Abstract
Glycan arrays have become a technique of choice to screen glycan-protein interactions in a high-throughput manner with high sensitivity and low sample consumption. Here, the synthesis of a new multifunctional fluorescent linker for glycan labelling via aminoxy ligation and immobilization is described; the linker features a fluorescent naphthalene group suitable for highly sensitive HPLC-based purification and an azido- or amino-modified pentanoyl moiety for the immobilization onto solid supports. Several glycoconjugates displaying small sugar epitopes via chemical or chemoenzymatic synthesis were covalently attached onto a microarray support and tested with lectins of known carbohydrate binding specificity. The glycan library was extended using glycosyltransferases (e.g. galactosyl-, sialyl- and fucosyltransferases); the resulting neoglycoconjugates, which are easily detected by mass spectrometry, mimic antennal elements of N- and O-glycans, including ABH blood group epitopes and sialylated structures. Furthermore, an example natural plant N-glycan containing core α1,3-fucose and β1,2-xylose was also successfully conjugated to the fluorescent linker, immobilised and probed with lectins as well as anti-horseradish peroxidase. These experiments validate our linker as being a potentially valuable tool to study glycozyme and lectin specificities, sensitive enough to allow purification of natural glycans.
Keywords: Chemo-enzymatic synthesis, fluorescent labelling, glycan arrays, natural glycans, N-glycoside
Introduction
All biological processes involve recognition either of ligands or substrates, regardless of whether the ‘partners’ are nucleic acids, proteins, lipids, glycans or smaller molecules. High throughput analyses have become established as a means to identify recognition partners, but, while nucleic acid and protein/polypeptide arrays have become routine for mass production, glycan array methodologies are still in their infancy; this is partially due to the inherent difficulties of oligosaccharide chemistry and the branched, non-template-driven nature of oligosaccharide structures and biosynthesis. Nevertheless, over the years a number of platforms, which use a variety of approaches to immobilize glycans, have been reported (Park, et al. 2013, Rillahan and Paulson 2011, Song, et al. 2014). Especially the covalently printed arrays of the Consortium for Functional Glycomics have been highly valuable in identifying potential glycan partners for a range of lectins and antibodies (Blixt, et al. 2004, Smith, et al. 2010); in the most current version of this glycan array, 609 mammalian and 153 pathogen glycan determinants are displayed. These glycan arrays present well-defined glycan structures that have been prepared by means of chemical or chemo-enzymatic approaches; insights into recognition of glycans by, e.g., plant lectins, mammalian galectins, influenza haemagglutinins and C-type lectins of the immune system (Smith, et al. 2010, Taylor and Drickamer 2009, Walther, et al. 2013) have resulted from using glycan arrays. Nevertheless, there are still many biologically-relevant glycans found in nature that are not represented in these glycan libraries; for instance, although blood group glycans on the Consortium array were found to be ligands for an oyster galectin (Feng, et al. 2013), the sulphated forms of these epitopes (as present in the oyster itself) were absent and so the positive or negative effect of the anionic modification on binding could not be studied. Therefore, as part of strategies to extend the number of glycans available on arrays, there is increasing interest in developing fluorescent tags that can be employed for purification, analysis and immobilization of oligosaccharides derived from natural sources or from enzymatic reactions.
For the elucidation of the glycome of a living organism, glycans are first released from glycoproteins or glycolipids using enzymatic or chemical methods; a common option is to then derivatise the glycans with a fluorescent tag and, purify the products by HPLC and characterize these by mass spectrometry (Ruhaak, et al. 2010). 2-aminobenzamide (2-AB) and 2-aminopyridine (2-AP) are still the most commonly used fluorescent tags for glycan labelling. This fluorescent derivatisation step proceeds in high yield via reductive amination and has been used to immobilize glycans on microarray supports (de Boer, et al. 2007, Song, et al. 2008). More recently, a novel 2-amino-N-(2-aminoethyl)-benzamide-based fluorescent tag (AEAB) was developed with higher immobilization efficiency and has been used for ‘shotgun glycomics’ in which the glycan partners for lectins are identified and then only ‘hit’ glycans are structurally characterized (Byrd-Leotis, et al. 2014). In all these cases (2-AB, 2-AP and AEAB), there is a reductive step, which results in loss of the biological “closed-ring” form at the reducing end of the glycan. Although this is not crucial for glycan analysis by HPLC or MS, the existence of an open-ring reduced form of a glycan can reduce or abolish its biological function (i.e., its capacity to bind), which is particularly relevant for short-chain glycans (Jiménez-Castells, et al. 2008, Liu, et al. 2007, Thygesen, et al. 2009, Wilson, et al. 1998). One approach to at least partially retain the cyclic nature of the reducing unit in glycan arrays is to prepare neoglycolipids via oxime ligation (Liu, et al. 2007). Although a portion of such conjugates is in the closed-ring form, the yield is not quantitative and remaining open-ring forms will complicate interpretation of binding results.
To overcome the limitations of many conjugation methods, Peri proposed the use of alkyl N,O-substituted hydroxylamines instead of regular hydroxylamine, which force the retention of the closed-ring form (Goff and Thorson 2014, Peri, et al. 1998). To date, a few glycan array platforms have been prepared using this N-substituted hydroxylamine (Bohorov, et al. 2006, Chen and Pohl 2008, Cló, et al. 2010) and a selection of the glycoconjugates on the Consortium array (those with spacer 21) were prepared with this method. Amongst these, only the linker developed by Cló with an UV-active aromatic chromophore allows purification and quantification of glycoconjugates by HPLC (Cló, et al. 2010). Another strategy to prepare glycan microarrays with a closed-ring structure at the reducing end was developed by Song et al. based on the AEAB linker. However, this strategy requires four reactions (i.e.: glycosylamine formation, reaction with acryloyl chloride, ozonolysis and reductive amination) to obtain the final fluorescent glycoconjugate (Song, et al. 2009b).
With the ultimate goal of producing fluorescent arrays with natural glycans solely in their closed-ring forms and in a low number of steps, we reappraised chemistry based on the use of N-substituted aminooxy linkers for glycan derivatization. Thereby, our goal was to develop a linker which (i) enables non-reductive reaction with non-protected saccharides, (ii) contains a fluorescent group for highly sensitive HPLC-based purification but with excitation and emission spectra different from those of labels used to detect lectin/antibody binding, (iii) features an azido- or amino-modified pentanoyl (C5) moiety for the immobilization onto solid supports via either alkyne-modified or N-hydroxysuccinimide (NHS)-activated surfaces, (iv) is easily detected by mass spectrometry and (v) is compatible with the use of glycosyltransferases. Indeed, all these characteristics were fulfilled; thereby, microarrays with glycans of different sizes (mono- to tetrasaccharides) were tested with plant lectins. We focused on mimicking selected aspects of mammalian glycosylation pathways especially N-acetyllactosamine and related antennal motifs (Cummings 2009); we also conjugated, immobilised and probed (with lectins and one antibody) a natural glycan derived from horseradish peroxidase (Yang, et al. 1996) to our bi-functional fluorescent linker in order to confirm its suitability for labelling and printing of natural glycans.
Results and Discussion
Chemical synthesis of fluorescent linkers
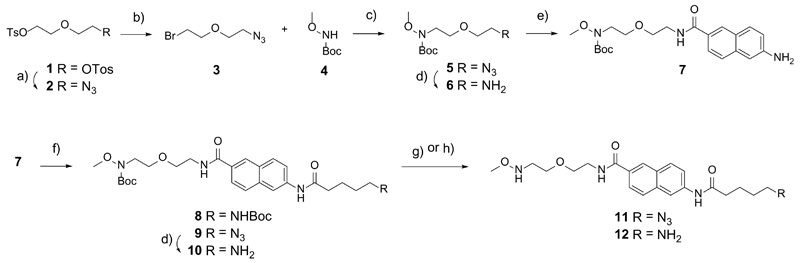
The linker design is based on three key elements: (i) a methoxylamino functionality for the chemoselective ligation with free reducing sugars and preservation of the closed-ring form, (ii) a fluorescent naphthalene ring to increase sensitivity during purification, and (iii) an azido- or amino-pentanoyl (C5) spacer for subsequent immobilization. The assembly of the fluorescent linker is shown in Scheme 1. The synthesis started with the commercially available diethylene glycol. First, the hydroxyl groups were tosylated to give compound 1 in high yield (90%). Then, 1 was treated with equimolar amounts of sodium azide to obtain the corresponding monoazide 2 in moderate yields (62%) with minimal formation of the diazido by-product (13%) and with recovery of the ditosylated starting material 1 (25%). Subsequently, the bromide group was introduced by treatment of 2 with lithium bromide, which gave compound 3 in 82% yield. After Boc-protection of methoxyamine (Kawase, et al. 1989), the resulting N-Boc-methoxyamine 4 was alkylated with compound 3 to deliver compound 5, which was subsequently reduced by catalytic hydrogenation to produce 6. Subsequently, the fluorescent moiety was introduced by amide bond formation between compound 6 and 6-amino-2-naphthoic acid to give compound 7 in 78% yield. Two linkers with different functionalization were prepared. The azido-functionalized linker was synthesized by amide bond formation with 5-azido-pentanoic acid in 77% yield. The same synthetic procedure was followed with Boc-5-aminopentanoic acid to prepare the amino-functionalized linker 8 in 92% yield. Eventually, cleavage of the Boc group delivered the azido-functionalized linker 11 and the amino-functionalized linker 12 in 92% and 94% yield, respectively. Hydrogenation of compound 9 before Boc deprotection gave the Boc-protected amino-functionalized linker 10 that is used afterwards for neoglycoconjugate quantification and as a reference surface on the glycan arrays.
Scheme 1. Synthesis of methoxy linkers.
Reagents and conditions: a) NaN3, DMF, 70 °C (62%); b) LiBr, DMF, 70 °C (82%); c) NaH, DMF (91%); d) H2, Pd/C 10% w/w, MeOH (74% for 6, 99% for 10); e) TBTU/HOBt/Et3N, THF/DMF (78%), f) HATU/DIPEA, DMF (92% for 8; 77% for 9); g) TFA, CH2CI2, rt (92%). h) 3 M HCl, dioxane (94%)
Chemoselective neoglycosylation with chemically synthesized glycans
Optimization of reaction conditions with azido-functionalized linker 11
To find the best conjugation condition for the chemoselective ligation, several mono- and disaccharides were conjugated under different reaction conditions (Table 1). For those small sugar epitopes, the difference in hydrophobicity between the azido-functionalized linker 11 and glycoconjugates 13-21 enabled their purif cation by silica gel column chromatography.
Table I. Neoglycosylation conditions and glycoside stereochemistry.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Linker [M] | Sugar [mM] | Solvent (v:v) | T (°C) | time (h) | Yield (%)a | Product | β-pyr:α-pyr:β-fur |
| 1 | 0.6 | GlcNAc (60) | DMF/AcOH 3:1 | 25 | 24 | 9 | 13, 22 | 100:0:0 |
| 2 | 0.6 | GlcNAc (60) | DMF/AcOH 3:1 | 60 | 24 | 24 | ||
| 3 | 0.6 | GlcNAc (60) | DMF/AcOH 50:1 | 60 | 24 | 21 | ||
| 4 | 1 | GlcNAc (100) | DMF/AcOH 3:1 | 40 | 72 | 64 | ||
| 5 | 1 | GlcNAc (100) | dioxane/aq buffer 1:1 | 40 | 72 | 93 | ||
| 6 | 1 | Gal (100) | dioxane/aq buffer 1:1 | 40 | 24 | 98 | 14, 23, 24 | 75:0:25 |
| 7 | 1 | Glc (100) | dioxane/aq buffer 1:1 | 40 | 24 | 97 | 15, 25 | 100:0:0 |
| 8 | 1 | Man (100) | dioxane/aq buffer 1:1 | 40 | 24 | 82 | 16, 26, 27 | 29:51:20b |
| 9 | 1 | GalNAc (100) | dioxane/aq buffer 1:1 | 40 | 24 | 98 | 17, 28, 29 | 50:0:50 |
| 10 | 1 | Fuc (100) | dioxane/aq buffer 1:1 | 40 | 24 | 98 | 18, 30 | 92:0:8 |
| 11 | 1 | Lac (100) | dioxane/aq buffer 1:1 | 40 | 24 | 80 | 19, 31 | 100:0:0 |
| 12 | 1 | LacNAc (100) | dioxane/aq buffer 1:1 | 40 | 72 | 71 | 20, 32 | 100:0:0 |
| 13 | 1 | GlcNAc2 (100) | dioxane/aq buffer 1:1 | 40 | 72 | 67 | 21, 33 | 100:0:0 |
Estimated by quantification of fluorescence intensity after purification.
The anomeric configuration could not be definitely proven.
Methoxyamine based neoglycosylation is often carried out under mildly acidic conditions, either in buffered aqueous (NaOAc/AcOH) solutions (Matsubara, et al. 2005) or in mixtures of organic solvents containing acetic acid (Langenhan, et al. 2013, Peri, et al. 2004). DMF/AcOH 3:1 has been frequently the choice due to its high dissolving power for both lipophilic and hydrophilic compounds. Therefore, we first performed the conjugation of linker 11 with GlcNAc under standard conditions in DMF/AcOH 3:1. Other parameters, such as temperature, incubation time, acidity and concentration of both reactants were screened in order to improve reaction yields (Table I, entries 1-4). In agreement with previously published results (Gudmundsdottir, et al. 2009, Munneke, et al. 2015), a considerably better yield was observed under highly concentrated solutions (1 M linker and 100 mM sugar). However, the limited solubility of complex glycans in DMF and the low pH led us to search for milder conditions compatible with natural glycans. Therefore it was necessary to find a solvent mixture, in which both the hydrophilic carbohydrate and the lipophilic linker were sufficiently soluble under highly concentrated solutions. A mixture of 1,4-dioxane/ammonium acetate buffer 1:1 (v:v) pH 4.6 provided the best results with longer incubation times required for free and substituted GlcNAc. With the combination of a highly concentrated solution and mild reaction conditions, the reaction proceeded in high yields with a 10-fold improvement over our initial trials (Table I, entries 5-13). Thereby, our conjugation efficiency with GlcNAc (93% also taking into account the purification step) exceeds that of Munneke et al (Munneke, et al. 2015) in their report describing the use of sodium acetate buffer for conjugation at room temperature.
After purification by silica gel column chromatography, all glycoconjugates listed in Table 1 were characterized by NMR. In agreement with previously-published work, the stereochemistry is influenced significantly by the sugar unit at the reducing end (Langenhan, et al. 2011, Peri, et al. 1998). NMR data showed that the gluco-configured saccharides (Glc, GlcNAc, Lac, LacNAc, chitobiose) derivatives are found exclusively in β-pyranose form, whereas galactose derivatives (Gal, GalNAc) were present as a mixture of β-pyranose and furanose products. This high diastereoselectivity towards the β-pyranosides can be rationalized to unfavorable steric factors, such as 1,3-diaxial interactions, present in α-pyranosides which are not compensated by the weak anomeric effect exerted by the aminoxy aglycon. Assignment of the β-anomeric configuration of the galactofuranose products was based on comparable published data (Langenhan, et al. 2011, Peri, et al. 1998) and additional NOESY experiments. The most complex mixture was observed for mannose (Man) products, with a mixture of α-pyranose (which corresponds to the natural eukaryotic O-Man-type linkage), β-pyranose and a furanose form. The values of the homonuclear JH1,H2 coupling constant seen in anomeric mannofuranoses are not very different and thus not a reliable diagnostic feature to assign the anomeric configuration. Steric considerations would suggest assigning the α-anomeric configuration for the mannofuranosyl conjugate. By contrast to glucose or galactose derivatives, the axial 2-OH of Man promotes the formation of α-pyranoside via favorable dipole/dipole interactions with the anomeric center. For all glycoconjugates listed in Table 1, it was possible to separate the isomers by HPLC with no anomeric re-equilibrium observed by NMR over time. Based on the screen of conjugation reactions with different mono- and disaccharides, we can demonstrate the synthesis of biologically-relevant forms of key glycan elements which reflect O-mannosyl and collagen-type O-glycans (Praissman and Wells 2014, Spiro 1967), LacNAc-type (LN) N-glycan antennae (Cummings 2009) and the lactose core of lactosylceramides (Jennemann, et al. 2005). Most importantly, the ‘closed-ring’ β-linkage of N-glycans can be mimicked.
After purification of neoglycoconjugates (13-21), the azido group was reduced to the corresponding amino group by catalytic hydrogenation without the need of any further purification step. After hydrogenation, the amino-functionalized neoglycoconjugates (22-33) were ready for printing on commercially available NHS-activated glass supports. The stability of amino-functionalized neoglycoconjugate 24 was tested at different pH values ranging from 2.0 to 12.0 for 48 h. Under neutral to basic conditions (pH >6) the glycoconjugate was stable and no loss of the sugar unit was observed. In agreement with previous results (Gudmundsdottir, et al. 2009, Langenhan, et al. 2011), the glycoconjugate is rapidly hydrolyzed at pH below 6. After 24 h, 60% and 70% of the neoglycoconjugates lost the sugar unit at pH 4 and pH 2, respectively. The lowest degree of deglycosylation (5%) was observed at pH 6. In addition, the medium-and long-term stability in aqueous solution was studied for Boc-protected linker 10 and neoglycoconjugates 22 and 24. For the three compounds, up to 80% is stable after 1-month storage at 4 °C or after 3-months storage at -20 °C.
Conjugation with amino-functionalized linker 12
With the aim of reducing the number of steps and facilitate the conjugation with larger and more complex glycans, the conjugation was carried out directly with the amino-functionalized linker 12. Linker 12 was conjugated with lactose under optimal reaction conditions described above to give the corresponding glycoconjugate in quantitative yields (i.e., >95% yield). NMR spectra demonstrated that the reaction is completely selective for the methoxyamino functionality and no reaction with the amino-modified pentanoyl chain takes place.
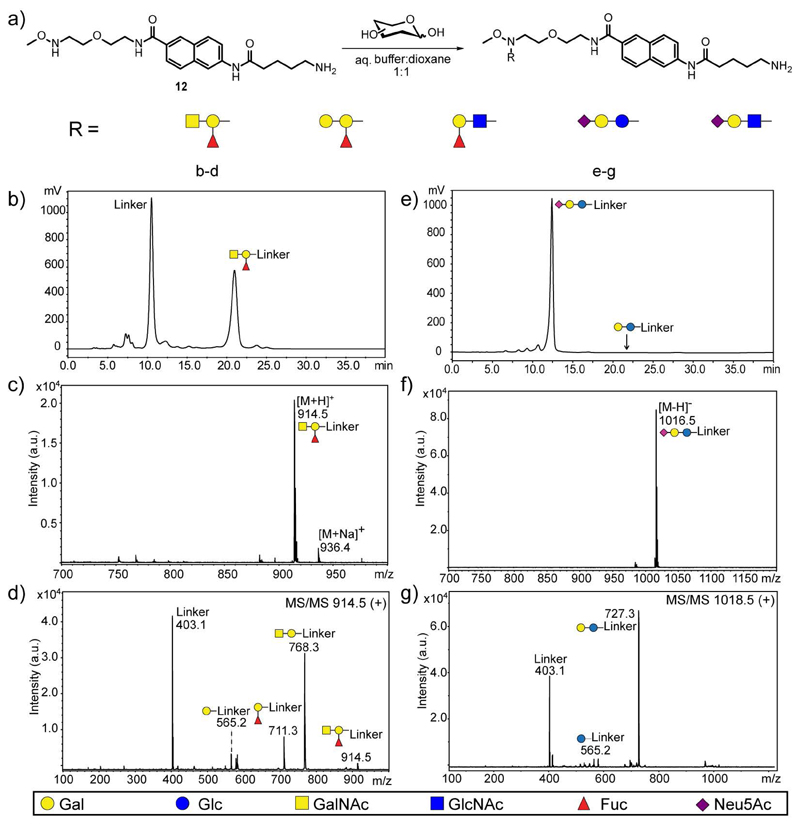
In order to confirm the applicability of this approach to branched trisaccharide glycan structures, ABH blood group trisaccharides were conjugated to 12. After clean-up with NH2-SPE to remove partially the excess of linker, glycoconjugates were purified by NP-HPLC and characterized by MALDI-TOF MS and MS/MS (Figure 1, panel a-d). All fragment ions from glycoconjugates with hexoses at the reducing end retain the linker moiety and the major ions are usually the result of glycosidic cleavages. By contrast, glycoconjugates with hexosamines at the reducing end results in 0,2X0 cross-ring fragment ions containing the aglycon moiety in addition to B glycan fragment ions.
Fig. 1.
Conjugation to amino-functionalized linker 12 with branched glycan structures and sialyl-containing trisaccharides. a) Schematic representation of conjugation and glycan structures tested. b-d) Conjugation with blood group A trisaccharide. (b) NP-HPLC chromatogram of crude mixture after NH2-SPE clean-up. c-d) MALDI-TOF MS and MS/MS analysis of blood group A-linked glycoconjugate (GalNAcα1,3(Fucα1,2)Galβ1-R). e-g) Conjugation with Neu5Ac-α2,6-lactose (NeuAcα2,6Galβ1,4Glcβ1-R). e) NP-HPLC chromatogram of crude mixture after NH2-SPE clean-up. f-g) MALDI-TOF MS and MS/MS analysis of Neu5Ac-α2,6-lactose-containing glycoconjugate. Glycan structures are shown using the symbol nomenclature for glycans (SNFG) adopted by the Consortium for Functional Glycomics and for Essentials of Glycobiology (Varki, et al. 2015).
As shown in Figure 1 (panel e-g), commercially available sialyl-α2,6-lactose was also conjugated to the amino-functionalized linker to test the suitability of this approach for acid-labile glycan modifications, such as sialic acid. After 72 hours of conjugation, glycoconjugates were cleaned up with NH2-SPE, purified by NP-HPLC and analyzed by MALDI-TOF MS and MS/MS. The HPLC chromatogram showed that no desialylation occurred during conjugation (Figure 1, panel e).
In solution enzymatic modification of neoglycoconjugates
In a further application for the chemoselectively-synthesized neoglycoconjugates, the possibility of their modification with different natural or recombinant enzymes was explored. Beginning with the common core structure found in N-glycan antennae (i.e. β-GlcNAcp) different glycan epitopes were enzymatically prepared. Due to the fluorescent naphthalene ring, the reactions could be monitored and quantified by HPLC equipped with a fluorescence detector. Glycoconjugates in the pmol range were straightforwardly monitored by HPLC prior to characterization by MALDI-TOF MS.
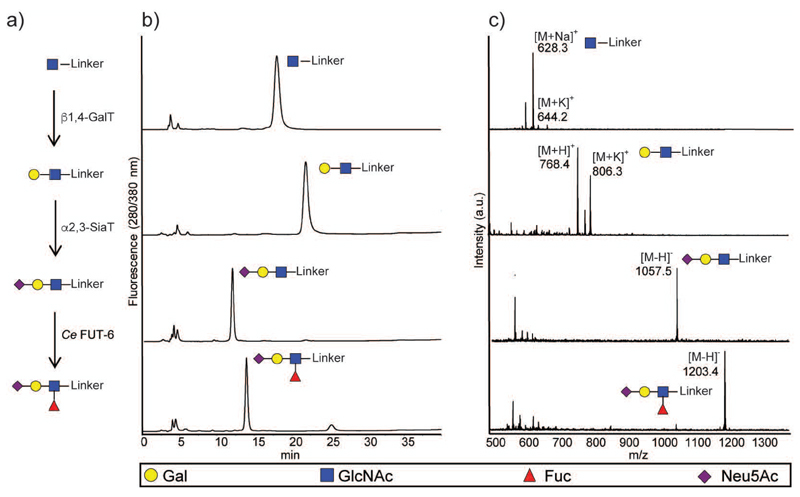
As shown in Figure 2, from compound 22 (with a β-GlcNAcp at the reducing end), the type 2 LN epitope (i.e. Gal-β1,4-GlcNAc) was built using the commercially-available bovine milk β1,4-galactosyltransferase (β1,4-GalT). After full conversion, the type 2 LN epitope was further elongated on the Gal residue by a recombinant α2,3-sialyltransferase from Neisseria meningitidis (α2,3-SiaT) (Gilbert, et al. 1996). Furthermore, the complex tetrasaccharide sialyl Lewis X (sLeX) was prepared from the GlcNAc-neoglycoconjugate in two steps: i) one-pot multiple reaction of β1,4-GalT and, α2,3-SiaT to made the type 2 3′-SLN, and ii) sequential addition of recombinant fucosyltransferase from Caenorhabditis elegans (Ce FUT-6) (Yan, et al. 2013). The chromatograms and MS spectra showed that all reactions proceeded to completion.
Fig. 2.
In solution chemo-enzymatic synthesis of sLeX epitope. a) Schematic representation of enzymatic modification of GlcNAc-neoglycoconjugate (30 μM) with bovine β1,4-galactosyltransferase, Neisseria α2,3-sialyltransferase and the Caenorhabditis FUT-6 α1,3-fucosyltransferase. b) NP-HPLC analysis of starting material, intermediates and final sialyl Lewis ✕ product NeuAcα2,3Galβ1,4(Fucα1,3)GlcNAcβ1-R with detection by fluorescence. c) MALDI-TOF analysis of crude reactions after each enzymatic modification in either positive or negative ion modes (a.u., arbitrary units). Symbols for each glycan are shown using SFNG nomenclature; glycosidic linkages and abbreviated names are given in Table 2.
Alternative starting points (see Supplementary Figure 1) were represented by the linkers displaying αManp or βGalpNAc at the reducing end; these could be respectively extended by a recombinant β1,2-N-acetylglucosaminyltransferase (POMGnT1) (Akasaka-Manya, et al. 2011) and β1,4-GalT or by either bacterial CgtB β1,3-galactosyltransferase (β1,3-GalT) (Persson, et al. 2001) or α2,6-sialyltransferase (α2,6-SiaT; Pd2,6ST) (Yamamoto, et al. 1998). In these small-scale experiments, at least 50% conversion was achieved showing the compatibility of the linker with a range of glycosyltransferases.
Microarray analysis of neoglycoconjugates displaying mono- to tetrasaccharides
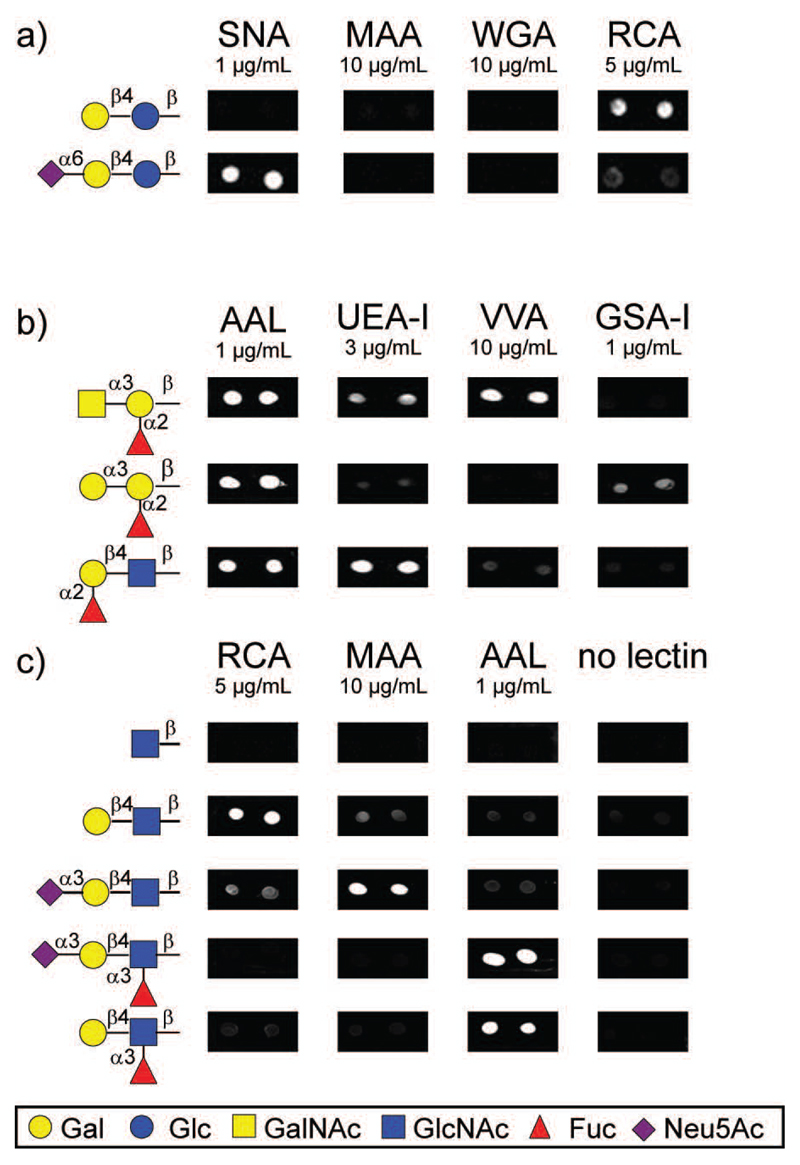
In order to test the applicability of these neoglycoconjugates for glycan arrays, those were immobilized onto NHS-activated glass slides and tested with several plant lectins. In these experiments, commercially available biotinylated lectins were employed, and the binding was detected after incubation with Alexa Fluor® 647-conjugated streptavidin. The naphthalene ring absorbs and re-emits at the UV light range (Ex 280/Em 380 nm). Therefore its fluorescence activity does not interfere with the detection of binding in the red light range (Ex 620/Em 675 nm). First, typical mono- and disaccharide anomers (β-Galp, α-Manp, β-GalpNAc, β-GlcpNAc, β-Lacp and β-LacpNAc) were spotted on the slide at three concentrations (33 μM, 250 μM and 1 mM) and the remaining free NHS-groups were blocked with ethanolamine. Subsequently, these glycan arrays were interrogated with lectins of different carbohydrate specificity at three concentrations (2, 10 and 20 μg/mL).
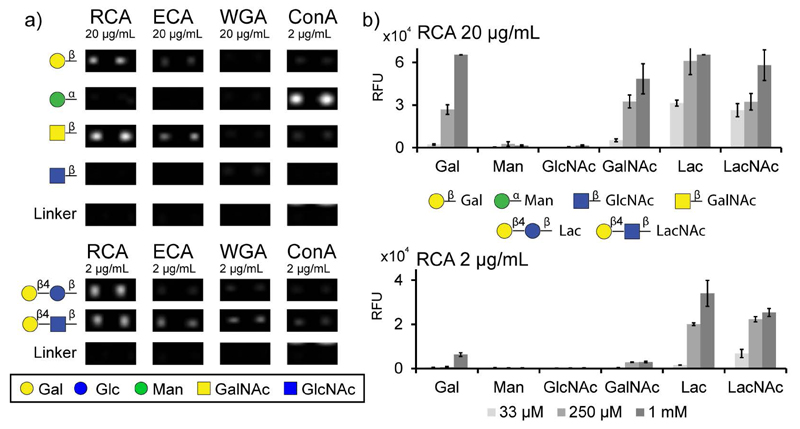
As shown in Figure 3, positive binding was observed for different monosaccharides according to the carbohydrate specificity of lectins. Ricinus communis agglutinin I (RCA I), a β-Gal specific lectin (Wu, et al. 2006), was clearly binding to β-Galp and β-GalpNAc but not to Man or GlcNAc; this contrasts with its inability to bind reduced forms of galactose (Liu, et al. 2007). β-Galp and β-GalpNAc were also recognized by Erythrina cristagalli agglutinin (ECA), another β-Gal specific lectin (Iglesias, et al. 1982). However, the fluorescence intensity was lower in comparison to RCA, which confirms their different affinities (Itakura, et al. 2007). For both lectins, high lectin concentrations (i.e. 20 μg/mL) or high immobilization levels (i.e. 1 mM) were necessary to detect lectin binding to monosaccharides. Only for Concanavalin A (ConA), a lectin generally considered specific for α-Man (Mandal, et al. 1994), bound well to αManp-glycoconjugate at 2 μg/mL. In contrast, wheat germ agglutinin (WGA) bind to any monosaccharide conjugate was low. The absence of a signal for the control (immobilized linker alone) proved that lectins bind exclusively to their sugar epitopes and not to the linker, even at high lectin concentrations.
Fig. 3.
Glycan microarray with chemically synthesized neoglycoconjugates displaying mono- and disaccharides. a) Image of lectin binding to robotically-printed glycoconjugates immobilized at 250 μM (1 nl; 100 μm diameter). Microarray was incubated with several biotinylated lectins, such as RCA, ConA, WGA and ECA at 2 and 20 μg/mL. Lectin binding was detected using Alexa Fluor® 647-conjugated streptavidin (at 2 μg/mL). Two representative spots from five replicates are shown. b) Histogram of quantified RCA binding at 2 and 20 μg/mL on different immobilization levels (33 μM, 250 μM, 1 mM). A full set of bar charts for ConA, ECA and WGA is shown in Supplementary Figure 2 (A-G).
For RCA, ECA and ConA, higher binding intensities were observed for disaccharides than for monosaccharides at the same lectin concentration, whereas of the mono- and disaccharide conjugates tested, wheat germ agglutinin (WGA) only significantly bound the β-LacpNAc disaccharide (Figure 3). Such results are not unexpected as the biological lectin-binding determinants are certainly more complex than a single residue and are in keeping with the older literature. For instance, RCA displays an increased affinity towards lactose as opposed to galactose (Podder, et al. 1974) or there are orders of magnitude difference in the inhibition of binding to WGA by GlcNAc di-, tri- and pentasaccharides as compared to GlcNAc alone (Allen, et al. 1973). This is reflected in more modern studies on WGA in which binding was observed to GlcNAc2-5 on the Consortium array, while an interaction with the monosaccharide was highly dependent on the type of glycoconjugate used (Wang, et al. 2014). In the current study, good binding was observed to disaccharides printed at 33 μM and detected with 20 μg/mL lectin.
The chemically synthesized neoglycoconjugate displaying α2,6-sialylated lactose was also immobilized on the array at 250 μM and interrogated with lectins that are specific for sialic acid. As shown in Figure 4a, clear binding was observed for Sambucus nigra agglutinin (SNA), a lectin that recognizes exclusively α2,6 sialic acid (Shibuya, et al. 1987). By contrast, no binding was observed for Maackia amurensis agglutinin (MAA II) (Knibbs, et al. 1991) or WGA (Iskratsch, et al. 2009), which bind α2,3-, but not α2,6-linked sialic acid. In addition, partial loss of the RCA binding was observed when lactose is capped with a terminal sialic acid.
Fig. 4.
Further examples of microarrays prepared in this study. Chemically-synthesized neoglycoconjugates (a, b) and chemo-enzymatically modified neoglycoconjugates (c) were immobilized at 250 μM on NHS-activated slides (0.2 μl hand-printed; 1 mm diameter). After blocking with ethanolamine, microarrays were interrogated with several lectins at different concentrations. Glycan-protein binding was detected using Alexa Fluor® 647-conjugated streptavidin (at 2 μg/mL). The structures are summarised in Table 2 and a full set of bar charts is shown in Supplementary Figure 2 (H-I).
Neoglycoconjugates with branched ABH histo-blood group trisaccharide structures were also immobilized on the array and tested with several lectins. Binding of Aleuria aurantia lectin (AAL), a lectin with a broad affinity against fucosylated glycans, to all three ABH determinants corroborates that the methoxy group is not a hindrance for recognition of branched structures at the reducing end. By contrast, Ulex europaeus agglutinin I (UEA-I), a lectin with described high specificity to blood group H antigen (Matsumoto and Osawa 1969), showed a different binding profile for the three fucosylated trisaccharides, with the strongest affinity to blood group H, a weak affinity to blood group A and almost no binding to blood group B (Figure 4, panel b). Binding according to their carbohydrate specificity was observed for Vicia villosa agglutinin (VVA), an α-GalNAc specific lectin, and Griffonia simplicifolia agglutinin I (GSA-I), an α-Gal specific lectin. The microarray data presented here demonstrates that immobilized small sugar epitopes, such as di- and trisaccharides with either linear or branched structures, are recognized by relevant specific lectins.
To determine the utility of the enzymatically-modified neoglycoconjugates for array preparation, the enzymatic reactions (shown by HPLC and mass spectrometry to be quantitative) were desalted using mini-C18 columns and spotted directly onto the slide. These arrays were then tested with different lectins. Figure 4 panel c shows the binding observed towards derivatives of LacNAc for lectins, such as RCA I, MAA II and AAL (Itakura, et al. 2007, Kochibe and Furukawa 1980, Wang and Cummings 1988). RCA I bound strongly to the LacNAc-conjugate, but this binding is partially lost when the type 2 LN disaccharide is elongated with a terminal sialic acid in α-2,3 linkage (Green, et al. 1987). However, also in keeping with a previous study using remodeled glycoproteins (Iskratsch, et al. 2009), the binding is lost when the GlcNAc moiety is fucosylated. A similar effect was observed for MAA II that binds to the trisaccharide Neu5Ac-α2,3-LacNAc but not to the fucosylated variants; this demonstrates that the binding of this lectin is indeed very context dependent (Geisler and Jarvis 2011) and is also compatible with results from an array based on reductive labelling (Byrd-Leotis, et al. 2014). On the other hand and as previously reported (Matsumura, et al. 2009), fucosylated epitopes (LeX and sLeX) were strongly recognized by the fucose-specific binding lectin AAL. The enzymatic modification performed on chip on GlcNAc-derivatized glycoconjugates also resulted in gain-of-epitope binding patterns (data not shown).
Conjugation and printing of a naturally derived glycan
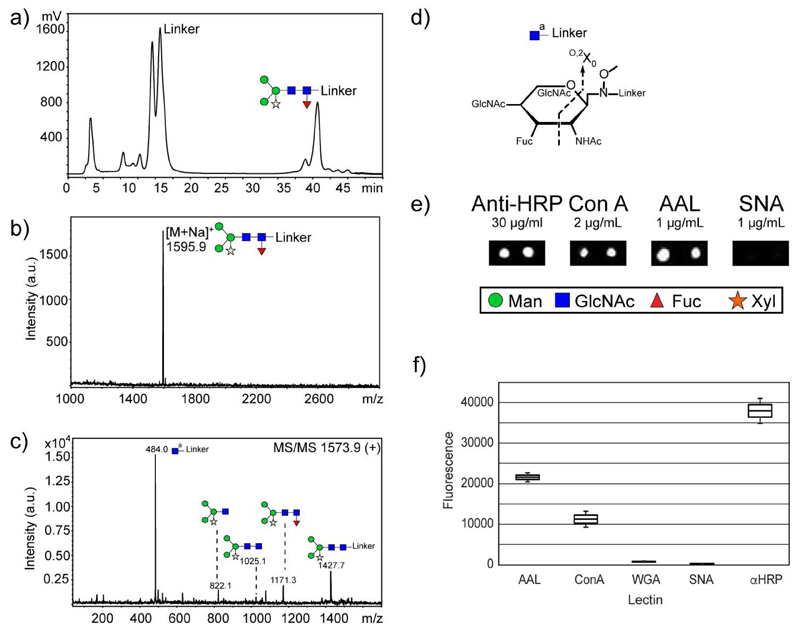
Our final aim was to test whether this approach is suitable for labelling of natural glycans and preparation of natural glycan arrays. As an example, the major glycan structure of the horseradish peroxidase (HRP) glycoprotein (Yang, et al. 1996) was released by treatment with PNGase A and conjugated with the amino-functionalized linker under optimum conditions described before. After conjugation, excess of linker was partially removed by precipitation of the crude mixture in 90% MeCN. However, two additional purification steps (i.e. G-10 size exclusion chromatography and NP-HPLC) were necessary to obtain the pure glycoconjugate (Figure 5, panel a). After concentration, the glycoconjugate was characterized by MALDI-TOF MS (Figure 5, panel b and c); subsequently immobilized onto a glass slide and interrogated with either lectins or an antibody against HRP; the latter is a well-known polyclonal which detects core α1,3-fucose and β1,2-xylose on N-glycans of a range of non-vertebrate organisms (Paschinger, et al. 2009). The larger size of the glycan chain allows lower immobilization level. As shown in Figure 5 (panel e), the glycan was strongly recognized by the antibody against HRP, even with an immobilization concentration of ~30 μM, which is lower than on other array platforms. Binding of AAL and ConA also demonstrated the ability of lectins to recognize either non-reducing or reducing parts of the immobilized glycan.
Fig. 5.
Conjugation of amino-functionalized linker 12 with major HRP-derived glycan. a) NP-HPLC chromatogram of an aliquot of the crude reaction of the linker with the PNGase A-released glycan after G-10 size-exclusion chromatography. b) MALDI-TOF MS spectrum of pure glycoconjugate. c) MALDI-TOF MS/MS analysis of HRP-containing glycoconjugate. d) Schematic representation of the observed cross-ring cleavage. e) A natural array was prepared with neoglycoconjugate immobilized at ~30 μM on NHS-activated slides (0.2 μl hand-printed; 1 mm diameter) and incubated with biotinylated lectins and a rabbit anti-HRP antibody. Binding was detected using Alexa Fluor® 647-conjugated streptavidin (at 2 μg/mL) or Alexa Fluor® 647-conjugated goat anti-rabbit IgG (at 2 μg/mL); SNA was used as a negative control. f) Box and whisker plot of the array data.
Conclusion
In this work, a derivatization strategy for free glycans is presented which combines, in the same straightforwardly-prepared linker, three important features not present together in other glycan array platforms: (a) one-step chemoselective reaction with free reducing glycans and preservation of the closed-ring form, (b) fluorescent derivatization for higher sensitivity and (c) a terminal azido- or amino- function for glycan immobilization on different surfaces. Several neoglycoconjugates covering the most common monosaccharides found in higher animals, as well as a natural glycan derived from HRP, were prepared, showing that the linker is compatible with both shorter and longer glycan chains. Not only were the conjugation conditions compatible with labile sugar residues (i.e., sialic acids), but the chemically-synthesized neoglycoconjugates could be extended enzymatically prior to testing with lectins. Upon testing the array, a not unexpected trend for lectins to bind more efficiently to di- or oligosaccharides was observed, an effect with can be examined in future experiments using natural N-glycans. With the ability to remodel, purify, characterize, immobilize specific small sugar epitopes and even natural glycans, we now have a tool, which promises to expand the existing range of glycan arrays to include structures previously inaccessible to such studies and so better reveal the variety of glycan-protein interactions in a range of biological systems.
Methods
Synthesis of linker and preparation of azido-functionalized neoglycoconjugates 13-21
Detailed synthetic procedures and characterization of linker (compounds 1 to 12) are described in the Supporting Information. For the conjugation with free reducing mono- and disaccharides, linker 11 (10 equiv.) and the sugar (1 equiv.) were placed in a reaction vial and dissolved in either DMF/AcOH mixture or dioxane/ammonium acetate buffer 1:1 (v:v) pH 4.6. The reaction mixture was stirred at rt, 40 or 60 °C for 24-72 h. The crude reaction was then concentrated under reduced pressure and purified by silica gel column chromatography. The following step gradients were used for purification: CHCl3/MeOH 20:1 for eluting the unreacted linker 11, CHCl3/MeOH 10:1 for eluting neoglycoconjugates 13-18 and CHCl3/MeOH 6:1 for eluting neoglycoconjugates 19-21. A second purification was needed for compound 14 (CH2Cl2/MeOH 10:1) and compound 16 (CHCl3/MeOH 5:1) to separate isomers. The fractions containing the glycoconjugate were identified by TLC, combined and concentrated to yield the pure neoglycoconjugates as yellowish syrups. Pure neoglycoconjugates were quantified by fluorescence and characterized by NMR.
In solution modification of neoglycoconjugates by glycosyltransferases
All enzymatic modifications started with a 25 μL solution of compound 22, 27 or 29 (0.45 μg, 0.75 nmol) in 80 mM MES buffer pH 6.5 supplemented with 20 mM MnCl2. Nucleotide sugars (50 nmol) and the corresponding glycosyltransferase (3 μL, 3 μg) were added to the reaction mixture prior to overnight incubation at 37 °C. Afterwards an aliquot (0.7 μL) was taken and diluted with MeCN for quantification by HPLC and characterization by MALDI-TOF MS.
Conjugation of amino-functionalized linker 12 with free reducing branched ABH blood group and sialylated trisaccharides
In a 1.5 mL Eppendorf tube, linker 12 (1.4 mg to 17.2 mg, 10 equiv., 1 M) in dioxane/ammonium acetate buffer 1:1 (v:v) pH 4.6 was added to the dried free reducing glycan (0.2 mg to 2.8 mg, 1 equiv., 100 mM). The mixture was stirred and incubated at 40 °C in a heating block for 72 h. The crude reaction was concentrated under vacuum (SpeedVac) and cleaned up using NH2-SPE cartridges. An isocratic elution with 85% MeCN was used for branched ABH blood group trisaccharides and a gradient elution from 85% to 50% MeCN for sialylated glycoconjugates. Fractions containing the glycoconjugates were lyophilized, purified by NP-HPLC on a Tosoh Amide-80 column with flow rate 1 mL/min and a linear gradient from 75 to 40% B in A over 40 min (A= 10 mM ammonium formate, pH 7; B= 95% MeCN) and characterized by MALDI-TOF MS; 10 pmol of conjugate is estimated to result in a signal of 80 mV.
Conjugation of amino-functionalized linker 12 with naturally-derived HRP-glycans
PNGase A-released glycans from the horseradish peroxidase glycoprotein (HRP; starting with 4.5 mg glycoprotein) were conjugated with the amino-functionalized linker. In a 1.5 mL Eppendorf tube, linker 12 (1.6 mg, 1 M solution in dioxane/ ammonium acetate buffer 1:1 (v:v) pH 4.6) was added to the dried free reducing glycan (maximally 90 μg) and the mixture was incubated at 40 °C for 24 h. Then, the mixture was concentrated under vacuum (SpeedVac). The glycoconjugate was purified by precipitation with 95% MeCN following the protocol previously described (Song, et al. 2009a). It was necessary to repeat the precipitation step at least three times in order to remove the excess of linker. No glycoconjugate was observed in any of the supernatant fractions. The pellet was dissolved in water and further purified by gel filtration (Sephadex G10) chromatography. Fractions were lyophilized and analyzed by MALDI-TOF MS prior to being subject to NP-HPLC on a Tosoh Amide-80 column with 10 mM ammonium formate pH 7.0 as buffer A, and 95% MeCN as buffer B. For naturally-derived glycoconjugates, the gradient of buffer B was applied as follows: 0-5 min, 70% B; 5-10 min, 70-65% B; 10-15 min, 65% B; 15-20 min, 65- 60% B; 20-25 min, 60% B; 25-30 min, 60-50% B; 30-35 min, 50-45% B; 35-40 min, 45-40% B: 40-45 min, 40% B. The glycoconjugate was collected, lyophilized and analyzed by MALDI-TOF MS; an estimated 2 nmol of pure glycoconjugate was obtained.
Printing of arrays and lectin binding assay
Linker 10 and chemically synthesized glycoconjugates (22, 24, 27, 29, 31 and 32) were spotted at three concentrations (1 nl of 33 μM, 250 μM and 1 mM) on NHS-coated slides. They were spotted in five replicates using a SpotBot3 Microarray (Arrayit, USA). Neoglycoconjugates with ABH trisaccharides, sialylated trisaccharides and chemo-enzymatically prepared neoglycoconjugates were spotted manually at 250 μM in two replicates. The HRP-derived neoglycoconjugate was spotted at ~30 μM in two replicates. The spot volume for the manually printing was 0.2 μL. All samples were printed in 150 mM sodium phosphate pH 8.5 + 0.0025% Tween-20. After printing, the slides were incubated overnight at rt in a humidity chamber to allow amide coupling of free amine to the NHS groups. The slides were blocked with 50 mM ethanolamine in 50 mM sodium borate pH 9.0 for 1 h, and afterwards washed successively with PBS + 0.5% Tween-20, PBS and H2O. For binding experiments, the slides were incubated with biotinylated lectins at 1-20 μg/mL or with anti-horseradish peroxidase (1:300 of 10 mg/ml) in PBS + 0.05% Tween for 1 h (no cations were added to the buffer; however, considering Ca2+ and/or Mn2+ in some lectin preparations, dilution with PBS resulted in submicromolar concentrations of one or both of these ions being present and sufficient for binding in the cases of ConA, ECA, GSA-I, SNA, UEA, VVA and WGA). Afterwards, the slides were washed again and lectin binding detected by incubation with Alexa Fluor® 647-conjugated streptavidin at 2 μg/mL for 1 h in the dark. Binding of anti-HRP antibody was detected using Alexa Fluor® 647-conjugated goat anti-rabbit IgG. Subsequently, the slides were washed as described above and scanned using the Agilent G2565CA Microarray scanner. The integrated spot intensities were determined using Imagene software (BioDiscovery; demonstration version) and converted from red colour to white. Dependent on the concentration of lectin used, the limit of detection of disaccharide conjugates is < 33 fmol for the robotically-printed array.
Supplementary Information
Further experimental details including synthetic procedures, preparation of recombinant enzymes, NMR data for chemically-synthesized compounds as well as further examples of enzymatic modification and a summary of fluorescent intensities in a bar chart format (Supplementary Figures 1 and 2).
This submission contains Supporting Information: Supplementary Results on other ‘in solution’ enzymatic modifications and a set of bar charts of fluorescent intensities (Figures S1 and S2); Supplementary Materials; General Synthetic Procedures; Structural characterization of linker 10 and neoglycoconjugates; Stability of amino-functionalized neoglycoconjugates; Preparation of recombinant enzymes; In solution modification of neoglycoconjugates by glycosyltransferases; Supplementary References.
Table II. Conjugate structures tested on arrays.
| Structure | Depiction | Figure | Reactivity |
| Galβ1-R | 3 | RCA+ | |
| Manα1-R | 3 | ConA+ | |
| GalNAcβ1-R | 3 | RCA+ | |
| GlcNAcβ1-R | 3, 4 | n.d. | |
| Galβ1,4Glcβ1-R (Lac) | 3, 4 | RCA+ | |
| Galβ1,4GlcNAcβ1-R (LacNAc) | 3, 4 | RCA/ECA/WGA+ | |
| NeuAcα2,6Galβ1,4Glcβ1-R (6’-SialylLac) | 4 | SNA+ | |
| GalNAcα1,3(Fucα1,2)Galβ1-R (Blood group A) | 4 | AAL/UEA/VVA+ | |
| Galα1,3(Fucα1,2)Galβ1-R (Blood group B) | 4 | AAL/GSA+ | |
| Fucα1,2Galβ1,4GlcNAcβ1-R (Blood group H) | 4 | AAL/UEA+ | |
| NeuAcα2,3Galβ1,4GlcNAcβ1-R (3’-SialylLacNAc) | 4 | MAA+ | |
| NeuAcα2,3Galβ1,4(Fucα1,3)GlcNAcβ1-R (SialylLeX) | 4 | AAL+ | |
| Galβ1,4(Fucα1,3)GlcNAcβ1-R (LeX) | 4 | AAL+ | |
| Manα1,6(Manα1,3)(Xylβ1,2)Manβ1,4GlcNAcβ1,4(Fucα1,3)GlcNAcβ1-R | 5 | AAL/ConA/αHRP+ |
Acknowledgments
The authors acknowledge Dr. Josef Voglmeir and Prof. Sabine Flitsch (University of Manchester) for providing the POMGnTI and sialyltransferase clones and Prof. Warren Wakarchuk (Ryerson University, Toronto) for the β1,3-GalT clone. We also thank to Dr. Jaroslav Katrlík and members of his laboratory for microarray printing and Dr. Jean-Baptiste Farcet for his comments.
Funding
This work was funded by a grant from the Austrian Science Fund (FWF; project P 23607 to IBHW).
Abbreviations
- anti-HRP
anti-horseradish peroxidase
- AAL
Aleuria aurantia lectin
- ConA
concanavalin A
- ECA
Erythrina cristagalli agglutinin
- GSA-I
Griffonia simplicifolia agglutinin I
- MAA
Maackia amurensis agglutinin
- RCA I
Ricinus communis agglutinin I
- SNA
Sambucus nigra agglutinin
- UEA-I
Ulex europaeus agglutinin I
- VVA
Vicia villosa agglutinin
- WGA
wheat germ agglutinin
Footnotes
Conflicts of Interest
The authors have no conflicts of interest.
References
- Akasaka-Manya K, Manya H, Mizuno M, Inazu T, Endo T. Effects of length and amino acid sequence of O-mannosyl peptides on substrate specificity of protein O-linked mannose β1,2-N-acetylglucosaminyltransferase 1 (POMGnT1) Biochemical and Biophysical Research Communications. 2011;410:632–636. doi: 10.1016/j.bbrc.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Allen AK, Neuberger A, Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochemical Journal. 1973;131:155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohorov O, Andersson-Sand H, Hoffmann J, Blixt O. Arraying glycomics: a novel bi-functional spacer for one-step microscale derivatization of free reducing glycans. Glycobiology. 2006;16:21C–27C. doi: 10.1093/glycob/cwl044. [DOI] [PubMed] [Google Scholar]
- Byrd-Leotis L, Liu R, Bradley KC, Lasanajak Y, Cummings SF, Song X, Heimburg-Molinaro J, Galloway SE, Culhane MR, Smith DF, Steinhauer DA, et al. Shotgun glycomics of pig lung identifies natural endogenous receptors for influenza viruses. Proceedings of the National Academy of Sciences. 2014;111:E2241–E2250. doi: 10.1073/pnas.1323162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-S, Pohl NL. Synthesis of Fluorous Tags for Incorporation of Reducing Sugars into a Quantitative Microarray Platform. Organic Letters. 2008;10:785–788. doi: 10.1021/ol702915e. [DOI] [PubMed] [Google Scholar]
- Cló E, Blixt O, Jensen KJ. Chemoselective Reagents for Covalent Capture and Display of Glycans in Microarrays. European Journal of Organic Chemistry. 2010;2010:540–554. [Google Scholar]
- Cummings RD. The repertoire of glycan determinants in the human glycome. Molecular BioSystems. 2009;5:1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- de Boer AR, Hokke CH, Deelder AM, Wuhrer M. General Microarray Technique for Immobilization and Screening of Natural Glycans. Analytical Chemistry. 2007;79:8107–8113. doi: 10.1021/ac071187g. [DOI] [PubMed] [Google Scholar]
- Feng C, Ghosh A, Amin MN, Giomarelli B, Shridhar S, Banerjee A, Fernandez-Robledo JA, Bianchet MA, Wang LX, Wilson IBH, Vasta GR. The galectin CvGal1 from the eastern oyster (Crassostrea virginica) binds to blood group A oligosaccharides on the hemocyte surface. J Biol Chem. 2013;288:24394–24409. doi: 10.1074/jbc.M113.476531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Jarvis DL. Letter to the Glyco-Forum: Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology. 2011;21:988–993. doi: 10.1093/glycob/cwr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Watson DC, Cunningham A-M, Jennings MP, Young NM, Wakarchuk WW. Cloning of the Lipooligosaccharide α-2,3-Sialyltransferase from the Bacterial Pathogens Neisseria meningitidis and Neisseria gonorrhoeae. Journal of Biological Chemistry. 1996;271:28271–28276. doi: 10.1074/jbc.271.45.28271. [DOI] [PubMed] [Google Scholar]
- Goff RD, Thorson JS. Neoglycosylation and neoglycorandomization: enabling tools for the discovery of novel glycosylated bioactive probes and early stage leads. MedChemComm. 2014;5:1036. doi: 10.1039/C4MD00117F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green ED, Brodbeck RM, Baenziger JU. Lectin affinity high-performance liquid chromatography. Interactions of N-glycanase-released oligosaccharides with Ricinus communis agglutinin I and Ricinus communis agglutinin II. Journal of Biological Chemistry. 1987;262:12030–12039. [PubMed] [Google Scholar]
- Gudmundsdottir AV, Paul CE, Nitz M. Stability studies of hydrazide and hydroxylamine-based glycoconjugates in aqueous solution. Carbohydrate Research. 2009;344:278–284. doi: 10.1016/j.carres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Iglesias JL, Lis H, Sharon N. Purification and Properties of a D-Galactose/N-Acetyl-D-galactosamine-Specific Lectin from Erythrina cristagalli. European Journal of Biochemistry. 1982;123:247–252. doi: 10.1111/j.1432-1033.1982.tb19760.x. [DOI] [PubMed] [Google Scholar]
- Iskratsch T, Braun A, Paschinger K, Wilson IBH. Specificity analysis of lectins and antibodies using remodeled glycoproteins. Analytical Biochemistry. 2009;386:133–146. doi: 10.1016/j.ab.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Itakura Y, Nakamura-Tsuruta S, Kominami J, Sharon N, Kasai K-i, Hirabayashi J. Systematic Comparison of Oligosaccharide Specificity of Ricinus communis Agglutinin I and Erythrina Lectins: a Search by Frontal Affinity Chromatography. Journal of Biochemistry. 2007;142:459–469. doi: 10.1093/jb/mvm153. [DOI] [PubMed] [Google Scholar]
- Jennemann R, Sandhoff R, Wang S, Kiss E, Gretz N, Zuliani C, Martin-Villalba A, Jäger R, Schorle H, Kenzelmann M, Bonrouhi M, et al. Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth. Proceedings of the National Academy of Sciences. 2005;102:12459–12464. doi: 10.1073/pnas.0500893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Castells C, de la Torre B, Andreu D, Gutiérrez-Gallego R. Neo-glycopeptides: the importance of sugar core conformation in oxime-linked glycoprobes for interaction studies. Glycoconj J. 2008;25:879–887. doi: 10.1007/s10719-008-9150-8. [DOI] [PubMed] [Google Scholar]
- Kawase M, Kitamura T, Kikugawa Y. Electrophilic aromatic substitution with N-methoxy-N-acylnitrenium ions generated from N-chloro-N-methoxy amides: syntheses of nitrogen heterocyclic compounds bearing a N-methoxy amide group. The Journal of Organic Chemistry. 1989;54:3394–3403. [Google Scholar]
- Knibbs RN, Goldstein IJ, Ratcliffe RM, Shibuya N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. Journal of Biological Chemistry. 1991;266:83–88. [PubMed] [Google Scholar]
- Kochibe N, Furukawa K. Purification and properties of a novel fucose-specific hemagglutinin of Aleuria aurantia. Biochemistry. 1980;19:2841–2846. doi: 10.1021/bi00554a004. [DOI] [PubMed] [Google Scholar]
- Langenhan JM, Endo MM, Engle JM, Fukumoto LL, Rogalsky DR, Slevin LK, Fay LR, Lucker RW, Rohlfing JR, Smith KR, Tjaden AE, et al. Synthesis and biological evaluation of RON-neoglycosides as tumor cytotoxins. Carbohydrate Research. 2011;346:2663–2676. doi: 10.1016/j.carres.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Langenhan JM, Mullarky E, Rogalsky DK, Rohlfing JR, Tjaden AE, Werner HM, Rozal LM, Loskot SA. Amphimedosides A–C: Synthesis, Chemoselective Glycosylation, And Biological Evaluation. The Journal of Organic Chemistry. 2013;78:1670–1676. doi: 10.1021/jo302640y. [DOI] [PubMed] [Google Scholar]
- Liu Y, Feizi T, Campanero-Rhodes MA, Childs RA, Zhang Y, Mulloy B, Evans PG, Osborn HMI, Otto D, Crocker PR, Chai W. Neoglycolipid Probes Prepared via Oxime Ligation for Microarray Analysis of Oligosaccharide-Protein Interactions. Chemistry & Biology. 2007;14:847–859. doi: 10.1016/j.chembiol.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Mandal DK, Bhattacharyya L, Koenig SH, Brown RD, Oscarson S, Brewer CF. Studies of the Binding Specificity of Concanavalin A. Nature of the Extended Binding Site for Asparagine-Linked Carbohydrates. Biochemistry. 1994;33:1157–1162. doi: 10.1021/bi00171a015. [DOI] [PubMed] [Google Scholar]
- Matsubara N, Oiwa K, Hohsaka T, Sadamoto R, Niikura K, Fukuhara N, Takimoto A, Kondo H, Nishimura S-I. Molecular Design of Glycoprotein Mimetics: Glycoblotting by Engineered Proteins with an Oxylamino-Functionalized Amino Acid Residue. Chemistry – A European Journal. 2005;11:6974–6981. doi: 10.1002/chem.200500531. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Osawa T. Purification and characterization of an anti-H(O) phytohemagglutinin of Ulex europeus. Biochim Biophys Acta. 1969;194:180–189. doi: 10.1016/0005-2795(69)90193-7. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Higashida K, Hata Y, Kominami J, Nakamura-Tsuruta S, Hirabayashi J. Comparative analysis of oligosaccharide specificities of fucose-specific lectins from Aspergillus oryzae and Aleuria aurantia using frontal affinity chromatography. Analytical Biochemistry. 2009;386:217–221. doi: 10.1016/j.ab.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Munneke S, Prevost JRC, Painter GF, Stocker BL, Timmer MSM. The Rapid and Facile Synthesis of Oxyamine Linkers for the Preparation of Hydrolytically Stable Glycoconjugate. Organic Letters. 2015 doi: 10.1021/ol503634j. [DOI] [PubMed] [Google Scholar]
- Park S, Gildersleeve JC, Blixt O, Shin I. Carbohydrate microarrays. Chemical Society Reviews. 2013;42:4310–4326. doi: 10.1039/c2cs35401b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Rendić D, Wilson IBH. Revealing the anti-HRP epitope in Drosophila and Caenorhabditis. Glycoconj J. 2009;26:385–395. doi: 10.1007/s10719-008-9155-3. [DOI] [PubMed] [Google Scholar]
- Peri F, Dumy P, Mutter M. Chemo- and stereoselective glycosylation of hydroxylamino derivatives: A versatile approach to glycoconjugates. Tetrahedron. 1998;54:12269–12278. [Google Scholar]
- Peri F, Jiménez–Barbero J, García–Aparicio V, Tvaroška I, Nicotra F. Synthesis and Conformational Analysis of Novel N(OCH3)-linked Disaccharide Analogues. Chemistry – A European Journal. 2004;10:1433–1444. doi: 10.1002/chem.200305587. [DOI] [PubMed] [Google Scholar]
- Persson K, Ly HD, Dieckelmann M, Wakarchuk WW, Withers SG, Strynadka NCJ. Crystal structure of the retaining galactosyltransferase LgtC from Neisseria meningitidis in complex with donor and acceptor sugar analogs. Nat Struct Mol Biol. 2001;8:166–175. doi: 10.1038/84168. [DOI] [PubMed] [Google Scholar]
- Podder SK, Surolia A, Bachhawat BK. On the specificity of carbohydrate-lectin recognition. The interaction of a lectin from Ricinus communis beans with simple saccharides and concanavalin A. Eur J Biochem. 1974;44:151–160. doi: 10.1111/j.1432-1033.1974.tb03468.x. [DOI] [PubMed] [Google Scholar]
- Praissman JL, Wells L. Mammalian O-Mannosylation Pathway: Glycan Structures, Enzymes, and Protein Substrates. Biochemistry. 2014;53:3066–3078. doi: 10.1021/bi500153y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillahan CD, Paulson JC. Glycan Microarrays for Decoding the Glycome. Annual Review of Biochemistry. 2011;80:797–823. doi: 10.1146/annurev-biochem-061809-152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhaak LR, Zauner G, Huhn C, Bruggink C, Deelder AM, Wuhrer M. Glycan labeling strategies and their use in identification and quantification. Anal Bioanal Chem. 2010;397:3457–3481. doi: 10.1007/s00216-010-3532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α2-6)Gal/GalNAc sequence. Journal of Biological Chemistry. 1987;262:1596–1601. [PubMed] [Google Scholar]
- Smith DF, Song X, Cummings RD. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 2010;480:417–444. doi: 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- Song X, Heimburg-Molinaro J, Cummings RD, Smith DF. Chemistry of natural glycan microarrays. Current Opinion in Chemical Biology. 2014;18:70–77. doi: 10.1016/j.cbpa.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Heimburg-Molinaro J, Smith DF, Cummings RD. Current Protocols in Chemical Biology. John Wiley & Sons, Inc; 2009a. Derivatization of Free Natural Glycans for Incorporation onto Glycan Arrays: Derivatizing Glycans on the Microscale for Microarray and Other Applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Lasanajak Y, Xia B, Smith DF, Cummings RD. Fluorescent Glycosylamides Produced by Microscale Derivatization of Free Glycans for Natural Glycan Microarrays. ACS Chemical Biology. 2009b;4:741–750. doi: 10.1021/cb900067h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xia B, Lasanajak Y, Smith D, Cummings R. Quantifiable fluorescent glycan microarrays. Glycoconj J. 2008;25:15–25. doi: 10.1007/s10719-007-9066-8. [DOI] [PubMed] [Google Scholar]
- Spiro RG. The Structure of the Disaccharide Unit of the Renal Glomerular Basement Membrane. Journal of Biological Chemistry. 1967;242:4813–4823. [PubMed] [Google Scholar]
- Taylor ME, Drickamer K. Structural insights into what glycan arrays tell us about how glycan-binding proteins interact with their ligands. Glycobiology. 2009;19:1155–1162. doi: 10.1093/glycob/cwp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen MB, Sauer J, Jensen KJ. Chemoselective Capture of Glycans for Analysis on Gold Nanoparticles: Carbohydrate Oxime Tautomers Provide Functional Recognition by Proteins. Chemistry – A European Journal. 2009;15:1649–1660. doi: 10.1002/chem.200801521. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T, Prestegard JJ, et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology. 2015;25:1323–1324. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther T, Karamanska R, Chan RW, Chan MC, Jia N, Air G, Hopton C, Wong MP, Dell A, Malik Peiris JS, Haslam SM, et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013;9:e1003223. doi: 10.1371/journal.ppat.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cummings RD, Smith DF, Huflejt M, Campbell CT, Gildersleeve JC, Gerlach JQ, Kilcoyne M, Joshi L, Serna S, Reichardt NC, et al. Cross-platform comparison of glycan microarray formats. Glycobiology. 2014;24:507–517. doi: 10.1093/glycob/cwu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked α-2,3 to penultimate galactose residues. Journal of Biological Chemistry. 1988;263:4576–4585. [PubMed] [Google Scholar]
- Wilson IBH, Harthill JE, Mullin NP, Ashford DA, Altmann F. Core α1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology. 1998;8:651–661. doi: 10.1093/glycob/8.7.651. [DOI] [PubMed] [Google Scholar]
- Wu AM, Wu JH, Singh T, Lai L-J, Yang Z, Herp A. Recognition factors of Ricinus communis agglutinin 1 (RCA1) Molecular Immunology. 2006;43:1700–1715. doi: 10.1016/j.molimm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakashizuka M, Terada I. Cloning and Expression of a Marine Bacterial β-Galactoside α2,6-Sialyltransferase Gene from Photobacterium damsela JT0160. Journal of Biochemistry. 1998;123:94–100. doi: 10.1093/oxfordjournals.jbchem.a021921. [DOI] [PubMed] [Google Scholar]
- Yan S, Serna S, Reichardt N-C, Paschinger K, Wilson IBH. Array-assisted Characterization of a Fucosyltransferase Required for the Biosynthesis of Complex Core Modifications of Nematode N-Glycans. Journal of Biological Chemistry. 2013;288:21015–21028. doi: 10.1074/jbc.M113.479147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BY, Gray JSS, Montgomery R. The glycans of horseradish peroxidase. Carbohydrate Research. 1996;287:203–212. doi: 10.1016/0008-6215(96)00073-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.