Abstract
The discovery that eosinophilia and steroid responsiveness are dominant signals in patients with asthma led to the conclusion that inflammation characterized by up-regulation of the type 2 cytokines that mediate eosinophilia (IL -4, -5, and -13) (type 2 inflammation) is central to asthma pathogenesis in all patients and resulted in a singular emphasis on animal models of type 2 inflammation to unravel disease mechanisms. This in turn led to great progress in identifying drug targets and in developing inhibitors of type 2 inflammation. Despite this significant and clinically important progress, there has been a growing body of evidence that airway type 2 inflammation is not a ubiquitous pathologic feature of asthma and a growing acceptance that the type 2–centric asthma paradigm has held back understanding of mechanisms of disease in patients who do not have type 2 inflammation (helper T-cell type 2 [Th2]-low asthma). This “tyranny of the dominant paradigm” effect means that asthma clinicians have no effective controller treatments to offer their many patients with Th2-low asthma. It also means that asthma researchers are struggling to understand the mechanisms of disease that operate in Th2-low asthma and how these mechanisms might be modeled in vitro and in vivo to identify novel drug targets and provide a broader range of asthma treatments.
Keywords: asthma, exacerbation, type 2 inflammation, Th2-high, Th2-low, asthma paradigm
Airway eosinophilia has long been known to be a pathologic feature of asthma, and the strong association between asthma and other allergic diseases such as allergic rhinitis, eczema, and eosinophilic esophagitis led to the solidification of a disease paradigm in which asthma has been considered a disease of type 2 inflammation. Type 2 inflammation occurs when upstream regulators such as IL-33, IL-25, or thymic stromal lymphopoietin activate innate or adaptive immune cells to secrete type 2 cytokines (IL-4, -5, and -13), which cause accumulation of inflammatory cells such as eosinophils, mast cells and basophils and remodeling of the airway epithelium and submucosa (mucous cell metaplasia and subepithelial fibrosis). This mechanism of disease was relatively easy to model in mice because mice develop typical asthma pathology (airway eosinophilia, goblet cell metaplasia, and peribronchial fibrosis) when sequentially sensitized and challenged with aeroallergens. Experiments in this mouse model have provided a detailed map of the molecular regulators of type 2 inflammation in the airway (1). We now have multiple drugs targeting these molecules in the type 2 pathway, including inhibitors of thymic stromal lymphopoietin and IL-4, -5, and -13. Apart from mepolizumab (an inhibitor of IL-5), none of these drugs are yet approved for treatment of asthma, but early-phase clinical trial data have consistently demonstrated their efficacy (2). At the moment, corticosteroids are the mainstay controller therapy for asthma because steroids improve outcomes of asthma control (symptoms, lung function, and susceptibility to exacerbations) in many patients. Consequently, asthma treatment guidelines around the world all consistently recommend that inhaled steroid be used to treat all but the mildest forms of asthma and that they be used in high doses to treat severe disease (3).
Asthma Was Talking, But We Weren’t Listening: Missed or Ignored Signals
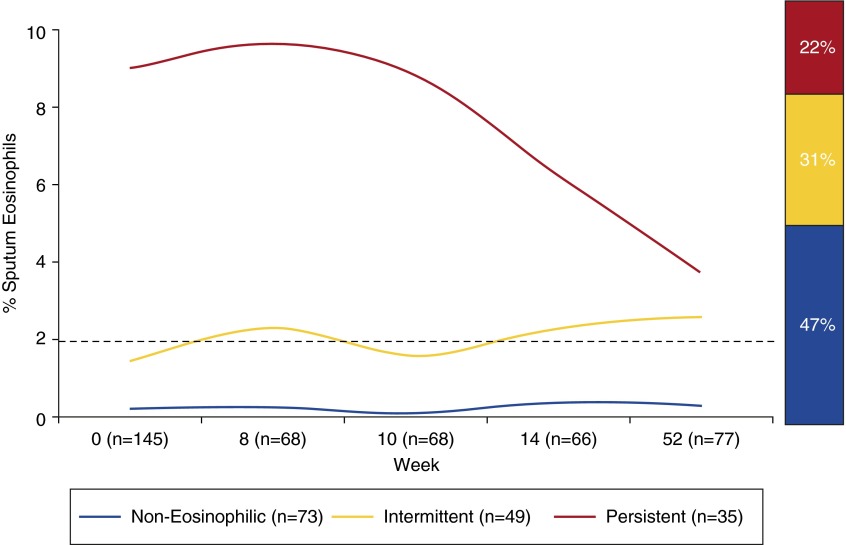
While the type 2 asthma disease paradigm was solidifying and controlling the research agenda in asthma, the emerging ability to target type 2 inflammation with precision, using protein therapeutics, provided an impetus to look more closely at the prevalence of airway eosinophilia and other markers of type 2 inflammation in patients. These studies showed that airway eosinophilia in asthma is heterogeneous (4, 5) and that a large subgroup of patients have persistent absence of eosinophils in their airways (Figure 1) (6). Although this heterogeneity in airway eosinophilia has been apparent for decades (asthma was talking), the research community was slow to acknowledge (listen to) it. The community began to pay more attention when upstream regulators of airway eosinophilia such as IL-13 were shown to be active in some subjects with asthma, but not in others (Figure 2) (7). This finding led to the concept of helper T-cell type 2 (Th2)–high asthma and Th2-low asthma, labels that describe patients with or without airway type 2 inflammation, and which explicitly acknowledge heterogeneity of disease mechanism in asthma.
Figure 1.
A large subgroup of patients has persistent absence of eosinophils in their airways. Two to four measures of eosinophil percentage in induced sputum from subjects with asthma were made over a period as long as 1 year. The subjects with asthma were not taking inhaled corticosteroids for disease management. The graph reveals three subgroups of subjects with asthma: (1) those with persistently eosinophilic asthma whose sputum eosinophil percentage is greater than or equal to 2% on every occasion it was measured; (2) those with intermittently eosinophilic asthma whose sputum eosinophil percentage is greater than 2% on at least one occasion but can be less than 2% on some occasions; and (3) a third subgroup whose sputum eosinophil percentage is persistently less than 2% on all occasions measured. Note that the number of subjects varies at each time point. Reproduced by permission from Reference 6.
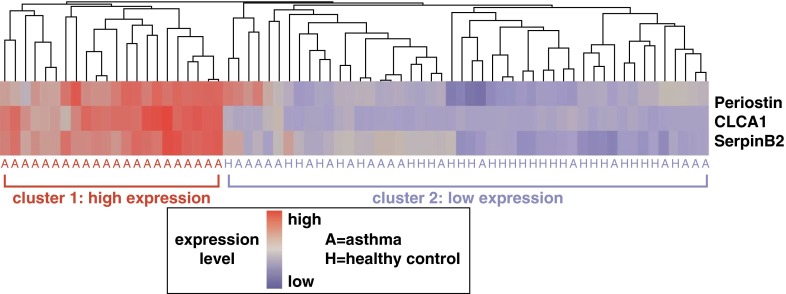
Figure 2.
Helper T-cell type 2 (Th2)-high and Th2-low asthma endotypes. Expression levels of three IL-13–induced genes in the airway epithelial brushings from subjects with asthma and healthy control subjects define two subgroups of patients with asthma. The heatmap depicts unsupervised hierarchical clustering (Euclidean complete) of POSTN, CLCA1, and SERPINB2 expression levels in bronchial epithelium across all subjects at baseline. Reproduced by permission from Reference 7.
Another way in which asthma has been “talking” is in the area of treatment response to corticosteroids. Although it had been clear for decades that treatment responses to corticosteroids in subjects with asthma are heterogeneous, the fact that many patients derive little if any benefit from corticosteroid treatment has not been acknowledged (listened to) in treatment guidelines for asthma. Instead, these guidelines promote a one-size-fits-all approach with recommendations to treat patients with more severe disease with higher doses of corticosteroids. Implicit in these guidelines is the dominance of the type 2 paradigm and the idea that a higher dose of type 2 inflammation demands a higher dose of corticosteroids to suppress it. But persistence of type 2 inflammation is not the only mechanism for severe asthma that is refractory to low- or medium-dose steroid treatment; at least as important is the possibility that lack of responsiveness to steroids is because of a lack of type 2 inflammation in the airways. Although prospective trials are needed to fully establish the efficacy of corticosteroids in Th2-low asthma, available evidence suggests that steroids have limited efficacy in this disease endotype (Figure 3) (6–8). It is hoped that future guidelines for asthma management will recommend corticosteroids only for patients who benefit from them. In fact, as an asthma community we should reexamine our relationship with corticosteroids to ensure that the many risks of corticosteroids are balanced by improvement in asthma control for individual patients. In particular, restraint in dosing corticosteroids should be shown for patients who have no markers of type 2 inflammation in their airways.
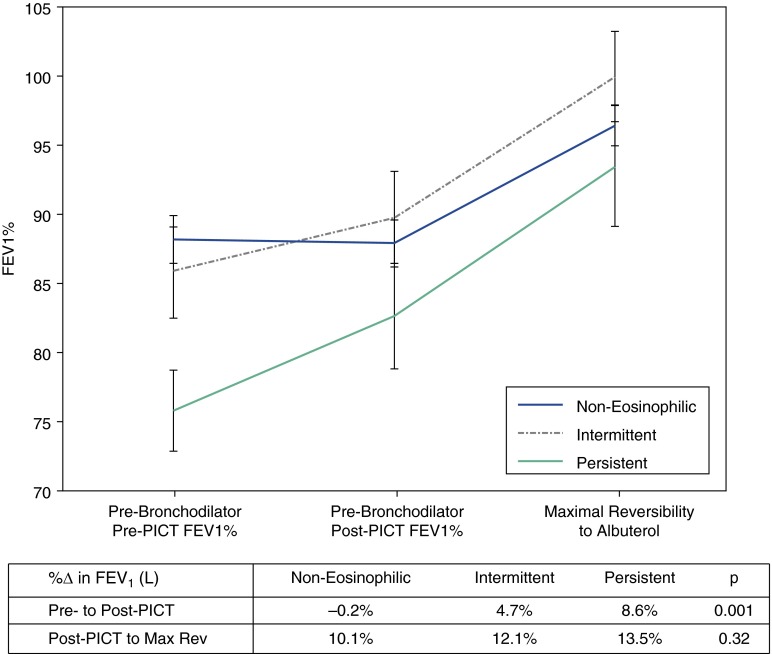
Figure 3.
Effects of asthma treatments in eosinophilic and noneosinophilic asthma: Change in FEV1 in a subgroup of the inhaled corticosteroid-negative group that received a period of intense combined therapy (PICT) for 10–14 days. The PICT consisted of 0.5 mg of prednisone/kg/day, 800 mg of budesonide twice per day, and 20 mg of zafirlukast twice per day. Prebronchodilator FEV1 was measured before and after the PICT. In addition, maximal bronchodilator reversibility (Max Rev) was measured after the PICT and involved measures of spirometry after up to 720 mg of inhaled albuterol. The data represent means and standard error. Reproduced by permission from Reference 6.
Tyranny of the Dominant Paradigm: Fealty to Dogma Can Impede Progress
The type 2 inflammatory pathway is a central mechanism of disease in many patients with asthma, and the identification of many molecules in this pathway that can be targeted with drugs is a success story of which the asthma community can be proud. Nevertheless, it is clear that it is perilous to allow a disease paradigm to dominate the field. Type 2 inflammation dominated research activity because asthmatic patients with type 2 inflammation in their airways represented a large subgroup of patients, and signals from this large subgroup drove summary descriptions of data, especially when data were summarized in mean statistics. A lesson here is the importance at looking at data ranges, not just means, so that subgroups other than the dominant subgroup can be identified and investigated for mechanism. Failure to do this is to succumb to the tyranny of the dominant paradigm, in which signals other than the one conforming to the dominant view of the disease are ignored.
Beyond the Dominant Paradigm: Rethinking Concepts of Disease Pathogenesis in Asthma
Because induction of type 2 inflammation in the airways of mice causes an asthma-like lung phenotype, it has been conceivable that type 2 inflammation in the airway causes asthma and that treating type 2 inflammation will effectively cure asthma. Although this remains possible in theory, available evidence argues strongly against it (9–17). The key physiological features of asthma are excessive tone in airway smooth muscle and exaggerated smooth muscle response to nonspecific agonists such as methacholine. Both of these physiological measures are not markedly improved by treatment with type 2 cytokine inhibitors or anti-IgE, and even corticosteroids have relatively limited effects in improving measures of airflow and bronchial hyperactivity. Type 2 cytokine inhibitors, anti-IgE, and corticosteroids have much greater effects on susceptibility to asthma exacerbation (2–4). This raises the possibility that airway smooth muscle dysfunction in asthma is not caused by type 2 inflammation, but is a fundamental disease abnormality that is independent of type 2 inflammation. In this conceptualization of asthma the core smooth muscle dysfunction is modified by type 2 inflammation to make asthma worse, and especially to make exacerbations more frequent. This concept would account for the findings that decreasing type 2 inflammation improves asthma control without large effects on airway smooth muscle outcomes. This concept also allows for other disease modifiers to operate (Figure 4) (2). At present, these modifiers are relatively poorly understood, but there is a growing recognition of the role of systemic inflammation associated with obesity in worsening asthma (18).
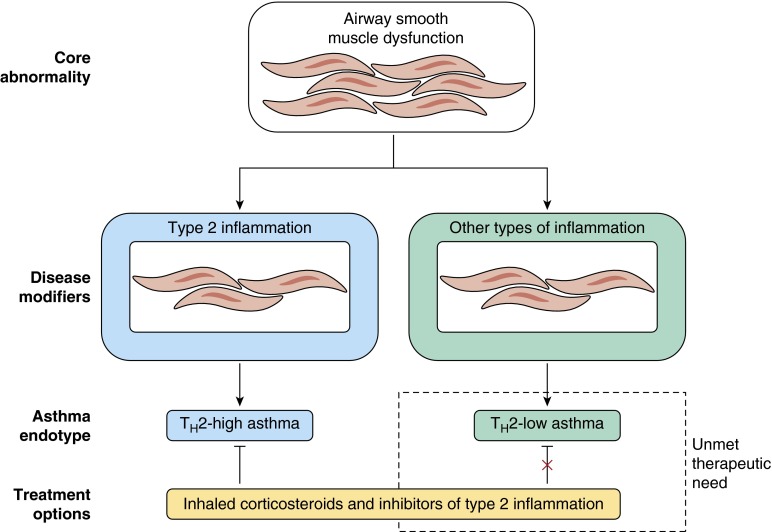
Figure 4.
Asthma as a core disease of smooth muscle that is modified by inflammation. Lessons learned from clinical trials of inhibitors of type 2 inflammation suggest a conceptualization of asthma as a disease with a core abnormality in airway smooth muscle function that can be modified by inflammation to worsen disease severity and to promote susceptibility to asthma exacerbations. Although type 2 inflammation has been shown to be an important disease modifier by the effects of its inhibition in clinical trials, these same clinical trials also highlight that additional types of inflammation must contribute to certain forms of asthma. These non–type 2 inflammatory pathways are not well understood but may include those associated with obesity, infection, or neutrophilia. Th2 = helper T-cell type 2. Reproduced by permission from Reference 2.
Summary
Type 2 inflammation is now recognized to be heterogeneous in asthma, and failure to acknowledge the heterogeneity in disease mechanisms in asthma has brought us to a place where we have multiple options to treat Th2-high asthma but few options to treat Th2-low asthma. As important as it has been to understand the details of type 2 inflammation in asthma and to bring novel type 2–specific treatments to many patients, there are also important lessons to be learned about how failure to acknowledge heterogeneity of disease mechanism can slow personalized treatment for patients. A priority for the future will be mechanism-oriented studies in patients with Th2-low asthma. On the one hand, these studies could use unbiased and powerful “-omic”-based technologies to uncover unsuspected disease mechanisms; on the other hand, investigators could use clues from clinical traits associated with more severe forms of asthma (e.g., obesity) to point them toward disease mechanisms, including how systemic inflammation can worsen lung function.
Footnotes
Supported by grants HL107202, HL109146, and HL080414 from the National Heart, Lung, and Blood Institute.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy AP, Gupta MR. Management of asthma: the current US and European guidelines. Adv Exp Med Biol. 2014;795:81–103. doi: 10.1007/978-1-4614-8603-9_6. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 5.Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, Monk P, Bradding P, Wardlaw AJ, Pavord ID, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–691. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185:612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2–driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353:2213–2214. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- 9.Solèr M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, Thirlwell J, Gupta N, Della Cioppa G. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 10.Milgrom H, Fick RB, Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, Metzger WJ rhuMAb-E25 Study Group. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med. 1999;341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 11.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, van As A, Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 12.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 13.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 14.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 16.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 17.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID SIRIUS Investigators. Oral glucocorticoid–sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 18.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42:1012–1019. doi: 10.1183/09031936.00124912. [DOI] [PubMed] [Google Scholar]