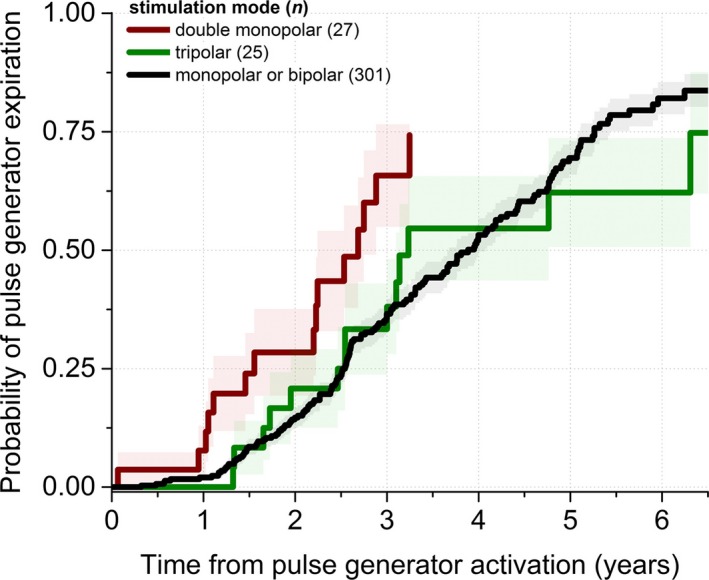
Double monopolar stimulation is associated with less battery longevity than conventional stimulation modes in patients with DBS for movement disorders. Double monopolar mode reduces battery longevity by approximately 1 year relative to conventional modes (monopolar and bipolar) and tripolar stimulation.
Regardless of stimulation mode, unilateral subthalamic DBS significantly improved motor function in a subset of 83 consecutive advanced PD patients from this sample (P < 0.001 for 5 of 5 measures of motor function; Fig. 5). Outpatient stimulator adjustment to optimal clinical benefit yielded 34 bipolar and 34 monopolar patients. At our level of power, we did not detect a significant effect or even a consistent trend favoring bipolar versus monopolar mode in four of the five motor outcomes (P > 0.4 for each), except that the number of steps walked in 1 minute with unilateral DBS at 6 months versus preoperative baseline favored bipolar stimulation at borderline significance (P = 0.04).
Figure 5.
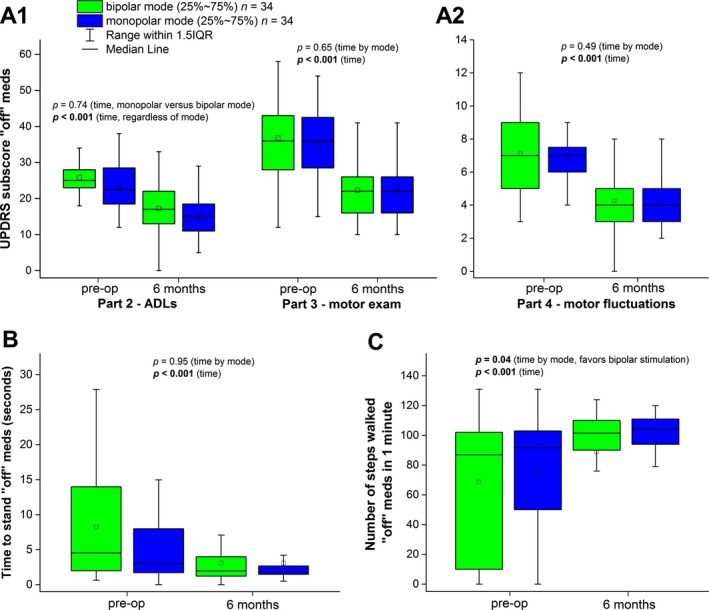
Clinical outcomes do not differ significantly between bipolar and monopolar DBS in a sample of consecutive patients who underwent unilateral subthalamic stimulation for PD. There was no statistically significant difference in postoperative outcome between monopolar and bipolar stimulation modes measured by the UPDRS Parts 2 and 3 off medications, Part 4, and the time to stand from the seated position. There was a borderline significant improvement in the number of steps walked comfortably favoring bipolar over monopolar stimulation (P = 0.04). Data presented as means ± standard deviation. IQR, interquartile range; ADLs, activities of daily living.
All patients were stimulated with a constant voltage DBS device. The tissue impedance at a given stimulation configuration contributes to the amount of current that is delivered by DBS over time. In a random cross‐section of 267 IPGs, the mean electrode impedance was greater during bipolar versus monopolar stimulation upon routine device interrogation (1,620.9 ± 394.2 vs. 1,198.2 ± 346.1 Ohm, respectively; P < 0.001, paired t test). Subdividing bipolar electrode pairings by the distance between the anode and cathode contacts showed mean impedances of 1,445.2 ± 413.8, 1,671.5 ± 371.7, and 1,753.6 ± 351.3 (at distances of 3, 6 and 9 mm, respectively).
Discussion
Our practice is to tailor stimulator settings to individually optimize efficacy, tolerability, and pulse generator longevity in patients who undergo DBS therapy. In this large, retrospective sample of 200 consecutive ET and PD patients, we found that bipolar stimulation with the 3387 Medtronic lead was associated with approximately 1 additional year of battery longevity versus monopolar stimulation. Furthermore, we found no significant differences in efficacy at 6 months follow‐up between monopolar and bipolar DBS across a battery of motor tests in a subset of 83 consecutive PD patients. Given that an individual patient experiences equivalent (or greater) efficacy or tolerability with bipolar versus monopolar stimulation, our results suggest that bipolar DBS would likely be associated with fewer battery replacement surgeries and lower cost over time. These findings are particularly important for patients who require DBS earlier in life and are expected to have a normal life span, in whom the implantation of nonrechargeable devices would result in numerous IPG replacement surgeries over time. Although we evaluated a large, homogeneous sample of patients with ET and PD to isolate the effects of stimulation mode on battery longevity, our findings may represent a common property of DBS devices, regardless of the indication for surgery.
Consistent with our results, Ondo et al.7 evaluated predictors for battery longevity in 61 DBS patients with PD, ET, dystonia, and cerebellar outflow tremor and found that bipolar stimulation was associated with greater battery life when compared to pooled results from all other stimulation modes (monopolar, double monopolar, and tripolar modes). Likewise, Blahak et al.11 evaluated DBS settings and battery longevity in 20 consecutive dystonia patients and found a mean IPG life of 25 months with mean bipolar stimulation parameters of 4.2 V, 210 μs, and 133 Hz. They observed a trend favoring improved battery life with bipolar stimulation at these high stimulation intensities and suggested that the total electrical energy delivered should be divided by 1.5 to more accurately represent energy delivery during bipolar stimulation.
Interestingly, the battery longevity advantage of bipolar over monopolar DBS in our study was largely driven by patients who (1) required low‐to‐moderate stimulation intensities for therapy (Fig. 2B) and (2) received chronic stimulation from more widely spaced anode and cathode contacts on the DBS electrode array (Fig. 3). This latter finding suggests that in addition to covering larger tissue volumes, more widely spaced DBS electrode contacts might also provide greater flexibility for programming strategies to optimize battery consumption. Although bipolar DBS is associated with higher impedances than monopolar DBS, the highest impedance within a given patient is almost always obtained from the most widely spaced anode‐cathode pair, presumably because of the larger tissue volume separating the contacts. This suggests that at least a portion of the battery longevity advantage of bipolar versus monopolar DBS may relate to the delivery of less current from the constant voltage device in stimulation modes with higher tissue impedances. Competing with this notion is the idea that activation of larger tissue volumes might confer greater efficacy at lower stimulation intensities within a given patient. Prospective studies with complementary modeling approaches using the VTA or other similar methods are required to better understand the relative contributions and the clinical importance of these potential phenomena.6, 12, 13, 14
Unconventional stimulation modes (double monopolar, tripolar) occasionally provide greater clinical improvement than monopolar or bipolar stimulation; however, they typically deplete the battery more rapidly because multiple cathode contacts deliver the stimulus. As expected, double monopolar stimulation decreased battery longevity by approximately 1 year versus conventional monopolar and bipolar stimulation. This agrees with findings from a previous study of 40 patients12 including 92% of the leads in monopolar mode and 8% in double monopolar lead, where the mean longevity was 83 months for all patients, but only 67 months for patients in double monopolar mode. Interestingly, tripolar stimulation had less impact on IPG longevity than double monopolar stimulation in the present study. Because of its energy inefficiency, our findings underscore that double monopolar stimulation in particular should be reserved for patients who respond specifically to this stimulation mode versus conventional stimulation configurations. With the development of new hardware, stimulation paradigms, and rechargeable devices, the concerns regarding the utilization of more inefficient stimulation modes and battery consumption might be diminished to some extent; however, rechargeable devices are currently limited by cost and the impact of recharging the device on the lifestyle of individ‐ual patients. These considerations will continue to impact the selection of hardware and programming parameters and should be the object of further studies.
This study has a number of strengths. First, we evaluate a larger sample of patients over a longer follow‐up interval than previous studies. Second, by specifically evaluating battery longevity in ET and PD patients, we control for potential disease‐ and target‐specific effects that have been shown to alter battery longevity.4 Third, the Soletra device does not allow patients to adjust their DBS settings at home; therefore, estimates of stimulator settings over time were based upon data from clinic visits rather than with battery estimators or other similar techniques. Given the enhanced capabilities for patient control with newer DBS devices, future battery longevity studies will be more technically challenging because of the potential for day‐to‐day changes in stimulation parameters in individuals.
This study has some potential limitations as well. In clinical practice, the choice of bipolar versus monopolar stimulation mode was always based on programming the device to maximize clinical efficacy in individual patients, without previous hypotheses about battery longevity. Although we found similar clinical improvement from monopolar and bipolar DBS in a subset of 83 consecutive PD patients, these patients were not randomized by stimulation mode. This emphasizes the need for prospective studies comparing these and other emerging strategies to shape the DBS electrical field to optimize battery longevity, clinical efficacy, and potential stimulation side effects, such as speech, gait, and cognitive dysfunction.13, 14, 15, 16, 17 Furthermore, more direct comparisons of monopolar and bipolar stimulation mode should be evaluated for both short‐ and longer‐term efficacy and tolerability. Finally, although our findings likely represent general properties of DBS devices, newer devices, stimulation modes, and electrode configurations might not show identical results.
In conclusion, we found that bipolar stimulation was associated with approximately 1 year of greater battery longevity versus monopolar stimulation in a large cohort of DBS patients with ET and PD. This effect was most pronounced in patients with low‐to‐moderate stimulation intensities and with more widely spaced anode and cathode DBS contacts. Because of its retrospective, nonrandomized design, our study does not indicate that bipolar stimulation should be preferred globally over monopolar stimulation in patients with PD and ET. Rather, if it provides identical (or better) efficacy and/or tolerability versus monopolar stimulation at otherwise similar settings, bipolar DBS will likely improve battery longevity. Emerging brain stimulation technologies will allow current steering and improvements in software and battery performance. In addition, the emergence of constant current, rather than more widely used constant voltage mode, is likely to have impact on battery longevity as well. Our results emphasize the need for prospective studies to directly compare DBS programming strategies with the potential to optimize the efficacy, tolerability, surgical morbidity, and cost associated with this intervention that can dramatically alter health‐related quality of life.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
L.A.: 1A, 1B, 1C, 3A, 3B
P.V.R.: 2C, 3B
B.D.: 1C
B.L.S.: 1B, 1C
H.H.: 1C
M.S.O.: 3A, 3B
B.L.G.: 3B
H.C.W.: 1A, 1B, 2A, 2B, 2C, 3A, 3B
Disclosures
Funding Sources and Conflicts of Interest: This work was supported by the National Institutes of Health/National Institutes of Neurological Disorders and Stroke (to H.C.W.) and the Bachmann Strauss Dystonia and Parkinson's Foundation (to H.C.W.). The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: H.C.W. receives grants from the National Institutes of Health (NIH)/National Institutes of Neurological Disorders and Stroke (K23‐NS067053), the Bachmann Strauss Dystonia and Parkinson's Foundation, and research funding from Medtronic Neuromodulation. M.S.O. serves as a consultant for the National Parkinson Foundation (NPF) and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkin‐son Alliance, Smallwood Foundation, the Bachmann‐Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Schuepbach WMM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med 2013;368:610–622. [DOI] [PubMed] [Google Scholar]
- 2. Kupsch A, Benecke R, Müller J, et al. Pallidal deep‐brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006;355:1978–1990. [DOI] [PubMed] [Google Scholar]
- 3. Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 2000;342:461–468. [DOI] [PubMed] [Google Scholar]
- 4. Rawal PV, Almeida L, Smelser LB, Huang H, Guthrie BL, Walker HC. Shorter pulse generator longevity and more frequent stimulator adjustments with pallidal DBS for dystonia versus other movement disorders. Brain Stimul 2014;7:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. twb .25wFakhar K, Hastings E, Butson CR, Foote KD, Zeilman P, Okun MS. Management of deep brain stimulator battery failure: battery estimators, charge density, and importance of clinical symptoms. PLoS One 2013;8:e58665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaturvedi A, Luján JL, McIntyre CC. Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. J Neural Eng 2013;10:056023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ondo WG, Meilak C, Vuong KD. Predictors of battery life for the Activa Soletra 7426 Neurostimulator. Parkinsonism Relat Disord 2007;13:240–242. [DOI] [PubMed] [Google Scholar]
- 8. Krack P, Fraix V, Mendes A, Benabid A‐L, Pollak P. Postoperative management of subthalamic nucleus stimulation for Parkinson's disease. Mov Disord 2002;17(Suppl 3):S188–S197. [DOI] [PubMed] [Google Scholar]
- 9. Walker HC, Lyerly M, Cutter G, et al. Weight changes associated with unilateral STN DBS and advanced PD. Parkinsonism Relat Disord 2009;15:709–711. [DOI] [PubMed] [Google Scholar]
- 10. Amara AW, Standaert DG, Guthrie S, Cutter G, Watts RL, Walker HC. Unilateral subthalamic nucleus deep brain stimulation improves sleep quality in Parkinson's disease. Parkinsonism Relat Disord 2012;18:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blahak C, Capelle H‐H, Baezner H, Kinfe TM, Hennerici MG, Krauss JK. Battery lifetime in pallidal deep brain stimulation for dystonia. Eur J Neurol 2011;18:872–875. [DOI] [PubMed] [Google Scholar]
- 12. Anheim M, Fraix V, Chabardès S, Krack P, Benabid A‐L, Pollak P. Lifetime of Itrel II pulse generators for subthalamic nucleus stimulation in Parkinson's disease. Mov Disord 2007;22:2436–2439. [DOI] [PubMed] [Google Scholar]
- 13. Butson CR, McIntyre CC. Current steering to control the volume of tissue activated during deep brain stimulation. Brain Stimul 2008;1:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep‐brain stimulation for Parkinson's disease. N Engl J Med 2010;362:2077–2091. [DOI] [PubMed] [Google Scholar]
- 15. Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty‐six‐month outcomes. Neurology 2012;79:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Odekerken VJJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 2013;12:37–44. [DOI] [PubMed] [Google Scholar]
- 17. Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol 2009;65:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]


