Abstract
Rationale: Although lung transplant recipient survival is better at higher volume centers, the effect of center volume on admission cost and early hospital readmission is unknown.
Objectives: To understand the association between transplant center volume and recipient risk-adjusted transplant admission cost, in-hospital mortality, and early hospital readmission in lung transplant recipients.
Methods: Medicare lung transplant recipients from May 4, 2005 to December 31, 2011 were identified through linkage of transplant registry and Medicare administrative claims. Transplant admission cost was extracted, adjusted for regional price variation, and compared across low-, intermediate-, and high-volume centers. A multivariable hierarchical generalized linear regression model was used to assess the effect of transplant center volume on recipient adjusted cost. Modified Poisson regression models were used to assess adjusted in-hospital mortality and early hospital readmission by transplant center volume.
Measurements and Main Results: There were 3,128 Medicare lung transplant recipients identified. Unadjusted transplant cost was lower at high-volume centers (mean, $131,352 [SD, ±$106,165]; median, $90,177; interquartile range [IQR], $79,165–$137,915) than at intermediate-volume (mean, $138,792 [SD, ±$106,270]; median, $93,024; IQR, $82,700–$154,857) or low-volume (mean, $143,609 [SD, ±$123,316]; median, $95,234; IQR, $83,052–$152,149) centers (P < 0.0001). After adjusting for recipient health risk, low-volume centers had an 11.66% greater transplant admission cost (P = 0.040), a 41% greater risk for in-hospital mortality (P = 0.015), and a 14% greater risk for early hospital readmission (P = 0.033) compared with high-volume centers. There was no significant difference in transplant cost, in-hospital mortality, or early hospital readmission between intermediate- and high-volume centers.
Conclusions: Lung transplant admission cost, in-hospital mortality, and early hospital readmission rate are lower at high-volume centers compared with low-volume centers.
Keywords: lung transplantation, economics, costs and cost analysis
Lung transplant is increasingly used to treat end-stage lung disease. While advancements in the field have led to improvements in recipient survival, the health-care resources associated with this procedure within the United States have risen, particularly after implementation of the lung allocation score (LAS) (1, 2). The resources attributed to this operation are considerable, with per-person Medicare costs averaging $236,450 over the first post-transplant year (3). Therefore, as we pursue improvements in transplant survival, there is also a growing need to understand and improve on cost and value.
The index transplant admission cost and early hospital readmission (EHR) cost are the highest components of resource use within the first postoperative year. Therefore, understanding the factors associated with these costs could enable transplant providers, centers, and stakeholders to implement care models that reduce cost and improve transplant value.
Previous kidney and liver transplant studies demonstrate significant variation in transplant admission cost and EHR among centers, with transplant center volume contributing to variation (4–12). For instance, concerning liver transplants, high-volume centers have lower transplant admission cost than intermediate- and low-volume centers (5). Concerning lung transplants, higher center volume is associated with better recipient survival; whether higher transplant center volume similarly improves lung transplant admission cost and decreases EHR remains to be determined (13–16).
The goal of this study was to understand the association between lung transplant center volume and recipient risk-adjusted transplant admission cost, in-hospital mortality, and EHR. Understanding the relationship between how busy a lung transplant center is and how resources are used may guide the development and implementation of lung transplant initiatives aimed at achieving better survival at lower cost.
Methods
The study population included adult Medicare lung transplant recipients who underwent transplant between May 4, 2005 and December 31, 2011. Recipients who underwent retransplant or multiorgan transplant were excluded. Lung transplant recipient information from the Scientific Registry of Transplant Recipients (SRTR) was linked to Medicare claims data. This linkage was performed by the SRTR and the Centers for Medicare and Medicaid Services, using a common patient identifier. All recipients with Medicare as the primary payer at the time of transplant, as represented by the presence of both Part A and Part B claims, were identified.
Descriptive variables were generated from SRTR data fields and compared between Medicare and non-Medicare recipients. This study used data from the SRTR. The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The Stanford University Institutional Review Board (Stanford, CA) approved this study.
Index Transplant Admission Cost
Paid Medicare cost—not hospital-submitted charges—was extracted from Medicare claims data and adjusted to measure payment only for patient care. The Medicare Provider Analysis and Review research identifiable file was used to extract inpatient diagnosis-related group (DRG) cost and DRG outlier cost during the time of index transplant admission. The Medicare carrier research identifiable file was used to extract provider cost from claims between the time of index transplant admission and discharge. Indirect medical education cost and disproportionate share cost were excluded as they represent non–patient care–related institutional cost variation. To account for intentional regional variation in Medicare payment, cost was adjusted using Medicare-reported core-based statistical area wage indices, as previously described (17, 18). Cost was adjusted to 2012 U.S. dollars to account for inflation (19).
Recipient and center volume associations with transplant admission cost were assessed, using a multivariable hierarchical generalized linear regression model with robust standard errors to account for center clustering. A gamma distribution with log-link was used to account for the right-skewed distribution of cost. Variables a priori selected for model inclusion included age, sex, underlying diagnosis, transplant procedure type (bilateral or single), lung allocation score (LAS) at transplant, pretransplant use of mechanical ventilation or extracorporeal membrane oxygenation (ECMO), and an indicator for transplant center volume.
Annual transplant center volume was calculated as the mean annual number of lung transplants performed within a center during the study period. On the basis of prior lung transplant literature, transplant center volume was a priori categorized into three groups: fewer than 20 annual lung transplants as a low-volume center, between 20 and 34 annual lung transplants as an intermediate-volume center, and at least 35 annual transplants as a high-volume center (13, 16). LAS at transplant was divided into functional categories according to previous literature and analyzed in continuous form in sensitivity analyses (1).
Resource Use, Complications, and Mortality by Center Volume
Recipient characteristics, unadjusted transplant admission cost, hospital and intensive care unit length of stay, frequency of DRG outlier payments, complications, and transplant admission mortality were compared by transplant center volume. Complications included duration of ventilator support and postoperative reintubation, tracheostomy, or dialysis. Continuous variables were compared by Kruskal–Wallis test, given their nonnormal distribution, and categorical variables were compared by χ2 or Fisher exact test.
A modified Poisson regression model with robust standard errors was used to estimate the adjusted relative risk (RR) of index admission death by transplant center volume (20). The risk for index admission death was adjusted for recipient age, sex, underlying diagnosis, transplant procedure type, LAS at transplant, and pretransplant use of mechanical ventilation or ECMO.
Early Hospital Readmission
EHR was defined as at least one readmission to an inpatient hospital, as identified by the presence of a Medicare claim, within 30 days of discharge from the index admission. The population at risk for EHR was limited to those alive at the time of hospital discharge. Recipient characteristics by EHR status were compared by Mann–Whitney U test for continuous variables and by either a χ2 or Fisher exact test for categorical variables.
A modified Poisson regression model with robust standard errors was used to estimate the adjusted RR of EHR by transplant center volume (6, 20). Recipient age, sex, ethnicity, underlying diagnosis, transplant procedure type, LAS at transplant, pretransplant use of mechanical ventilation or ECMO, and length of stay were included. Length of stay was categorized as a binary variable by a split at the median length of stay (16 d).
Results
There were 9,726 first-time isolated lung transplant recipients within the study period, of which 3,128 (32.2%) had Medicare as their primary payer at the time of transplant. Of these, 13 recipients were excluded from model analyses because of missing data. The characteristics of Medicare and non-Medicare recipients are shown in Table 1.
Table 1.
Characteristics of Medicare and non-Medicare lung transplant recipients
| Medicare |
Non-Medicare |
P Value | |
|---|---|---|---|
| (n = 3,128) | (n = 6,598) | ||
| Age, yr | 63 (55–67) | 56 (46–62) | <0.0001 |
| Male, n (%) | 1,839 (58.8) | 3,879 (58.8) | 0.9992 |
| Ethnicity, n (%) | 0.0210 | ||
| White | 2,677 (85.6) | 5,514 (83.6) | |
| Black | 250 (8.0) | 589 (8.9) | |
| Hispanic | 143 (4.6) | 357 (5.4) | |
| Asian | 31 (1.0) | 97 (1.5) | |
| Other | 27 (0.9) | 41 (0.6) | |
| Diagnosis, n (%) | <0.0001 | ||
| Group A, COPD | 1,331 (42.7) | 1,978 (30.2) | |
| Group B, pulmonary hypertension | 103 (3.3) | 225 (3.4) | |
| Group C, cystic fibrosis | 293 (9.4) | 945 (14.4) | |
| Group D, pulmonary fibrosis | 1,388 (44.6) | 3,411 (52.0) | |
| Bilateral transplant type, n (%) | 1,822 (58.3) | 4,560 (69.1) | <0.0001 |
| Life support, n (%) | 173 (5.5) | 469 (7.1) | 0.0034 |
| Lung allocation score, n (%) | <0.0001 | ||
| <35 | 1,096 (35.0) | 1,855 (28.1) | |
| 35–39 | 760 (24.3) | 1,635 (24.8) | |
| 40–49 | 679 (21.7) | 1,574 (23.9) | |
| ≥50 | 593 (19.0) | 1,534 (23.2) | |
| Transplant center volume, n (%) | 0.0001 | ||
| Low | 604 (19.3) | 1,285 (19.5) | |
| Intermediate | 800 (25.6) | 1,944 (29.5) | |
| High | 1,724 (55.1) | 3,369 (51.0) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Medicare lung transplant recipients were of older age (median, 63 vs. 56 yr; P < 0.0001), more likely to have an underlying diagnosis of chronic obstructive pulmonary disease (COPD; 42.7 vs. 30.2%), and less likely to have cystic fibrosis (9.4 vs. 14.4%) or pulmonary fibrosis (44.6 vs. 52.0%) than non-Medicare recipients (P < 0.0001). They were less likely to undergo bilateral transplant (58.3 vs. 69.1%; P < 0.0001) and their illness was less severe (median LAS, 37.8 vs. 39.3; P < 0.0001) than non-Medicare recipients.
Index Transplant Admission Cost
The mean total cost of the index lung transplant admission was $135,622 (SD, ±$109,788; median, $92,109; interquartile range [IQR], $80,620–$147,198). The results of a hierarchical multivariable regression model with total admission cost as the outcome and the percent change in admission cost by recipient and center volume variables is shown in Table 2. Recipient age and sex did not affect transplant admission cost. Recipients with pulmonary hypertension have an adjusted 18.64% (95% confidence interval [CI], 2.00–35.29%) increase in cost whereas recipients with cystic fibrosis have an adjusted 26.52% (95% CI, 8.00–45.04%) decrease in cost compared with recipients with COPD. There was no significant cost difference for recipients with pulmonary fibrosis.
Table 2.
Recipient and center volume associations with transplant admission cost
| Percent Change* | 95% Confidence Interval | |
|---|---|---|
| Age (per year change) | −0.30 | −0.74 to 0.15 |
| Female sex | 1.46 | −2.86 to 5.77 |
| Diagnosis group | ||
| Group A, COPD | Reference | Reference |
| Group B, pulmonary hypertension | 18.64 | 2.00–35.29 |
| Group C, cystic fibrosis | –26.52 | –8.00 to –45.04 |
| Group D, pulmonary fibrosis | 5.31 | −3.11 to 13.73 |
| Single transplant procedure | –26.23 | –18.66 to –33.80 |
| Pretransplant life support | 40.02 | 24.37–55.67 |
| Lung allocation score | ||
| <35 | Reference | Reference |
| 35–40 | 10.21 | 0.5–19.92 |
| 40–50 | 14.93 | 3.86–26.01 |
| >50 | 25.04 | 11.27–38.81 |
| Center volume | ||
| Low | 11.66 | 0.5–22.79 |
| Intermediate | 11.67 | −1.74 to 25.07 |
| High | Reference | Reference |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Significant associations are presented in boldface.
Reflects percent change from the reference model cost of $144,770.
Undergoing a single lung transplant reduced adjusted cost by 26.23% (95% CI, 18.66–33.80%). The need for pretransplant mechanical ventilation and/or ECMO increased adjusted cost by 40.02% (95% CI, 24.37–55.67%). Similarly, a higher lung allocation score was associated with incremental increases in cost as shown in Table 2. Transplant recipients at low-volume centers had an adjusted 11.66% (95% CI, 0.5–22.79%) higher transplant admission cost as compared with high-volume centers. There was no significant difference in cost at intermediate-volume centers compared with high-volume centers. In sensitivity analyses, the findings were robust to use of LAS as a continuous variable.
Resource Use, Complications, and Mortality by Center Volume
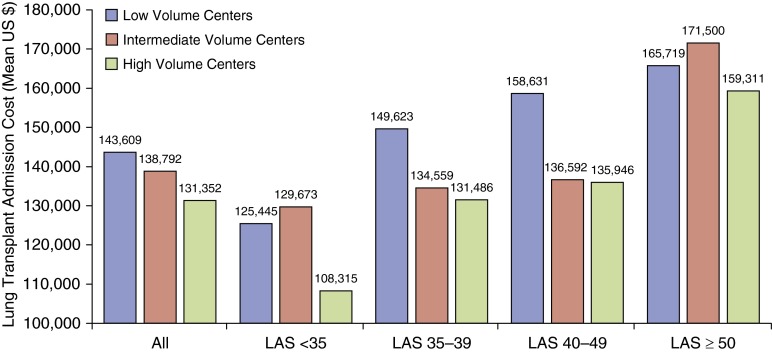
The characteristics of low-, intermediate-, and high-volume centers are shown in Table 3. There were 29 centers in the low-volume group, 16 in the intermediate-volume group, and 14 in the high-volume group. Recipients at high-volume centers were of older age and had a higher median LAS at transplant. There was an inverse relationship between center volume and unadjusted total cost (P < 0.0001), with lower cost at high-volume centers (mean, $131,352; SD, ±$106,165; median, $90,177; IQR, $79,165–$137,915) than at intermediate-volume (mean, $138,792; SD, ±$106,270; median, $93,024; IQR, $82,700–$154,857) or low-volume (mean, $143,609; SD, ±$123,316; median, $95,234; IQR, $83,052–$152,149) centers. This relationship was seen across most LAS thresholds, except for recipients with LAS greater than 50, who had similar cost across center volume (Figure 1).
Table 3.
Recipient characteristics, resource use, and complications by transplant center volume
| |
Low Volume |
Intermediate Volume |
High Volume |
P Value* |
|---|---|---|---|---|
| (n = 604) | (n = 800) | (n = 1,724) | ||
| Recipient characteristic | ||||
| Age (yr), median (IQR) | 61.5 (53–66) | 60 (51–65) | 65 (56–67) | <0.0001 |
| Bilateral transplant, n (%) | 369 (61.09) | 418 (52.25) | 1,035 (60.03) | 0.0003 |
| LAS at transplant, median (IQR) | 36.46 (33.22–43.23) | 37.28 (33.82–43.91) | 38.58 (33.84–48.77) | <0.0001 |
| Pretransplant life support, n (%) | 26 (4.3) | 40 (5.0) | 107 (6.21) | <0.1591 |
| Admission resource use | ||||
| Total costs, mean (±SD) | $143,609 (±$123,316) | $138,792 (±$106,270) | $131,352 (±$106,165) | <0.0001 |
| Total costs, median (IQR) | $95,234 ($83,052–$152,149) | $93,024 ($82,700–$154,857) | $90,177 ($79,165–$137,915) | <0.0001 |
| Hospital length of stay, median (IQR) | 17 (11–29) | 15 (11–27) | 16 (11–29) | 0.0430 |
| ICU length of stay, median (IQR) | 14 (9–23) | 11 (6–20) | 10 (4–18) | <0.0001 |
| Outlier cost payments, n (%) | 272 (45.03) | 251 (31.38) | 570 (33.06) | <0.0001 |
| Admission complications | ||||
| Reintubation, n (%) | 0.0790 | |||
| Yes | 123 (20.4) | 130 (16.25) | 332 (19.26) | |
| Unknown | 21 (3.48) | 12 (1.50) | 15 (0.87) | |
| Ventilator support duration, n (%) | 0.0154 | |||
| 0–47 h | 337 (55.79) | 513 (64.13) | 1,157 (67.11) | |
| 48 h to 5 d | 98 (16.23) | 108 (13.50) | 232 (13.46) | |
| 5 d or longer | 112 (18.54) | 132 (16.50) | 273 (15.84) | |
| Unknown | 57 (9.44) | 47 (5.88) | 62 (3.60) | |
| Tracheostomy, n (%) | 0.5597 | |||
| Yes | 13 (2.15) | 12 (1.5) | 36 (2.09) | |
| Unknown | 16 (2.65) | 2 (0.25) | 4 (0.23) | |
| Dialysis, n (%) | 0.0020 | |||
| Yes | 9 (1.49) | 3 (0.38) | 4 (0.23) | |
| Unknown | 1 (0.17) | 0 (0.0) | 27 (1.57) |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range; LAS = lung allocation score.
P value reflects significance of comparison across all volume groups.
Figure 1.
Cost of lung transplant admission by transplant center volume. The mean unadjusted cost for the index lung transplant admission is shown for low-, intermediate-, and high-volume centers stratified by all recipients and by recipients at various lung allocation score (LAS) thresholds. P values for within-group comparisons are as follows: All, P < 0.0001; LAS < 35 subgroup, P < 0.0001; LAS 35–39 subgroup, P = 0.0036; LAS 40–49 subgroup, P = 0.0142; LAS ≥ 50 subgroup, P = 0.1109.
The cost for recipients receiving life support before transplant was significantly less (P = 0.0020) at high-volume centers (mean, $194,465; SD, ±$117,921; median, $166,924; IQR, $89,528–$252,019) than at low-volume centers (mean, $336,785; SD, ±$227,644; median, $259,224; IQR, $191,929–$460,801), with no difference in cost between intermediate- and high-volume centers (P = 0.4911).
Hospital length of stay, particularly intensive care unit (ICU) length of stay, differed by center volume, with longer ICU stay at low-volume centers (median, 14 d; IQR, 9–23) compared with intermediate-volume (median, 11 d; IQR, 6–20) or high-volume centers (median, 10 d; IQR, 4–18) (P < 0.0001).
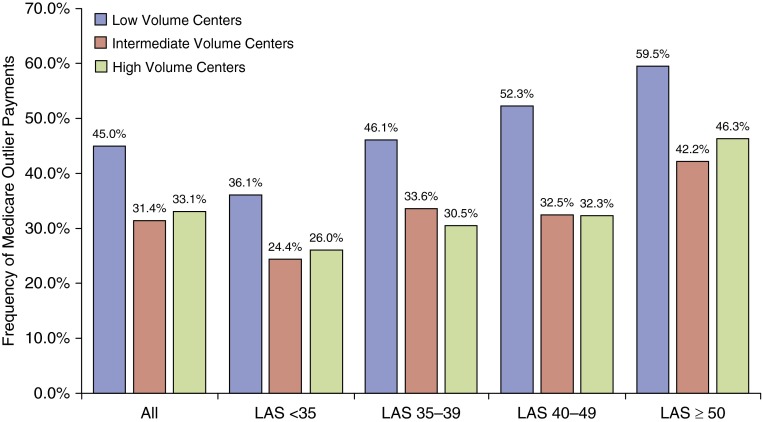
The frequency of Medicare DRG outlier payments was higher at low-volume centers (45.0%) then at intermediate-volume (31.4%) or high-volume (33.1%) centers (P < 0.0001). This difference in DRG outlier payments by center volume was significant across all LAS thresholds (Figure 2). Ventilator duration was longer (P = 0.0154) and postoperative dialysis (P = 0.002) greater at low-volume centers. There was no significant difference in reintubation or tracheostomy by center volume.
Figure 2.
Medicare outlier payments by transplant center volume. The frequency of Medicare diagnosis related group outlier payment for the index transplant admission is shown for low-, intermediate-, and high-volume centers stratified by all recipients and by recipients at various lung allocation score (LAS) thresholds. P values for within-group comparisons are as follows: All, P < 0.0001; LAS < 35 subgroup, P < 0.0038; LAS 35–39 subgroup, P = 0.0.0036; LAS 40–49 subgroup, P = 0.0001; LAS ≥ 50 subgroup, P = 0.0461.
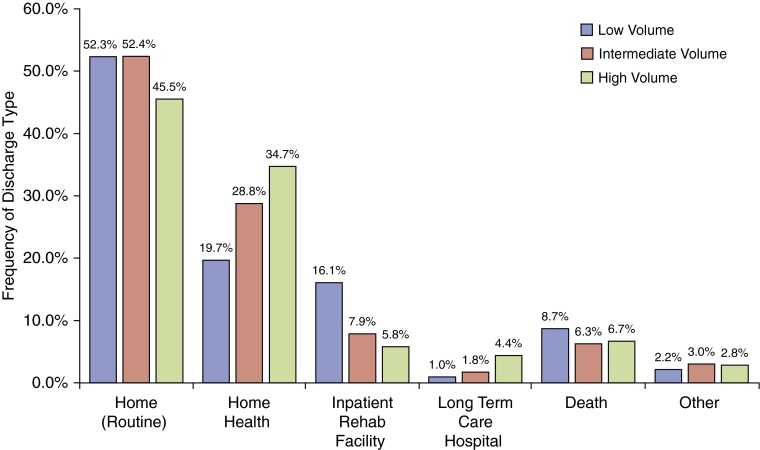
There was a nonsignificant trend of increased unadjusted in-hospital mortality at low-volume centers (8.77%) compared with intermediate-volume (6.25%) and high-volume (6.67%) centers (P = 0.1414). After adjusting for recipient risk, low-volume centers had a 41% increased risk of in-hospital death (RR, 1.41; 95% CI, 1.08–1.96) compared with high-volume centers. There was no difference in the risk of in-hospital death at intermediate-volume centers (RR, 1.05; 95% CI, 0.70–1.58). As shown in Figure 3, discharge to an inpatient rehabilitation facility was more frequent at low-volume centers (P < 0.0001), whereas discharge to a long-term acute care hospital (LTAC) was more frequent at high-volume centers (P < 0.0001).
Figure 3.
Discharge disposition by transplant center volume. The frequency of specific discharge dispositions for the index transplant admission is shown for low-, intermediate-, and high-volume centers stratified by all recipients and by recipients at various lung allocation score thresholds. P values for within-group comparisons are as follows: Home (routine), P < 0.0007; home health, P < 0.0001; inpatient rehabilitation facility, P < 0.0001; long-term care hospital, P < 0.0001; death, P = 0.1414; other, P = 0.5919.
Early Hospital Readmission
Thirty-nine percent of lung transplant recipients (1,137 of 2,911) had an EHR. The average inpatient cost of an EHR was $27,233 (SD, ±$40,612; median, $16,109; IQR, $9,097–$27,837). EHR occurred more frequently in older recipients (median age, 64 vs. 62 yr; P < 0.0001) and recipients with pulmonary fibrosis (46.9 vs. 41.9%) and less frequently in recipients with cystic fibrosis (7.4 vs. 10.9%) (P = 0.0036). There was no difference in unadjusted EHR by sex, ethnicity, transplant procedure type, use of pretransplant life support, LAS at transplant, or transplant center volume (Table 4). EHR was more frequent in those with an index hospital stay greater than 16 days (50.2%; P < 0.0001).
Table 4.
Characteristics of lung transplant recipients with early hospital readmission
| EHR |
No EHR |
P Value | |
|---|---|---|---|
| (n = 1,137) | (n = 1,773) | ||
| Age (yr), median (IQR) | 64 (56–67) | 62 (53–66) | <0.0001 |
| Male, n (%) | 666 (58.6) | 1,037 (58.5%) | 0.963 |
| Ethnicity, n (%) | 0.1571 | ||
| White | 980 (86.2) | 1,520 (85.7) | |
| Black | 96 (8.4) | 131 (7.4) | |
| Hispanic | 47 (4.1) | 80 (4.5) | |
| Asian | 6 (0.5) | 24 (1.4) | |
| Other | 8 (0.7) | 18 (1.0) | |
| Diagnosis, n (%) | 0.0036 | ||
| Group A, COPD | 483 (42.8) | 775 (43.8) | |
| Group B, pulmonary hypertension | 32 (2.8) | 60 (3.4) | |
| Group C, cystic fibrosis | 84 (7.4) | 193 (10.9) | |
| Group D, pulmonary fibrosis | 530 (46.9) | 741 (41.9) | |
| Bilateral transplant type, n (%) | 679 (59.7) | 1,005 (56.7) | 0.1057 |
| Life support, n (%) | 67 (5.9) | 78 (4.4) | 0.0709 |
| Lung allocation score, median (IQR) | 38.1 (33.65–46.1) | 37.3 (33.6–45.4) | 0.1237 |
| Transplant center volume | |||
| Low | 227 (20.0) | 324 (18.3) | 0.4595 |
| Intermediate | 295 (25.9) | 455 (25.7) | |
| High | 615 (54.1) | 994 (56.1) | |
| Length of stay > 16 d | 571 (50.2) | 1,090 (61.5) | <0.0001 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; IQR = interquartile range; EHR = early hospital readmission.
In adjusted analysis, older age was associated with increased risk of EHR (RR, 1.15 per 10-yr increase in age; 95% CI, 1.08–1.23). Sex, ethnicity, underlying diagnosis, LAS at transplant, and use of pretransplant life support did not affect the risk of EHR. Single lung transplant recipients had a 14% decreased risk of EHR (RR, 0.86; 95% CI, 0.78–0.96), and recipients with an index transplant admission exceeding 16 days (the median length of stay) had a 30% increased risk for EHR (RR, 1.30; 95% CI, 1.19–1.43). Recipients at low-volume centers had a 14% increased risk of EHR (RR, 1.14; 95% CI, 1.01–1.28) compared with those at high-volume centers. There was no significant difference in EHR risk between intermediate-volume (RR, 1.10; 95% CI, 0.98–1.23) and high-volume centers.
Discussion
In the present era of increased emphasis on health-care cost containment and in light of the recent recognition of increasing resource use in lung transplantation, there is a need to identify how to reduce lung transplant cost without sacrificing lung transplant access, quality, or outcomes (2). In this study, we have shown that low-volume transplant centers provide transplant admission care at a greater cost than high-volume centers while also demonstrating higher adjusted in-hospital mortality and excess risk for early hospital readmission. Overall, lung transplant value, in terms of admission cost and survival, was worse at low-volume centers compared with high-volume centers.
The presence of a volume–outcome relationship in lung transplantation is well established, with prior studies demonstrating improved survival at higher volume centers compared with low-volume centers (13–16). However, it was unknown whether these improved outcomes came at a cost of increased resource use or resulted from improved care delivery and value. Through the novel linkage of lung transplant registry data to administrative Medicare data, our study builds on this volume–outcome relationship by demonstrating that these better outcomes occur in the setting of lower health-care cost.
There is an incomplete understanding of what drives these volume–outcome differences. Beyond cost, we found increased ICU length of stay and a higher frequency of outlier payments in low-volume centers. Possible explanations for this finding include increased extraneous use of ICU resources, such as a reluctance to transfer patients out of the favorable staffing and monitoring setting of the ICU, versus increased “appropriate” use of ICU resources due to more frequent complications or a diminished ability to recognize and rescue recipients from complications. Dialysis use was more frequent and ventilator duration was longer in low-volume centers than in intermediate- or high-volume centers, suggesting “appropriate” ICU use from complications is more likely. Outlier payments are administered for unusually expensive admissions, which after cardiac surgery are often secondary to complications (21). Previous literature suggests that whereas postoperative lung transplant complications do not vary by center volume, higher volume centers are able to recognize and “rescue” complications and thereby improve outcomes (13).
Notably, we found that volume-related cost and outlier payment differences become less significant when recipient LAS is greater than 50 but remain significant in the most ill recipients being bridged to transplant on mechanical ventilation or ECMO. The use of ambulatory ECMO and rehabilitation before transplant within a high-volume center reduced admission costs by more than 10% compared with nonambulatory ECMO recipients (22). Process sharing and successful implementation of similar interventions across high-, intermediate-, and low-volume centers could improve lung transplant cost and outcomes. Interestingly, the cost difference between high- and low-volume centers is most pronounced in the least severe recipient subgroup (LAS, <35). We speculate that this may reflect more refined post-transplant care processes in high-volume centers.
The response to the volume–outcome finding in lung transplantation could include transplant regionalization based on a volume threshold or process sharing and implementation of best care processes that improve lung transplant value. Although transplanting candidates in high-volume centers may improve recipient outcomes and cost, there are important considerations to any regionalization discussion. First, our results are specific to the Medicare lung transplant population and may not be generalizable to the entire lung transplant population. Second, lung transplantation would be available only to patients who could access these high-volume centers. Lung transplant access varies by geography within the United States, and an effort to regionalize lung transplant has the potential to worsen access to this life-saving treatment (23). Regionalization could delay candidate referral, which can increase recipient illness severity and thereby cost. Third, there are low-volume centers that provide high-value care, and thoughtful yet arbitrary volume thresholds could penalize centers that provide high-value care and fail to exclude those that do not. Fourth, regionalization could overburden the infrastructure and care system of centers, and make it difficult for centers to maintain high-value care at even higher volumes. Finally, the presence of fewer centers under regionalization would reduce competition, increase institutional market power, and could ultimately increase lung transplant cost.
An alternative response to the volume–outcome relationship is to understand and implement best center practices that improve lung transplant value. Unlike regionalization, this response is rooted in the belief that improvements in care quality, outcomes, and resource use can result from cumulative or indirect experience rather than the direct experience that high volume provides. Current efforts to improve center-specific care have focused on the measurement and reporting of risk-adjusted program outcomes, specifically survival, without guidance on best center practice(s) or care process measurement.
This approach is not unlike the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) (24). Analyses of the ACS NSQIP demonstrated that hospital participation in the program did not result in a more significant reduction in mortality, complications, early hospital readmissions, or cost compared with nonparticipating (control) hospitals (25, 26). This reveals the difficulty in translating reported data to surgical care processes that improve resource use and outcomes. Although scrutiny of a center’s risk-adjusted outcomes by governing transplant bodies can prompt change within a transplant center, this needs to be harnessed with constructive guidance on evidence-based best care practices that improve transplant care quality, cost, and outcomes. Further work is needed in this area if we are to improve lung transplant value.
We have previously demonstrated increased transplant admission resource use within the post-LAS era (2). This study builds on that work by describing recipient factors associated with increased resource use. Lung transplant registry data demonstrate increasing bilateral procedures and recipient illness severity, as reflected by increasing median LAS and life support use, within the LAS era (1). Not unsurprisingly, these factors are highly associated with increased transplant admission cost. Importantly, while the lung transplant recipient population is older, age was not associated with increased transplant cost in this study population.
The finding of reduced cost and readmission with single lung transplant is relevant. In COPD, single lung transplant recipients have comparable survival to bilateral lung transplant recipients (27). Therefore, policies directed at standardizing single lung transplant in COPD could improve the cost of lung transplant without sacrificing recipient survival. Further study of the cost-effectiveness of single versus bilateral lung transplant in COPD that accounts for the health-related quality of life differences between procedure types and incorporates the societal benefit of increased organ supply is needed.
Limitations
Our study was limited to Medicare lung transplant recipients, which differ from the general lung transplant population. Although the relationship between transplant center volume and resource use was robust to adjusting for recipient age, diagnosis, and illness adjustment, whether the center volume and resource use relationship is generalizable to non-Medicare recipients is unknown. Because of the cost-leveling flat rate payment structure within the Medicare inpatient payment system, we hypothesize that this relationship may differ for other payers, and further study of this relationship using all-payer data is needed.
Although volume is thought to drive outcomes, a reverse direction of causality, that outcomes drive volume, is plausible. Patients may be selectively referred to transplant centers with better outcomes, and these superior outcomes may already reflect reduced complications and thereby cost.
The costs reported underrepresent the true cost paid by Medicare for lung transplant. The intentional exclusion of indirect medical education cost and disproportionate share cost and the adjustment for regional price variation all result in a lower overall cost than the actual cost paid by Medicare. High-volume centers had increased LTAC discharges, perhaps obtaining cost-savings by transferring cost from transplant admission to LTAC. However, when accounting for LTAC discharge as a readmission, EHR remained higher at low-volume centers and discharges to inpatient rehabilitation facilities were higher at low-volume centers.
Although high-volume centers generally have lower cost and EHR than low-volume centers, there is variability across all center volume categories. Volume does not fully explain the processes within centers that contribute to resource use and outcomes, and further work is needed to understand variation in transplant center care processes.
Conclusions
High-volume lung transplant centers have better recipient survival while using fewer hospital resources than low-volume centers. This finding persisted after adjustments for regional price variation and recipient risk and highlights the need to understand the underpinnings of the volume–outcome relationship in lung transplantation. Understanding the transplant center care processes and best practices that might be responsible for lower cost and better outcomes is essential to improving the overall value of lung transplantation.
Acknowledgments
Acknowledgment
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. government.
Footnotes
Supported by a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum NIH KL2 TR 001083 (J.J.M.).
Author Contributions: J.J.M. contributed to the conception and design of the study, data acquisition, and analysis and interpretation, and wrote the initial draft and subsequent revisions of the manuscript. D.W., J.H.B., and M.R.N. contributed to study design, data interpretation, and critical shaping and revision of the manuscript. J.B. and G.S.D. contributed equally to the conception and design of the study, data interpretation, and critical shaping and revising of the manuscript. All authors approved the final version before submission. J.J.M. takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Valapour M, Skeans MA, Heubner BM, Smith JM, Hertz MI, Edwards LB, Cherikh WS, Callahan ER, Snyder JJ, Israni AK, et al. OPTN/SRTR 2013 Annual Data Report: lung. Am J Transplant. 2015;15:1–28. doi: 10.1111/ajt.13200. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell BG, Mooney JJ, Lee PH, Levitt JE, Chhatwani L, Nicolls MR, Zamora MR, Valentine V, Weill D, Dhillon GS. Increased resource use in lung transplant admissions in the lung allocation score era. Am J Respir Crit Care Med. 2015;191:302–308. doi: 10.1164/rccm.201408-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnitzler MA, Skeans MA, Axelrod DA, Lentine KL, Tuttle-Newhall JE, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2013 Annual Data Report: economics. Am J Transplant. 2015;15:1–24. doi: 10.1111/ajt.13201. [DOI] [PubMed] [Google Scholar]
- 4.Showstack J, Katz PP, Lake JR, Brown RS, Jr, Dudley RA, Belle S, Wiesner RH, Zetterman RK, Everhart J NIDDK Liver Transplantation Database Group. Resource utilization in liver transplantation: effects of patient characteristics and clinical practice. JAMA. 1999;281:1381–1386. doi: 10.1001/jama.281.15.1381. [DOI] [PubMed] [Google Scholar]
- 5.Macomber CW, Shaw JJ, Santry H, Saidi RF, Jabbour N, Tseng JF, Bozorgzadeh A, Shah SA. Centre volume and resource consumption in liver transplantation. HPB (Oxford) 2012;14:554–559. doi: 10.1111/j.1477-2574.2012.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant. 2012;12:3283–3288. doi: 10.1111/j.1600-6143.2012.04285.x. [DOI] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, Segev DL. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlakis M. Transplantation: early hospital readmissions after kidney transplantation. Nat Rev Nephrol. 2014;10:188–189. doi: 10.1038/nrneph.2014.32. [DOI] [PubMed] [Google Scholar]
- 9.Nassir BA, Dean CE, Li S, Salkowski N, Solid CA, Schnitzler MA, Snyder JJ, Kim SJ, Kasiske BL, Linzer M, et al. Variation in cost and quality in kidney transplantation. Transplantation. 2015;99:2150–2157. doi: 10.1097/TP.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 10.Ellimoottil C, Ye Z, Chakrabarti AK, Englesbe MJ, Miller DC, Wei JT, Mathur AK. Understanding inpatient cost variation in kidney transplantation: implications for payment reforms. Urology. 2016;87:88–94. doi: 10.1016/j.urology.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englesbe MJ, Dimick JB, Fan Z, Baser O, Birkmeyer JD. Case mix, quality and high-cost kidney transplant patients. Am J Transplant. 2009;9:1108–1114. doi: 10.1111/j.1600-6143.2009.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiting JF, Martin J, Zavala E, Hanto D. The influence of clinical variables on hospital costs after orthotopic liver transplantation. Surgery. 1999;125:217–222. [PubMed] [Google Scholar]
- 13.Kilic A, George TJ, Beaty CA, Merlo CA, Conte JV, Shah AS. The effect of center volume on the incidence of postoperative complications and their impact on survival after lung transplantation. J Thorac Cardiovasc Surg. 2012;144:1502–1508, discussion 1508–1509. doi: 10.1016/j.jtcvs.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304:53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]
- 15.Weiss ES, Allen JG, Meguid RA, Patel ND, Merlo CA, Orens JB, Baumgartner WA, Conte JV, Shah AS. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg. 2009;88:1062–1070. doi: 10.1016/j.athoracsur.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Scarborough JE, Bennett KM, Davis RD, Lin SS, Tracy ET, Kuo PC, Pappas TN. Temporal trends in lung transplant center volume and outcomes in the United States. Transplantation. 2010;89:639–643. doi: 10.1097/TP.0b013e3181ceecf7. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb J, Zhou W, Song Y, Andrews K, Skinner J, Sutherland J. A standardized method for adjusting Medicare expenditures for regional differences in prices. Hanover, NH: Dartmouth Institute for Health Policy and Clinical Practice, Center for Health Policy Research, Dartmouth College; 2010. [Google Scholar]
- 18.Gottlieb DJ, Zhou W, Song Y, Andrews KG, Skinner JS, Sutherland JM. Prices don’t drive regional Medicare spending variations. Health Aff (Millwood) 2010;29:537–543. doi: 10.1377/hlthaff.2009.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bureau of Labor StatisticsConsumer Price Index detailed report. 2015[cited 2015 Oct 14]. Available from: http://www.bls.gov/cpi/tables.htm
- 20.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Baser O, Fan Z, Dimick JB, Staiger DO, Birkmeyer JD. Outlier payments for cardiac surgery and hospital quality. Health Aff (Millwood) 2009;28:1154–1160. doi: 10.1377/hlthaff.28.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bain JC, Turner DA, Rehder KJ, Eisenstein EL, Davis RD, Cheifetz IM, Zaas DW. Economic outcomes of extracorporeal membrane oxygenation with and without ambulation as a bridge to lung transplantation. Respir Care. 2016;61:1–7. doi: 10.4187/respcare.03729. [DOI] [PubMed] [Google Scholar]
- 23.Thabut G, Munson J, Haynes K, Harhay MO, Christie JD, Halpern SD. Geographic disparities in access to lung transplantation before and after implementation of the lung allocation score. Am J Transplant. 2012;12:3085–3093. doi: 10.1111/j.1600-6143.2012.04202.x. [DOI] [PubMed] [Google Scholar]
- 24.Maggard-Gibbons M. The use of report cards and outcome measurements to improve the safety of surgical care: the American College of Surgeons National Surgical Quality Improvement Program. BMJ Qual Saf. 2014;23:589–599. doi: 10.1136/bmjqs-2013-002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborne NH, Nicholas LH, Ryan AM, Thumma JR, Dimick JB. Association of hospital participation in a quality reporting program with surgical outcomes and expenditures for Medicare beneficiaries. JAMA. 2015;313:496–504. doi: 10.1001/jama.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etzioni DA, Wasif N, Dueck AC, Cima RR, Hohmann SF, Naessens JM, Mathur AK, Habermann EB. Association of hospital participation in a surgical outcomes monitoring program with inpatient complications and mortality. JAMA. 2015;313:505–511. doi: 10.1001/jama.2015.90. [DOI] [PubMed] [Google Scholar]
- 27.Schaffer JM, Singh SK, Reitz BA, Zamanian RT, Mallidi HR. Single- vs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need. JAMA. 2015;313:936–948. doi: 10.1001/jama.2015.1175. [DOI] [PubMed] [Google Scholar]