Abstract
Rationale: Little is known about the effectiveness of noninvasive ventilation for patients hospitalized with asthma exacerbation.
Objectives: To assess clinical outcomes of noninvasive (NIV) and invasive mechanical ventilation (IMV) and examine predictors for NIV use in patients hospitalized with asthma.
Methods: This was a retrospective cohort study at 97 U.S. hospitals using an electronic medical record database. We developed a hierarchical regression model to identify factors associated with the choice of initial ventilation and used the Laboratory Acute Physiological Score to adjust for differences in the severity of illness. We assessed the outcomes of patients treated with initial NIV or IMV in a propensity-matched cohort.
Measurements and Main Results: Among 13,930 subjects, 73% were women and 54% were white. The median age was 53 years. Overall, 1,254 patients (9%) required ventilatory support (NIV or IMV). NIV was the initial ventilation method for 556 patients (4.0%) and IMV for 668 (5.0%). Twenty-six patients (4.7% of patients treated with NIV) had to be intubated (NIV failure). The in-hospital mortality was 0.2, 2.3, 14.5, and 15.4%, and the median length of stay was 2.9, 4.1, 6.7, and 10.9 days among those not ventilated, ventilated with NIV, ventilated with IMV, and with NIV failure, respectively. Older patients were more likely to receive NIV (odds ratio, 1.06 per 5 yr; 95% confidence interval [CI], 1.01–1.11), whereas those with higher acuity (Laboratory Acute Physiological Score per 5 units: odds ratio, 0.85; 95% CI, 0.82–0.88) and those with concomitant pneumonia were less likely to receive NIV. In a propensity-matched sample, NIV was associated with a lower inpatient risk of dying (risk ratio, 0.12; 95% CI, 0.03–0.51) and shorter lengths of stay (4.3 d less; 95% CI, 2.9–5.8) than IMV.
Conclusions: Among patients hospitalized with asthma exacerbation and requiring ventilatory support (NIV or IMV), more than 40% received NIV. Although patients successfully treated with NIV appear to have better outcomes than those treated with IMV, the low rate of NIV failure suggests that NIV was being used selectively in a lower risk group. The increased risk of mortality for patients who fail NIV highlights the need for careful monitoring to avoid possible delay in intubation.
Keywords: respiratory insufficiency, outcomes research, mortality, length of stay, electronic medical records
Asthma is a common disorder that affects upward of 1 in every 10 adults in the United States. It accounts for nearly 2 million emergency department visits per year, and 20 to 30% of these patients require hospitalization (1). Asthma exacerbations are characterized by progressive worsening of dyspnea and wheezing, and most exacerbations respond to treatment with supplemental oxygen, bronchodilators, and steroids. Despite aggressive management, some patients fail to improve; intensive care admissions and intubation rates vary from 10 to 30%, depending on the population studied (2, 3), and among those intubated, 8 to 22% die (4, 5).
The use of noninvasive ventilation (NIV) in patients with acute respiratory failure has increased dramatically during the last decade (6), although the evidence for NIV as first-line therapy varies widely depending on the primary underlying condition. For patients hospitalized with moderate to severe COPD exacerbation, numerous systematic reviews and metaanalyses suggest that NIV is effective in reducing the risk of intubation and short-term mortality (7). However, the evidence supporting the efficacy of NIV in patients with an acute exacerbation of asthma is far less clear. A Cochrane review of five trials and 206 patients found that compared with usual care alone, NIV improved respiratory rate and lung function and increased the number of patients discharged from emergency departments but did not show a reduction in the risk of intubation or mortality (8). Despite the paucity of data on efficacy and safety, a recent analysis of the Nationwide Inpatient Sample showed that rates of NIV use among patients with asthma had increased fivefold between 2000 and 2008 (9).
Therefore, using data from a large multihospital electronic medical record (EMR) database that contains results of laboratory data, we sought to examine factors associated with the choice of ventilation in patients hospitalized with asthma exacerbation and compare the clinical outcomes of NIV and invasive mechanical ventilation (IMV) in these patients.
A limited set of results from this study has been previously reported in the form of an abstract (10).
Methods
Data Source
We conducted a retrospective cohort study using a comprehensive EMR dataset, Cerner HealthFacts (Cerner Corporation, Kansas City, MO), from January 2009 to December 2012. In addition to the information contained in a traditional hospital claim file, Health Facts contains detailed, time-stamped, clinical, pharmacy, and laboratory results. Health Facts is a Health Information Portability and Accountability Act–compliant comprehensive source of deidentified data, and Cerner aggregates the data provided by participating facilities and uses stringent quality assurance processes to ensure the ongoing integrity of information. In 2012, the database comprised approximately 125 geographically and structurally diverse hospitals throughout United States; details have been described elsewhere (11–13). The database does not contain any information about treatments the patients had in the ambulatory setting before being admitted (i.e., NIV use, inhaled steroids, or bronchodilators).
Study Cohort
We included patients 18 years or older with a principal diagnosis of asthma (International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9] codes 493.0x, 493.1x, 493.2x, 493.8x, 493.9x) or principal diagnosis of acute respiratory failure (ICD-9 codes: 518.81, 518.82, 518.84, 518.4, 786.09) combined with a secondary diagnosis of asthma (14). Status asthmaticus was defined based on the ICD-9 subcategory codes for asthma. These subcategories are descriptive and do not assign the level of severity but do state if the asthma was with status asthmaticus, was with acute exacerbation, or was unspecified.
To ensure that included patients were treated for an asthma exacerbation of at least moderate intensity, we restricted the analysis to patients treated with short-acting bronchodilators and systemic steroids within 48 hours of admission. As such, patients without available medication data were excluded, as were patients without laboratory results, because these data were used to calculate an acuity score. We also excluded patients with obstructive sleep apnea, because it would not be possible to differentiate chronic use of NIV from treatment specifically for acute respiratory failure, and patients with a contraindication for NIV including cardiac arrest, acute myocardial infarction, facial trauma, significant arrhythmia, and hemodynamic instability present on admission. We further excluded patients who were transferred to or from another facility, because their initial form of ventilation and their outcomes could not be ascertained.
Independent Variables
For each hospitalization, data were recorded on patient age, sex, race, and insurance status. We calculated an overall combined comorbidity score on the basis of the method described by Gagne and colleagues (15), which is based in part on the Charlson Score and elements from the Elixhauser Comorbidities. The Gagne score has been shown to offer improvements in comorbidity summarization over existing scores (15). We used software provided by the Healthcare Costs and Utilization Project of the Agency for Healthcare Research and Quality to classify comorbidities on the basis of the method described by Elixhauser (16).
To assess severity of illness at the time of hospital admission, we applied the Laboratory Acute Physiology Score (LAPS), which was developed by Escobar and colleagues, in an EMR dataset to predict in-hospital mortality (17). The LAPS uses data from admission and integrates 14 laboratory tests (albumin, anion gap, arterial pH, bicarbonate, bilirubin, blood urea nitrogen, creatinine, glucose, hematocrit, lactate, PaCO2, PaO2, sodium, troponin, total white blood cell count), into a single continuous variable, which ranges between 0 and a theoretical 256; higher LAPS is associated with increased mortality. Of note, the LAPS was not developed for a specific condition but on a large cohort of hospitalized patients. The variables included in computing this score are collected directly from the EMR and do not require chart abstraction. The LAPS is similar to many existing severity-of-illness scores but uses an algorithm to compute values on the basis of an initial assessment of predicted mortality, and it has been validated externally and used in other studies (17, 18).
Type and Sequence of Ventilation
We used ICD-9 procedure codes to identify patients receiving NIV (code 93.90) or IMV (codes 96.7x and 96.04) (19). We recorded initial form of ventilation, defined as the first method of ventilation started after hospital admission, as initial NIV or initial IMV. In rare instances where NIV and IMV were recorded the same day and no other ventilation information was recorded, we considered those as indeterminate cases and excluded them from the cohort. Analyses with these patients included in the initial NIV or initial IMV category did not change the results substantially.
The dataset does not contain information about the location were the NIV was initiated (intensive care unit or general medical floor) or about the specialist who is involved in the care of a patient treated with NIV (e.g., pulmonary specialist).
Outcomes
The primary outcomes of the study were in-hospital case fatality and length of stay. Secondary outcomes included initial method of ventilation and rates and outcomes of NIV failure. NIV failure was defined as treatment with IMV after a trial of NIV (i.e., NIV followed by IMV).
In all analyses that assessed mortality, if a patient had multiple eligible admissions during the study period, we randomly chose one encounter to avoid survival bias.
Statistical Analysis
To describe the study population, we calculated counts and percentages for categorical variables and means, medians, and percentiles for continuous variables. We used the chi-square test to examine the association of patient or hospital characteristics with ventilation strategies: no ventilation, initial NIV, or initial IMV. For cell counts less than 5, we used the Fisher exact test. When comparing means, we used a one-way analysis of variance, and when comparing medians, we used the Kruskal-Wallis test.
To assess the impact of choice of ventilation on in-hospital risk of death and length of stay, we first developed a hierarchical logistic regression model to estimate the probability that patients who were ventilated would initially receive NIV. Propensities for NIV were obtained from a hierarchical (multilevel) mixed effects logistic regression model, where hospitals were treated as random effects, and hospital characteristics, patient demographics, comorbidities, and the LAPS were treated as fixed effects. Each patient treated with NIV was matched to a patient of similar propensity who was treated with IMV using a greedy match algorithm. Our primary analysis was performed in the propensity-matched cohort.
We performed several other sensitivity analyses to assess the association between ventilation choice and outcomes. First, propensity scores from a logistic regression model with no hierarchical structure were used to estimate treatment effects using inverse probability weighting, which estimates treatment effects in a population with risk factor distribution similar to the full study population (20, 21). Second, we developed a hierarchical generalized linear model adjusting for patient characteristics and including a random hospital effect to assess the effect of NIV on the study outcomes. We used logit link models for mortality and logarithmic link function for length of stay. The intraclass correlation statistic was derived to quantify the percent of variation explained by hospitals. Third, we explored how the presence of a hypothetical unmeasured confounder associated with both NIV use and mortality might influence the effect estimates for NIV treatment. We hypothesized a range of relative risks for mortality associated with unmeasured confounder from 1.5 to 3 and varied its prevalence among the IMV group. Using the method by Lin and colleagues, we explored the combination of prevalence and effect sizes that would result in a nonsignificant relative risk (22).
Finally, we identified factors predictive of NIV failure by first restricting the cohort to those started on NIV, while using the same hierarchical regression model structure and the same predictors as in the other models.
In a sensitivity analysis, we restricted the cohort to patients without concomitant pneumonia at admission, because prior studies have shown that patients with pneumonia have worse outcomes, including NIV failure (23). The diagnosis of pneumonia was established based on the ICD-9 codes present on admission and receipt of antibiotic treatment within 48 hours of admission and not on the basis of the result of chest X-rays.
Results
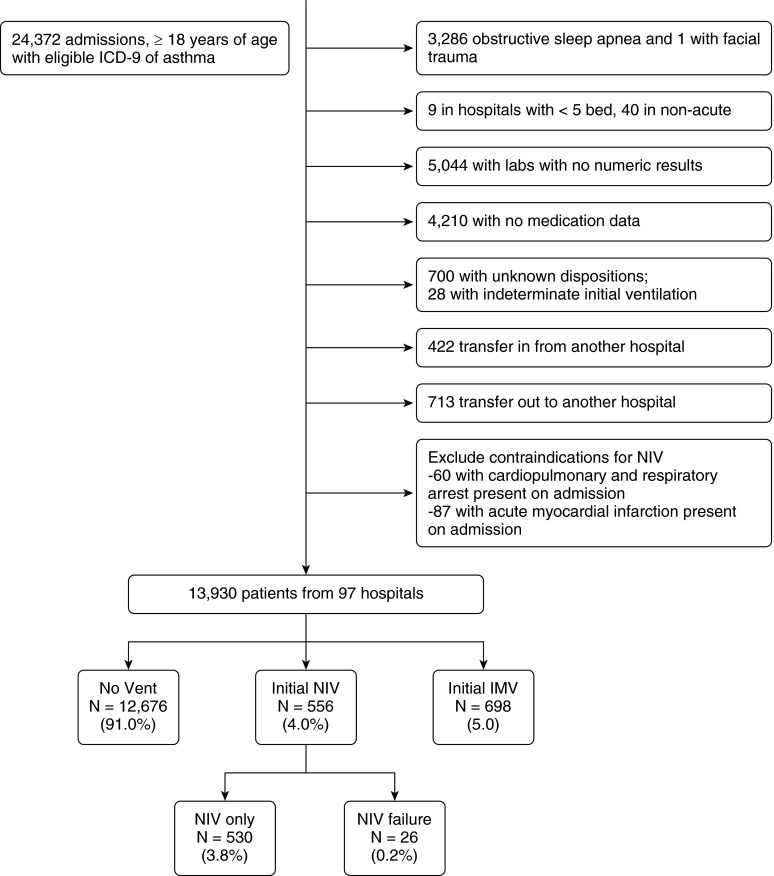
We identified a total of 13,930 admissions at 97 hospitals that met our inclusion criteria (Figure 1). The median (interquartile range [IQR]) age of the study population was 53 years (42–67), 73.0% were women, 54.3% were white, and 34.9% were black. The median LAPS was 26 (IQR, 20–38), and comorbidity score was 1 (IQR, 1–2); a secondary diagnosis of pneumonia present on admission was identified in 1,253 cases (9.0%). The majority of the 97 hospitals were urban (97.3%), half of them were teaching hospitals, and 44.3% had between 200 and 499 beds.
Figure 1.
Study cohort flow chart (criteria are not mutually exclusive). ICD-9 = International Classification of Disease, Ninth Revision, Clinical Modification; IMV = invasive mechanical ventilation; NIV = noninvasive ventilation.
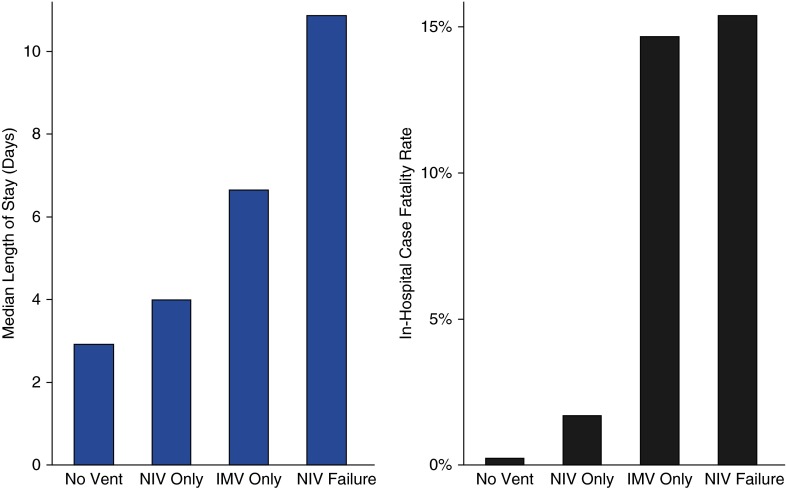
Among all 13,930 admissions, 1,254 were ventilated with NIV or IMV; initial NIV was used in 556 patients (4.0% of all patients and 44.3% of all ventilated patients) and initial IMV in 698 patients (5.0% of all patients and 55.7% of all ventilated patients). NIV failure (IMV after a trial of NIV) was recorded in 26 patients (4.7% of those treated with initial NIV) (Table 1). The in-hospital case-fatality rate and median length of stay were 0.2, 2.3, 14.5, and 15.4% and 2.9 days, 4.1 days, 6.7 days, and 10.9 days among those not ventilated, initially ventilated with NIV, initially ventilated with IMV, and with NIV failure respectively (Figure 2).
Table 1.
Characteristics and outcomes of patients included in the study
| Variable | No Ventilation (n = 12,676; 91.0%) | Initial NIV (n = 556; 4.0%) | Initial IMV (n = 698; 5.0%) | P Value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, median (IQR), yr | 53 (42–67) | 53 (43–68) | 53 (40–64) | 0.09 |
| Female, % | 73.6 | 69.2 | 65.8 | <0.001 |
| Race/ethnicity, % | ||||
| White | 54.8 | 51.3 | 48.9 | |
| Black | 34.5 | 37.9 | 41.6 | |
| Hispanic | 4.3 | 4.0 | 3.2 | <0.001 |
| Asian/other | 4.8 | 6.7 | 4.6 | |
| Unknown | 1.7 | 0.2 | 1.9 | |
| Smoking | 36.8 | 47.1 | 39.3 | <0.001 |
| Combined score | 1 (1–2) | 2 (1–3) | 2 (1–4) | <0.001 |
| Comorbidities, %* | ||||
| CHF | 10.5 | 23.0 | 24.4 | <0.001 |
| Valvular disease | 3.3 | 6.7 | 4.3 | <0.001 |
| Pulm. circ. disease | 0.7 | 6.7 | 6.0 | <0.001 |
| Neuro disorders | 5.3 | 4.5 | 13.5 | <0.001 |
| Diabetes mellitus | 24.6 | 27.2 | 23.9 | 0.35 |
| Hypothyroidism | 9.3 | 9.0 | 5.9 | 0.009 |
| Renal failure | 5.6 | 7.6 | 9.9 | <0.001 |
| Obesity | 16.6 | 19.1 | 16.6 | 0.30 |
| Morbid obesity | 6.5 | 9.2 | 5.6 | 0.03 |
| Anemia | 9.9 | 13.7 | 17.3 | <0.001 |
| Alcohol abuse | 2.4 | 5.9 | 6.5 | <0.001 |
| Drug abuse | 5.8 | 11.5 | 14.0 | <0.001 |
| Psychoses | 5.4 | 7.6 | 7.2 | 0.02 |
| Hypertension | 40.2 | 39.0 | 35.5 | 0.05 |
| Pneumonia POA | 8.0 | 13.1 | 24.1 | <0.001 |
| Status asthmaticus | 0.8 | 5.6 | 14.6 | <0.001 |
| Principal diagnosis of ARF | 7.4 | 38.1 | 72.3 | <0.001 |
| Principal diagnosis of asthma | 92.6 | 61.9 | 27.7 | |
| ICD-9 of chronic obstructive asthma | 43.7 | 58.3 | 48.7 | <0.001 |
| No ICD-9 of chronic obstructive asthma | 56.3 | 41.7 | 51.3 | |
| LAPS | ||||
| 0–19 | 12.9 | 7.2 | 2.4 | |
| 20–24 | 27.6 | 15.0 | 11.9 | |
| 25–30 | 22.0 | 10.9 | 6.9 | <0.001 |
| 31–41 | 22.7 | 22.0 | 12.9 | |
| 42+ | 14.9 | 44.9 | 65.9 | |
| Admitted to ICU, % | 12.7 | 16.0 | 39.8 | |
| Baro/volutrauma, %† | 0.2 | 0.5 | 3.4 | <0.001 |
| AMI not present at admission, % | 0.2 | 0.5 | 3.0 | <0.001 |
| LOS, d | 2.9 (1.8–4.7) | 4.1 (2.7–6.9) | 6.7 (3.8–11.5) | <0.001 |
| Inpatient case-fatality rate | 0.2% | 2.3% | 14.5% | <0.001 |
Definition of abbreviations: AMI = acute myocardial infarction; ARF = acute respiratory failure; CHF = congestive heart failure; ICD-9 = International Classification of Disease, Ninth Revision, Clinical Modification; ICU = intensive care unit; IMV = invasive mechanical ventilation; IQR = interquartile range; LAPS = Laboratory Acute Physiology Score; LOS = length of stay; NIV = noninvasive ventilation; POA = present on admission.
Additional comorbidities present in less than 5% of the sample: peripheral vascular disease, liver disease, peptic ulcer disease with bleeding, AIDS, lymphoma, metastatic cancer, solid tumor without metastasis, rheumatoid arthritis, coagulopathy.
Complications include: pneumothorax, subcutaneous emphysema, and subcutaneous emphysema from procedure.
Figure 2.
Hospital mortality and length of stay for patients nonventilated, treated with noninvasive ventilation (NIV), or treated with invasive mechanical ventilation (IMV).
Those with a principal diagnosis of acute respiratory failure were more likely to be ventilated than those with a principal diagnosis of asthma (43.2 vs. 4.4%, P < 0.001). Patients with a principal diagnosis of acute respiratory failure also had higher in-hospital case-fatality rate (6.3 vs. 0.3%, P < 0.001) and longer median lengths of stay (4.6 vs. 2.9 d, P < 0.001). Overall, 6,198 (44.4%) of the patients had an ICD-9 of chronic obstructive asthma. These patients were older (61 vs. 46 yr) and more likely to be ventilated noninvasively (5.2 vs. 3%) and invasively (5.5 vs. 4.6%) than those without this ICD-9 code, which may be used for patients with asthma-COPD overlap.
Predictors for Initial Use of NIV
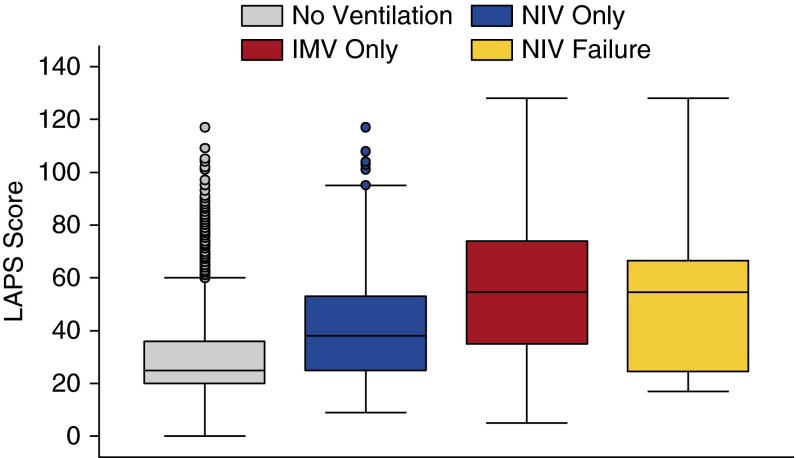
Figure 3 shows that the LAPS was significantly higher in admissions initially receiving IMV than admissions receiving NIV. In the regression analysis including only ventilated patients, we found that older patients were slightly more likely to receive initial NIV (odds ratio [OR], 1.06 per 5 yr; 95% confidence interval [CI], 1.01–1.11), whereas those with higher acuity (LAPS per 5 units: OR, 0.85; 95% CI, 0.82–0.88) and those with concomitant pneumonia (OR, 0.52; 95% CI, 0.35–0.78) were less likely to receive NIV. Patients with prior use of NIV and those with more than two prior admissions were also more likely to receive initial NIV, whereas patients with status asthmaticus, prior IMV use, comorbid weight loss, and neurological disorders were less likely to receive NIV (Table 2).
Figure 3.
Ventilation strategies by Laboratory Acute Physiology Score. IMV = invasive mechanical ventilation; NIV = noninvasive ventilation; vent = ventilation.
Table 2.
Predictors for initial noninvasive versus invasive mechanical ventilation for the patients included in the study
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age (per 5 yr) | 1.06 (1.01–1.11) | 0.02 |
| Prior admission within past 12 mo | ||
| 0 | Referent | |
| 1 | 0.90 (0.60–1.35) | 0.61 |
| 2+ | 1.77 (1.10–2.84) | 0.02 |
| Prior NIV within past 12 mo | 2.73 (1.51–4.94) | 0.001 |
| Prior IMV within past 12 mo | 0.36 (0.20–0.64) | 0.001 |
| LAPS (per 5 units) | 0.85 (0.82–0.88) | <0.001 |
| Status asthmaticus | 0.30 (0.18–0.52) | <0.001 |
| Pneumonia POA | 0.52 (0.35–0.78) | 0.002 |
| Neurological disorders | 0.23 (0.13–0.41) | <0.001 |
| Weight loss | 0.20 (0.08–0.52) | 0.001 |
| Diabetes mellitus (with and without complications) | 1.45 (1.00–2.08) | 0.05 |
Definition of abbreviations: CI = confidence interval; IMV = invasive mechanical ventilation; LAPS = Laboratory Acute Physiology Score; NIV = noninvasive ventilation; POA = present on admission.
NIV initial refers to the first method of ventilation during hospitalization. NIV initial includes NIV failure.
A likelihood ratio test comparing the hierarchical model to a logistic regression model without hospitals was highly significant (P < 0.001), indicating that the hospital where the patient was treated had a major association with the type of ventilation received. The proportion of variance explained by hospitals was 54% (95% CI, 39–68%).
Adjusted Outcomes Associated with Choice of Ventilation Strategy
We were able to match 211 patients (37.9% of the NIV cohort) who were initially treated with NIV with a person of similar propensity who was treated with IMV to assess mortality and 256 patients (46.0%) to assess length of stay. The LAPS, hospital characteristics (i.e., bed size and teaching status), demographics, and comorbidities were nonsignificantly different between NIV and IMV admissions after matching. Use of NIV was associated with lower inpatient risk of dying (relative risk ratio, 0.12; 95% CI, 0.03–0.51) and shorter lengths of stay (4.3 d less; 95% CI, 2.9–5.8). The results from the analyses that used hierarchical regression modeling with hospitals as random effects and with inverse-probability weighting yielded similar results (Table 3).
Table 3.
Adjusted outcomes of initial noninvasive and invasive ventilation for the patients included in the study
| Statistical Method | NIV Initial Case-Fatality Rate (%) | IMV Initial Case-Fatality Rate (%) | Relative Risk Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Greedy matched (211 matched pairs) | 1.0 | 8.1 | 0.12 (0.03–0.51) | 0.004 |
| Hierarchical logistic regression model | 2.5 | 14.6 | 0.17 (0.06–0.29) | <0.001 |
| Inverse probability weighting | 2.3 | 15.0 | 0.15 (0.04–0.26) | 0.006 |
| NIV mean LOS (d) | IMV Mean LOS (d) | Difference in LOS (95% CI) | ||
| Greedy matched (256 matched pairs) | 5.6 | 10.0 | 4.3 (2.9–5.8) | <0.001 |
| Hierarchical generalized linear regression model | 4.3 | 9.4 | 5.1 (4.2–6.0) | <0.001 |
| Inverse probability weighting | 5.8 | 9.7 | 3.8 (2.7–5.0) | <0.001 |
Definition of abbreviations: CI = confidence interval; IMV = invasive mechanical ventilation; LOS = length of stay; NIV = noninvasive ventilation.
NIV or IMV initial refers to the first method of ventilation during hospitalization. NIV initial includes NIV failure. Relative risks < 1 mean that NIV is protective (is associated with less mortality than IMV).
We also explored how our estimates of NIV effectiveness might have been influenced by a residual unmeasured confounder. We found that for the results to become nonsignificant, an unmeasured confounder had to have a risk ratio of 3 and be present in 80% of the initial IMV group if it was absent in the initial NIV group, which is very unlikely.
Failure of NIV
NIV failure (IMV after a trial of NIV) was uncommon, as only 26 out of 556 patients (4.7%) treated with initial NIV were later intubated. Figure 2 shows that patients with NIV failure had admission LAPS that more closely approximated the values of patients treated initially with IMV than those treated with NIV. The characteristics of patients with NIV failure compared with those treated with NIV success and initial IMV are in Table E1 in the online supplement. These patients had mortality rates slightly higher than patients treated with IMV initially (15.4 vs. 14.7%, P = 0.92) but longer median lengths of stay (10.9 vs. 6.7 d, P = 0.007). In the multivariable adjustment analysis, factors that were associated with NIV failure were: admission for asthma within the prior 12 months, diabetes mellitus, and the coexistence of pneumonia (Table 4).
Table 4.
Predictors for noninvasive ventilation failure
| Variable | Odds Ratio (95% CI) (N = 26; 4.7%) | P Value |
|---|---|---|
| No. of prior admissions within 1 yr | ||
| 0 | Referent | |
| 1 | 0.51 (0.10–2.59) | 0.42 |
| 2+ | 2.76 (1.04–7.27) | 0.04 |
| Congestive heart failure | 2.23 (0.86–6.81) | 0.10 |
| Diabetes mellitus | 2.73 (1.10–6.81) | 0.03 |
| Pneumonia POA | 5.68 (2.04–15.86) | 0.001 |
Definition of abbreviations: CI = confidence interval; POA = present on admission.
NIV failure indicates invasive mechanical ventilation after NIV. Odds ratios > 1 mean that if the variable is present, the patient is more likely to have NIV failure (the odds of NIV failure are 2.74 times greater, for example, with congestive heart failure than without).
Patients with and without Concomitant Pneumonia
Patients with a diagnosis of asthma exacerbation and pneumonia were more likely to be ventilated than patients without pneumonia, and, furthermore, NIV failure rate was higher (12.3 vs. 3.5%) among them (Table E3). We also saw higher case-fatality rates and longer lengths of stay in patients with pneumonia than in those without concomitant pneumonia. Consistent with the results in the full cohort, case-fatality rate and length of stay were lower in patients treated with NIV than in those treated with IMV in the sample restricted to patients with and without pneumonia (mortality: 6.9 vs. 16.7% and 1.7 vs. 13.8%, respectively). Tables E3 and E4 show results in patients with and without pneumonia.
Discussion
In this large observational study of almost 14,000 patients with asthma at 97 U.S. hospitals, we found that NIV was used in 4.0% of all hospitalizations and accounted for more than 4 out of every 10 ventilator starts (NIV or IMV). Compared with patients treated with IMV, those treated with NIV were older, were less likely to have concomitant pneumonia, and had a lower severity of illness score at admission. After adjusting for differences between patients using propensity matching and inverse probability of treatment weighting, we observed that patients successfully treated with NIV had lower mortality and a shorter length of stay than those receiving IMV. The large difference in mortality and length of stay between the groups treated with NIV and IMV raises the possibility of residual confounding by indication; because NIV and IMV were not randomly assigned, patients who received IMV were therefore sicker, as reflected in the LAPS. We found that although NIV failure was uncommon, it was associated with much higher mortality and resource use than NIV success but roughly comparable with IMV therapy. Patients with concomitant pneumonia were 2.5 times more likely to be ventilated than those without pneumonia and had worse outcomes.
Over the last 15 years, NIV has become standard of care in the management of acute exacerbation of COPD and cardiogenic pulmonary edema (24, 25). Nevertheless, NIV use has increased regardless of the etiology of the acute respiratory failure, including use for conditions such as asthma, where the supporting evidence is weak (6, 9, 26). One of the rationales for the applicability of NIV in severe asthma is likely to be a faster resolution of the attack and shorter hospital length of stay. Five small randomized controlled studies in adults with asthma suggest that compared with usual care, NIV may have a beneficial role through improving respiratory rate and reducing the need for hospitalization (8). We also found that patients treated with NIV had shorter length of stay than those treated with IMV. In a recent study of hospitalizations with asthma exacerbation at 58 U.S. hospitals, we found that there is a large variation in the hospital use of NIV, and hospitals in the highest quartile of NIV use had a small but significantly shorter length of stay, but higher hospital NIV rates were not associated with lower risk-adjusted case-fatality rates (27).
Despite the lack of evidence, a recent large study that used the Nationwide Inpatient Dataset reported that the proportion of admissions with asthma exacerbation for which NIV was used increased from 0.3% in 2000 to 1.9% in 2008 (9). Our study shows a continuous increase in NIV use from 2.3% in 2009 to 4.7% in 2012. There are few explanations for this increase in NIV use in asthma. First, clinicians may decide to use NIV, recognizing the pathophysiologic similarities between an asthma attack and COPD exacerbation. Second, the improved familiarity and comfort of physicians and respiratory therapists with NIV might also contribute to its use outside of the evidence-supported indications. Third, the fact that NIV is used more and more outside the intensive care unit may encourage practitioners to use this strategy for treating patients with less severe respiratory failure.
Compared with patients who were intubated (initial IMV or intubation after a trial of NIV), patients successfully treated with NIV had much better outcomes. In practice, NIV can be used in three different situations: in severe acute respiratory failure as an alternative to invasive mechanical ventilation, in mild to moderate respiratory failure without need for immediate respiratory support, or to prevent acute respiratory failure in patients without significant gas exchange abnormalities. One explanation for the marked difference in mortality in our study is that NIV may have been used mainly in patients with lower severity of illness and not in borderline patients who failed standard medical therapy (28, 29). The low rate of NIV failure in our study (4.7%) is in contrast with the rate of 19.4% reported in a study of 98 patients with a severe acute asthma exacerbation who failed routine treatment treated with NIV in the emergency department (30). The low rate of NIV failure also provided additional evidence that NIV was being used selectively in a lower-risk group.
Consistent with studies in patients with COPD, we found that patients with NIV failure had worse outcomes than patients with NIV success and slightly worse outcomes than patients who were initially intubated (23, 31, 32). Patients who failed NIV were more similar in their characteristics to those who were initially intubated, raising the possibility that NIV may have been used inappropriately in some patients, and it is possible that those patients who had early NIV failure should not have received NIV initially. Thus, our study supports the need to carefully choose which patients would be well suited for being treated initially with NIV rather than IMV. Patients with asthma can deteriorate rapidly, and those at high risk for NIV failure require close monitoring in an intensive care unit. Patients with asthma and concurrent pneumonia had higher risk of NIV failure, and for this group, caution in the use of NIV and vigilant monitoring if NIV is used appears prudent.
Strengths and Limitations
This study has several strengths. This is the largest cohort of patients with asthma treated with mechanical ventilation studied to date and provides insights into current ventilation practices and their outcomes. Using data from the EMRs of ∼100 hospitals allowed us to take advantage of detailed laboratory data and provide better adjustment for the severity of illness than in other observational studies with large administrative datasets. Given the lack of national clinical registries in patients hospitalized with asthma, these results give a glimpse into real-world practice patterns.
However, the study has also several limitations. First, the nonrandom nature of treatment assignment means that selection bias may have been present; nevertheless, we took advantage of rich clinical data obtained from the EMR of participating hospitals to produce a validated illness severity score (LAPS). Although we used robust statistical methods to reduce the threat of confounding by indication, there is still the possibility of residual bias. Second, we used ICD-9 diagnosis and procedure codes to define our cohort and the type of ventilation. There may be variability in the way hospitals use these codes. We supplemented the diagnosis codes with the requirement for specific treatments (i.e., steroids and bronchodilators) to ensure that patients included in the study were being treated for asthma exacerbation. In a few cases, we were unable to accurately define the sequence of NIV and IMV, and these patients were excluded, but this was uncommon (n = 28) and unlikely to bias our results. Third, we were able to match only 38% of patients treated with NIV with patients with similar propensity treated with IMV, and as such the results from the propensity-matched analysis apply only to those patients. Fourth, we had information only on the treatments the patients received during hospitalization and not before being hospitalized and hence could not assess if some patients were using NIV before admission. However, we excluded patients with a diagnosis of obstructive sleep apnea, which probably accounts for a large proportion of those on home NIV. In addition, the dataset does not contain information about the location where the NIV was initiated (intensive care unit or general medical floor) or about the specialist who was involved in the care of a patient treated with NIV (e.g., a pulmonary specialist), which may have affected mortality and length of stay.
Fourth, the diagnosis of pneumonia was established based on the ICD-9 codes present on admission and if the patient had antibiotic treatment within 48 hours of admission rather than chest X-rays. Thus, it is not possible to conclude that pneumonia was the trigger for the severe asthma exacerbation. Finally, although Cerner hospitals are diverse in their size and include both teaching and nonteaching hospitals, a majority are urban, and all have an EMR system. Consequently, they are not representative of all hospitals in the United States.
Conclusions
In this large observational study, we found that despite limited evidence regarding its efficacy in asthma, NIV was used in more than 40% of patients started on some form of ventilation (NIV or IMV). Patients successfully treated with NIV had better outcomes than those treated with IMV and patients who fail NIV. Although these results are hypothesis generating, they also highlight the need for large multicenter clinical trials before routine clinical use of NIV can be recommended. Future research should aim to identify criteria for patients with asthma exacerbation most likely to benefit from NIV.
Footnotes
Supported by National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health grant 1K01HL114631-01A1 (M.S.S.), and NHLBI grant 1R18HL108810-0131 (P.K.L.). The funders had no role in data collection, management, or analysis; study design, conduct, or interpretation of study findings; or the preparation, review, or approval of the manuscript for submitted for publication.
Author Contributions: M.S.S., T.L., P.S.P., J.S.S., N.S.H., and P.K.L. conceived and designed the study. M.S.S. acquired the data used in the analysis. M.S.S., B.H.N., T.L., A.P., J.S.S., N.S.H., R.J.G., D.M.K., and P.K.L. were involved in the analysis and interpretation of the data. M.S.S. drafted the manuscript, and B.H.N., T.L., A.P., P.S.P., J.S.S., N.S.H., R.J.G., D.M.K., and P.K.L. reviewed and contributed to revisions before submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Hospital Ambulatory Medical Care survey: 2010 emergency summary tables. 2010 [accessed 2015 May 29]. Available from: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf
- 2.McFadden ER., Jr. Acute severe asthma. Am J Respir Crit Care Med. 2003;168:740–759. doi: 10.1164/rccm.200208-902SO. [DOI] [PubMed] [Google Scholar]
- 3.Pendergraft TB, Stanford RH, Beasley R, Stempel DA, Roberts C, McLaughlin T. Rates and characteristics of intensive care unit admissions and intubations among asthma-related hospitalizations. Ann Allergy Asthma Immunol. 2004;93:29–35. doi: 10.1016/S1081-1206(10)61444-5. [DOI] [PubMed] [Google Scholar]
- 4.Gupta D, Nath A, Agarwal R, Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir Care. 2010;55:536–543. [PubMed] [Google Scholar]
- 5.Shapiro JM. Intensive care management of status asthmaticus. Chest. 2001;120:1439–1441. doi: 10.1378/chest.120.5.1439. [DOI] [PubMed] [Google Scholar]
- 6.Walkey AJ, Wiener RS.Utilization of non-Invasive ventilation in patients with acute respiratory failure from 2000–2009: a population-based study[abstract]. Am J Respir Crit Care Med 2012185A6488 [Google Scholar]
- 7.Ram FS, Lightowler JV, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003;(1):CD004104. doi: 10.1002/14651858.CD004104. [DOI] [PubMed] [Google Scholar]
- 8.Lim WJ, Mohammed Akram R, Carson KV, Mysore S, Labiszewski NA, Wedzicha JA, Rowe BH, Smith BJ. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12:CD004360. doi: 10.1002/14651858.CD004360.pub4. [DOI] [PubMed] [Google Scholar]
- 9.Nanchal R, Kumar G, Majumdar T, Taneja A, Patel J, Dagar G, Jacobs ER, Whittle J. Utilization of mechanical ventilation for asthma exacerbations: analysis of a national database. Respir Care. 2014;59:644–653. doi: 10.4187/respcare.02505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefan MS, Nathanson BH, Lindenauer PK, Priya A. Comparative effectiveness of noninvasive and invasive mechanical ventilation in patients hospitalized with asthma exacerbation [abstract] Am J Respir Crit Care Med. 2015;191:A3425. [Google Scholar]
- 11.Amin AP, Salisbury AC, McCullough PA, Gosch K, Spertus JA, Venkitachalam L, Stolker JM, Parikh CR, Masoudi FA, Jones PG, et al. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med. 2012;172:246–253. doi: 10.1001/archinternmed.2011.1202. [DOI] [PubMed] [Google Scholar]
- 12.Kosiborod M. Blood glucose and its prognostic implications in patients hospitalised with acute myocardial infarction. Diab Vasc Dis Res. 2008;5:269–275. doi: 10.3132/dvdr.2008.039. [DOI] [PubMed] [Google Scholar]
- 13.Kosiborod M, Inzucchi SE, Krumholz HM, Masoudi FA, Goyal A, Xiao L, Jones PG, Fiske S, Spertus JA. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med. 2009;169:438–446. doi: 10.1001/archinternmed.2008.593. [DOI] [PubMed] [Google Scholar]
- 14.International Classification of Diseases, 9th Revision, Clinical Modification. Salt Lake City: Medicode; 2004. [Google Scholar]
- 15.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 18.van Walraven C, Escobar GJ, Greene JD, Forster AJ. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63:798–803. doi: 10.1016/j.jclinepi.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 19.American Hospital Association, American Medical Record Association, Health Care Financing Administration, National Center for Health Statistics. ICD-9-CM coding and reporting official guidelines. J Am Med Rec Assoc. 1990;61:1–17. [PubMed] [Google Scholar]
- 20.Brookhart MA, Stürmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48:S114–S120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–963. [PubMed] [Google Scholar]
- 23.Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982–1993. doi: 10.1001/jamainternmed.2014.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med. 2007;35:2402–2407. doi: 10.1097/01.CCM.0000284587.36541.7F. [DOI] [PubMed] [Google Scholar]
- 25.Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, Mehta S, Cook DJ, Ayas N, Adhikari NK, Hand L, et al. Canadian Critical Care Trials Group/Canadian Critical Care Society Noninvasive Ventilation Guidelines Group. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183:E195–E214. doi: 10.1503/cmaj.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walkey AJ, Wiener RS. Use of noninvasive ventilation in patients with acute respiratory failure, 2000-2009: a population-based study. Ann Am Thorac Soc. 2013;10:10–17. doi: 10.1513/AnnalsATS.201206-034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefan MS, Nathanson BH, Priya A, Pekow PS, Lagu T, Steingrub JS, Hill NS, Goldberg RJ, Kent DM, Lindenauer PK. Hospitals’ patterns of use of noninvasive ventilation in patients with asthma exacerbation. Chest. 2016;149:729–736. doi: 10.1016/j.chest.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scala R. Noninvasive ventilation in severe acute asthma? Still far from the truth. Respir Care. 2010;55:630–637. [PubMed] [Google Scholar]
- 29.Soroksky A, Klinowski E, Ilgyev E, Mizrachi A, Miller A, Ben Yehuda TM, Shpirer I, Leonov Y. Noninvasive positive pressure ventilation in acute asthmatic attack. Eur Respir Rev. 2010;19:39–45. doi: 10.1183/09059180.00006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganesh A, Shenoy S, Doshi V, Rishi M, Molnar J. Use of noninvasive ventilation in adult patients with acute asthma exacerbation. Am J Ther. 2015;22:431–434. doi: 10.1097/MJT.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 31.Stefan MS, Bannuru RR, Lessard D, Gore JM, Lindenauer PK, Goldberg RJ. The impact of COPD on management and outcomes of patients hospitalized with acute myocardial infarction: a 10-year retrospective observational study. Chest. 2012;141:1441–1448. doi: 10.1378/chest.11-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA., Jr Comparative effectiveness of noninvasive ventilation vs invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013;8:165–172. doi: 10.1002/jhm.2014. [DOI] [PubMed] [Google Scholar]