Abstract
Abnormalities in mucus production and qualitative properties such as mucus hydration are central to the pathophysiology of airway disease including cystic fibrosis, asthma, and chronic bronchitis. In vitro air–liquid interface epithelial cell cultures demonstrate direct relationships between mucociliary transport, periciliary liquid (PCL) height, and mucus concentration (expressed as percent solids or partial osmotic pressure). In health, the osmotic modulus/pressure of the PCL exceeds that of the mucus layer, resulting in efficient, low-friction movement of mucus. In disease, through multiple mechanisms, the osmotic pressure of the mucus begins to exceed basal PCL values, resulting in compression of the cilia and slowing of mucus transport. The in vivo data in both cystic fibrosis and chronic bronchitis parallel in vitro data demonstrating that when mucus osmotic pressure is increased, mucociliary clearance is decreased. In chronic bronchitis, there is a direct correlation between FEV1 and percent solids of mucus, demonstrating a strong relationship between disease progression and mucus abnormalities. Animal models, based mechanistically on raised sodium absorption (and therefore water absorption) from airway surfaces, mimic the pathophysiology of chronic obstructive pulmonary disease. Collectively, these data suggest the importance of mucus concentration in the pathogenesis of airway disease. It is important to understand the precise mechanisms that result in mucus hyperconcentration, for example, mucin overproduction versus abnormal regulation of ion/water transport, which may be unique to and characteristic of each disease phenotype. The measurement of mucus concentration may be a simple method to diagnose chronic bronchitis, monitor its progression, and serve as a biomarker for development of new therapies.
Keywords: chronic bronchitis, mucus, osmotic pressure, mucociliary clearance
Chronic bronchitis is defined epidemiologically as chronic sputum production for 3 months for two consecutive years. Under this definition, the chronic bronchitic syndrome would include genetic forms of chronic bronchitis, for example, cystic fibrosis and primary ciliary dyskinesia, and the environmentally driven forms of chronic bronchitis, typically due to cigarette smoke. Indeed, to the extent that asthma often is characterized by chronic mucus production, it also can be included as a form of chronic bronchitis; but it will not be an emphasis of this review.
As implied by the definition of chronic bronchitis, abnormalities in mucus production and abnormal quantitative and qualitative mucus properties are central to the pathophysiology of chronic bronchitis. It is clear that abnormalities in the mucus production/clearance system can produce all of the aspects of chronic bronchitis, including chronic cough, sputum production, inflammation, and infection (1, 2). In this review, we cover the topic of how the mucus clearance system can be perturbed to produce the chronic bronchitic phenotype and how we may better define the progression and specific chronic bronchitic endotypes in the future.
The Airway Mucus Clearance System in Health and Disease
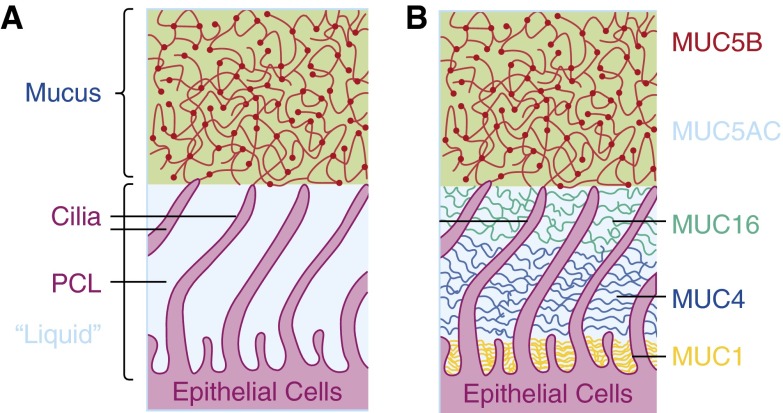
The normal airway mucus system was traditionally defined in the context of three elements: (1) a mobile mucus layer composed of the secreted mucins MUC5AC and MUC5B; (2) motile cilia, beating with a ciliary beat frequency of approximately 10 Hz; and (3) a watery periciliary layer in which the cilia beat in a low-viscosity environment (Figure 1A). However, this formulation did not accurately predict why there would be a single mucus (gel) layer on the airway surface, and it did not produce quantitative predictions of mucus clearance rates in health or disease. The organization of the mucus ciliary transport apparatus on the human airway surface has been revisited. Importantly, a series of biochemical, immunologic, and biophysical studies established that there is a “second gel” on the airway surface (3) (Figure 1B). This gel occupies the periciliary and cell surface environment and is composed of the tethered mucins MUC1, MUC4, MUC16, and possibly MUC20. Importantly, the density of the periciliary gel exhibits gradient-like features, with the more densely packed gel occurring toward the epithelial cell surface.
Figure 1.
Anatomy of the mucociliary apparatus. (A) Traditional view of system as composed of a mucus layer comprised of secreted mucins that resides over a water periciliary liquid (PCL) layer. (B) Two-gel (gel on brush) model of mucociliary apparatus. A mucus layer composed of MUC5B and MUC5AC secreted mucins resides over a periciliary and cell surface environment occupied by a relatively high density of tethered mucins, including MUC1, MUC4, and MUC16.
The observation that there are two gels on the airway surface has immense implications with respect to the distribution of water, that is, hydration, on the airway surface. Both the mucus and the periciliary gels are “hydrogels” with a density of mucins that produces a network of interpenetrating high molecular weight mucin polymers. In polymer physics terms, this concentration of mucin molecules produces a “semidilute” solution. The water-drawing power of hydrogels is proportional to their mucin polymer concentration. Under conditions in which the polymers are not penetrating or overlapping, the mucin osmotic water-drawing power, π, is linearly proportional to mucin number (n), that is, π = n ⋅ kT. However, polymers under semidilute, interpenetrating conditions exhibit a water-drawing power proportional to the second to third power of the concentration, that is, π ≅ kT/ξ2.5. Note that ξ is defined as the correlation coefficient, which is a measure of the distance between the nearest neighboring mucin molecules, that is, a measure of concentration.
Although mucus viscosity has often been measured in relation to mucus transport, we do not for the sake of space consider it in our analyses of mucus transport. We would note, however, that depending on the absence or presence of interactions between mucin polymers, mucus concentration is also the dominant variable controlling mucus viscosity, with viscosities ranging from the 2.5th to 8.5th power of concentration (4).
The periciliary gel contributes two important functions to the airway surface: (1) It acts as a molecular barrier, preventing penetration of large particles to the airway surface; and (2) it acts as a lubricant layer over which the mucus layer slides during surface transport. The lubricant powers of the periciliary liquid (PCL) heavily depend on its hydration status. Thus in health, the mucus transport system works most efficiently when the osmotic water-drawing power of the PCL, πPCL, exceeds that of the mucus layer. The normal airway epithelium possesses regulatory feedback mechanisms whereby motile cilia sense the hydration status of the mucus layer (5). In this scenario, cilia sense mucus layer osmotic modulus/concentration properties and, via mechanical transduction mechanisms, regulate release of ATP onto the extracellular surface. Extracellular ATP concentrations control the relative rates of fluid absorption (sodium-dependent) versus fluid secretion (chloride-dependent) to provide the appropriate amount of salt/water (hydration) on the airway surface. Thus, airway epithelia are configured in health to maintain an airway surface on which the osmotic modulus/pressure in the PCL exceeds that in the mucus layer (πPCL = ∼500 Pa; πML = ∼100 Pa), so that efficient low-friction movement of the mucus layer over the PCL surface is maintained.
In disease, there is an imbalance of the osmotic pressures of the mucus layer and the PCL, with the osmotic pressure of the mucus layer rising to values equal to and exceeding the basal PCL osmotic pressure value. This situation is achieved in cystic fibrosis (CF) by virtue of an imbalance of salt and water transport at the CF airway surface (6). Abnormal active epithelial transport of salt and water from the CF epithelial surface removes water from the gel with the lowest water-drawing power, that is, osmotic pressure. Thus, water is initially extracted from the mucus layer, increasing its mucin concentration and osmotic pressure.
In acquired disease, such as chronic obstructive pulmonary disease (COPD), the situation is most likely more complex. Cigarette smoke produces abnormalities in salt and water transport due to disruption of cystic fibrosis transmembrane conductance regulator (CFTR) transcription and degradation of extracellular ATP signals by increased ecto-ATPase expression (7–9). However, there is also likely a contribution of increased mucin secretion, without appropriate salt and water secretion to the pathogenesis of hyperconcentrated mucus (10–12).
The functional effect of having a mucus layer osmotic pressure that exceeds basal PCL osmotic pressure is osmotic compression of the PCL by the mucus layer. When it is mild, the osmotic imbalance will produce compression of the cilia and slow mucus transport due to inefficient cilial beat (13). As the disease progresses, virtually all of the extractable water will be removed from the PCL and the mucus layer will adhere to a flattened cilial surface. It is hypothesized that it is mucus layer adhesion to the cell surface that is the initiating factor in both genetic forms of bronchitis (e.g., CF) and the acquired forms (e.g., COPD).
The “Cart” versus “Horse” Problem: The Role of Mucus Abnormalities Producing Airway Disease versus Resulting from Airway Disease
In a recent report, there was a strong relationship between the degree of mucus concentration and osmotic pressure, and the severity of lung disease in COPD (10). The natural question that arises is whether the degree of mucus dehydration drives the severity of lung disease or is a reflection of the other pathways that produce disease, with mucus dehydration being secondary. To address this question, animal models of selective dehydration of airway surfaces have been produced. As reviewed elsewhere in this symposium, approximately a decade ago Mall and colleagues produced a transgenic model of epithelial Na+ channel β subunit (βENaC) overexpression (14). The model was based on the fact that the major pathway controlling absorption of sodium from the airway surface is the ENaC. Airway epithelial overexpression of each of the three ENaC genes that contribute polypeptides to the functional ENaC, that is, αENaC, βENaC, and γENaC, revealed that selective overexpression of βENaC produced a phenotype of accelerated sodium absorption. The importance of βENaC in this phenotype was consistent with data from others, suggesting that in heterologous oocyte expression systems selective expression of αENaC and βENaC mRNAs produced channels that were constitutively activated, that is, with an open probability (Po) of approximately 1 (15). Pharmacologic studies of amiloride inhibition of βENaC-mediated Na+ currents in wild-type versus βENaC mice were consistent with the notion of constitutively activated α/βENaC subunits in βENaC mice (16).
The unregulated absorption of sodium, without any molecular perturbation of Cl– secretory pathways, produced airway surface liquid volume hyperabsorption in the βENaC mice. This conclusion emanated from in vitro studies of airway surface liquid volume homeostasis by confocal microscopy and in vivo studies of freshly harvested mucus concentration, showing that mucus concentrations were raised as indexed by a greater percent solids content (14, 16). A series of studies were then designed to explore the phenotype of a murine model in which selective dehydration of the airway surface by an ion channel dysregulation mechanism was achieved. Importantly, airway surface dehydration appeared to produce the entire spectrum associated with chronic bronchitis (17). The features of chronic bronchitis recapitulated by this model included the following:
First, there was evidence of widespread mucus adhesion to, and plugging of, intrapulmonary airways (18). Direct measures of extracted mucus revealed an increase in concentration; bronchoalveolar lavage studies revealed increased mucin levels; and electron microscopy studies indicated the presence of osmotic compression/flattening of the cilia by an adherent mucus layer (17).
Second, there was evidence of airway inflammation (19). This evidence was derived from bronchoalveolar lavage studies of βENaC mice that revealed a persistent increase in neutrophil numbers, the presence of macrophage activation, and an increased concentration of proinflammatory cytokines/chemokines, including keratinocyte chemoattractant (KC), LIX, macrophage inflammatory protein (MIP)-1α, and tumor necrosis factor (TNF)-α. Histologic evaluation of βENaC mouse lungs also was consistent with the presence of neutrophilic inflammation in airway lumens and demonstrated the presence of bronchial-associated lymphoid tissue, an index of chronic inflammation.
Third, there was evidence of airway remodeling. Specifically, there was an increase in the airway epithelial height, consistent with epithelial proliferation. Importantly, there was also an increase in the density of metaplastic goblet cells with increased stores of both MUC5AC and MUC5B.
Fourth, there was evidence of infection. The infection was characterized by a spectrum of microaerophilic gram-positive and gram-negative organisms most prominently seen in the neonatal period (19). These organisms were also identified in the oropharynx of neonatal mice. Thus, it was speculated that the infection resulted from the aspiration of oropharyngeal contents that were poorly cleared in the βENaC mouse.
Finally, a number of novel observations were made with respect to the pathogenesis the bronchitis exhibited by the βENaC mouse model. First, the pulmonary inflammation appeared to persist in βENaC mice raised in gnotobiotic facilities (19). This observation suggests that mucostasis per se, over and above the effects of bacterial infection of mucus, can produce the chronic bronchitic inflammatory phenotype noted in humans. Second, longitudinal studies noted the dramatic gene and functional responses to airway mucus dehydration provided by airway and alveolar macrophages (20). Similar to the neutrophilia in the βENaC mice, the macrophage activation persisted, and indeed was perhaps more robust, in macrophages derived from βENaC mice raised under gnotobiotic conditions.
Collectively, it appears from these studies that mucus dehydration and the predicted osmotically induced mucus stasis alone can produce the phenotype of bronchitic lung disease. Of course, disease in humans is likely not so simple, with superimposed mechanisms resulting from environmental conditions that may worsen the airway mucus dehydration and produce positive inflammatory feedbacks loops and accelerated disease progression.
Relevance of Mucus Dehydration Properties in Human Disease
As noted previously, cystic fibrosis is characterized by abnormal ion transport pathways. In human CF airways, it is likely that the absence of salt and water secretion, mediated by defective CFTR chloride/bicarbonate secretory activity in the superficial epithelium and in the glands, contributes to a depletion of airway surface liquid volume (21, 22). This abnormality is complemented by the regulatory abnormality in CF of the epithelial sodium channel that produces persistent and inappropriate sodium and liquid absorption (6). These two problems in combination produce the severe dehydration of airway surfaces in CF that leads to CF airway disease. Note that this airway surface depletion is heterogeneous in CF, and reflects the heterogeneity of CF lung disease. We have speculated that the CF dehydration phenotype is triggered by exogenous insults, for example, viral infection and aspiration, at airway epithelial surfaces that reveal the CF-limited adaptive responses to such insults (23).
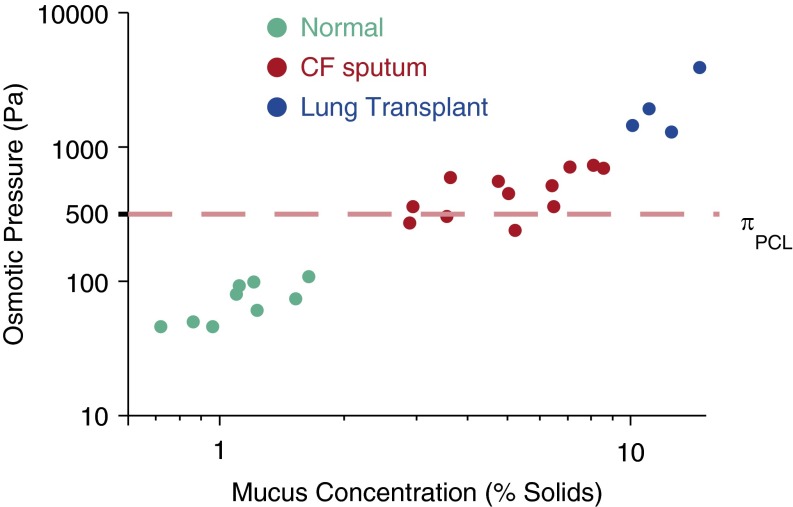
The generation of dehydrated, hyperconcentrated mucus in patients with CF has been predicted from in vitro studies but has recently been confirmed by in vivo data. Using sputum induced from normal subjects, sputum obtained from subjects with CF during periods of clinical stability, and mucus obtained from CF lungs at the time of transplantation (i.e., the mucus that could not be coughed out as sputum), a relationship was established between mucus osmotic pressures and previously measured PCL osmotic pressures (24) (Figure 2). These data revealed that the normal human mucus osmotic pressure was indeed approximately 100 Pa, a value well below that of the osmotic pressure of the PCL (500 Pa). The osmotic pressures of normal subjects were associated with mucus percent solids concentrations of approximately 1.5–2%. In CF sputum, the percent solids contents rose to approximately 7% and were associated with osmotic pressures that exceeded basal PCL values, that is, 600–700 Pa. Importantly, the mucus that could not be coughed out by subjects with CF, that is, mucus obtained at transplantation, showed percent solids in excess of 10% and mucus osmotic pressures in the 3,000- to 4,000-Pa range. It is particularly in this range of mucus concentration/osmotic pressures that mucus adhesion is predicted to occur. Thus it appears likely that mucus dehydration is a fundamental component of CF lung disease pathogenesis, and further studies in earlier and progressive forms of CF lung disease are warranted.
Figure 2.
Osmotic pressure of normal versus cystic fibrosis (CF) mucus. Percent solids and osmotic pressure of induced sputum from normal subjects, spontaneous sputum for subjects with stable CF, and mucus obtained from CF lungs at the time of transplantation are shown. The dashed line depicts the basal periciliary liquid (PCL) osmotic pressure value from Button and colleagues (3).
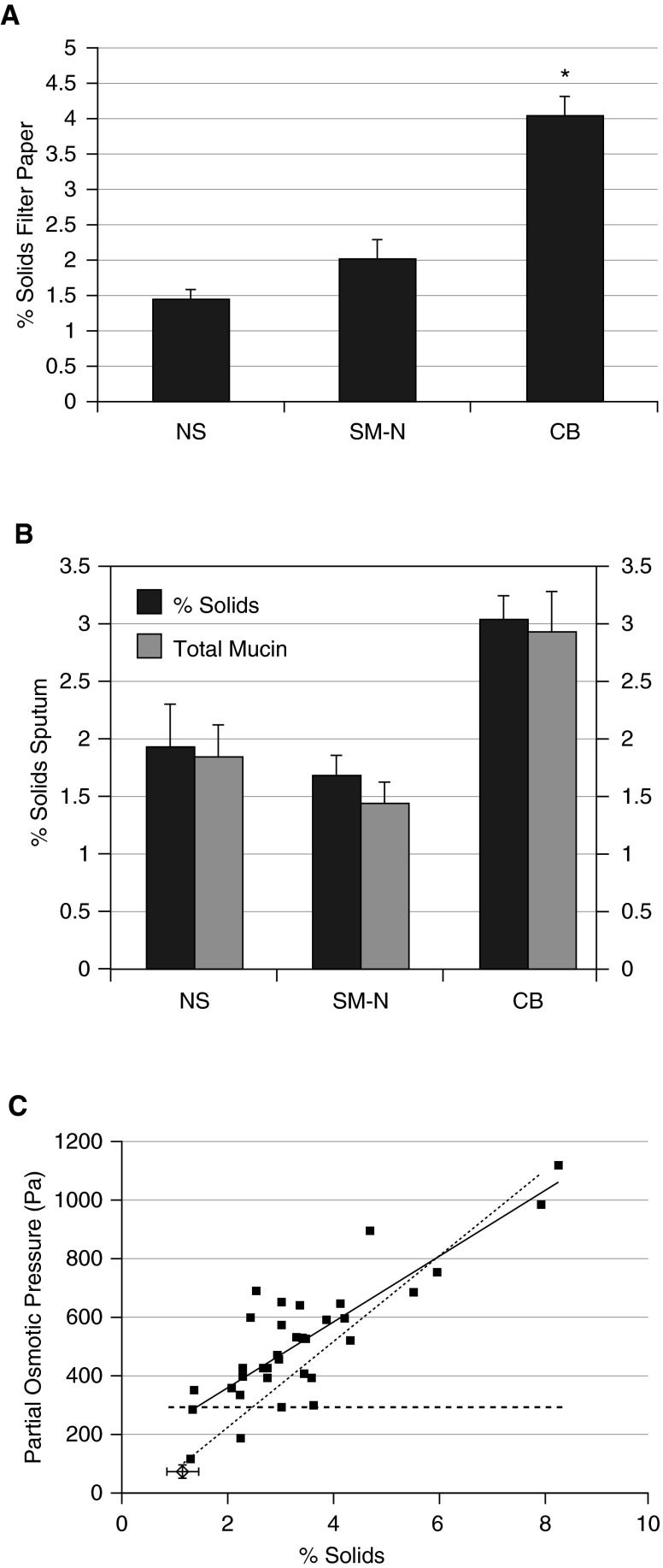
Similarly, studies have reported the mucus hydration and mucin concentrations in normal subjects, smokers without lung disease, and subjects with cigarette smoke–induced chronic bronchitis with a spectrum of FEV1 values and COPD severity (10). Using direct filter paper sampling to measure mucus percent solids, it was found that subjects with cigarette smoke–induced chronic bronchitis had percent solids concentration values that were approximately 2.5-fold higher than those of normal subjects or smokers with no lung disease (Figure 3A). Induced sputum collected from these same subjects revealed a smaller but significant increase in percent solids for subjects with CB versus normal subjects, and the increase in percent solids was paralleled by an increase in the total mucin content of CB induced sputum as compared with normal subjects or smokers without lung disease (Figure 3B). Further, the relationship between percent solids and the osmotic pressure of sputum from subjects with CB was assessed, and it was found that there was a proportionate increase in osmotic pressure as a function of the increase in percent solids (Figure 3C). The relationship between mucus concentration and mucus transport was tested in vitro, and a direct relationship between an increase in mucus solids content and a reduction in mucus transport rates was observed (10). This relationship was paralleled by studies in which in vivo mucus transport, measured by inhaled radiotracer clearance techniques, was found to be deceased as a direct function of the increase in mucus percent solids measured by the filter paper technique.
Figure 3.
Mucus concentration (expressed as percent solids) for normal subjects (NS), cigarette smokers with normal lung transfer (SM-N), and subjects with chronic bronchitis (CB). (A) Samples obtained by the filter paper technique. (B) Sputum percent solids and total mucin concentrations of induced sputum for NS, SM-N, and CB subjects. (C) Relationship between sputum percent solids and measured osmotic pressure for subjects with CB. Dashed line depicts basal periciliary liquid (PCL) osmotic pressure.
The percent solids versus osmotic pressure relationship for both CF and COPD sputum samples was compared with the relationship reported for mucus collected from sterile human bronchial epithelial cultures. The relationships between percent solids and osmotic pressures for both CF and COPD sputum approximated that of human bronchial epithelial mucus. This observation suggests that the inflamed and proteolytic environment of CF, and to a lesser extent COPD, airways did not dramatically alter the osmotic properties of the mucins and large molecules in diseased sputum (25). Further, to the extent that there may be small pH differences between CF and COPD sputum, these comparisons suggest that pH effects on osmotic pressure are small compared with the effects of concentration.
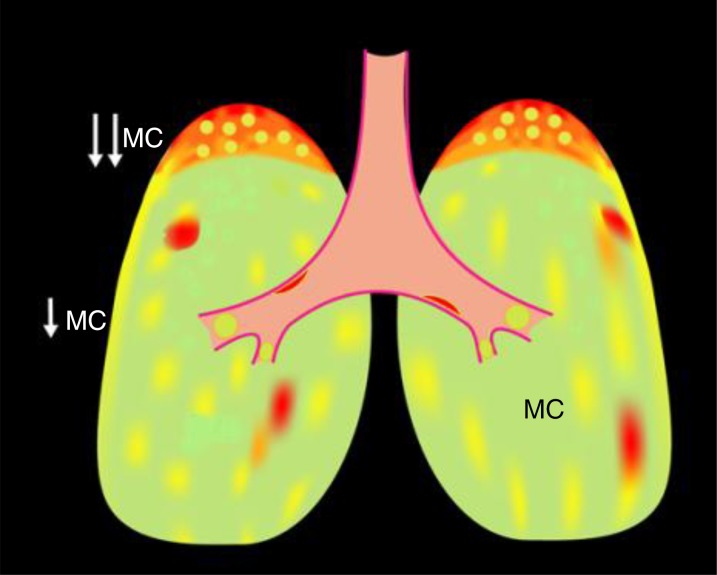
It should be noted that neither direct transbronchoscopic filter paper sampling nor sputum techniques for sampling mucus for study are optimal in patients with chronic bronchitis that heterogeneously affects lung surfaces (Figure 4). Direct filter paper measures avoid oral contamination but require bronchoscopy and often are limited to samples from one designated site, for example, the right main stem bronchus. The spontaneous sputum technique likely samples multiple diseased, but not normal, areas of the central lung. In contrast, induced sputum, which generates samples from “normal” areas and diseased areas of the central lung, may give a better estimate of average lung mucus properties. Depending on the experimental question, a measure of the diseased areas or of average lung areas, may be advantageous. However, both sputum techniques suffer from variable contamination with oral saliva that contains predominantly MUC5B and is of low ionic strength. Exhaled breath condensates have been adapted to sample small volumes of microaerosols collected from the expired airstream (26). It is difficult to directly measure mucin concentrations in such small samples, but measurement of the ratio of a mucin component, sialic acid, to urea may allow estimates of mucin concentrations in such samples. However, exhaled breath condensate samples may sample exclusively distal airway sites and, again, suffer from oral contamination.
Figure 4.
Heterogeneity of muco-obstructive lung disease with respect to mucin hyperconcentration and decreased mucus clearance. Green depicts areas of normal mucus concentrations and clearance; yellow depicts areas of increased mucus concentrations and decreased clearance; and red depicts areas of very concentrated mucus and mucus adhesion. The heterogeneity of disease presents broad challenges with respect to mucus sampling, that is, with techniques that harvest samples from discrete areas of disease and/or region (central vs. peripheral). MC = mucus clearance.
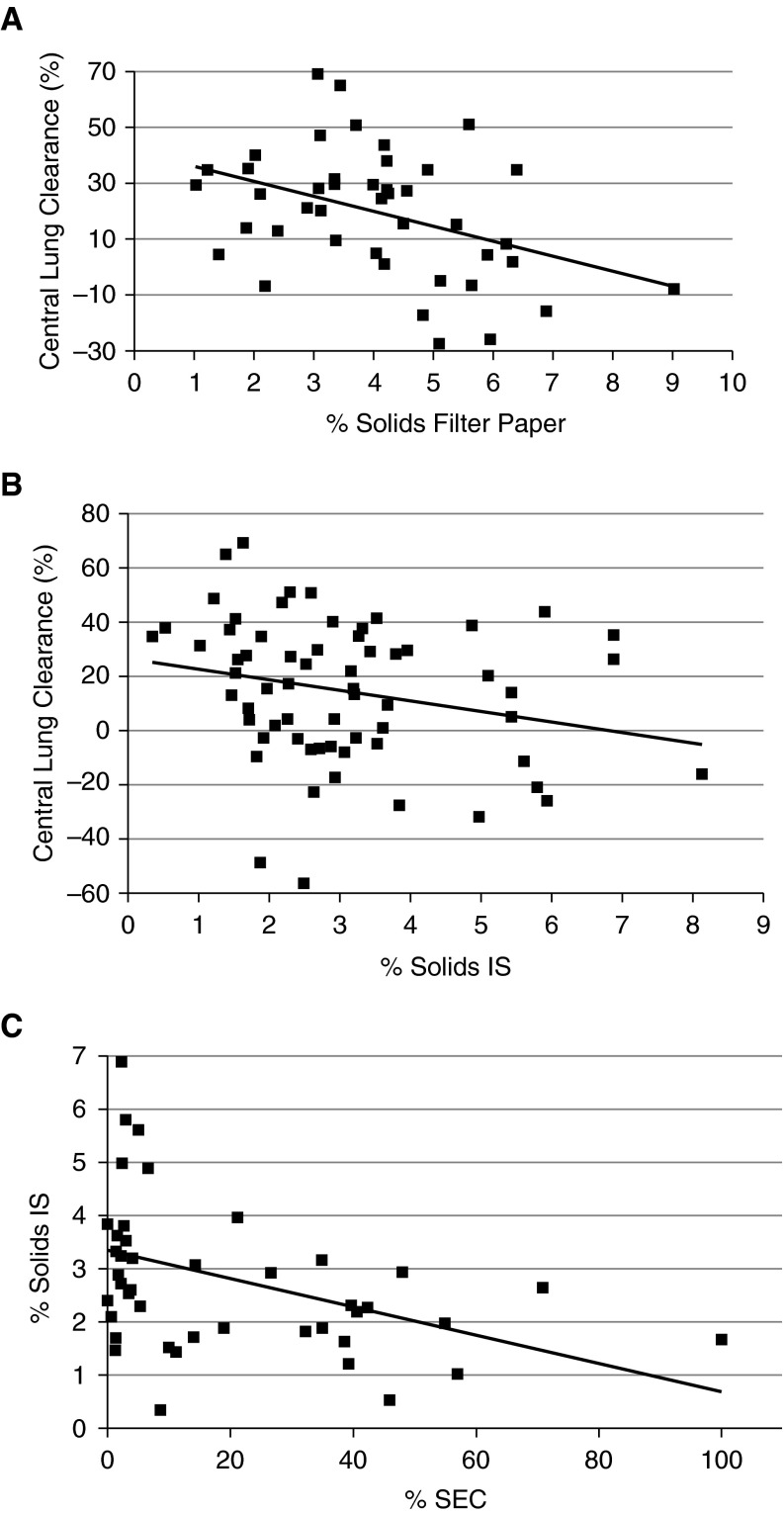
We have compared induced sputum with filter paper samples with respect to measured parameters pertinent to CB. As noted previously, sputum samples exhibited a smaller fold percent solids increase in subjects with CB versus normal subjects as compared with percent solids filter paper samples. Predictably, the negative correlation between percent solids and in vivo measurements of mucus clearance was less strong for induced sputum than for filter paper samples (Figures 5A and 5B). Variable oral contamination may have contributed to the decrement in correlation for induced sputum. As a test of the contribution of oral contamination to sputum percent solids, we examined the relationship between a marker of oral contamination, squamous cells, and sputum percent solids. As predicted from the dilute nature of saliva, a strong negative correlation between this marker of salivary contamination and “sputum” percent solids was observed (Figure 5C).
Figure 5.
Correlation of mucus via sample type versus central lung clearance and induced sputum (IS) contamination. (A) Bronchoscopic filter paper–obtained percent solids versus central clearance for subjects with chronic bronchitis (CB) (R = –0.387; P = 0.01). (B) Induced sputum values for percent solids versus central clearance for the same subjects with CB (R = –0.271; P = 0.03). (C) The relationship between percentage of squamous epithelial cells (SECs) in sputum versus percent solids in induced sputum (R = –0.427; P = 0.005).
The observation of large variability with induced sputum samples is not unique to percent solids measurements and has been a common problem in sputum studies of inflammatory markers and infection. Regardless of the limitations of sample collection, it appears that CB secretions are characterized by increased percent solids, and this increase may contribute directly to the decrements in mucus clearance, and likely the generation of the mucostasis that characterizes these subjects.
Conclusions
The data from an animal model and from the study of two different forms of chronic bronchitis, that is, the genetic disease CF and the environmentally induced COPD, point to the importance of mucus concentration in the pathogenesis of airway diseases. Ironically, mucus concentration has not been a widely explored property of mucus. This oversight likely reflects the absence of a biophysical framework to predict the mechanisms that control mucus transport and the great importance of mucus concentration, via the second- to third power–dependent relationships between critical biophysical properties (osmotic pressure), and mucus transport rates. The recognition that mucus concentration may be an important, pathophysiological driver of the bronchitic phenotype has three implications for future studies.
First, it will be important to understand the precise mechanisms that are unique and characteristic to each disease phenotype that is characterized by mucus hyperconcentration. For example, as noted previously, CF likely reflects early in its pathogenesis the effects of ion transport defects that produce volume (liquid) depletion. Later in CF, airway remodeling with mucus cell hyperplasia and mucin hypersecretion may worsen mucus hyperconcentration. Similarly, the causes of airway mucus hyperconcentration in CB are likely multifactorial. We would note that mucin secretion typically is associated with a proportionate release of ATP and adenosine that provides a paracrine hydration stimulus for normal hydration of secreted mucins (27). The observation that COPD secretions were associated with decreased ATP and adenosine levels points to the likely importance of ecto-ATPases induced by inflammation as contributors to the dehydration phenotype in COPD (10).
Second, the recognition that percent solids (hydration) of mucus is important in the pathogenesis of CB types of lung disease suggests that the hydration status of mucus might be an important biomarker to identify/characterize patients with CB. Studies of mucus hydration have thus far been performed only in patients who were clinically selected for CB and, hence, it will be informative to study broader patient populations in the future. Specifically, it will be important to study large populations of subjects with COPD with a spectrum of CB versus emphysema phenotypes regarding mucus concentration properties.
Finally, the projected importance of mucus concentration in CB predicts that novel therapies should be developed to normalize the concentration of mucus on airway surfaces of patients with CB disease. This goal can be achieved by increasing the salt and water content on the airway surface, decreasing mucin secretion rates, or a combination of both approaches.
Footnotes
Supported by NIH grants: SCCOR (Specialized Centers of Clinically Oriented Research; grant 5P50HL084934), TPPG (Translational Program Project Grant; grant P01HL108808), and MTCC (Molecular Therapy Core Centers; grant P30DK065988); and by a CFFT (Cystic Fibrosis Foundation Therapeutics) grant (BUTTON07XX0).
Author Contributions: B.B., W.H.A., and R.C.B. were involved in one or more of the following: design, acquisition of data, analysis, and interpretation. All authors were involved in drafting and revising, and have approved the final manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wanner A, Salathé M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 2.Munkholm M, Mortensen J. Mucociliary clearance: pathophysiological aspects. Clin Physiol Funct Imaging. 2014;34:171–177. doi: 10.1111/cpf.12085. [DOI] [PubMed] [Google Scholar]
- 3.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinstein M, Colby RH. Polymer physics. Oxford: Oxford University Press; 2003. [Google Scholar]
- 5.Button B, Okada SF, Frederick CB, Thelin WR, Boucher RC. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci Signal. 2013;6:ra46. doi: 10.1126/scisignal.2003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 7.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 9.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med. 2015;192:182–190. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 12.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis–like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 15.Chalfant ML, Denton JS, Langloh AL, Karlson KH, Loffing J, Benos DJ, Stanton BA. The NH2 terminus of the epithelial sodium channel contains an endocytic motif. J Biol Chem. 1999;274:32889–32896. doi: 10.1074/jbc.274.46.32889. [DOI] [PubMed] [Google Scholar]
- 16.Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O’Neal WK, Pradervand S, et al. Airway surface liquid volume regulation determines different airway phenotypes in Liddle compared with βENaC-overexpressing mice. J Biol Chem. 2010;285:26945–26955. doi: 10.1074/jbc.M110.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, et al. Development of chronic bronchitis and emphysema in β-epithelial Na+ channel–overexpressing mice. Am J Respir Crit Care Med. 2008;177:730–742. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livraghi-Butrico A, Grubb BR, Kelly EJ, Wilkinson KJ, Yang H, Geiser M, Randell SH, Boucher RC, O’Neal WK. Genetically determined heterogeneity of lung disease in a mouse model of airway mucus obstruction. Physiol Genomics. 2012;44:470–484. doi: 10.1152/physiolgenomics.00185.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O’Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol. 2012;5:397–408. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saini Y, Dang H, Livraghi-Butrico A, Kelly EJ, Jones LC, O’Neal WK, Boucher RC. Gene expression in whole lung and pulmonary macrophages reflects the dynamic pathology associated with airway surface dehydration. BMC Genomics. 2014;15:726. doi: 10.1186/1471-2164-15-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol. 2014;307:L917–L923. doi: 10.1152/ajplung.00326.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke LW, Myerburg MM, Markovetz MR, Parker RS, Weber L, Czachowski MR, Harding TJ, Brown SL, Nero JA, Pilewski JM, et al. Quantitative imaging of airway liquid absorption in cystic fibrosis. Eur Respir J. 2014;44:675–684. doi: 10.1183/09031936.00220513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, et al. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, DeMaria GC, Matsui H, Donaldson S, Davis CW, et al. Mucins are abnormally concentrated in CF respiratory secretions: role in disease pathogenesis. J Clin Invest. 2014 doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esther CR, Jr, Coakley RD, Henderson AG, Zhou YH, Wright FA, Boucher RC. Metabolomic evaluation of neutrophilic airway inflammation in cystic fibrosis. Chest. 2015;148:507–515. doi: 10.1378/chest.14-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreda SM, Boucher RC, Lazarowski ER. Nucleotide release and airway epithelial physiology. Physiology News. 2008;71:19–22. [Google Scholar]