Abstract
Rationale: The presence of obstructive sleep apnea (OSA) in patients with chronic obstructive pulmonary disease (COPD) is referred to as the OSA-COPD overlap syndrome. While lung inflation has been shown to be an important factor in determining upper airway stability, its role in determining OSA severity in smokers, including those with emphysema, has not been evaluated.
Objectives: To evaluate the importance of lung inflation on OSA severity (apnea–hypopnea index [AHI]) in smokers with suspected OSA.
Methods: Fifty-one smokers (18 males; mean [±SD] age, 59 ± 9 yr; body mass index [BMI], 32 ± 9 kg/m2) who were part of the Genetic Epidemiology of COPD (COPDGene) project were studied. Patients underwent a full-night polysomnography for suspected OSA. Other testing included spirometry and volumetric chest computed tomography (CT) for quantitative measurement of CT-derived percent emphysema and CT-derived percent gas trapping.
Measurements and Main Results: For the group overall, there was evidence of obstructive airway disease by spirometry (FEV1, 1.4 ± 0.5 L, 58 ± 14% predicted) and emphysema by quantitative CT (CT-derived percent emphysema, 11 ± 13%; CT-derived percent gas trapping, 31.6 ± 24.1%). Twenty-nine (57%) of the patients had OSA (AHI, 18 ± 12 events/h). Patients with OSA had a higher BMI but were younger than those without OSA (BMI, 35 ± 9 kg/m2 vs. 29 ± 7 kg/m2, respectively [P = 0.007]; age, 56 ± 8 yr vs. 62 ± 9 yr, respectively [P = 0.01]). There was an inverse correlation between the AHI and the CT-derived percent emphysema and CT-derived percent gas trapping, both for the entire group (r = −0.41 [P < 0.01] and r = −0.44 [P < 0.01], respectively) and when just those patients with OSA were evaluated (r = −0.43 [P = 0.04] and r = −0.49 [P = 0.03], respectively). Multiple linear regression revealed that, in addition to CT-derived percent emphysema and CT-derived percent gas trapping, sex and BMI were important in determining the AHI in these patients.
Conclusions: In smokers with OSA, increased gas trapping and emphysema as assessed by CT are associated with a decreased AHI. Along with sex and BMI, these measurements may be important in determining the severity of OSA in patients with COPD and may offer a protective mechanism in patients with more advanced disease.
Keywords: emphysema, chronic obstructive pulmonary disease, obstructive sleep apnea, overlap syndrome, lung volume
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation and is the most common smoking-related illness (1, 2). With a reported prevalence greater than 10% in adults 40–79 years of age, COPD is now reported as the third leading cause of mortality in Western countries (1, 3, 4). Obstructive sleep apnea (OSA), defined by recurrent upper airway obstructive events during sleep in the presence of daytime sleepiness, is also highly prevalent in the general population (3–17%) and has been associated with the development of significant cardiovascular disease (5–10). The prevalence of moderate to severe OSA appears to be only increasing with the ongoing obesity epidemic (6).
Coexistence of COPD and OSA has been referred to as the OSA-COPD overlap syndrome (11). The true prevalence remains uncertain, with one report noting 11% of patients with known OSA having an obstructive pattern on spirometry (12). While researchers in another study stated that the prevalence of OSA in patients with obstructive lung disease was no higher than in the general population, they noted that patients with overlap syndrome had more disturbed sleep and greater nocturnal oxygen desaturation than those with either disease alone (13). In addition, patients with overlap syndrome have a higher mortality and are more likely to experience an exacerbation that leads to hospitalization than patients with COPD alone (14). Continuous positive airway pressure (CPAP) therapy appears to reverse this effect, with improved survival noted in treated patients with overlap syndrome (14, 15).
The normal upper airway behaves like a collapsible tube and is therefore influenced by forces tending to collapse it and those that dilate and maintain its patency (16). The critical closing pressure (Pcrit), measured as the amount of negative pressure applied nasally required to collapse the upper airway, reflects the balance between these collapsing and dilating forces, with a more negative number indicating a more stable and less collapsible airway (16, 17). Collapsing forces include the surrounding bony and soft tissue structures of the upper airway and the intraluminal negative pressure generated by the diaphragm during inspiration (16). Dilating forces include those generated by upper airway pharyngeal dilator muscles. In addition, the importance of lung volume on upper airway patency has been demonstrated, with increasing lung volume stabilizing the airway and decreasing upper airway resistance (18–21). Proposed mechanisms include caudal traction on the trachea and/or an increasing transpulmonary pressure gradient (18–21). In patients with OSA, it has been shown that lower lung volume is an important factor that contributes to upper airway collapsibility and obstruction (22–24). However, no previous study has evaluated the impact that lung inflation has on the severity of OSA in smokers, including those with emphysema (overlap patients).
Genetic Epidemiology of COPD (COPDGene) is a multicenter observational study designed to identify genetic factors associated with COPD (25). It also uses high-resolution computed tomography (CT) to characterize disease-related phenotypes. With specialized software that is able to calculate both the CT-derived percent emphysema and CT-derived percent gas trapping in the lung, we were able to use these measurements in a subgroup of patients with suspected OSA. We hypothesized that patients with a higher percentage of emphysema would have a lower apnea–hypopnea index (AHI) because of the effects of lung inflation and gas trapping on upper airway stability. Some of the results of the present study have been reported previously in the form of an abstract (26).
Methods
Patient Selection
The sample was derived from 10,192 smokers with and without emphysema from the COPDGene project (25). Patients enrolled were non-Hispanic whites and African Americans between the ages of 45 and 80 years with a minimum smoking history of 10 pack-years. Inclusion and exclusion criteria for the study have been described previously (25). The present study included 51 patients from COPDGene who had been referred to the Sleep Clinic at Temple University Hospital for suspected OSA and had completed an overnight polysomnogram. The study was approved by the Temple University School of Medicine Institutional Review Board for Human Research (Philadelphia, PA).
Spirometry
All spirometric data were collected using the EasyOne spirometer (ndd Medical, Zurich, Switzerland). Subjects performed spirometry according to the American Thoracic Society standards, with the predictive values based on the Third National Health and Nutrition Examination Survey reference values (27). The reported values are the post-bronchodilator measurements following 180 mg of albuterol.
High-Resolution Computed Tomography
Multidetector CT scanners were used for volumetric chest CT acquisitions that were obtained at full inspiration (220 mA) and at the end of normal expiration (50 mA) as previously described (25, 28). To enhance spatial resolution, scans were reconstructed with thin-slice collimation with slice thickness and intervals of less than 1 mm. Quantitative analysis of emphysema severity was performed using 3DSlicer (http://www.slicer.org/) on segmented lung images. The percent emphysema was defined as the total percentage of both lungs with attenuation values less than −950 Hounsfield units on inspiratory images. The percent gas trapping was defined as the total percentage of both lungs with attenuation values less than −856 Hounsfield units on expiratory images (25, 28).
Polysomnograms
Polysomnography was performed while the patient was breathing room air according to the American Academy of Sleep Medicine guidelines. Sleep was staged and arousals defined using established criteria (29). Obstructive apneas were defined by lack of airflow for longer than 10 seconds, associated with the presence of rib cage and abdominal movement (29). Obstructive hypopneas were defined by a 30% decrease in airflow for longer than 10 seconds, associated with the presence of rib cage and abdominal movement and accompanied by an oxygen desaturation of greater than or equal to 3% or an arousal (29). Apneas were defined as central if there was a lack of respiratory effort during the period of absent airflow (29). The AHI was calculated as the number of apneic and hypopnic events per hour of sleep. All of the polysomnograms were initially scored by a single senior technologist. The same author (S.L.K.) reviewed each study.
Statistical Analysis
Data are displayed as the mean ± SD for continuous variables and the count and percentage for categorical variables. Comparisons of patients with versus without OSA were performed using Fisher’s exact test or Wilcoxon rank-sum test. The relationships between physiological variables or other covariates were assessed using Spearman’s correlation coefficients. P values less than 0.05 were considered statistically significant. Multiple linear regression models were used to evaluate the contribution of the various parameters in the prediction of AHI using all available patients. The significance level for retention of a variable in a model was less than 0.10. All statistical analyses were performed using SAS version 9.3 software (SAS Institute, Cary, NC).
Results
Patient Characteristics
Fifty-one patients (18 males; mean [±SD] age, 59 ± 9 yr; body mass index [BMI], 32 ± 9 kg/m2) who were enrolled in COPDGene and had completed a full-night polysomnogram for suspected OSA were included in the study. There was evidence of obstructive airway disease (FEV1, 1.4 ± 0.5 L, 58 ± 14% predicted) and CT-derived evidence of emphysema and gas trapping (11 ± 13% and 31.6 ± 24.1%, respectively). An accurate CT measurement of the percent emphysema and percent gas trapping could not be obtained in 9 and 15 of the 51 patients, respectively. There were no significant differences between those patients in whom percent emphysema could and could not be calculated, in regard to both BMI and degree of airflow obstruction (BMI, 32 ± 8 kg/m2 vs. 33 ± 11 kg/m2 [P = 0.7]; FEV1 [% predicted], 1.5 ± 0.6 L [58 ± 22%] vs. 1.4 ± 0.4 L [56 ± 18%] [P = 0.6, P = 0.9], respectively). Similar findings were noted in regard to those patients in whom the percent gas trapping could and could not be determined (BMI, 32 ± 8 kg/m2 vs. 38 ± 10 kg/m2 [P = 0.8]; FEV1 [% predicted], 1.5 ± 0.6 L [58 ± 22%] vs. 1.3 ± 0.4 L [55 ± 20%] [P = 0.3, P = 0.6], respectively). Table 1 describes the baseline characteristics of the 51 patients who completed the study.
Table 1.
Baseline characteristics of all patients (n = 51)
| Variable | Overall (n = 51) | OSA (n = 29) | No OSA (n = 22) | P Value* |
|---|---|---|---|---|
| Sex, female/male | 33/18 | 17/12 | 16/6 | 0.38 |
| Age, yr | 59 ± 9 | 56 ± 8 | 62 ± 9 | 0.01 |
| BMI,† kg/m2 | 32 ± 9 | 35 ± 9 | 29 ± 7 | 0.007 |
| Pack-years‡ | 43 ± 24 | 42 ± 28 | 45 ± 19 | 0.37 |
| BODE index | 3.0 ± 1.7 | 3.4 ± 1.8 | 2.5 ± 1.4 | 0.04 |
| FEV1, % predicted, L | 1.4 ± 0.5 (57 ± 21) | 1.4 ± 0.6 (54 ± 24) | 1.5 ± 0.5 (63 ± 17) | 0.61 |
| FVC, % predicted, L | 2.4 ± 0.7 (76 ± 18) | 2.4 ± 0.7 (71 ± 19) | 2.6 ± 0.8 (83 ± 13) | 0.52 |
| FEV1/FVC, % | 58 ± 14 | 58 ± 16 | 58 ± 12 | 0.91 |
| CT-derived percent emphysema† | 11.1 ± 13.1 | 10.2 ± 14.1 | 12.3 ± 12.1 | 0.11 |
| CT-derived percent gas trapping† | 31.6 ± 24.1 | 28.1 ± 26.3 | 35.6 ± 21.4 | 0.11 |
| AHI, events/h | 11 ± 12 | 18 ± 12 | 2 ± 1 | <0.0001 |
| Non–REM AHI, events/h | 9 ± 14 | 15 ± 16 | 2 ± 2 | <0.0001 |
| REM AHI, events/h | 22 ± 25 | 33 ± 26 | 4 ± 7 | 0.0001 |
| TST, min | 296 ± 74 | 293 ± 83 | 299 ± 62 | 0.95 |
| Sleep efficiency, % | 72 ± 19 | 69 ± 22 | 76 ± 14 | 0.28 |
| Arousal index, events/h | 20 ± 19 | 25 ± 23 | 15 ± 11 | 0.04 |
| Mean SaO2, % | 93 ± 3 | 93 ± 3 | 94 ± 3 | 0.17 |
| Lowest SaO2, % | 83 ± 8 | 81 ± 7 | 85 ± 8 | 0.02 |
| Percent TST SaO2 <90% | 13 ± 23 | 15 ± 25 | 10 ± 21 | 0.10 |
| Sleep architecture, % TST | ||||
| Stage N1 | 13.5 ± 8.7 | 12.8 ± 7.5 | 14.3 ± 10.2 | 0.82 |
| Stage N2 | 61.5 ± 14.1 | 62.5 ± 13.4 | 60.2 ± 15.1 | 0.68 |
| Stage N3 | 7.7 ± 9.7 | 8.7 ± 11.1 | 6.4 ± 7.6 | 0.37 |
| Stage REM | 17.4 ± 8.9 | 16.0 ± 8.7 | 19.2 ± 8.9 | 0.35 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; BODE = body mass index, airflow obstruction, dyspnea, and exercise capacity; CT = computed tomography; OSA = obstructive sleep apnea; REM = rapid eye movement; TST = total sleep time.
Data are presented as the mean ± SD unless otherwise indicated.
Based on Fisher’s exact test or Wilcoxon rank-sum test.
Missing values for BMI, CT-derived percent emphysema, and CT-derived percent gas trapping for the OSA/no OSA groups: 1/0, 5/4, and 10/5, respectively.
According to American Thoracic Society guidelines.
Prevalence of Obstructive Sleep Apnea
Twenty-nine (57%) of the 51 patients had OSA on the basis of their polysomnogram (AHI, 18 ± 12 events/h in the OSA group vs. 2 ± 1 events/h in the group without OSA). The BMI was higher but the age younger in the OSA group than among those patients without OSA (BMI, 35 ± 9 kg/m2 vs. 29 ± 7 kg/m2, respectively, P = 0.007; age, 56 ± 8 yr vs. 62 ± 9 yr, respectively, P = 0.01). While the CT-derived percent emphysema and CT-derived percent gas trapping trended higher in the patients without OSA than in those with OSA (CT-derived percent emphysema, 12.3 ± 12.1% vs. 10.2 ± 14.1%, P = 0.11; CT-derived percent gas trapping, 35.6 ± 21.4% vs. 28.1 ± 26.3%, P = 0.11), the differences were not statistically significant (Table 1). Other variables that were significantly different between patients with versus without OSA included the arousal index and the lowest SaO2 during the night (Table 1).
Correlates of Sleep-disordered Breathing and CT Measurements
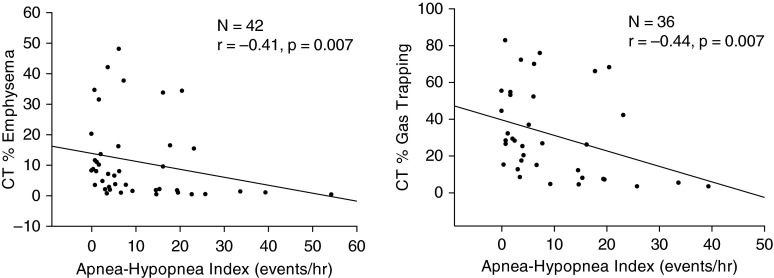
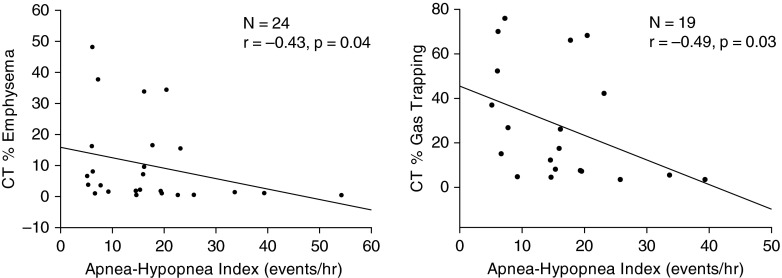
For the group as a whole, there was a significant inverse correlation between the AHI and the CT-derived percent emphysema (r = −0.41; P < 0.01) and CT-derived percent gas trapping (r = −0.44; P < 0.01) (Figure 1). When just those patients diagnosed with OSA were included in the analysis, a similar relationship was seen, with a significant inverse correlation between the AHI and CT-derived percent emphysema (r = −0.43; P = 0.04) and the CT-derived percent gas trapping (r = −0.49; P = 0.03) (Figure 2). For the group as a whole, there was a significant inverse correlation noted between the REM AHI and the CT-derived percent emphysema (r = −0.43; P = 0.01) and CT-derived percent gas trapping (r = −0.39; P = 0.03) but no correlation between the non–REM AHI and CT-derived percent emphysema (r = −0.25; P = 0.16) and CT-derived percent gas trapping (r = −0.12; P = 0.54). While there was a significant inverse correlation seen between the BMI and CT-derived percent gas trapping for the group as a whole (r = −0.49; P < 0.01), there was no correlation noted when just those patients with diagnosed OSA were included (r = −0.29; P = 0.23). A correlation was also noted between the BODE index (body mass index, airflow obstruction, dyspnea, and exercise capacity) and CT-derived percent gas trapping, both for the group as a whole (r = 0.33; P = 0.048) and for just those patients with OSA (r = 0.48; P = 0.04). Physiological variables that were correlated with the AHI, both for the total group and for the OSA group, are reported in Table 2. Multiple linear regression demonstrated that, in addition to the CT-derived percent emphysema and CT-derived percent gas trapping, sex and BMI appeared to be responsible for determining the AHI (Table 3).
Figure 1.
For the entire group (those with and without obstructive sleep apnea), there was a significant inverse correlation between both the computed tomography (CT)–derived percent emphysema and CT–derived percent gas trapping and the apnea–hypopnea index.
Figure 2.
For those patients with obstructive sleep apnea (apnea–hypopnea index, >5 events/h), there was a significant inverse correlation between both the computed tomography (CT)–derived percent emphysema and CT–derived percent gas trapping and the apnea–hypopnea index.
Table 2.
Spearman’s correlation coefficients between physiological variables and the apnea–hypopnea index, both overall and for patients with obstructive sleep apnea only
| Variable | Apnea–Hypopnea Index Overall (n = 51) | Apnea–Hypopnea Index OSA Group (n = 29) |
|---|---|---|
| Age, yr | −0.28* | 0.12 |
| BMI,† kg/m2 | 0.48‡ | 0.29 |
| Pack-years§ | −0.03 | 0.21 |
| CT-derived percent emphysema† | −0.41‡ | −0.42* |
| CT-derived percent gas trapping† | −0.44‡ | −0.49* |
| TST, min | −0.21 | −0.40* |
| Sleep efficiency, % | −0.31* | −0.37* |
| Arousal index, events/h | 0.47‡ | 0.47‡ |
| Mean SaO2, % | −0.16 | 0.03 |
| Lowest SaO2, % | −0.29* | −0.01 |
| Percent TST SaO2 <90% | 0.22 | 0.16 |
| Sleep architecture, % TST | ||
| Stage N1 | 0.16 | 0.47‡ |
| Stage N2 | −0.02 | −0.02 |
| Stage N3 | 0.11 | −0.14 |
| Stage REM | −0.18 | −0.21 |
Definition of abbreviations: BMI = body mass index; CT=computed tomography; OSA = obstructive sleep apnea; REM = rapid eye movement; TST = total sleep time.
P < 0.05.
Missing values for BMI, CT-derived percent emphysema, and CT-derived percent gas trapping for the OSA/no OSA groups: 1/1, 9/5, and 15/10, respectively.
P < 0.01.
According to American Thoracic Society guidelines.
Table 3.
Multiple linear regression analyses of apnea–hypopnea index (n = 36)
| Independent Variable | Regression Coefficient | Standard Error | P Value |
|---|---|---|---|
| Dependent variable AHI* | |||
| Female sex | −7.47 | 2.97 | 0.017 |
| BMI | 0.57 | 0.20 | 0.008 |
| CT-derived percent emphysema | 0.45 | 0.24 | 0.069 |
| CT-derived percent gas trapping | −0.31 | 0.13 | 0.022 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CT = computed tomography.
Regression equation: Predicted AHI = 0.73–7.47 × Female + 0.57 × BMI + 0.45 × CT % Emphysema − 0.31 × CT % Gas Trapping. Standard error of the estimate, 8.32; r2 = 0.39.
Discussion
While the overlap syndrome describes the coexistence of OSA in patients with COPD, the physiological variables that determine the severity of OSA in these patients has not previously been investigated. Our study is the first to demonstrate that the degree of emphysema and gas trapping, as assessed by CT, are important determinants of OSA severity in patients with COPD. There are three major findings in this study: (1) the prevalence of OSA is high in smokers and patients with emphysema with clinically suspected OSA; (2) there is an inverse relationship between the severity of emphysema and gas trapping and the AHI; and (3) factors in addition to the severity of emphysema and gas trapping, such as sex and BMI, are important in determining the severity of OSA in these patients.
The true prevalence of the overlap syndrome is still debated. While researchers in prior studies have reported prevalence rates greater than 10%, epidemiological studies have noted either much lower rates (1–3%) or rates of OSA that are similar in those with and without obstructive airway disease (12–14, 30). However, in the present study, we did not attempt to evaluate the prevalence of the overlap syndrome. All of our patients had suspected OSA clinically and therefore underwent an in-laboratory polysomnogram study, with 57% having an AHI of more than five events per hour (AHI, 18 ± 12 events/h). Sharma and colleagues (31) reported that 24 (92%) of 26 of their patients with obstructive airway disease and a Berlin Questionnaire score that put them at high risk for OSA had a positive polysomnogram study (mean AHI, 17.1 events/h). In contrast, Machado and colleagues (15) reported that only 95 (16%) of their patients with COPD on long-term oxygen had symptoms of OSA and an AHI of more than 15 events per hour in overnight testing.
The most significant finding in our study was the inverse relationship noted between the CT-derived percent emphysema and CT-derived percent gas trapping, and the AHI. In addition, CT-derived percent gas trapping was noted to be an important determinant for the AHI based on multiple linear regression. In previous studies, lung volume has been shown to be an important determinant for upper airway stability in both normal animal and human studies, as well as in patients with OSA (18–24, 32). Potential mechanisms that have been investigated to explain how an increase in lung volume increases upper airway patency include (1) mediastinal caudal traction generated by the diaphragm with inspiration that results in stabilization of the airway wall and (2) an increase in the transmural distending pressure in the upper airway induced by thoracic expansion during inspiration.
Van de Graaff and coworkers (18, 19) initially demonstrated that, during inspiration in anesthetized dogs, the thoracic traction and stabilization of the trachea were the result of both the caudal pull of mediastinal structures and a transthoracic pressure gradient that was generated (18, 19). In a study of 19 healthy individuals, Stanchina and coworkers (32) demonstrated that, during non–REM sleep, manipulated decreases in end-expiratory lung volume (EELV) resulted in increased upper airway collapsibility and resistance. While increases in EELV had the opposite effect of decreasing resistance and collapsibility, these changes were not significant. Squier and colleagues (20) examined the relationship between EELV and the negative pressure required to collapse the upper airway (Pcrit) in 18 healthy subjects during non–REM sleep. By manipulating EELV with a negative pressure ventilator, they found that Pcrit varied inversely with EELV, with a more negative Pcrit indicative of a less collapsible airway.
More recently, Hillman and colleagues (21) used phrenic nerve–stimulated diaphragmatic contraction to evaluate the effect of lung volume changes on upper airway stability in 10 anesthetized healthy subjects. Peak inspiratory flow increased only when diaphragmatic contraction was associated with an increase in lung volume. These findings suggest that lung volume–induced transthoracic pressure changes, and not caudal traction on the mediastinum, is important in upper airway stabilization during inspiration.
While an increase in lung volume appears to help stabilize the upper airway, a decrease in lung volume has been shown to be important in the development of upper airway obstruction in patients with OSA (22–24). Heinzer and coworkers (22) evaluated the effect of changes in lung volume on CPAP requirements in 17 patients with OSA. With patients sleeping in a head-out rigid shell to manipulate extrathoracic pressure and thus lung volume, a decrease in lung volume by 570 ml was associated with an increase in the CPAP required (from 12 to 17 cm H2O) to prevent flow limitation. In comparison, an increase in lung volume by 420 ml led to a decrease in CPAP required (from 12 to 5 cm H2O) to maintain flow.
In a subsequent study in 12 patients with OSA, Heinzer and colleagues (23) examined how the AHI is affected when there is a change in lung volume. While again lung volume was externally manipulated during sleep, the baseline AHI of 62 events per hour decreased to 37 events per hour when lung volume was increased by the amount noted when the patient was placed on the prescribed CPAP and decreased further to 31 events per hour when lung volume was increased an additional 500 ml. Finally, Owens and coworkers (24) measured the effect of increasing EELV by 500 ml on Pcrit measurements in 15 patients with OSA and 7 healthy controls. In both groups, Pcrit decreased to a similar degree, which supports the importance of EELV in the pathogenesis of OSA.
In our present study, we demonstrated an inverse correlation between the AHI and the CT-derived percent emphysema and CT-derived percent gas trapping in patients who smoked and had emphysema. In addition, we showed the importance of these parameters in determining the AHI by multiple linear regression, suggesting that variables that may influence lung volume and thus airway stability may also be important in determining which patients with COPD develop OSA (overlap syndrome). While CT-derived percent emphysema demonstrated only marginal independent predictive significance for the AHI on multiple linear regression, we believe this is secondary and compensatory to the dominant relationship noted between CT-derived percent gas trapping and the AHI.
With regard to the difference in REM versus non–REM AHI as they relate to correlations with CT measurements of lung volume, it is difficult to know if a change in lung volume during sleep was responsible for these findings, especially without dynamic monitoring of lung volumes during the night (non–REM vs. REM). The findings may reflect the increased number of apneas and hypopneas noted during REM sleep in our patients (Table 1), which may be the result of an increase in upper airway resistance during REM sleep in patients with COPD (33). The fewer number of events during non–REM sleep may not allow a correlation to be seen without a larger number of patients.
While prior studies have shown that lung volume appears to be important in determining upper airway stability, there are other recognized factors that are also involved, including the BMI, sex, and age (31, 34). Kirkness and colleagues (34) examined the importance of these factors in airway stability as assessed by Pcrit measurements in a group of patients with OSA and healthy control subjects. Pcrit was found to be higher in men than in women, and it was directly correlated with BMI in both groups.
Age was also noted to be a factor in men and in perimenopausal women. In a group of patients with obstructive airways disease, Sharma and colleagues (31) found that BMI but not FEV1 percent predicted was correlated with OSA risk. These findings are consistent with our results, where sex and BMI, in addition to measurements of CT-derived percent emphysema and CT-derived percent gas trapping, were important determinants for the AHI (Table 3).
While a BMI effect on EELV may have influenced the relationship between AHI and CT-derived percent gas trapping in our study, the lack of a correlation between BMI and CT-derived percent gas trapping in those patients with diagnosed OSA despite a continued correlation between AHI and CT-derived percent gas trapping suggests that other factors, such as the effects of their obstructive lung disease on CT-derived percent gas trapping, were playing a role.
Limitations
Our study has a number of limitations that need to be discussed. First, not all of the patients who had a polysomnogram demonstrated OSA, despite a clinical suspicion for the disorder (43%). However, the fact that the patients with an AHI of fewer than five events per hour had greater CT-derived percent emphysema and greater CT-derived percent gas trapping (Figure 1) is in keeping with our hypothesis that an increase in these variables would stabilize the upper airway and therefore be protective of obstructive events.
Second, other factors, such as sex and BMI, were noted determinants for the AHI in our patients. However, in addition to these factors, CT-derived percent gas trapping was also found to be an important determinant on multiple linear regression.
Finally, the CT of the chest was done with the patients awake, while the polysomnography and the determination of the AHI were done during sleep. However, both tests were performed with the patient recumbent, which can affect lung volume and upper airway cross-sectional area (35). In addition, EELV during non–REM sleep and FRC during non–REM and REM sleep have been shown to remain unchanged as compared with wakefulness in patients with emphysema (33, 36).
Conclusions
In smokers with OSA, the degree of gas trapping and emphysema have a significant impact on the severity of OSA, with more severe disease assessed by CT measurements being associated with a lower AHI. Whether these variables have a stabilizing effect on the upper airway in these patients awaits further study.
Footnotes
Supported by National Institutes of Health (NIH) grants R01 HL089856 and R01 HL089897. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions: S.L.K.: takes responsibility for the study as a whole; R.T., M.E.V., X.S., F.J., V.K., I.S., G.E.D’A., and G.J.C.: contributed to study design, data collection, data analysis, statistical analysis, and manuscript preparation; and D.Y.: contributed to data analysis, statistical analysis, and manuscript preparation.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Rostron BL, Chang CM, Pechacek TF. Estimation of cigarette smoking-attributable morbidity in the United States. JAMA Intern Med. 2014;174:1922–1928. doi: 10.1001/jamainternmed.2014.5219. [DOI] [PubMed] [Google Scholar]
- 3.Tilert T, Dillon C, Paulose-Ram R, Hnizdo E, Doney B. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Respir Res. 2013;14:103. doi: 10.1186/1465-9921-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doney B, Hnizdo E, Dillion CF, Paulose-Ram R, Tilert T, Wolz M, Beeckman-Wagner LA. Prevalence of airflow obstruction in U.S. adults aged 40–79 years: NHANES data 1988–1994 and 2007–2010. COPD. 2015;12:355–365. doi: 10.3109/15412555.2014.948998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 10.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 11.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6:651–661. [PubMed] [Google Scholar]
- 12.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151:82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 13.Sanders MH, Newman AB, Haggerty CL, Redline S, Lebowitz M, Samet J, O’Connor GT, Punjabi NM, Shahar E Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 14.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 15.Machado M-CL, Vollmer WM, Togeiro SM, Bilderback AL, Oliveira M-VC, Leitão FS, Queiroga F, Jr, Lorenzi-Filho G, Krishnan JA. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. 2010;35:132–137. doi: 10.1183/09031936.00192008. [DOI] [PubMed] [Google Scholar]
- 16.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol (1985) 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 18.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol (1985) 1988;65:2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 19.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol (1985) 1991;70:1328–1336. doi: 10.1152/jappl.1991.70.3.1328. [DOI] [PubMed] [Google Scholar]
- 20.Squier SB, Patil SP, Schneider H, Kirkness JP, Smith PL, Schwartz AR. Effect of end-expiratory lung volume on upper airway collapsibility in sleeping men and women. J Appl Physiol (1985) 2010;109:977–985. doi: 10.1152/japplphysiol.00080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillman DR, Walsh JH, Maddison KJ, Platt PR, Schwartz AR, Eastwood PR. The effect of diaphragm contraction on upper airway collapsibility. J Appl Physiol (1985) 2013;115:337–345. doi: 10.1152/japplphysiol.01199.2012. [DOI] [PubMed] [Google Scholar]
- 22.Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–117. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo Y-L, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol (1985) 2010;108:445–451. doi: 10.1152/japplphysiol.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swift I, Thwari R, Vega ME, Yu D, Soler X, Jaffe F, Criner GC, Krachman SL. Effect of emphysema severity on the apnea–hypopnea index in smokers with obstructive sleep apnea [abstract] Am J Respir Crit Care Med. 2014;189:A5845. doi: 10.1513/AnnalsATS.201511-765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 28.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, Criner GJ, Kim V, Bowler RP, Hanania NA, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.0. Darien, IL: American Academy of Sleep Medicine; 2012. [Google Scholar]
- 30.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration. 2005;72:142–149. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 31.Sharma B, Feinsilver S, Owens RL, Malhotra A, McSharry D, Karbowitz S. Obstructive airway disease and obstructive sleep apnea: effect of pulmonary function. Lung. 2011;189:37–41. doi: 10.1007/s00408-010-9270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, White DP. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 33.Ballard RD, Clover CW, Suh BY. Influence of sleep on respiratory function in emphysema. Am J Respir Crit Care Med. 1995;151:945–951. doi: 10.1164/ajrccm.151.4.7697271. [DOI] [PubMed] [Google Scholar]
- 34.Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol (1985) 2008;104:1618–1624. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fouke JM, Strohl KP. Effect of position and lung volume on upper airway geometry. J Appl Physiol (1985) 1987;63:375–380. doi: 10.1152/jappl.1987.63.1.375. [DOI] [PubMed] [Google Scholar]
- 36.O’Donoghue FJ, Catcheside PG, Eckert DJ, McEvoy RD. Changes in respiration in NREM sleep in hypercapnic chronic obstructive pulmonary disease. J Physiol. 2004;559:663–673. doi: 10.1113/jphysiol.2004.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]