Abstract
Rationale: One in 12 adults has chronic obstructive pulmonary disease or asthma. Acute exacerbations of these chronic lower respiratory diseases (CLRDs) are a major cause of morbidity and mortality. Valid approaches to classifying cases and exacerbations in the general population are needed to facilitate prevention research.
Objectives: To assess the feasibility, reproducibility, and performance of a protocol to identify CLRD cases and exacerbations triggering emergency department (ED) visits or hospitalizations in cohorts of patients derived from general populations of adults.
Methods: A protocol was developed to classify CLRD cases and severe exacerbations on the basis of review of medical records. ED and inpatient medical records were ascertained prospectively in the Hispanic Community Health Study/Study of Latinos, and inpatient records were retrospectively identified by administrative codes in the Multi-Ethnic Study of Atherosclerosis. “Probable” exacerbations were defined as a physician’s diagnosis of CLRD with acute respiratory symptoms. “Highly probable” exacerbations additionally required systemic corticosteroid therapy, and “definite” exacerbations required airflow limitation or evidence of CLRD on imaging studies. Adjudicated results were compared with CLRD cases identified by spirometry and self-report, and with an administrative definition of exacerbations.
Measurements and Main Results: Protocol-based classification was completed independently by two physicians for 216 medical records (56 ED visits and 61 hospitalizations in the Hispanic Community Health Study/Study of Latinos; 99 hospitalizations in the Multi-Ethnic Study of Atherosclerosis). Reviewer disagreement occurred in 2–5% of cases and 4–8% of exacerbations. Eighty-nine percent of records were confirmed as at least probable CLRD cases. Fifty-six percent of confirmed CLRD cases had airflow limitation on the basis of baseline study spirometry. Of records that described CLRD as the primary discharge diagnosis code, an acute exacerbation was confirmed as at least probable for 96% and as highly probable or definite for 77%. Only 50% of records with CLRD as a secondary code were confirmed, although such records accounted for over half of all confirmed exacerbations.
Conclusions: CLRD cases and severe exacerbations without preceding documentation of airflow limitation are identified frequently in population-based cohorts of persons. A primary discharge diagnosis of CLRD is specific but insensitive for defining exacerbations. Protocol-based classification of medical records may be appropriate to supplement and to validate identification of CLRD cases and exacerbations in general population studies.
Clinical trials registered with www.clinicaltrials.gov (NCT00005487 and NCT02060344).
Keywords: administrative data, asthma, chronic obstructive pulmonary disease, disease progression, incidence
Chronic lower respiratory disease (CLRD), which affects approximately 1 in 12 people worldwide, is the third leading cause of death (1). As defined by the CDC and the World Health Organization, CLRD encompasses four frequently overlapping chronic lung diseases: chronic obstructive pulmonary disease (COPD), chronic bronchitis, emphysema, and asthma (2–4). The major cause of CLRD morbidity and mortality is CLRD exacerbations, or episodic worsening of respiratory symptoms (5). Severe CLRD exacerbations, which require an emergency department (ED) visit or hospitalization, account for more than 50% of CLRD costs (6).
Population-based research in epidemiologic cohort studies and administrative databases such as electronic health records is essential to informing primary prevention of CLRD, a current priority of the National Heart, Lung, and Blood Institute. However, standard approaches to identifying and classifying CLRD cases and CLRD exacerbations in cohort studies or administrative databases are lacking. Spirometric definitions of COPD may be insufficient to detect persons at risk of CLRD exacerbations and CLRD-related mortality (6–13), and the validity of administrative definitions for CLRD exacerbations remains uncertain (14–16). Central physician adjudication of medical records for major adverse cardiac events is a routine approach to minimizing bias in cardiovascular cohort studies and randomized clinical trials (17, 18), and yet no similar standard has been applied to CLRD events, even in major randomized clinical trials (19–22).
We therefore developed a protocol for ascertainment and physician adjudication of CLRD cases and CLRD exacerbations in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) and further evaluated it in the Multi-Ethnic Study of Atherosclerosis (MESA). We compared protocol-based classifications with self-report, spirometry, and administrative definitions for CLRD cases and CLRD exacerbations. Preliminary results of these studies were previously reported in abstract form (23).
Methods
Approval and Consent
The protocols and all studies described herein were approved by the institutional review boards of all collaborating institutions and the National Heart, Lung, and Blood Institute, and all participants provided written informed consent for all study procedures.
Ascertainment
HCHS/SOL
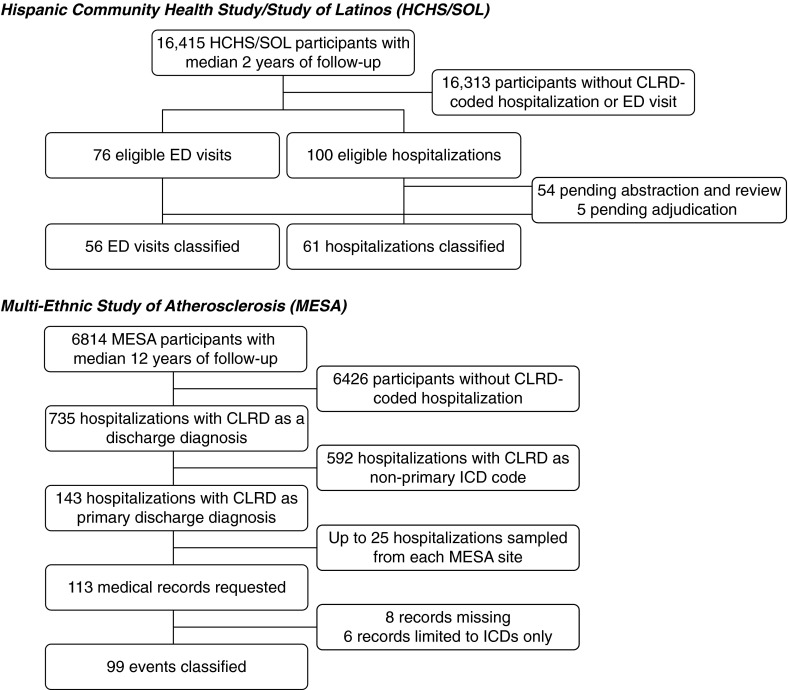
HCHS/SOL is a multicenter, prospective, population-based cohort study of Hispanic/Latino Americans that enrolled 16,415 participants 18–74 years of age who self-identified as Hispanic or Latino in the 2008–2011 period. Study visits were conducted in English or Spanish in San Diego, California; Chicago, Illinois; Bronx, New York; and Miami, Florida (24). This analysis includes all events occurring during the first 3 years of HCHS/SOL that were classified before March 1, 2015.
Participants were called every 12 months to identify ED visits for CLRD exacerbations and hospitalizations for any cause. CLRD exacerbations not requiring an ED visit or hospitalization were not ascertained. All reported events were investigated and processed through the HCHS/SOL Events Classification Committee.
Events were eligible for CLRD adjudication if they were assigned an International Classification of Diseases (ICD) discharge diagnosis code, in any code position, for asthma (ICD-9 code 493 and ICD-10 codes J45–J46), chronic airway obstruction (ICD-9 code 496 and ICD-10 code J44), emphysema (ICD-9 code 492 and ICD-10 code J43), chronic obstructive bronchitis (ICD-9 codes 490–491 and ICD-10 codes J40–J42), bronchiectasis (ICD-9 code 494 and ICD-10 code J47), pulmonary heart disease (ICD-9 codes 415 and 416.9 and ICD-10 codes I26 and I27.9), or respiratory failure (ICD-9 code 518 and ICD-10 code J96), or if the discharge summary included relevant keywords (see Table E1 in the online supplement).
For eligible events, the field center attempted to obtain the following additional information: physician, triage, and nurse notes; treatments; blood count and arterial blood gases; temperature; arterial oxyhemoglobin saturation; chest radiology reports; peak flow; and pulmonary function test results. Records were blinded by the field center before transmission to the coordinating center for centralized abstraction and adjudication.
MESA
MESA is a multicenter, prospective, population-based cohort study that enrolled 6,814 participants 45–84 years of age who were free of clinical cardiovascular disease and self-classified as non-Hispanic white, African American, Hispanic, or Chinese in the 2000–2002 period (25). Participants were recruited in Forsyth County, North Carolina; northern Manhattan and the Bronx in New York, New York; Baltimore, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles, California. This analysis includes eligible events occurring before July 1, 2013, of which no more than 25 were randomly sampled from each study site.
MESA participants were called every 9 months to ascertain all hospital admissions. Complete medical records, including discharge diagnoses, were requested for all hospitalizations and were investigated and processed through the MESA Events Classification Committee. In the present study, we retrospectively ascertained events eligible for CLRD classification, which were defined as hospitalizations assigned a primary discharge diagnosis of a CLRD (ICD-9 codes 490–493 or 496 or ICD-10 codes J40–J46).
Protocol
The classification protocol was developed in 2011 in HCHS/SOL by an expert panel of pulmonologists, allergists, pulmonary epidemiologists, and internists, taking into account discussions by an earlier expert panel (see Acknowledgment section before the References). Medical records were evaluated for (1) whether the participant had CLRD and (2) if so, whether the hospitalization or ED visit was caused by CLRD exacerbation. The draft protocol was piloted on a random subset of MESA medical records by a single reviewer, who recommended refinements to improve efficiency, accuracy, and reproducibility.
The final protocol defined CLRD cases and CLRD exacerbations as summarized in Table 1. (For the full protocol, see the online supplement.) Major CLRD subphenotypes were systematically evaluated according to standard definitions (3, 4, 26).
Table 1.
Classification of chronic lower respiratory disease cases and severe chronic lower respiratory disease exacerbations
| Classification | Criteria |
|---|---|
| CLRD case (subphenotypes*) | |
| Probable | Physician documentation in the medical record of at least one of the following: |
| • Past medical history or new diagnosis of COPD, emphysema, chronic bronchitis, and/or asthma (defines probable subphenotypes). | |
| • Outpatient prescription of inhaled long- or short-acting anticholinergic agent, inhaled corticosteroid (excluding intranasal administration), long-acting β-agonist, combination inhaled long-acting β-agonist and corticosteroid, theophylline, or roflumilast. Isolated inhaled short-acting β-agonist therapy does not qualify. | |
| Highly probable | Confirmation of probable CLRD case, plus physician documentation of at least one of the following diagnostic test results, qualitatively or quantitatively, which may have been obtained during or before the current event: |
| • Spirometry showing incompletely reversible airflow limitation (COPD). | |
| • Chest radiography or CT demonstrating emphysema (emphysema). | |
| • Spirometry or PEF showing reversible airflow limitation (asthma). | |
| • History of bronchitis event within the past 2 yr (chronic bronchitis) | |
| Definite | Formal diagnostic test results from the current event in the medical record: |
| • Spirometry with incompletely reversible airflow limitation, defined as post-bronchodilator FEV1/FVC ratio <0.70 or the LLN (COPD). | |
| • Chest radiography or CT demonstrating emphysema (emphysema), hyperinflation, or flattened diaphragm. | |
| • Spirometry showing reversible airflow limitation or PEF <70% predicted, with or without reversibility (defined by an observed improvement in PEF ≥30%), or, in the absence of repeated PEF, by a clear improvement in clinical status (if reversible and age of onset <45 yr, asthma). | |
| • Clinical history of bronchitis 3 mo/yr for the past 2 yr (chronic bronchitis). | |
| CLRD exacerbation | |
| Probable | Confirmation of probable CLRD case, combined with at least one of the following: |
| • Self-report of new onset or worsening of dyspnea, cough, sputum, or wheeze. | |
| • Physical examination with use of accessory muscles, respiratory distress, wheezing, or prolonged expiration. | |
| Highly probable | Confirmation of probable CLRD exacerbation, plus administration and/or prescription of systemic corticosteroids. |
| Definite | Confirmation of highly probable or definite CLRD case and highly probable CLRD exacerbation. |
Definition of abbreviations: CLRD = chronic lower respiratory disease; COPD = chronic obstructive pulmonary disease; CT = computed tomography; LLN = lower limit of normal; PEF = peak expiratory flow.
Classification of CLRD subphenotypes was performed after establishment of whether and with what certainty CLRD was present. Subphenotype correspondence is indicated in brackets. Additional information is included in the online supplement.
Reviewers
Reviewers included pulmonologists, allergists, and internists trained via conferences and online documentation. The first 23 events in HCHS/SOL were adjudicated by all eight HCHS/SOL reviewers. Disagreements were discussed, and final determinations were achieved by consensus. The remaining 193 records were independently reviewed by two physicians blinded to results from the pilot phase, study data, and ICD coding.
Disagreements between reviewers for confirmation of CLRD cases and CLRD exacerbations triggered independent adjudication by a third reviewer, the referee, for 27 HCHS/SOL records and 7 MESA records. Two reviewers and one referee were common to both studies.
Other Measures
Study spirometry was performed at the baseline examination (2008–2011) for all consenting HCHS/SOL participants and in 2004–2006 for all MESA-Lung participants (27) using the same protocol (28, 29). Airflow limitation was defined on the basis of prebronchodilator spirometry as a ratio of FEV1 to FVC less than 0.70 (3). Restriction on spirometry was defined as an FVC percent predicted less than 80%, calculated using Hankinson reference equations (28) without airflow limitation.
Baseline age, sex, race and/or ethnicity, physician-diagnosed CLRD, and tobacco use were self-reported. Never smoking was defined as a lifetime smoking history of less than 100 cigarettes, and current smoking was defined as cigarette use within the preceding 30 days. Pack-years were calculated as (cigarettes per day divided by 20) multiplied by years of smoking.
Comparisons
Protocol-confirmed CLRD cases and CLRD exacerbations were defined as those classified as probable, highly probable, or definite. For nonrefereed classifications, in cases where reviewers disagreed regarding the certainty of classification, the more conservative decision is reported.
Protocol classifications at two levels of certainty—“at least probable” and “highly probable or definite”—were compared with two alternative CLRD case definitions (airflow limitation on study spirometry and self-reported CLRD) and, in HCHS/SOL, with an administrative definition of CLRD exacerbations as hospitalization or ED visit with CLRD as the primary discharge diagnosis. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated using the protocol classification as the reference standard.
Statistical Analysis
The characteristics of events eligible for and undergoing classification were evaluated and compared using χ2 and Student’s t tests. Interrater agreement was assessed via positive agreement, negative agreement, and the Cohen’s κ-statistic (30). All statistical analyses were performed in SAS version 9.3 software (SAS Institute, Cary, NC).
Results
Events
HCHS/SOL
Of 176 eligible ED visits and hospitalizations accrued by the cutoff date, there were no differences in event or participant characteristics between the 117 (66%) classified events and those pending classification (Figure 1, Tables E2 and E3). Approximately one-third of classified ED visits and one-fifth of hospitalizations were assigned a primary discharge diagnosis code for CLRD, most commonly asthma (Table 2). The majority of participants were women and ever smokers; 52% were obese; and 80% had self-reported CLRD, of whom 92% had self-reported asthma. Approximately one-third had airflow limitation and one-fourth had restriction on the basis of study spirometry (Table 2).
Figure 1.
Classification samples for the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) and the Multi-Ethnic Study of Atherosclerosis (MESA), 2000–2013. CLRD = chronic lower respiratory disease; ED = emergency department; ICD = International Classification of Diseases.
Table 2.
Baseline characteristics of participants with classified emergency department visits or hospitalizations
| HCHS/SOL |
MESA | ||
|---|---|---|---|
| ED Visits | Hospitalizations | Hospitalizations | |
| Classified events, n | 56 | 61 | 99 |
| Primary discharge ICD diagnosis code | |||
| COPD (ICD-9 codes 496, 506.4; or ICD-10 code J44) | 0 | 0 | 15 (15%) |
| Emphysema (ICD-9 code 492 or ICD-10 code J43) | 0 | 0 | 3 (3%) |
| Bronchitis (ICD-9 codes 490–491 or ICD-10 codes J40–J42) | 3 (5%) | 1 (2%) | 40 (40%) |
| Asthma (ICD-9 code 493 or ICD-10 codes J45–J46) | 17 (30%) | 12 (20%) | 41 (41%) |
| Any CLRD | 20 (36%) | 13 (21%) | 99 (100%) |
| Participants, n | 50 | 56 | 75 |
| Age, yr | 51 (13) | 54 (12) | 67 (8.8) |
| Male sex | 6 (15%) | 17 (34%) | 29 (39%) |
| Body mass index, kg/m2 | 31 (6) | 32 (9) | 30 (8) |
| Race/ethnicity | |||
| White | — | — | 29 (39%) |
| African American | — | — | 24 (32%) |
| Hispanic/Latino | 50 (100%) | 56 (100%) | 15 (20%) |
| Asian | — | — | 7 (9%) |
| Smoking status | |||
| Never | 23 (47%) | 23 (43%) | 19 (25%) |
| Former | 16 (33%) | 14 (26%) | 31 (41%) |
| Current | 10 (20%) | 17 (32%) | 25 (33%) |
| Pack-years of smoking | 15 (15) | 20.0 (17) | 36.5 (26) |
| Self-reported CLRD | |||
| Asthma | 36 (77%) | 36 (68%) | 36 (36%) |
| COPD, emphysema, or chronic bronchitis | 17 (36%) | 21 (40%) | 12 (16%) |
| Baseline spirometry | |||
| FEV1, % predicted, L | 74 (25) | 76 (22) | 68 (32) |
| FEV1/FVC ratio | 0.75 (0.11) | 0.73 (0.12) | 0.58 (0.17) |
| Airflow limitation | 14 (35%)* | 15 (30%)* | 24 (65%)† |
| Restrictive pattern | 9 (23%)* | 13 (26%)* | 2 (5%)† |
Definition of abbreviations: CLRD = chronic lower respiratory disease; COPD = chronic obstructive pulmonary disease; ED = emergency department; HCHS/SOL = Hispanic Community Health Study/Study of Latinos; ICD = International Classification of Diseases; MESA = Multi-Ethnic Study of Atherosclerosis.
Airflow limitation is defined as a prebronchodilator FEV1/FVC ratio <0.70. A restrictive pattern is defined as a prebronchodilator FEV1/FVC ratio ≥0.70 and an FVC percent predicted <80%.
Values are presented as mean (SD) or frequency (percent), with the percentages calculated out of participants with nonmissing data.
In HCHS/SOL, 88 of 102 participants with classified events had valid spirometric measures.
In MESA, 37 of 75 participants with classified events had valid spirometric measures that were performed in 2004–2006 (approximately 4 yr after baseline examination and initiation of event follow-up).
MESA
Of 143 eligible hospitalizations, 113 were randomly sampled, of which 99 (88%) were sufficiently complete for classification (Figure 1). There were no differences in event or participant characteristics between classified and nonclassified eligible hospitalizations (Tables E3 and E4).
All MESA hospitalizations were assigned a primary discharge diagnosis code for CLRD, with asthma, bronchitis, and COPD being the most common codes (Table 2). The majority of participants were women and smokers, and 48% had self-reported CLRD, of whom 67% had self-reported asthma. Approximately two-thirds had airflow limitation and 5% had restriction on the basis of study spirometry (Table 2).
Reliability
HCHS/SOL
There was excellent agreement in ED records for classification of CLRD cases (κ = 0.88; 95% confidence interval [CI], 0.71–1.00]) and CLRD exacerbations (κ = 0.90; 95% CI, 0.78–1.00]), with 4% reviewer disagreement. There was also excellent agreement in hospitalization records for CLRD cases (κ = 0.85; 95% CI, 0.69–1.00) and CLRD exacerbations (κ = 0.84; 95% CI, 0.70–0.97), with a slightly greater frequency of disagreement (Table 3).
Table 3.
Interrater agreement for determination of chronic lower respiratory disease cases and exacerbations
| Classified Events | Agreement* |
Disagreement† | κ-Statistic | 95% CI | |||
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| HCHS/SOL |
|||||||
| Emergency department | |||||||
| CLRD cases | 56 | 45 (80%) | 9 (16%) | 2 (4%) | 0.88 | 0.71–1.00 | |
| CLRD exacerbations | 56 | 41 (73%) | 13 (23%) | 2 (4%) | 0.90 | 0.78–1.00 | |
| Hospitalizations | |||||||
| CLRD cases | 61 | 47 (77%) | 11 (18%) | 3 (5%) | 0.85 | 0.69–1.00 | |
| CLRD exacerbations | 61 | 29 (48%) | 27 (44%) | 5 (8%) | 0.84 | 0.70–0.97 | |
| MESA |
|||||||
| Hospitalizations (ICD primary discharge diagnosis‡) | |||||||
| CLRD cases | 99 | 94 (95%) | 3 (3%) | 2 (2%) | 0.74 | 0.40–1.00 | |
| CLRD exacerbations | 99 | 89 (90%) | 3 (3%) | 7 (7%) | 0.42 | 0.07–0.78 | |
Definition of abbreviations: CI = confidence interval; CLRD = chronic lower respiratory disease; HCHS/SOL = Hispanic Community Health Study/Study of Latinos; ICD = International Classification of Diseases; MESA = Multi-Ethnic Study of Atherosclerosis.
Percentages are calculated out of the total number of eligible events of that type (emergency department visit or hospitalization) in that cohort.
Positive agreement is defined as both reviewers confirming the event as at least a probable CLRD case or CLRD exacerbation; negative agreement is defined as both reviewers determining that the event was not a CLRD case or CLRD exacerbation.
Disagreement is defined as one reviewer classifying the event as at least a probable CLRD case or CLRD exacerbation and the other determining that it was not consistent with a CLRD case or CLRD exacerbation.
All classified events in MESA had an ICD code for CLRD as the primary discharge diagnosis.
MESA
Relatively low κ-values—0.74 (95% CI, 0.40–1.00) and 0.42 (95% CI, 0.07–0.78) for CLRD cases and CLRD exacerbations, respectively—were observed due to high expectation of agreement (31, 32). Reviewers disagreed on classification of 2% of cases and 7% of exacerbations.
Confirmation
HCHS/SOL
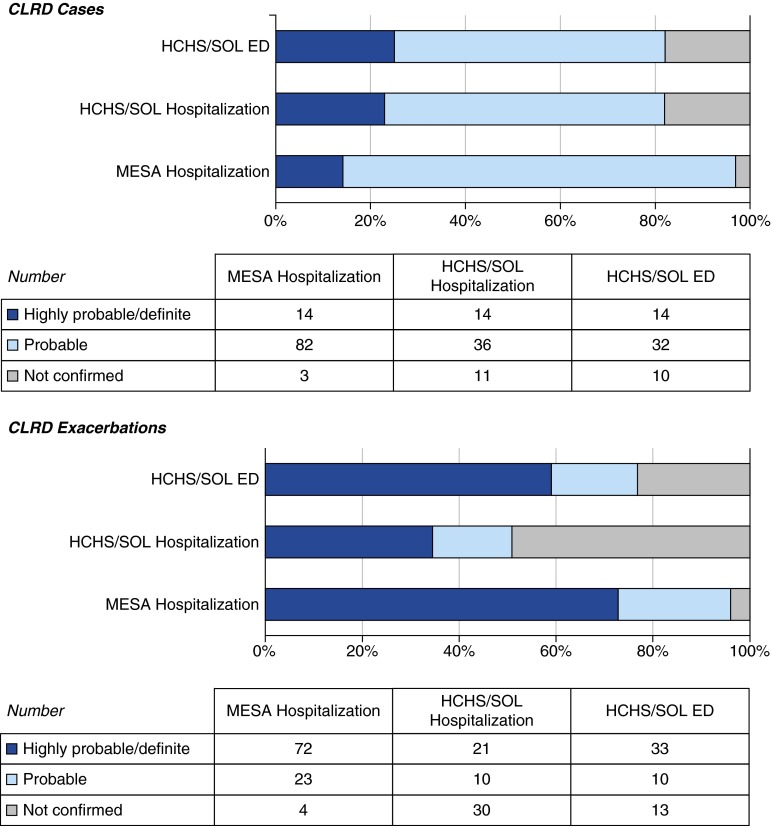
Eighty-two percent of HCHS/SOL classified records were confirmed as demonstrating at least probable CLRD cases (Figure 2, Table E5). Cases were subclassified mainly as asthma. Among the 46 ED visits and 50 hospitalizations confirmed as CLRD cases, 77% were confirmed as at least probable CLRD exacerbations and 56% as highly probable or definite CLRD exacerbations.
Figure 2.
Confirmation of medical records as chronic lower respiratory disease (CLRD) cases and CLRD exacerbations, by cohort. ED = emergency department; HCHS/SOL = Hispanic Community Health Study/Study of Latinos; MESA = Multi-Ethnic Study of Atherosclerosis.
Limiting to the analysis to records with CLRD as the primary discharge diagnosis, 95% of ED records were confirmed as at least probable CLRD exacerbations and 85% as highly probable or definite CLRD exacerbations. All hospitalization records with a primary discharge diagnosis of CLRD were confirmed as highly probable or definite CLRD exacerbations.
MESA
Almost all (97%) classified MESA records were confirmed as at least probable CLRD cases (Figure 2), the majority of which were subclassified as COPD. Ninety-six percent of classified MESA hospitalizations were confirmed as at least probable CLRD exacerbations and 73% as highly probable or definite CLRD exacerbations.
Comparison with Study Spirometry
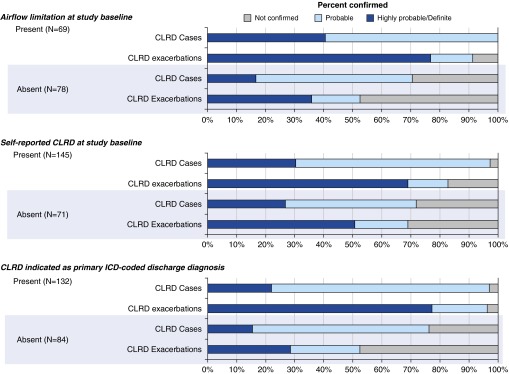
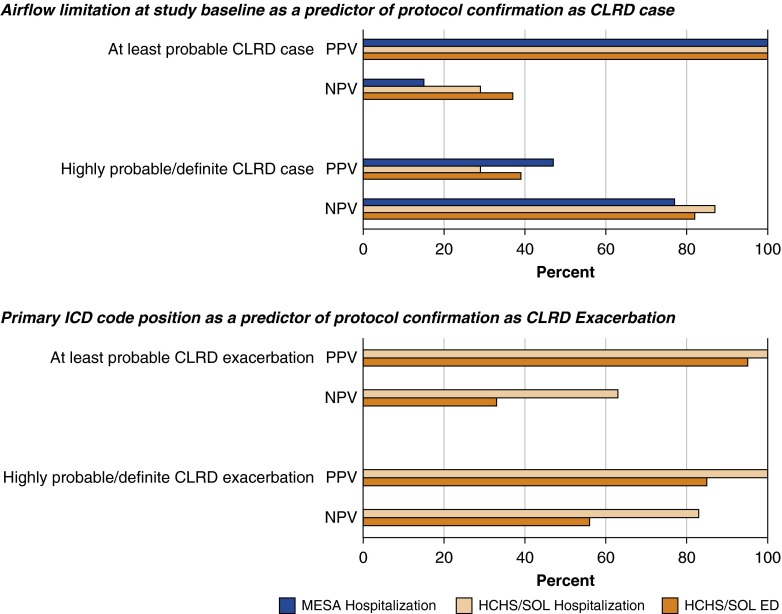
A greater percentage of events were confirmed as CLRD cases and CLRD exacerbations in persons with airflow limitation compared with those without airflow limitation on the basis of study spirometry (Figure 3, Table E6). However, only 56% of probable CLRD cases occurred in participants with airflow limitation on the basis of study spirometry. Consistent with these results, airflow limitation on the basis of study spirometry showed a high PPV for protocol-defined CLRD cases, but NPV was low (Figure 4, Table E7). Percentages of events confirmed as CLRD cases and CLRD exacerbations did not differ by the presence or absence of restriction on the basis of spirometry (Table E8).
Figure 3.
Confirmation of chronic lower respiratory disease (CLRD) cases and CLRD exacerbations according to selected characteristics in the pooled cohort sample. ICD = International Classification of Diseases.
Figure 4.
Positive predictive value (PPV) and negative predictive value (NPV) of airflow limitation at study baseline and primary International Classification of Diseases (ICD) code position with respect to protocol-defined endpoints, by cohort. CLRD = chronic lower respiratory disease; ED = emergency department; HCHS/SOL = Hispanic Community Health Study/Study of Latinos; MESA = Multi-Ethnic Study of Atherosclerosis.
Comparison with Self-reported CLRD at Study Baseline
In persons with self-reported CLRD at study baseline, a greater percentage of events were confirmed as CLRD cases and CLRD exacerbations (Figure 3, Table E6); yet, confirmed new diagnoses of CLRD cases and CLRD exacerbations were frequent in both cohorts. In MESA, which had a longer follow-up period, 41% of probable CLRD exacerbations and 36% of highly probable or definite CLRD exacerbations occurred among persons without self-reported CLRD at study baseline. Of 19% of events occurring in both cohorts among persons with neither airflow limitation nor self-reported CLRD at baseline, 29% were confirmed as CLRD exacerbations.
Comparison with an Administrative Definition of Exacerbation
A great percentage of events assigned a primary discharge diagnosis of CLRD were confirmed as CLRD cases and CLRD exacerbations (Figure 3, Table E6), whereas half of events without a primary discharge diagnosis of CLRD were not confirmed as CLRD exacerbations. The PPV of primary discharge diagnosis code for protocol-confirmed CLRD exacerbation was excellent (Figure 4, Table E9). However, the NPV was 50%, and restriction of event ascertainment in HCHS/SOL to the 36% of ED visits and 21% of hospitalizations with a primary ICD code for CLRD would have reduced the number of confirmed CLRD exacerbations by 57%.
Subphenotypes
Reviewer agreement was similar for CLRD subphenotypes of COPD, emphysema, chronic bronchitis, and asthma (Table E10). Asthma was confirmed in approximately three-fourths of HCHS/SOL records versus one-third of MESA records, whereas COPD was confirmed in one-fifth of HCHS/SOL records and two-thirds of MESA records (Table E11). Comorbid asthma and COPD were confirmed in 5%. In the pooled cohort sample, the PPV of airflow limitation was similar for asthma and COPD, whereas the NPV of airflow limitation was greater for COPD than for asthma (Table E12). One-fourth of confirmed COPD cases occurred among participants without airflow limitation on the basis of study spirometry.
Discussion
CLRD cases and CLRD exacerbations were defined in two population-based cohorts of adults in the United States by using a new classification protocol based upon review of medical records of ED visits and hospitalizations. Interrater agreement was excellent. Almost all medical records with a primary discharge diagnosis code for a CLRD were confirmed as CLRD exacerbations, compared with approximately half of those with CLRD as a nonprimary discharge diagnosis code. Our results suggest that the protocol is a practical and reliable approach to defining incident CLRD cases and exacerbations in general population studies, which is an important step toward valid epidemiologic research on primary and secondary prevention of CLRD.
We confirmed a substantial number of CLRD cases and CLRD exacerbations among persons without airflow limitation on the basis of study spirometry or self-reported disease at baseline who would not be captured by current standard epidemiologic definitions (33, 34). In persons without previously documented airflow limitation, many of the confirmed CLRD cases were subphenotyped as asthma, which is not defined by fixed airflow limitation; however, persons without airflow limitation also gave rise to one-fourth of confirmed COPD cases. These findings are consistent with recent reports of the clinical importance of respiratory exacerbations and related events in persons without documented baseline airflow limitation in the CanCOLD (35), MESA (7), COPDGene (8), and SPIROMICS (9) studies. Such events may correspond to dynamic airflow limitation attributable to emphysematous lung changes or asthma–COPD overlap (2), which were not uncommon in the present study. Confirmed CLRD cases in persons without baseline airflow limitation on the basis of study spirometry may also correspond to incident airflow limitation. Any of these potential explanations support consideration of incident CLRD exacerbations as sentinel events and thus as promising targets for primary prevention research and interventions.
To our knowledge, this is the first standardized protocol for adjudication of CLRD exacerbations. In population-based and other large studies of CLRD risk, administrative data are commonly used to classify severe exacerbations, despite ambiguities regarding the validity of this approach (36). In the Copenhagen City Heart Study, ICD-defined COPD-related deaths showed evidence for both under- and overdiagnosis compared with baseline spirometry measures (15). In a study of 200 hospitalizations at two U.S. urban academic medical centers, several ICD-based algorithms used to classify COPD exacerbations had very low sensitivity but high specificity compared with physician-report of COPD plus hospitalization for an acute respiratory symptom (14). With respect to asthma, the authors of a recent systematic review identified only three studies reporting any validation metrics for identification of bronchospasm, and all three studies were in pediatric populations (16).
Among events with CLRD as the primary discharge diagnosis code, the protocol confirmed almost all as CLRD exacerbations. In HCHS/SOL, in which events were ascertained by CLRD in any ICD code position as well as by relevant keywords, events without CLRD as the primary discharge code were almost three times as common as those with primary coding; yet, only approximately one-half and one-third were confirmed as probable and highly probable or definite CLRD exacerbations, respectively. These findings support use of primary discharge diagnosis codes as a relatively efficient approach to capturing severe CLRD exacerbations, although the broader strategy used in HCHS/SOL more than doubled the total number of confirmed CLRD.
Findings were relatively consistent across these two studies, even though HCHS/SOL used prospective ascertainment of CLRD events and its sample was markedly younger, comprised exclusively of Hispanic/Latino participants, and had more asthma, whereas MESA collected CLRD events retrospectively and its sample was older, multiethnic, and had more COPD. This between-cohort heterogeneity may be seen as a limitation, especially for pooled analyses. Nonetheless, the consistency of the results suggests that the protocol is likely to be applicable to other general population studies as well as to disease-based studies such as SPIROMICS (38), which is collecting hospitalization records using methodology similar to that of HCHS/SOL.
Strengths and Limitations
Strengths of our study include systematic application and evaluation of a protocol developed by CLRD experts in two well-characterized, population-based cohorts with standardized event follow-up, strong minority representation, and complementary age distributions. The major limitation was the lack of a clear gold standard for classifying CLRD exacerbations. Although spirometry is the current standard for diagnosis of COPD and provides evidence of airflow limitation (3), it is not recommended in the acute setting and does not capture emphysema or chronic bronchitis. Peak expiratory flow rates were more commonly measured during presumed asthma exacerbations, in which context they have acceptable diagnostic accuracy (4). Neither measure was attempted systematically as part of the study protocol; thus, data confirming dynamic airflow limitation, which is a quantitative physiologic correlate of COPD and asthma exacerbations (4, 38), were available in only a subset of events. Nonetheless, the protocol made use of a large amount of clinical data in an attempt to provide an alloyed gold standard. The prevalence of restriction on the basis of spirometry was high in HCHS/SOL, which may have been due to the high prevalence of obesity or possible misclassification of reference equations. Regardless of these considerations, exclusion of persons with restriction on the basis of spirometry did not alter protocol performance.
Ascertainment of medical records was limited to those coded with CLRD-related ICD codes or keywords. We were therefore unable to characterize the performance of our protocol for events in which the diagnosis of CLRD was missed. Nonetheless, the ascertainment criteria for CLRD exacerbations in HCHS/SOL included keywords for standard symptoms (cough) and ICD codes for related diagnoses (pulmonary heart failure, respiratory failure). Also, the number of charts evaluated was modest but sufficient to demonstrate protocol feasibility and favorable performance.
The protocol is appropriate only to detect clinically significant CLRD cases and “severe” CLRD exacerbations in persons presenting for ED or hospital care. Ascertainment of CLRD cases and CLRD exacerbations in persons who do not pursue or require this level of health care use entails use of alternative methods. The protocol is similar in this regard to protocols used for heart failure (39) and myocardial infarction (40), which do not ascertain heart failure exacerbations treated in the outpatient setting and “silent” myocardial infarcts.
Conclusions
The protocol described in this article is a practical and reliable approach to defining CLRD cases and CLRD exacerbations in epidemiologic studies, in which valid measures of CLRD endpoints are important for evaluating primary and secondary prevention strategies. Protocol-based classification encompasses evolving definitions of CLRD cases and confirms a substantial percentage of CLRD cases in persons without airflow limitation or self-reported CLRD. CLRD exacerbations were confirmed in the vast majority of records with a primary discharge diagnosis of CLRD, compared with only half of records with CLRD listed as a nonprimary code. Our results justify further examination of CLRD adjudication in population-based cohorts, in other cohorts collecting medical records for at-risk smokers (e.g., SPIROMICS), and potentially in electronic medical records to promote large-scale CLRD prevention research.
Acknowledgments
Acknowledgment
The authors acknowledge and thank Drs. Gerald Criner, Frank Sciurba, Byron Thomashow, Gerard Turino, Robert Wise, and others for intellectual input into an earlier version of this protocol at a working group meeting that was organized by the COPD Foundation.
Footnotes
This work was supported primarily by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health. The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) was performed as a collaborative study supported by contracts from the NHLBI to the University of North Carolina (N01-HC65233), the University of Miami (N01-HC65234), the Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes, centers, and offices contributed to the baseline HCHS/SOL funding period through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and National Institutes of Health Office of Dietary Supplements. Multi-Ethnic Study of Atherosclerosis (MESA) research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the NHLBI and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources. The MESA Lung Study was supported by NHLBI grants R01-HL077612 and R01-HL093081. The study was additionally supported by NHLBI grants R21-HL121457 (R.G.B.) and R21-HL129924 (E.C.O.), as well as by NHLBI grant HHSN268200900013C-20C.
Author Contributions: E.C.O.: was primarily responsible for the statistical analysis, which was independently verified by statisticians from the Hispanic Community Health Study/Study of Latinos, and for the drafting of the manuscript; E.C.O., L.R.L., K.M.D., P.L.E., W.D.R., and R.G.B.: contributed substantively to study design and adjudication protocol development; and E.C.O., L.R.L., A.G.H., K.M.D., P.L.E., R.K., C.M.L.C., A.R., N.S., B.M.S., and R.G.B.: actively participated in protocol-based chart review in one or both of the involved cohorts. All authors participated in manuscript review.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health OrganizationThe top 10 causes of death [updated May 2014; accessed 2016 May 2]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- 2.Postma DS, Rabe KF. The asthma–COPD overlap syndrome. N Engl J Med. 2015;373:1241–1249. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 3.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute (NHLBI), National Asthma Education and Prevention ProgramExpert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: NHLBI, National Institutes of Health; 2007 [accessed 2016 May 2]. http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf [Google Scholar]
- 5.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P, Curren K, Balter MS, Bhutani M, Camp PG, et al. Executive summary: prevention of acute exacerbation of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:883–893. doi: 10.1378/chest.14-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAllister DA, Ahmed FS, Austin JH, Henschke CI, Keller BM, Lemeshow A, Reeves AP, Mesia-Vela S, Pearson GD, Shiau MC, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9:e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, Kauczor HU, Bailey WC, DeMeo DL, Casaburi RH, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, Gouskova NA, Hansel NN, Hoffman EA, Kanner RE, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oelsner EC, Hoffman EA, Folsom AR, Carr JJ, Enright PL, Kawut SM, Kronmal R, Lederer D, Lima JA, Lovasi GS, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161:863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbehairy AF, Raghavan N, Cheng S, Yang L, Webb KA, Neder JA, Guenette JA, Mahmoud MI, O’Donnell DE. Physiologic characterization of the chronic bronchitis phenotype in GOLD grade IB COPD. Chest. 2015;147:1235–1245. doi: 10.1378/chest.14-1491. [DOI] [PubMed] [Google Scholar]
- 12.Martinez CH, Kim V, Chen Y, Kazerooni EA, Murray S, Criner GJ, Curtis JL, Regan EA, Wan E, Hersh CP, et al. COPDGene Investigators. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014;108:491–499. doi: 10.1016/j.rmed.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, Falsey AR, Mathur SK, Ramsdell JW, Rogers L, et al. Asthma in Elderly workshop participants. Asthma in the elderly: current understanding and future research needs—a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011;128(3) Suppl:S4–S24. doi: 10.1016/j.jaci.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein BD, Bautista A, Schumock GT, Lee TA, Charbeneau JT, Lauderdale DS, Naureckas ET, Meltzer DO, Krishnan JA. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141:87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen HH, Godtfredsen NS, Lange P, Vestbo J. Potential misclassification of causes of death from COPD. Eur Respir J. 2006;28:781–785. doi: 10.1183/09031936.06.00152205. [DOI] [PubMed] [Google Scholar]
- 16.Sharifi M, Krishanswami S, McPheeters ML. A systematic review of validated methods to capture acute bronchospasm using administrative or claims data. Vaccine. 2013;31:K12–K20. doi: 10.1016/j.vaccine.2013.06.091. [DOI] [PubMed] [Google Scholar]
- 17.Canner PL, Krol WF, Forman SA. The Coronary Drug Project: external quality control programs. Control Clin Trials. 1983;4:441–466. doi: 10.1016/0197-2456(83)90028-4. [DOI] [PubMed] [Google Scholar]
- 18.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tashkin D, Celli B, Kesten S, Lystig T, Decramer M. Effect of tiotropium in men and women with COPD: results of the 4-year UPLIFT trial. Respir Med. 2010;104:1495–1504. doi: 10.1016/j.rmed.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 21.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 23.Oelsner EC, Loehr LR, Henderson AG, Enright P, Donohue KM, Kalhan R, Ries AL, Shah NA, Smith BM, Lo Cascio CM, et al. Adjudication protocol for chronic lower respiratory disease events in two population-based cohorts: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) and the Multi-Ethnic Study of Atherosclerosis (MESA)[abstract]Am J Respir Crit Care Med 2014189A2941 [Google Scholar]
- 24.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 26.Definition and classification of chronic bronchitis for clinical and epidemiological purposes: a report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet. 1965;1:775–779. [PubMed] [Google Scholar]
- 27.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152:201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 30.Chen G, Faris P, Hemmelgarn B, Walker RL, Quan H. Measuring agreement of administrative data with chart data using prevalence unadjusted and adjusted kappa. BMC Med Res Methodol. 2009;9:5. doi: 10.1186/1471-2288-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43:543–549. doi: 10.1016/0895-4356(90)90158-l. [DOI] [PubMed] [Google Scholar]
- 32.Cicchetti DV, Feinstein AR. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol. 1990;43:551–558. doi: 10.1016/0895-4356(90)90159-m. [DOI] [PubMed] [Google Scholar]
- 33.Barr RG, Herbstman J, Speizer FE, Camargo CA., Jr Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155:965–971. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 34.Diaz-Guzman E, Mannino DM. Airway obstructive diseases in older adults: from detection to treatment. J Allergy Clin Immunol. 2010;126:702–709. doi: 10.1016/j.jaci.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Tan WC, Bourbeau J, Hernandez P, Chapman KR, Cowie R, FitzGerald JM, Marciniuk DD, Maltais F, Buist AS, O’Donnell DE, et al. CanCOLD Collaborative Research Group. Exacerbation-like respiratory symptoms in individuals without chronic obstructive pulmonary disease: results from a population-based study. Thorax. 2014;69:709–717. doi: 10.1136/thoraxjnl-2013-205048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsia DC, Krushat WM, Fagan AB, Tebbutt JA, Kusserow RP. Accuracy of diagnostic coding for Medicare patients under the prospective-payment system. N Engl J Med. 1988;318:352–355. doi: 10.1056/NEJM198802113180604. [DOI] [PubMed] [Google Scholar]
- 37.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, et al. SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 39.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]