Abstract
Many protein families are common to all cellular organisms, indicating that many genes have ancient origins. Genetic variation is mostly attributed to processes such as mutation, duplication, and rearrangement of ancient modules. Thus it is widely assumed that much of present-day genetic diversity can be traced by common ancestry to a molecular "big bang." A rarely considered alternative is that proteins may arise continuously de novo. One mechanism of generating different coding sequences is by "overprinting," in which an existing nucleotide sequence is translated de novo in a different reading frame or from noncoding open reading frames. The clearest evidence for overprinting is provided when the original gene function is retained, as in overlapping genes. Analysis of their phylogenies indicates which are the original genes and which are their informationally novel partners. We report here the phylogenetic relationships of overlapping coding sequences from steroid-related receptor genes and from tymovirus, luteovirus, and lentivirus genomes. For each pair of overlapping coding sequences, one is confined to a single lineage, whereas the other is more widespread. This suggests that the phylogenetically restricted coding sequence arose only in the progenitor of that lineage by translating an out-of-frame sequence to yield the new polypeptide. The production of novel exons by alternative splicing in thyroid receptor and lentivirus genes suggests that introns can be a valuable evolutionary source for overprinting. New genes and their products may drive major evolutionary changes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Bond C. T., Douglass J., Herbert E. Two mammalian genes transcribed from opposite strands of the same DNA locus. Science. 1987 Mar 20;235(4795):1514–1517. doi: 10.1126/science.3547652. [DOI] [PubMed] [Google Scholar]
- Argos P. Analysis of sequence-similar pentapeptides in unrelated protein tertiary structures. Strategies for protein folding and a guide for site-directed mutagenesis. J Mol Biol. 1987 Sep 20;197(2):331–348. doi: 10.1016/0022-2836(87)90127-6. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Barany F., Slatko B., Danzitz M., Cowburn D., Schildkraut I., Wilson G. G. The corrected nucleotide sequences of the TaqI restriction and modification enzymes reveal a thirteen-codon overlap. Gene. 1992 Mar 1;112(1):91–95. doi: 10.1016/0378-1119(92)90307-b. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Air G. M., Hutchison C. A., 3rd Overlapping genes in bacteriophage phiX174. Nature. 1976 Nov 4;264(5581):34–41. doi: 10.1038/264034a0. [DOI] [PubMed] [Google Scholar]
- Beremand M. N., Blumenthal T. Overlapping genes in RNA phage: a new protein implicated in lysis. Cell. 1979 Oct;18(2):257–266. doi: 10.1016/0092-8674(79)90045-x. [DOI] [PubMed] [Google Scholar]
- Biebricher C. K., Eigen M., Luce R. Template-free RNA synthesis by Q beta replicase. Nature. 1986 May 1;321(6065):89–91. doi: 10.1038/321089a0. [DOI] [PubMed] [Google Scholar]
- Bozarth C. S., Weiland J. J., Dreher T. W. Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology. 1992 Mar;187(1):124–130. doi: 10.1016/0042-6822(92)90301-5. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q Rev Biol. 1971 Jun;46(2):111–138. doi: 10.1086/406830. [DOI] [PubMed] [Google Scholar]
- Casino A., Cipollaro M., Guerrini A. M., Mastrocinque G., Spena A., Scarlato V. Coding capacity of complementary DNA strands. Nucleic Acids Res. 1981 Mar 25;9(6):1499–1518. doi: 10.1093/nar/9.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. A., Terwilliger E. F., Sodroski J. G., Haseltine W. A. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988 Aug 11;334(6182):532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of human immunodeficiency virus replication. Annu Rev Microbiol. 1991;45:219–250. doi: 10.1146/annurev.mi.45.100191.001251. [DOI] [PubMed] [Google Scholar]
- Curran J., Boeck R., Kolakofsky D. The Sendai virus P gene expresses both an essential protein and an inhibitor of RNA synthesis by shuffling modules via mRNA editing. EMBO J. 1991 Oct;10(10):3079–3085. doi: 10.1002/j.1460-2075.1991.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
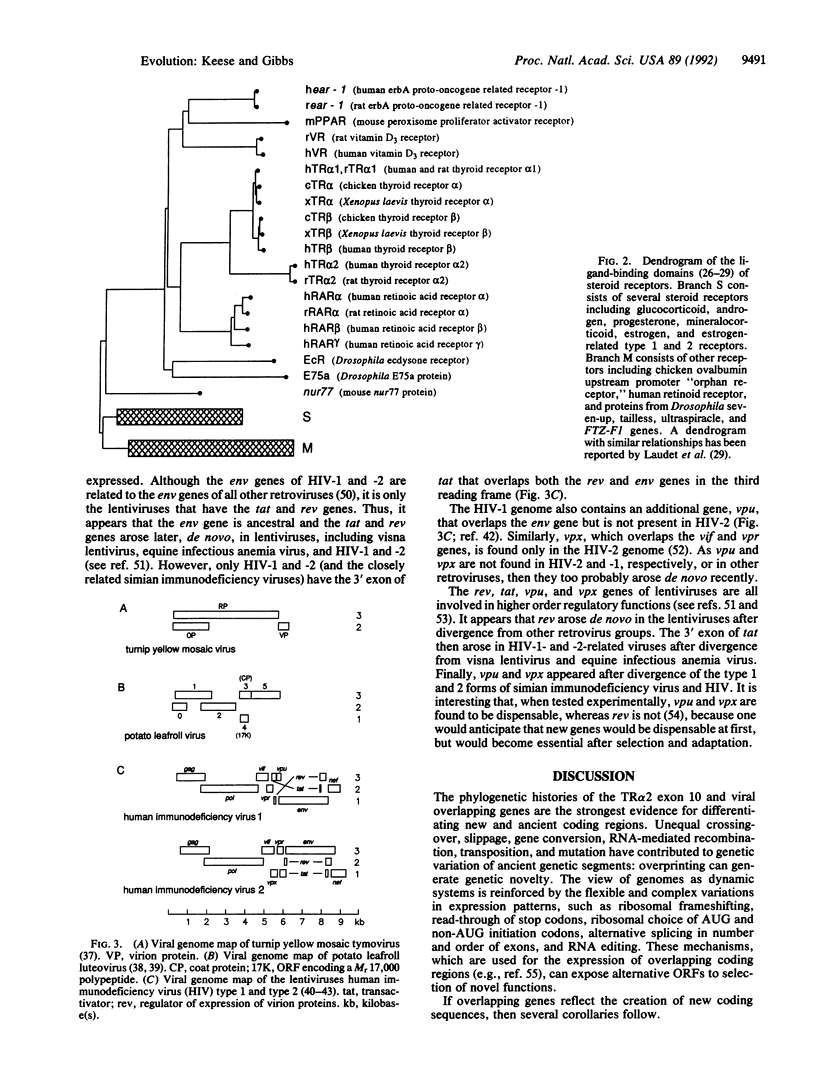
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley I. M., Walker J. E. Two overlapping genes in bovine mitochondrial DNA encode membrane components of ATP synthase. EMBO J. 1986 Aug;5(8):2003–2008. doi: 10.1002/j.1460-2075.1986.tb04456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Forrest D., Sjöberg M., Vennström B. Contrasting developmental and tissue-specific expression of alpha and beta thyroid hormone receptor genes. EMBO J. 1990 May;9(5):1519–1528. doi: 10.1002/j.1460-2075.1990.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R., Wong-Staal F., Montagnier L., Haseltine W. A., Yoshida M. HIV/HTLV gene nomenclature. Nature. 1988 Jun 9;333(6173):504–504. doi: 10.1038/333504a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hu W., Vander Heyden N., Ratner L. Analysis of the function of viral protein X (VPX) of HIV-2. Virology. 1989 Dec;173(2):624–630. doi: 10.1016/0042-6822(89)90574-6. [DOI] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990 Oct 18;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Jankowski J. M., Krawetz S. A., Walczyk E., Dixon G. H. In vitro expression of two proteins from overlapping reading frames in a eukaryotic DNA sequence. J Mol Evol. 1986;24(1-2):61–71. doi: 10.1007/BF02099952. [DOI] [PubMed] [Google Scholar]
- Kavaler J., Davis M. M., Chien Y. Localization of a T-cell receptor diversity-region element. Nature. 1984 Aug 2;310(5976):421–423. doi: 10.1038/310421a0. [DOI] [PubMed] [Google Scholar]
- Keese P., Mackenzie A., Gibbs A. Nucleotide sequence of the genome of an Australian isolate of turnip yellow mosaic tymovirus. Virology. 1989 Oct;172(2):536–546. doi: 10.1016/0042-6822(89)90196-7. [DOI] [PubMed] [Google Scholar]
- Keese P., Martin R. R., Kawchuk L. M., Waterhouse P. M., Gerlach W. L. Nucleotide sequences of an Australian and a Canadian isolate of potato leafroll luteovirus and their relationships with two European isolates. J Gen Virol. 1990 Mar;71(Pt 3):719–724. doi: 10.1099/0022-1317-71-3-719. [DOI] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kurosky A., Barnett D. R., Lee T. H., Touchstone B., Hay R. E., Arnott M. S., Bowman B. H., Fitch W. M. Covalent structure of human haptoglobin: a serine protease homolog. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3388–3392. doi: 10.1073/pnas.77.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
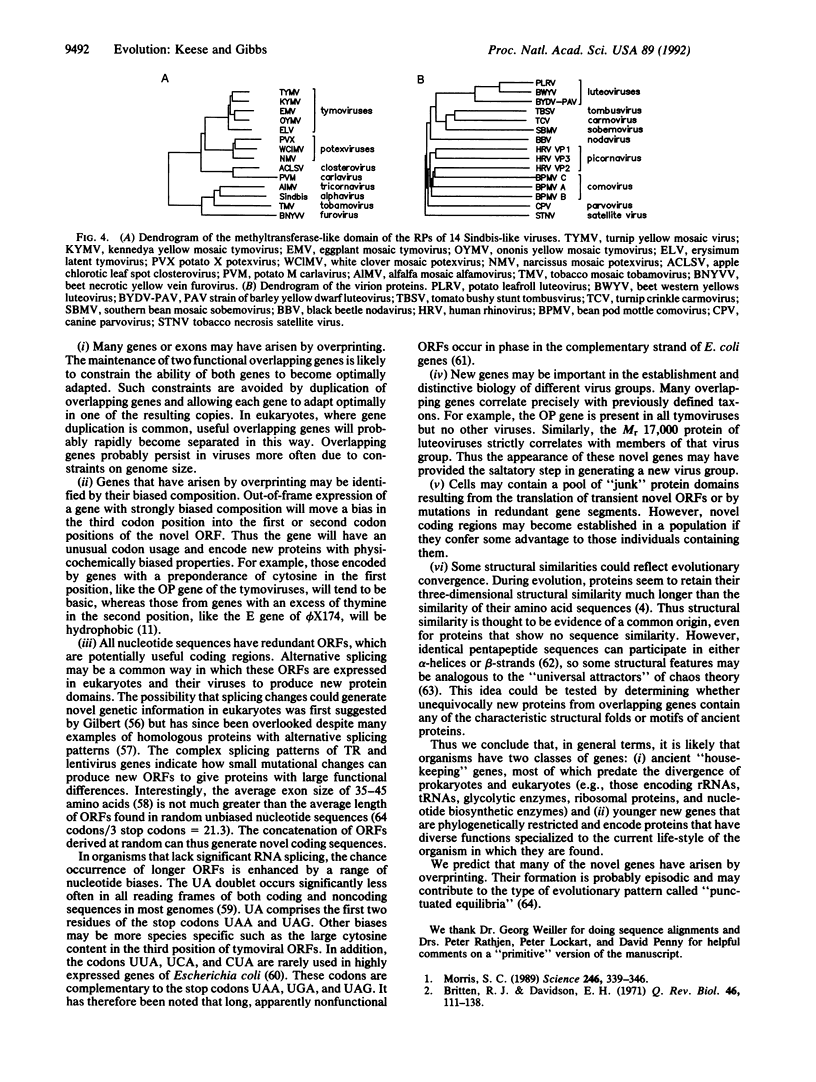
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989 Mar;9(3):1128–1136. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. W. Corporate medicine for profit. Sci Am. 1986 Dec;255(6):38–45. doi: 10.1038/scientificamerican1286-38. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Böhnlein S., Hauber J., Cullen B. R. Functional dissection of the HIV-1 Rev trans-activator--derivation of a trans-dominant repressor of Rev function. Cell. 1989 Jul 14;58(1):205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Rossmann M. G. Comparison of protein structures. Methods Enzymol. 1985;115:397–420. doi: 10.1016/0076-6879(85)15029-9. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Johnson M. S., Feng D. F., Doolittle R. F. Sequence comparisons of retroviral proteins: relative rates of change and general phylogeny. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2469–2473. doi: 10.1073/pnas.85.8.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima N., Horiuchi R., Shibuya Y., Fukushige S., Matsubara K., Toyoshima K., Yamamoto T. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell. 1989 Apr 7;57(1):31–39. doi: 10.1016/0092-8674(89)90169-4. [DOI] [PubMed] [Google Scholar]
- Morris S. C. Burgess shale faunas and the cambrian explosion. Science. 1989 Oct 20;246(4928):339–346. doi: 10.1126/science.246.4928.339. [DOI] [PubMed] [Google Scholar]
- Nussinov R. Doublet frequencies in evolutionary distinct groups. Nucleic Acids Res. 1984 Feb 10;12(3):1749–1763. doi: 10.1093/nar/12.3.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Birth of a unique enzyme from an alternative reading frame of the preexisted, internally repetitious coding sequence. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2421–2425. doi: 10.1073/pnas.81.8.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak B., Lusky M., Hable M. Expression of two proteins from overlapping and oppositely oriented genes on transposable DNA insertion element IS5. Nature. 1982 May 13;297(5862):124–128. doi: 10.1038/297124a0. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Johnson J. E. Icosahedral RNA virus structure. Annu Rev Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Polycistronic animal virus mRNAs. Prog Nucleic Acid Res Mol Biol. 1989;37:127–153. doi: 10.1016/S0079-6603(08)60697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Benko D. M., Fenyö E. M., Pavlakis G. N. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves W. A. Something old, some things new: the steroid receptor superfamily in Drosophila. Cell. 1991 Oct 18;67(2):225–228. doi: 10.1016/0092-8674(91)90172-u. [DOI] [PubMed] [Google Scholar]
- Sharp P. M. Does the 'non-coding' strand code? Nucleic Acids Res. 1985 Feb 25;13(4):1389–1397. doi: 10.1093/nar/13.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. C., Walker J. E., Northrop F. D., Barrell B. G., Godson G. N., Fiddes J. C. Gene K, a new overlapping gene in bacteriophage G4. Nature. 1978 Apr 6;272(5653):510–515. doi: 10.1038/272510a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Thisted T., Gerdes K. Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok antisense RNA regulates hok gene expression indirectly through the overlapping mok gene. J Mol Biol. 1992 Jan 5;223(1):41–54. doi: 10.1016/0022-2836(92)90714-u. [DOI] [PubMed] [Google Scholar]
- Thomas C. M., Smith C. A. The trfB region of broad host range plasmid RK2: the nucleotide sequence reveals incC and key regulatory gene trfB/korA/korD as overlapping genes. Nucleic Acids Res. 1986 Jun 11;14(11):4453–4469. doi: 10.1093/nar/14.11.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T. W. Do exons code for structural or functional units in proteins? Proc Natl Acad Sci U S A. 1988 May;85(9):2944–2948. doi: 10.1073/pnas.85.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav Y. N., Wong-Staal F. The biochemistry of AIDS. Annu Rev Biochem. 1991;60:577–630. doi: 10.1146/annurev.bi.60.070191.003045. [DOI] [PubMed] [Google Scholar]
- Vellard M., Soret J., Sureau A., Perbal B. A novel type of RNA-binding protein is potentially encoded by the opposite strand of the trans-spliced c-myb coding exon. C R Acad Sci III. 1991;313(13):591–597. [PubMed] [Google Scholar]
- Yaoita Y., Shi Y. B., Brown D. D. Xenopus laevis alpha and beta thyroid hormone receptors. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7090–7094. doi: 10.1073/pnas.87.18.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]



