Abstract
In allergic asthma, aeroallergen exposure of sensitized individuals mobilizes robust innate and adaptive airway immune responses, stimulating eosinophilic airway inflammation and the activation and infiltration of allergen-specific CD4+ T cells into the airways. Allergen-specific CD4+ T cells are thought to be central players in the asthmatic response as they specifically recognize the allergen and initiate and orchestrate the asthmatic inflammatory response. In this article, we briefly review the role of allergen-specific CD4+ T cells in the pathogenesis of human allergic airway inflammation in allergic individuals, discuss the use of allergen–major histocompatibility complex class II tetramers to characterize allergen-specific CD4+ T cells, and highlight current gaps in knowledge and directions for future research pertaining to the role of allergen-specific CD4+ T cells in human asthma.
Keywords: allergens, helper T type 2 cells, CD4-positive T lymphocytes, inflammation, humans
CD4+ T Cells in Subjects with and without Allergic Asthma
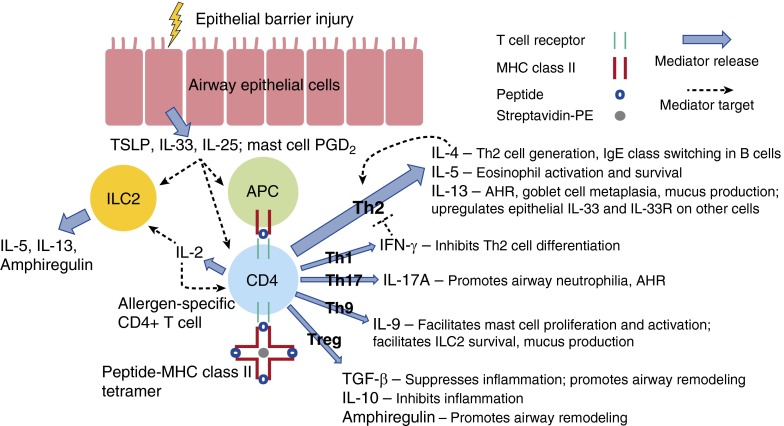
Over the last several decades, the rising prevalence of allergic diseases and asthma in the developed world has encouraged research into the underlying environmental and immunologic mechanisms responsible for this common disease. Since the important dichotomy between CD4+ helper T-cell type 1 (Th1) and Th2 immune responses was first elucidated (1), investigators have established that Th2 cells play a critical role in allergic asthma (2, 3). An atopic milieu and respiratory infections early in life may predispose certain individuals to develop asthma later in life. Prescott and colleagues found that allergen-specific Th2 immune responses combined with defective Th1 immune responses during early childhood may contribute to the persistence of atopy (4). More recent research suggests that impaired type I interferon responses to upper respiratory viruses may lead to increased production of innate type 2 cytokines, such as thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, which can promote allergic airway inflammation (5). The role of other helper T subsets in asthma has also been elucidated: aberrant Th17 or Th2/Th17 immune responses, which promote IL-17A production and airway neutrophilia (6); Th9 immune responses, which promote IL-9 production, facilitating mast cell proliferation and activation as well as airway remodeling (7); and impaired regulatory responses due to inadequate production of immunoregulatory cytokines (e.g., transforming growth factor-β and IL-10), inadequate contact-dependent immune regulation (e.g., through cytotoxic T-lymphocyte antigen-4), or conversion of regulatory T cells (Tregs) to Th2 effector cells (8) may exacerbate the development and persistence of type 2 inflammation. The role of these various T-cell subsets and their interactions with structural, innate, and regulatory cells are summarized in Figure 1 and have been reviewed by Lambrecht and Hammad (9).
Figure 1.
Allergen-specific CD4+ T-cell subsets and their interactions with structural, innate, and regulatory cells in the asthmatic airway. In response to epithelial barrier injury, airway epithelial cells release innate type 2 cytokines, including thymic stromal lymphopoietin (TSLP), IL-33, and/or IL-25, which promotes allergic airway inflammation by acting on helper T type 2 (Th2) cells, type 2 innate lymphoid cells (ILC2s), and antigen-presenting cells (APCs), including dendritic cells (DCs) and macrophages. Activated mast cells release numerous mediators, including prostaglandin D2 (PGD2), which promotes airway hyperresponsiveness (AHR) and facilitates the migration and activation of Th2 cells, ILC2s, and other cells. Allergen that breaches the damaged epithelium can be processed and presented by APCs to prime allergen-specific CD4+ T cells. Depending on the cytokine milieu, allergen-specific CD4+ T cells may become Th2-polarized or may proceed down other differentiation pathways: Th1, Th17, Th9, or regulatory T cell (Treg). Allergen-specific CD4+ T cells can be identified with peptide–MHC class II tetramers, which consist of four MHC class II molecules associated with an antigenic peptide bound to streptavidin linked to phycoerythrin (PE). Activated allergen-specific CD4+ T cells produce IL-2, which acts as a growth factor for T cells and ILC2s. Th2 cells specifically produce IL-4, which promotes the generation of more Th2 cells in a positive feedback loop and class switching in B cells to produce allergen-specific IgE, IL-5, which stimulates eosinophil activation and survival, and IL-13, which promotes AHR, goblet cell metaplasia, mucus production, and may also up-regulate epithelial IL-33 and IL-33 receptor (IL-33R) on other cells. Th1 cells produce IFN-γ, which can counteract Th2-cell differentiation. Th17 cells produce IL-17A, which promotes the production of cytokines and chemokines that recruit neutrophils to the airway and may also promote AHR by enhancing smooth muscle cell contractility. Th9 cells produce IL-9, which facilitates mast cell proliferation and activation, facilitates ILC2 survival, and may also promote mucus production and airway remodeling. Tregs produce IL-10, which can dampen inflammation, and transforming growth factor (TGF)-β, which can suppress inflammation and, along with ILC2-derived amphiregulin, may promote airway remodeling. Impaired regulatory responses may exacerbate the development and persistence of type 2 inflammation. Thick blue arrows indicate the production of a cytokine or mediator by a particular cell; thin dotted black lines indicate the activity of a cytokine or mediator on a particular cell.
Studies in humans have demonstrated that levels of Th2 cytokines, including IL-4, IL-5, and IL-13, are elevated in the airways of subjects with asthma at baseline and after allergen challenge (10). Increased expression of Th2 transcription factors STAT6 (signal transducer and activator of transcription-6) and GATA3 (GATA-binding protein-3) is detected in bronchial biopsies of patients with asthma (11, 12). Allergen challenge of human subjects with allergic asthma induces airway inflammation characterized by enhanced eosinophil activity (13), mast cell activation (14), and leukotriene release (15). Elevation of Th2-associated cytokines also correlates with disease activity and bronchial hyperresponsiveness (16). Subjects with allergic asthma typically exhibit enhanced Th2 immune responses compared with healthy control subjects (3, 17–20), allergic subjects without asthma (17, 18, 20), or subjects with nonallergic asthma (2, 18, 19). However, some studies have identified increased Th2 immune responses in all subjects with asthma regardless of atopic status (21–23). This divergence in findings is not contradictory; it merely highlights the complexity of asthma due to the existence of various asthma phenotypes/endotypes (24), and the limitation of assessing immune responses in human asthma because of the heterogeneity in subjects, study end points, and time points.
The pathophysiologic and immunologic mechanisms that distinguish subjects with allergic asthma from allergic subjects without asthma remain incompletely elucidated. Airway hyperresponsiveness (AHR) represents a key difference between subjects with allergic asthma and allergic subjects without asthma (25), and serves as an objective criterion for defining asthma. Patients with allergic rhinitis have a greater than twofold risk of developing AHR (26); indeed, there may be a “continuum” of AHR whereby nonatopic subjects have no AHR, those with allergic rhinitis have minimal or only nasal reactivity, and subjects with asthma have marked AHR (27), and this AHR continuum may correlate with levels of airway inflammation (i.e., those with allergic rhinitis have minimal or intermediate airway inflammation; those with asthma have more marked airway inflammation) (28). This suggests that Th2 immune responses may play an important role in mediating an “atopic march” to asthma. Because AHR is a defining feature of asthma, the airway epithelium and its interaction with innate and adaptive immune cells and airway structural cells have become an important focus of research. Impaired airway epithelial barrier function or damage to the airway epithelium may lead to the release of innate type 2 cytokines, including TSLP, IL-25, and IL-33, which in turn enhances IL-4, IL-5, and IL-13 production from CD4+ T cells and ILCs, promotes the activation and survival of B cells, eosinophils, mast cells, ILCs, and basophils, and increases goblet cell hyperplasia, mucus hypersecretion, and airway smooth muscle cell reactivity (29). Thus, in allergic asthma, epithelial and innate immune responses potentiate activation of the adaptive immune system, which is likely propagated in an allergen-specific manner by CD4+ T cells, and these responses eventually result in persistent structural changes due to airway remodeling and variable airflow obstruction.
Understanding the differences in CD4+ T-cell immune responses between subjects with allergic asthma and allergic subjects without asthma, and how they interact with the airway epithelium and innate immune cells, may shed light on the fundamental immunologic factors that underlie asthma.
Several studies comparing subjects with allergic asthma and allergic subjects without asthma have revealed key differences in type 2 immune responses (17, 18, 30, 31). Studies have revealed that both subjects with allergic asthma and allergic subjects without asthma exhibited enhanced allergen-specific CD4+ T-cell activation and IL-4 production from stimulated peripheral blood mononuclear cells (PBMCs) compared with healthy control subjects (17, 30). However, subjects with allergic asthma generated more IL-5 from stimulated PBMCs or bronchoalveolar lavage (BAL) than either allergic subjects without asthma or healthy control subjects (17, 31). Tang and colleagues also found that IL-5 production by CD4+ T cells could be enhanced by alveolar macrophages from subjects with allergic asthma but not by those from allergic subjects without asthma (32, 33). In another study, healthy individuals with detectable levels of allergen-specific IgG had more circulating IL-10–secreting CD4+ T cells (compared with IL-4+CD4+ or IFN-γ+CD4+ T cells), whereas allergic individuals had more IL-4–secreting CD4+ T cells (34). These studies highlight differences in CD4+ T-cell immune responses between those with allergic asthma, allergic subjects without asthma, and healthy control subjects.
Studies have diverged regarding findings related to Tregs. Some have found that there were similar numbers of circulating Tregs in subjects with asthma (whether in exacerbation or not) compared with healthy control subjects (35), and in atopic individuals compared with nonatopic individuals (36). One study found that allergen-specific Tregs were detectable and functionally active in the peripheral blood of both nonatopic control subjects and subjects with asthma (37); however, other studies have found that the total number of airway Tregs was lower in subjects with asthma (38), and the suppressive function of Tregs (39) and IL-10 production (40) may also be decreased in subjects with asthma compared with healthy control subjects. These divergent findings highlight the fact that there are still unresolved questions regarding blood and BAL Treg frequency and function in allergic asthma that require further investigation to clarify (1) how regulatory mechanisms may attenuate Th2-associated allergic airway inflammation, and (2) whether such regulatory mechanisms occur in an allergen-specific manner.
Allergen–Major Histocompatibility Complex Class II Tetramers Identify Allergen-Specific CD4+ T Cells
Before the availability of allergen–major histocompatibility complex (MHC) class II tetramers, the identification of allergen-specific T cells relied on their ex vivo response to allergen stimulation, such as T-cell proliferation and cytokine expression, or induction of activation markers, such as CD25 or CD40 ligand (CD40L). Because of the scarcity of allergen-specific CD4+ T cells in peripheral blood or BAL, ex vivo stimulation was required to induce clonal expansion to facilitate the detection of these CD4+ T cells. However, this approach does not provide precise quantitation of the actual number of allergen-specific CD4+ T cells in blood or tissue, nor does it allow for the phenotypic and functional characterization of allergen-specific CD4+ T cells without ex vivo stimulation. Bonvalet and colleagues found that there was no consistent combination of activation markers (e.g., CD25, CD30, CD39, CD69, CD137, CD154, GITR [glucocorticoid-induced tumor necrosis factor receptor], HLA-DR [human leukocyte antigen-DR], and ICOS [inducible costimulator]) that could be used to identify the same populations of allergen-specific CD4+ T cells as those identified using tetramers, suggesting that bystander (non–allergen-specific) T cells may be activated after allergen stimulation and that some tetramer-positive allergen-specific T cells may not be activated and may be anergic (41). This finding highlights the fact that the general insights provided by any surrogate activation marker approach may lack the precise characterization afforded by tetramer staining of allergen-specific CD4+ T cells.
The availability of allergen-specific tetramer staining has the potential to greatly enhance our understanding of allergen-specific CD4+ T-cell immune responses within the blood and airways of asthmatics, providing a unique opportunity to investigate the in vivo frequency and phenotype of allergen-specific CD4+ T cells at baseline and after allergen exposure while avoiding the possible confounding factors associated with ex vivo stimulation. Allergen–MHC class II tetramers are composed of complexes of four MHC class II molecules associated with a specific peptide and bound to a fluorochrome (42). This is more challenging than the design of MHC class I tetramers because the affinity between CD4+ T cells and MHC class II is lower than that between CD8+ T cells and MHC class I. One key benefit (and limitation) of allergen–MHC class II tetramers is that each tetramer is specific to only one peptide of an allergen; furthermore, epitopes recognized by these tetramers are HLA-restricted, which means that HLA typing of human subjects is a necessary component of research that uses this tool. Evaluation of allergen-specific CD4+ T cells can be streamlined by (1) identifying the most common allergens that have an impact on allergic asthma, including cat allergen, house dust mite allergen, and cockroach allergen (43–47), and (2) designing and using tetramers that incorporate the most common HLA types.
Studies have identified the major cat (Fel d 1) T-cell epitopes and have also compared cat-allergic subjects and healthy control subjects in terms of their T-cell responses to Fel d 1 protein and peptides (48–50). Cat allergen–derived peptides that do not cross-link IgE are able to stimulate T-cell proliferation and IL-5 production, which highlights the importance of MHC class II–restricted Th2 immune responses in allergen-specific late asthmatic reactions in sensitized subjects with asthma (51). Fel d 1–specific HLA class II tetramers have also been used to investigate cat allergen–specific T-cell function (52–54). Using Fel d 1 class II DRB1*0101 tetramers, one group found that circulating Fel d 1–specific DRB1*0101-restricted CD4+ T cells from patients with atopic dermatitis maintain a central memory phenotype, expressing high levels of CCR7, CD62L, CD27, and CD28, suggesting that this pool of cells may contribute to persistent atopic disease (55). Another study investigated DR1-restricted Fel d 1–specific T cells in a DR1 transgenic mouse allergic asthma model and found that peptide immunotherapy led to an increase in IL-10+ T cells and reduced the recruitment, proliferation, and effector function of allergen-specific Th2 cells (52). This highlights the potential usefulness of immunomodulatory peptide immunotherapy for the treatment of allergic asthma and of monitoring allergen-specific CD4+ T cells longitudinally as a readout of disease activity and control. Although a similarly detailed characterization of T cells has not been performed in humans yet, treatment of subjects with allergic asthma with low doses of peptides containing T-cell epitopes from Fel d 1 did reduce allergic sensitization and improve surrogate markers of disease (56). A more recent study found that more than 90% of Fel d 1–specific CD4+ T cells in cat-allergic subjects were CD45RO+, CD28+, CD62L+, and CCR4+; CRTH2+/− and CCR7+/−; and mostly CXCR3– and CCR6– (as opposed to influenza-specific CD4+ T cells, which were CXCR3+, CCR4–, and CRTH2–), indicating that Fel d 1–specific T cells exhibit a distinct Th2 memory phenotype compared with other lung antigen–specific T cells (such as virus-specific T cells) (53). These studies suggest that cat allergen–specific CD4+ T cells can be identified in subjects with allergic diseases, produce mediators that promote allergic inflammation, and may exhibit a distinct Th2 memory phenotype; however, further studies are needed to investigate this in human asthma.
Research has identified major dust mite (Der p 1) epitopes, and investigated T-cell immune responses in dust mite–associated allergy (17, 57–60). DCs from dust mite–sensitive patients exposed to Der p 1 generated enhanced Th2 immune responses compared with those from healthy subjects (59). Th2 cytokines were increased in PBMCs derived from subjects with allergic asthma (including children) (58) and BAL (17) after stimulation with dust mite allergen when compared with nonatopic control subjects. Dust mite–specific Th2 cells are increased in the skin and blood of patients with atopic dermatitis (57) and dermatitis can be exacerbated by bacterial superantigen in an MHC class II–restricted, IL-4+CD4+ T cell–dependent manner (61). Wambre and colleagues found that peripheral seasonal allergen-specific CD4+ T cells (e.g., Bet v 1/birch-specific) exhibited a higher avidity than perennial allergen-specific CD4+ T cells (e.g., Der p 1/dust-mite specific); the former exhibited a mix of effector (CD62L–CCR7–) and central memory (CD62L+CCR7+) phenotypes, whereas the latter were mostly central memory (62). In nonallergic subjects, both birch- and dust-specific T cells produced IFN-γ and IL-10, whereas in allergic subjects, birch-specific immune responses were mainly Th2, while dust-specific responses varied (Th1, Th2/Th1, or Th2/Th17) (62). These studies suggest that dust mite–specific CD4+ T cells can be identified and phenotyped in subjects with allergic diseases and exhibit a distinct memory phenotype. However, unlike cat allergen–specific CD4+ T cells, which exhibited more of a Th2 memory phenotype (53), dust mite–specific CD4+ T cells exhibited a more heterogeneous memory phenotype, which could have important implications for the impact of these T cells on different phenotypes of asthma (e.g., eosinophilic asthma in the case of more Th2-predominant T-cell responses or neutrophilic asthma in the case of Th17). HLA class II tetramers have emerged as an important tool for quantitative analysis of allergen-specific T-cell immune responses in allergy associated with exposure to animals (cat, cow, horse), dust mites, trees (alder, birch), grasses (blue, rye, timothy), and weeds (mugwort), but further research is needed to investigate the use of tetramers to quantitatively assess the function and phenotype of allergen-specific T cells in asthma.
Knowledge Gaps and Directions for Future Research
Allergen-specific CD4+ T cells play an important role in the pathogenesis of allergic airway inflammation. However, there are gaps in our current knowledge of allergen-specific CD4+ T cells in how they interface with the airway epithelium and other structural cells, mast cells, ILCs, and B cells to coordinate airway inflammation and AHR.
While studies have investigated allergen-specific CD4+ T cells in the peripheral blood of atopic subjects, to our knowledge, allergen-specific CD4+ T cells in the airway of human subjects with asthma have not been characterized using allergen–MHC class II tetramers. The combination of tetramer, activation marker (e.g., CD25, CD154), and memory marker (CD45RA, CD62L, CCR7, CD27) staining by flow cytometry will help reveal whether there are phenotypic differences that differentiate subjects with allergic asthma from allergic subjects without asthma. Similarly, flow cytometry to determine the expression of IL-25R, IL-33R, CRTH2 (PGD2 receptor), TSLP-R, and other Th2 markers could provide insight into how allergen-specific T cells interface with innate cells, including mast cells and airway epithelial cells. Studies by Wambre and colleagues identified CRTH2+CD27– allergen-specific CD4+ T cells as a “proallergic” T-cell phenotype found in allergic subjects but not in healthy control subjects in alder and timothy grass allergy; these cells decreased after specific immunotherapy (63, 64). Thus, allergen-specific CD4+ T cells might be a key target for specific immunotherapy, and their frequency and phenotype could be tracked longitudinally as possible biomarkers of disease activity and resolution. There is also emerging interest in the use of immunomodulators (e.g., Toll-like receptor agonists) and biologics (e.g., dupilumab [anti–IL-4Rα] and omalizumab [anti-IgE]) to inhibit allergic inflammation and how these might alter the frequency and phenotype of allergen-specific CD4+ T cells (e.g., rendering them less sensitive to innate type 2 cytokines, dampening their activation, or modifying their effector function from Th2-polarized to Th1 or T regulatory).
There are few studies looking at Tregs within the lung in human asthma (38); they have been better characterized in at least one study in the nasal epithelium (65), which contains local Tregs that increase in frequency after specific immunotherapy and correlate with suppression of seasonal allergen-associated symptoms. Whether lung Tregs play a similar role as nasal mucosal Tregs in allergen-specific tolerance remains to be elucidated.
Finally, the involvement of allergen-specific T cells in response to non–allergen-specific stimuli, including viruses, cold air, and irritants (e.g., diesel exhaust particles and cigarette smoke), remains unclear. Viruses and other noxious stimuli may result in defects in the airway epithelial barrier, which may lead to the release of innate mediators, including IL-25, IL-33, and TSLP, all of which can promote allergic airway inflammation. However, the relative contribution of T cells, ILC2s, and other ILC subsets in response to these nonspecific stimuli and innate mediators and how these cells interact with the airway epithelium during asthma exacerbations remain unknown and warrant more detailed characterization in terms of their frequency and function in humans.
To achieve a greater mechanistic understanding of allergic airway inflammation in asthma and to develop novel and more effective preventative and therapeutic strategies, allergen–MHC class II tetramers can be used to more extensively characterize allergen-specific CD4+ T cells and how they interact with innate, regulatory, and structural cells of the airway.
Footnotes
Supported by grants from the National Institutes of Health (F32 AI108125, U19 AI095261, and R37 AI040618).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 4.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 5.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, Jerico Del-Rosario, Telcian AG, Nikonova A, Zhu J, et al. IL-33–dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–1186.e7. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127:450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010;3:216–229. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 10.Kelly EA, Rodriguez RR, Busse WW, Jarjour NN. The effect of segmental bronchoprovocation with allergen on airway lymphocyte function. Am J Respir Crit Care Med. 1997;156:1421–1428. doi: 10.1164/ajrccm.156.5.9703054. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura Y, Ghaffar O, Olivenstein R, Taha RA, Soussi-Gounni A, Zhang DH, Ray A, Hamid Q. Gene expression of the GATA-3 transcription factor is increased in atopic asthma. J Allergy Clin Immunol. 1999;103:215–222. doi: 10.1016/s0091-6749(99)70493-8. [DOI] [PubMed] [Google Scholar]
- 12.Taha R, Hamid Q, Cameron L, Olivenstein R. T helper type 2 cytokine receptors and associated transcription factors GATA-3, c-MAF, and signal transducer and activator of transcription factor-6 in induced sputum of atopic asthmatic patients. Chest. 2003;123:2074–2082. doi: 10.1378/chest.123.6.2074. [DOI] [PubMed] [Google Scholar]
- 13.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- 14.Wenzel SE, Fowler AA, III, Schwartz LB. Activation of pulmonary mast cells by bronchoalveolar allergen challenge: in vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis. 1988;137:1002–1008. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel SE, Larsen GL, Johnston K, Voelkel NF, Westcott JY. Elevated levels of leukotriene C4 in bronchoalveolar lavage fluid from atopic asthmatics after endobronchial allergen challenge. Am Rev Respir Dis. 1990;142:112–119. doi: 10.1164/ajrccm/142.1.112. [DOI] [PubMed] [Google Scholar]
- 16.Robinson DS, Bentley AM, Hartnell A, Kay AB, Durham SR. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26–32. doi: 10.1136/thx.48.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang C, Rolland JM, Ward C, Quan B, Walters EH. IL-5 production by bronchoalveolar lavage and peripheral blood mononuclear cells in asthma and atopy. Eur Respir J. 1997;10:624–632. [PubMed] [Google Scholar]
- 18.Magnan AO, Mély LG, Camilla CA, Badier MM, Montero-Julian FA, Guillot CM, Casano BB, Prato SJ, Fert V, Bongrand P, et al. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma: increased IFN-γ–producing CD8+ T cells in asthma. Am J Respir Crit Care Med. 2000;161:1790–1796. doi: 10.1164/ajrccm.161.6.9906130. [DOI] [PubMed] [Google Scholar]
- 19.Walker C, Virchow JC, Jr, Bruijnzeel PL, Blaser K. T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol. 1991;146:1829–1835. [PubMed] [Google Scholar]
- 20.Leonard C, Tormey V, Burke C, Poulter LW. Allergen-induced cytokine production in atopic disease and its relationship to disease severity. Am J Respir Cell Mol Biol. 1997;17:368–375. doi: 10.1165/ajrcmb.17.3.2797. [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Durham SR, Ying S, Kimmitt P, Barkans J, Assoufi B, Pfister R, Menz G, Robinson DS, Kay AB, et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med. 1996;154:1497–1504. doi: 10.1164/ajrccm.154.5.8912771. [DOI] [PubMed] [Google Scholar]
- 22.Ying S, Humbert M, Barkans J, Corrigan CJ, Pfister R, Menz G, Larché M, Robinson DS, Durham SR, Kay AB. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1997;158:3539–3544. [PubMed] [Google Scholar]
- 23.Truyen E, Coteur L, Dilissen E, Overbergh L, Dupont LJ, Ceuppens JL, Bullens DM. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006;61:202–208. doi: 10.1136/thx.2005.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42:650–658. doi: 10.1111/j.1365-2222.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 25.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138(2) Suppl:4S–10S. doi: 10.1378/chest.10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaaban R, Zureik M, Soussan D, Antó JM, Heinrich J, Janson C, Künzli N, Sunyer J, Wjst M, Burney PG, et al. Allergic rhinitis and onset of bronchial hyperresponsiveness: a population-based study. Am J Respir Crit Care Med. 2007;176:659–666. doi: 10.1164/rccm.200703-427OC. [DOI] [PubMed] [Google Scholar]
- 27.Prieto L, Gutiérrez V, Morales C, Marín J. Differences in sensitivity, maximal response and position of the concentration–response curve to methacholine between asthmatics, patients with allergic rhinitis and healthy subjects. Respir Med. 1998;92:88–94. doi: 10.1016/s0954-6111(98)90038-5. [DOI] [PubMed] [Google Scholar]
- 28.Brown JL, Behndig AF, Sekerel BE, Pourazar J, Blomberg A, Kelly FJ, Sandström T, Frew AJ, Wilson SJ. Lower airways inflammation in allergic rhinitics: a comparison with asthmatics and normal controls. Clin Exp Allergy. 2007;37:688–695. doi: 10.1111/j.1365-2222.2007.02695.x. [DOI] [PubMed] [Google Scholar]
- 29.DeKruyff RH, Yu S, Kim HY, Umetsu DT. Innate immunity in the lung regulates the development of asthma. Immunol Rev. 2014;260:235–248. doi: 10.1111/imr.12187. [DOI] [PubMed] [Google Scholar]
- 30.Park CS, Ra DJ, Lee SM, Jeong SW, Uh S, Kim HT, Kim YH. Interleukin-4 and low-affinity receptor for IgE on B cells in peripheral blood of patients with atopic bronchial asthma. J Allergy Clin Immunol. 1996;97:1121–1128. doi: 10.1016/s0091-6749(96)70267-1. [DOI] [PubMed] [Google Scholar]
- 31.Kenyon NJ, Kelly EA, Jarjour NN. Enhanced cytokine generation by peripheral blood mononuclear cells in allergic and asthma subjects. Ann Allergy Asthma Immunol. 2000;85:115–120. doi: 10.1016/S1081-1206(10)62450-7. [DOI] [PubMed] [Google Scholar]
- 32.Tang C, Rolland JM, Li X, Ward C, Bish R, Walters EH. Alveolar macrophages from atopic asthmatics, but not atopic nonasthmatics, enhance interleukin-5 production by CD4+ T cells. Am J Respir Crit Care Med. 1998;157:1120–1126. doi: 10.1164/ajrccm.157.4.9706118. [DOI] [PubMed] [Google Scholar]
- 33.Tang C, Rolland JM, Ward C, Li X, Bish R, Thien F, Walters EH. Modulatory effects of alveolar macrophages on CD4+ T-cell IL-5 responses correlate with IL-1β, IL-6, and IL-12 production. Eur Respir J. 1999;14:106–112. doi: 10.1034/j.1399-3003.1999.14a18.x. [DOI] [PubMed] [Google Scholar]
- 34.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pumputiene I, Emuzyte R, Siaurys A, Tamosiunas V, Valiulis A. CD4+CD25high Treg cells in peripheral blood during remission and exacerbation of allergic asthma in children. Acta Paediatr. 2011;100:1006–1010. doi: 10.1111/j.1651-2227.2011.02241.x. [DOI] [PubMed] [Google Scholar]
- 36.Maggi L, Santarlasci V, Liotta F, Frosali F, Angeli R, Cosmi L, Maggi E, Romagnani S, Annunziato F. Demonstration of circulating allergen-specific CD4+CD25highFoxp3+ T-regulatory cells in both nonatopic and atopic individuals. J Allergy Clin Immunol. 2007;120:429–436. doi: 10.1016/j.jaci.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, Michel S, Tost J, Liu J, Genuneit J, et al. Protection against Allergy: Study in Rural Environments Study Group. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. 2014;133:551–559. doi: 10.1016/j.jaci.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 39.Boulet LP, Turcott H, Plante S, Chakir J. Airway function, inflammation and regulatory T cell function in subjects in asthma remission. Can Respir J. 2012;19:19–25. doi: 10.1155/2012/347989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 41.Bonvalet M, Wambre E, Moussu H, Horiot S, Kwok WW, Louise A, Ebo D, Hoarau C, Van Overtvelt L, Baron-Bodo V, et al. Comparison between major histocompatibility complex class II tetramer staining and surface expression of activation markers for the detection of allergen-specific CD4⁺ T cells. Clin Exp Allergy. 2011;41:821–829. doi: 10.1111/j.1365-2222.2011.03708.x. [DOI] [PubMed] [Google Scholar]
- 42.Moon JJ, Chu HH, Hataye J, Pagán AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Custovic A, Taggart SC, Francis HC, Chapman MD, Woodcock A. Exposure to house dust mite allergens and the clinical activity of asthma. J Allergy Clin Immunol. 1996;98:64–72. doi: 10.1016/s0091-6749(96)70227-0. [DOI] [PubMed] [Google Scholar]
- 44.Sporik R, Squillace SP, Ingram JM, Rakes G, Honsinger RW, Platts-Mills TA. Mite, cat, and cockroach exposure, allergen sensitisation, and asthma in children: a case–control study of three schools. Thorax. 1999;54:675–680. doi: 10.1136/thx.54.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platts-Mills TA, Thomas WR, Aalberse RC, Vervloet D, Champman MD. Dust mite allergens and asthma: report of a second international workshop. J Allergy Clin Immunol. 1992;89:1046–1060. doi: 10.1016/0091-6749(92)90228-t. [DOI] [PubMed] [Google Scholar]
- 46.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood: a prospective study. N Engl J Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 47.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 48.Mark PG, Segal DB, Dallaire ML, Garman RD. Human T and B cell immune responses to Fel d 1 in cat-allergic and non–cat-allergic subjects. Clin Exp Allergy. 1996;26:1316–1328. [PubMed] [Google Scholar]
- 49.Haselden BM, Syrigou E, Jones M, Huston D, Ichikawa K, Chapman MD, Kay AB, Larché M. Proliferation and release of IL-5 and IFN-γ by peripheral blood mononuclear cells from cat-allergic asthmatics and rhinitics, non–cat-allergic asthmatics, and normal controls to peptides derived from Fel d 1 chain 1. J Allergy Clin Immunol. 2001;108:349–356. doi: 10.1067/mai.2001.117461. [DOI] [PubMed] [Google Scholar]
- 50.van Neerven RJ, van de Pol MM, van Milligen FJ, Jansen HM, Aalberse RC, Kapsenberg ML. Characterization of cat dander–specific T lymphocytes from atopic patients. J Immunol. 1994;152:4203–4210. [PubMed] [Google Scholar]
- 51.Haselden BM, Kay AB, Larché M. Immunoglobulin E–independent major histocompatibility complex–restricted T cell peptide epitope–induced late asthmatic reactions. J Exp Med. 1999;189:1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WL, Stern LJ, Grönlund H, van Hage M, Reynolds CJ, Boyton RJ, et al. Peptide immunotherapy in allergic asthma generates IL-10–dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206:1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwok WW, Roti M, Delong JH, Tan V, Wambre E, James EA, Robinson D. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol. 2010;125:1407–1409.e1. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bateman EA, Ardern-Jones MR, Ogg GS. Identification of an immunodominant region of Fel d 1 and characterization of constituent epitopes. Clin Exp Allergy. 2008;38:1760–1768. doi: 10.1111/j.1365-2222.2008.03098.x. [DOI] [PubMed] [Google Scholar]
- 55.Bateman EA, Ardern-Jones MR, Ogg GS. Persistent central memory phenotype of circulating Fel d 1 peptide/DRB1*0101 tetramer-binding CD4+ T cells. J Allergy Clin Immunol. 2006;118:1350–1356. doi: 10.1016/j.jaci.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 56.Alexander C, Tarzi M, Larché M, Kay AB. The effect of Fel d 1–derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy. 2005;60:1269–1274. doi: 10.1111/j.1398-9995.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 57.Neumann C, Gutgesell C, Fliegert F, Bonifer R, Herrmann F. Comparative analysis of the frequency of house dust mite specific and nonspecific Th1 and Th2 cells in skin lesions and peripheral blood of patients with atopic dermatitis. J Mol Med (Berl) 1996;74:401–406. doi: 10.1007/BF00210634. [DOI] [PubMed] [Google Scholar]
- 58.Macaubas C, Sly PD, Burton P, Tiller K, Yabuhara A, Holt BJ, Smallacombe TB, Kendall G, Jenmalm MC, Holt PG. Regulation of T-helper cell responses to inhalant allergen during early childhood. Clin Exp Allergy. 1999;29:1223–1231. doi: 10.1046/j.1365-2222.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 59.Hammad H, Charbonnier AS, Duez C, Jacquet A, Stewart GA, Tonnel AB, Pestel J. Th2 polarization by Der p 1–pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood. 2001;98:1135–1141. doi: 10.1182/blood.v98.4.1135. [DOI] [PubMed] [Google Scholar]
- 60.Lordan JL, Bucchieri F, Richter A, Konstantinidis A, Holloway JW, Thornber M, Puddicombe SM, Buchanan D, Wilson SJ, Djukanović R, et al. Cooperative effects of Th2 cytokines and allergen on normal and asthmatic bronchial epithelial cells. J Immunol. 2002;169:407–414. doi: 10.4049/jimmunol.169.1.407. [DOI] [PubMed] [Google Scholar]
- 61.Ardern-Jones MR, Black AP, Bateman EA, Ogg GS. Bacterial superantigen facilitates epithelial presentation of allergen to T helper 2 cells. Proc Natl Acad Sci USA. 2007;104:5557–5562. doi: 10.1073/pnas.0700733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wambre E, Bonvalet M, Bodo VB, Maillère B, Leclert G, Moussu H, Von Hofe E, Louise A, Balazuc AM, Ebo D, et al. Distinct characteristics of seasonal (Bet v 1) vs. perennial (Der p 1/Der p 2) allergen-specific CD4+ T cell responses. Clin Exp Allergy. 2011;41:192–203. doi: 10.1111/j.1365-2222.2010.03641.x. [DOI] [PubMed] [Google Scholar]
- 63.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129:544–551, 551.e1–7. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wambre E, DeLong JH, James EA, Torres-Chinn N, Pfützner W, Möbs C, Durham SR, Till SJ, Robinson D, Kwok WW. Specific immunotherapy modifies allergen-specific CD4+ T-cell responses in an epitope-dependent manner. J Allergy Clin Immunol. 2014;133:872–879.e7. doi: 10.1016/j.jaci.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–1472, 1472.e1. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]