Abstract
Physiological and cellular functions operate in a 24-hour cyclical pattern orchestrated by an endogenous process known as the circadian rhythm. Circadian rhythms represent intrinsic oscillations of biological functions that allow for adaptation to cyclic environmental changes. Key clock genes that affect the persistence and periodicity of circadian rhythms include BMAL1/CLOCK, Period 1, Period 2, and Cryptochrome. Remarkable progress has been made in our understanding of circadian rhythms and their role in common medical conditions. A critical review of the literature supports the association between circadian misalignment and adverse health consequences in sepsis, obstructive lung disease, obstructive sleep apnea, and malignancy. Circadian misalignment plays an important role in these disease processes and can affect disease severity, treatment response, and survivorship. Normal inflammatory response to acute infections, airway resistance, upper airway collapsibility, and mitosis regulation follows a robust circadian pattern. Disruption of normal circadian rhythm at the molecular level affects severity of inflammation in sepsis, contributes to inflammatory responses in obstructive lung diseases, affects apnea length in obstructive sleep apnea, and increases risk for cancer. Chronotherapy is an underused practice of delivering therapy at optimal times to maximize efficacy and minimize toxicity. This approach has been shown to be advantageous in asthma and cancer management. In asthma, appropriate timing of medication administration improves treatment effectiveness. Properly timed chemotherapy may reduce treatment toxicities and maximize efficacy. Future research should focus on circadian rhythm disorders, role of circadian rhythm in other diseases, and modalities to restore and prevent circadian disruption.
Keywords: chronobiology disorders, sepsis, obstructive lung disease, obstructive sleep apnea, neoplasms
Endogenous rhythms that approximate 24 hours play important regulatory functions in human biology and physiology (1). Because these rhythms likely evolved as a response to the environmental changes between day and night, these rhythms have been given the name circadian (i.e., circa = around, dia = day) (2). A key oscillation of these circadian rhythms in humans is the sleep-wake cycle, which not only emerges endogenously but also provides feedback to the circadian system (1). Central influences to this system are governed by a central pacemaker in the suprachiasmatic nuclei in the hypothalamus and subsidiary peripheral pacemakers in nearly every cell of the body (3, 4). Many circadian rhythms are autonomous and self-sustained by transcriptional–translational antiregulatory loops that generate molecular oscillations of clock genes, such as BMAL1, CLOCK, Period 1, Period 2, and Cryptochrome (4). It is evident that interactions among clock components vary in a tissue-specific manner.
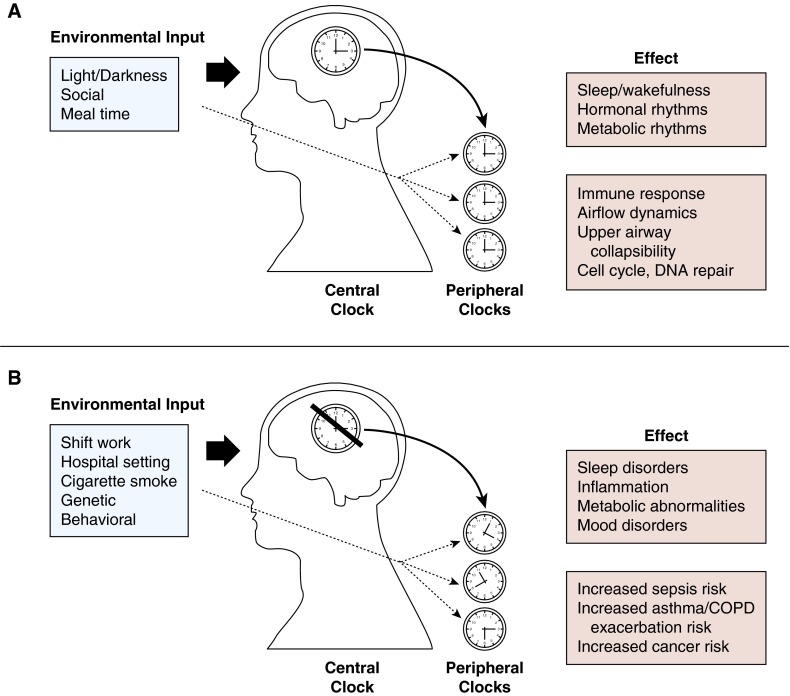
Environmental cues such as light, feeding-fasting cycles, and social interactions exert strong influences on both central and peripheral clocks (5, 6). The rhythmic oscillation of sleep and wakefulness, in the context of other circadian processes, represents an important physiologic phenomenon that serves to synchronize biologic functions with each other and with the external environment (Figure 1A).
Figure 1.
Simplified depiction of (A) normal circadian rhythm and its inputs and effects, and (B) disrupted circadian rhythm and its inputs and effects. Environmental inputs such as light/darkness, socialization, and meals influence central and peripheral circadian clocks. Disrupted circadian clocks may lead to various negative health outcomes. COPD = chronic obstructive pulmonary disease.
In addition to synchronizing the sleep–wake cycle, circadian rhythms play an important role in the onset and severity of diseases. Many diseases have well-documented diurnal variability. Myocardial infarction, ischemic stroke, and sudden cardiac death all peak in frequency between the hours of 8:00 a.m. and 10:00 a.m., compared with the hours between 9:00 p.m. and 11:00 p.m. (7–9). Many other diseases, including hypertension, seizures, and asthma, have been shown to exhibit a day–night variation in disease presentation as well (10).
Much of the current evidence demonstrating negative health outcomes from circadian rhythm disruption in real-world situations is from studies of shift workers (Figure 1B). By virtue of the difficulty of maintaining nonstandard schedules, shift workers are often sleep deprived in addition to being exposed to a misalignment between internal and external circadian cues. Sleep loss affects neurocognitive performance (11, 12), behavior (13), and cardiometabolic disease risk (14). Furthermore, sleep loss leads to changes in circadian rhythms in central and peripheral tissues in association with disruptions of energy metabolism (15). In short- and long-term studies of shift workers, unfavorable alterations in lipid and carbohydrate metabolism, insulin resistance, growth hormone, and corticosteroid secretion patterns (16–18) and increased risk of cancer have been reported (19–21). Similar to shift workers, patients in the intensive care unit (ICU) also experience circadian rhythm disruption due to noise, patient care interaction, mechanical ventilation, pain, medications, artificial light, and the illness itself (22, 23).
The goal of this review is to describe the circadian rhythmic processes related to common medical conditions: sepsis, obstructive lung diseases (asthma and chronic obstructive pulmonary disease [COPD]), obstructive sleep apnea (OSA), and malignancy, with the hope of improving therapeutic efficiencies and patient care. We briefly review the normal circadian rhythm physiology related to the disease and the clinical relevance of circadian rhythm disruption during disease state. The review also highlights the therapeutic implications of the sleep–wake cycle and chronotherapy—a concept of delivering treatment on the basis of the circadian timing of the disease process to maximize therapeutic efficacy and minimize toxicity.
Sepsis
Normal Circadian Rhythm of Immune Cells
Like other cell types in the body, immune cells have an endogenous circadian clock. Light and dark cycles influence the immune functions of natural killer cell activity, lymphocyte proliferation, and monocyte proliferation (24, 25). Levels of inflammatory mediators such as IL-6, tumor necrosis factor, and IFN-γ have a diurnal variation (26). Eight percent of all mRNA transcripts in macrophages have a 24-hour period oscillating expression. This translates to a concept called “circadian gating.” The capacity of a cellular function, such as the magnitude of immune response to pathogen, becomes time-dependent throughout the day (27, 28). Toll-like receptor 9, a recognition receptor for DNA of bacterial and viral pathogens, has been observed to peak during activity phases of mammals and reach a nadir during sleep. When sepsis was induced in mice by colon puncture, more severe forms of sepsis, increased number of bacterial burden, and earlier mortality were observed when toll-like receptor 9 expression was highest (29).
Circadian Rhythm and Sepsis
Sepsis is a complex syndrome of systemic inflammatory response when the host immune system recognizes pathogen-specific molecules. This process leads to rapid activation of proinflammatory cascades in promoting pathogen clearance through complement pathways, mobilization of leukocytes, phagocytosis, and release of reactive oxygen species and antimicrobial peptides. Activation of antiinflammatory processes is equally important, as unchecked inflammation leads to hypotension, multiorgan failure, and death (30).
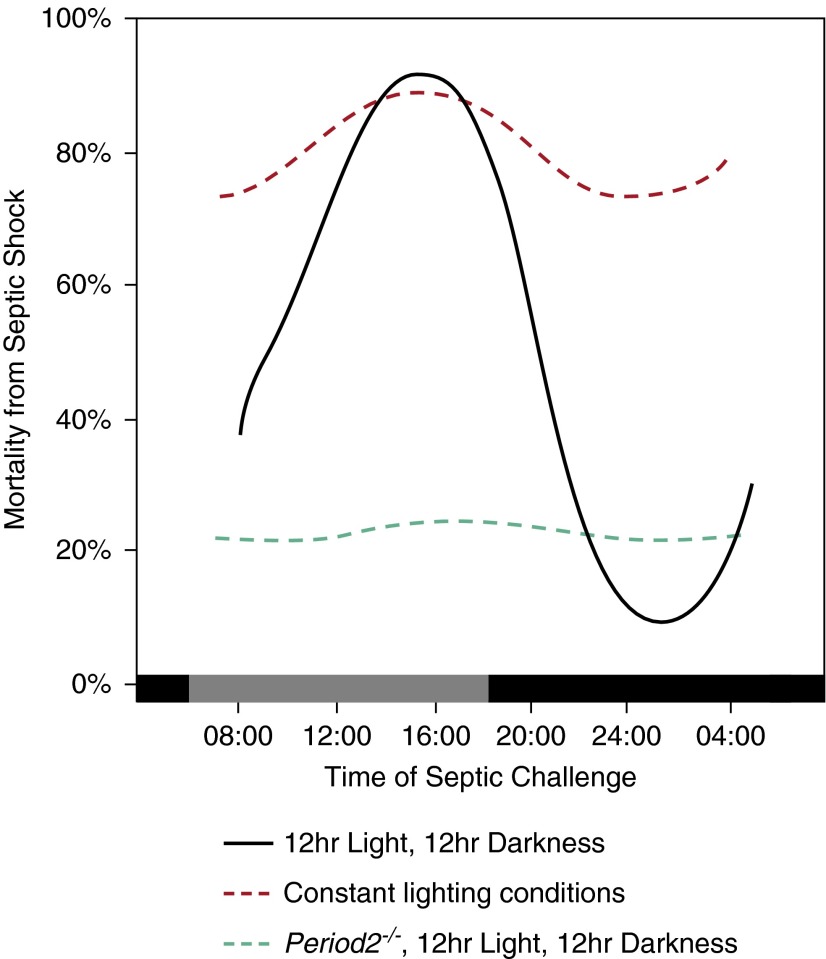
The circadian effect on sepsis is well documented in controlled animal experiments. Halberg and colleagues (31) tested the circadian effect of sepsis by inoculating equivalent doses of endotoxin in mice at different times of the day. When endotoxin was injected at 4:00 p.m., the survival from septic shock was less than 20%. When the same dose of endotoxin was given at 12:00 a.m., the survival was greater than 90% (31) (Figure 2). This finding seems to be dependent on the circadian physiology, because genetic disruption of Period 2, a key circadian clock gene, abolishes the diurnal effect of endotoxin on survival (26). Melatonin, a major hormone of circadian rhythm, is also an important mediator of the inflammatory response seen in sepsis. Deletion of melatonin receptors in experimental sepsis generated a greater inflammatory response in mice (32). Exogenous melatonin increased survival of sepsis in a melatonin receptor–dependent fashion (33).
Figure 2.
Circadian effect on mortalities from animal model of septic shock. Mortality rate from septic shock exhibited a strong diurnal pattern dependent on the timing of the septic challenge (black line) (31). The diurnal variation diminished when subjects were housed in conditions with constant lighting (red dashed line) (39). Knockout of Period 2 also abolished the diurnal variation even when the subjects were housed in normal 12-hour light/12-hour dark conditions (26).
How sepsis affects the circadian clock is still under investigation. Circadian rhythm may be suppressed during acute inflammatory illness, as evidenced by suppression of circadian clock genes in peripheral leukocytes for at least 17 hours after inoculation of endotoxin in mice (34). This finding may explain why diurnal variation of melatonin was blunted in ICU patients with sepsis compared with ICU patients without sepsis (35). Furthermore, injection of endotoxin disrupted sleep by decreasing non-REM sleep and doubling the amount of wakefulness during the night (36).
Genome-wide transcriptional profiling of lung tissues showed cytokine production and leukocyte trafficking exhibited a circadian rhythm (37). Genetic knockouts of BMAL1, a prototypical circadian clock gene, altered the rhythmic expression of IFN-γ and myeloperoxidase in granulocytes. BMAL1 is also important for the circadian pattern of granulocyte infiltration into the endotoxemic lungs (26, 37).
Sleep deprivation may also impact risk and severity of sepsis (Figure 1B). Animals undergoing septic challenge are less likely to recover when their 12/12 hour light/dark cycle is replaced by constant light or constant darkness (38, 39). In humans, systemic C-reactive protein, IL-1, and IL-6 were increased in sleep-deprived individuals (40–42). Individuals deprived of 24 to 88 hours of sleep had impaired immediate antibody response to hepatitis and influenza vaccines, suggesting an attenuated antigen-specific immune defense with sleep disruption (43, 44). Collectively, these studies highlight circadian clock realignment and sleep quality improvement as a crucial role in therapy in sepsis.
Implications on Treatment of Sepsis
Restoring the circadian rhythm in patients is an underused aspect in sepsis treatment. Melatonin is one of the main endogenous mediators of the circadian rhythm, with beneficial antioxidative and antiinflammatory effects. A randomized controlled trial among 24 ICU patients with respiratory failure showed oral melatonin to increase sleep time and quality (45).
Melatonin also has effective antioxidative and antiinflammatory effects. In a small randomized controlled trial of 20 infants with sepsis, Gitto and colleagues (46) assessed the antiinflammatory effect of melatonin. Blood leukocyte count and absolute neutrophil count were significantly decreased in infants with sepsis receiving 20 mg melatonin (47). Infants with sepsis treated with melatonin had an 80% decrease in C-reactive protein compared with control infants. A higher proportion of melatonin-treated infants had full recovery from sepsis within 48 hours. In a second study, IL-6, IL-8, and tumor necrosis factor-α levels were significantly lower in melatonin-treated newborns with respiratory distress syndrome, further supporting the antiinflammatory effects of melatonin (48). Melatonin has no major toxicity and is rapidly cleared at high doses after oral administration (46–49). Melatonin may be a highly promising tool in the ICU to promote healthy circadian rhythm, improve immunity, and improve sepsis outcomes.
Regarding light therapy in the ICU, various studies have failed to show objective benefit. Wunsch and colleagues (50) showed that the presence of a window in an ICU did not improve functional status, length of stay, or mortality in 789 critically ill patients with subarachnoid hemorrhage. However, the authors mentioned limitations of their study included not taking into account sedation, delirium, limited light exposure after transferring out of the ICU, and a study cohort with acute brain injury, which may make external stimuli less important. Another study reported that in patients with severe sepsis, outdoor light did not entrain melatonin secretion pattern (51). The authors noted that the environmental light levels were low (maximum of 200 lux). The white light intensity most reliably shown to entrain the circadian rhythm is 10,000 lux for at least 30 minutes or 2,500 lux for at least 1 hour (52–54).
Obstructive Lung Disease
Circadian Rhythm in Asthma
Nighttime worsening of asthma has been historically recognized. A Roman physician in the fifth century, Caelius Aurelianus, noted: “On the heavy breathing and wheezing which is called Asthma by the Greeks, this disease is a burden…during the winter and at night more than during the day or the spring” (55). Clinically, asthma exacerbations frequently occur in the early hours of the morning, around 4:00 a.m. Dyspnea-induced nighttime awakening occurs in more than 75% of respondents in a large survey of individuals with asthma (56). In a 1-year review and a 2.5-year review of deaths due to asthma in adults, approximately 70% of asthma-related deaths occurred between 12:00 a.m. and 6:00 a.m. (57, 58).
Physiologically, airway caliber and inflammation also follow circadian patterns. Peak expiratory flow (PEF) as a measure of airway obstruction has been shown to fluctuate over a 24-hour period in both healthy patients and patients with asthma. Airway obstruction worsens during the night, with patients with asthma having a 51% larger change in PEF during nighttime than control patients (59). In addition, Bonnet and colleagues (60) revealed the circadian variation of airway responsiveness, with maximum bronchial responsiveness to methacholine and histamine bronchial challenge at 3:00 a.m. and 4:00 a.m. When transbronchial biopsies were performed in patients with and without asthma at 4:00 p.m. (when lung function is optimal) and 4:00 a.m. (when airflow limitation is highest), the tissue biopsies of nocturnal patients with asthma had a pronounced circadian variation in alveolar eosinophil number per unit volume, with a significantly higher eosinophil number at 4:00 a.m. than 4:00 p.m. (61). Bronchoalveolar lavage (BAL) studies show higher numbers of macrophages, neutrophils, lymphocytes, and CD4+ T cells have also been reported in alveolar tissue at 4:00 a.m. than at 4:00 p.m. (62, 63). The increase in inflammatory cells correlates with the overnight increase in airflow obstruction.
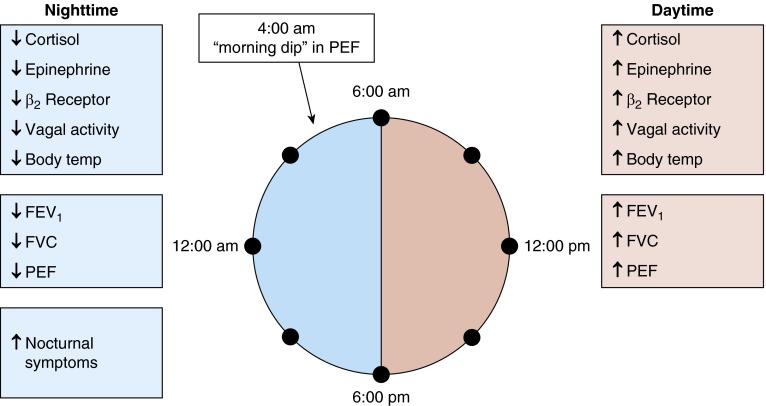
Sleep deprivation alone does not have an effect on circadian variation of lung function, suggesting that an endogenous circadian pacemaker is responsible for the diurnal variations (64). Many other factors are associated with the nocturnal exacerbation of asthma (Figure 3). The robust circadian rhythms of cortisol dip during the night and the vagal tone increase during sleep are believed to contribute significantly to the diurnal variation in airway inflammation and reactivity (65, 66). Cortisol binding and steroid responsiveness appear impaired in nocturnal asthma, resulting in impaired endogenous antiinflammatory processes (67). Other factors, such as late phase response to allergen exposure, nighttime predominance of gastroesophageal reflux, sleep apnea, and lung volume changes during sleep, may also play a role in nocturnal asthma symptoms (68–70).
Figure 3.
Diurnal depiction of neuroendocrine factors and airway dynamics associated with nocturnal asthma exacerbations. PEF = peak expiratory flow; Temp = temperature.
Chronotherapy for Asthma
Chronotherapy provides a reasonable approach to treatment of asthma by taking into account the diurnal nature of the disease (Table 1). Systemic corticosteroid therapy has been found to be most efficacious for nocturnal asthma when administered at 3:00 p.m. In a study by Beam and colleagues (71), prednisone at 50 mg was administered to patients with asthma at 8:00 a.m., 3:00 p.m., or 8:00 p.m., and FEV1 and BAL fluid were studied. The 3:00 p.m. administration of prednisone was associated with significantly increased nocturnal FEV1 and decreased neutrophils, eosinophils, lymphocytes, and macrophages in the BAL fluid. The 8:00 a.m. and 8:00 p.m. prednisone administration were ineffective in improving FEV1. Another study administered methylprednisolone at a dose of 40 mg to patients with asthma at 3:00 a.m., 7:00 a.m., 3:00 p.m., or 7:00 p.m. and found that administration at 3:00 p.m. led to greatest increase in PEF (72).
Table 1.
Evidence for chronotherapy in asthma by medication class
| Class | Medication | Suggested Time | Effect | Reference |
|---|---|---|---|---|
| Systemic steroids | Oral prednisone | 3:00 p.m. | Significantly increased nocturnal FEV1 when compared with 8:00 a.m. and 8:00 p.m. administration | 71 |
| 3:00 p.m. | Decreased inflammatory cells in BAL fluid | 71 | ||
| Intravenous methylprednisolone | 3:00 p.m. | Greatest increase in PEF when compared with 3:00 a.m., 7:00 a.m., and 7:00 p.m. administration | 72 | |
| Inhaled steroids | Triamcinolone | 3:00 p.m. | Once per day at 3:00 p.m. equivalent to four times daily | 73, 74 |
| Beclomethasone | Afternoon | Single dose in afternoon same efficacy as twice daily | 75 | |
| Mometasone | Evening | Significantly increased FEV1, morning PEF, FVC, FEF25–75%; morning dose and placebo showed no difference | 76, 77 | |
| Long-acting β2-agonist | Salmeterol | Evening | One dose at night is equivalent to twice-daily dosing | 78 |
| Leukotriene receptor antagonist | Montelukast | Evening | Improved FEV1 when compared with morning administration | 79 |
| Inhaled antimuscarinic | Tiotropium | N/A | No difference between morning and evening administration | 81 |
Definition of abbreviations: BAL = bronchoalveolar lavage; FEF25–75% = forced expiratory flow between 25% and 75% of vital capacity; N/A = not applicable; PEF = peak expiratory flow.
Several studies have looked at chronotherapy of inhaled corticosteroids. Triamcinolone of 800 μg administered at 3:00 p.m. to 5:30 p.m. was found to be equivalent to conventional 200 μg four times per day (73, 74). Furthermore, triamcinolone of 800 μg at 8:00 a.m. did not improve morning or evening peak expiratory flow rate (74). Despite giving a higher dose at one time, there were no differences in adrenocortical suppression or systemic side effects between the groups. Similarly, the efficacy and safety profile of inhaled beclomethasone dipropionate for patients with moderate asthma is the same when administered twice per day (conventional) or as a single dose in the afternoon or at bedtime (75). For mometasone furoate, the effect of evening regimen at 200 μg on FEV1, morning PEF, FVC, and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75%) was equivalent to 200 μg twice daily. Of note, when mometasone furoate was given in the morning at 200 μg, it demonstrated no differences compared with placebo (76, 77).
The concept of chronotherapy has also been shown in other treatment agents for asthma. For long-acting β2-agonist salmeterol, 100 μg given at night is as effective as 50 μg twice a day in averting nighttime bronchial asthma attacks in otherwise difficult-to-control patients (78). The leukotriene receptor antagonist montelukast improves FEV1 when dosed in the evening compared with the morning (79). Once-daily dosing in the evening of theophylline, compared with twice-daily and round-the-clock dosing, improves both asthma symptoms and PEF (80). Inhaled tiotropium showed no significant differences in effect on airway caliber when administered once daily in the morning versus the evening (81). However, the long half-life of tiotropium may mask its circadian-dependent effects.
Understanding the diurnal nature of the pathophysiology and the diurnal effect of treatment is important in management of asthma. When assessing response to therapy, knowing the timing of when patients take their medications is crucial, as treatment at certain times of the day may not be effective (74, 76). Furthermore, having to take only one dose per day significantly simplifies treatment regimen. This approach helps patients with adherence.
Circadian Rhythm in COPD
The link between circadian rhythm and chronotherapy in COPD is less established, especially given the heterogeneous nature of the disease. Similar to asthma, the diurnal variation in symptom severity has been observed during COPD exacerbations, with elevated risk for intubation during early morning hours in the emergency department (82). Exciting research is emerging showing environmental risks of COPD, such as tobacco smoking, can affect components of the circadian clock (83), which may lead to chronic inflammatory responses (84). As future studies elucidate the interrelationship between circadian rhythm and COPD, new therapeutic targets and approaches may emerge in COPD treatment.
OSA
OSA affects conservatively 10% of the U.S. population and is defined by obstruction of the upper airway during sleep, leading to chronic intermittent hypoxemia and sleep fragmentation. OSA has also been linked to metabolic syndrome, hypertension, stroke, and cancer. Animal models that mimic conditions of OSA show altered metabolic, inflammatory, and autonomic changes with associated autonomic risk (85). The impact of the circadian system on OSA is unclear, but it may influence apnea occurrence and duration in the early morning compared with late afternoon. In two separate studies, higher upper airway critical closing pressure (86) and prolonged apnea events (87) were consistently found to be greater in the morning than in the evening or afternoon. The mechanism is still unclear, but this knowledge provides a possible novel therapeutic modality for sleep apnea. Future studies to examine circadian rhythms in patients with OSA and timing of cardiometabolic risk incidence in OSA are needed (88).
Cancer
Circadian Rhythm Disruption and Tumorigenesis
Cell cycle regulation, apoptosis, and DNA repair, among other important processes for tumorigenesis, follow circadian rhythms. This notion has been shown in rapidly dividing cells of skin, gut, pancreas, reproductive organs, and bone marrow in humans and animals (89–94). Disruption of the circadian rhythm is linked with deregulated cell proliferation and the progression of cancer (95, 96). A large number of animal studies have shown cancer development in animal models of circadian disruption (94, 97–100). It was reported that both pancreatic carcinoma and osteosarcoma xenografts grew at a faster rate in animals with suprachiasmatic nuclei lesions (101). In humans, BMAL1/CLOCK, Period 1, and Period 2 are human circadian clock genes found to maintain a rhythmic pattern of cell proliferation and repair of DNA damage (94, 100–102).
A recent case-cohort study looking at lung cancer did not reveal increased risk among rotating night shift workers (103); however, multiple international prospective cohort studies have shown an increased risk in breast cancer and prostate cancer in night shift workers (104–107). The World Health Organization’s International Agency for Research on Cancer concludes, “shiftwork that involves circadian disruption is probably carcinogenic to humans” (Group 2A classification) (108). In 2009, night shift workers with breast cancer were awarded compensation in Denmark (107). More recently, an American Medical Association policy statement recognized circadian disruption due to occupational light as a cancer risk and has encouraged lighting technologies at home and at work to minimize circadian disruption (109).
Future prospective investigations are important to solidify current knowledge and shed light on pathophysiology of circadian disruption on cancer risk in humans.
Chronotherapy for Cancer
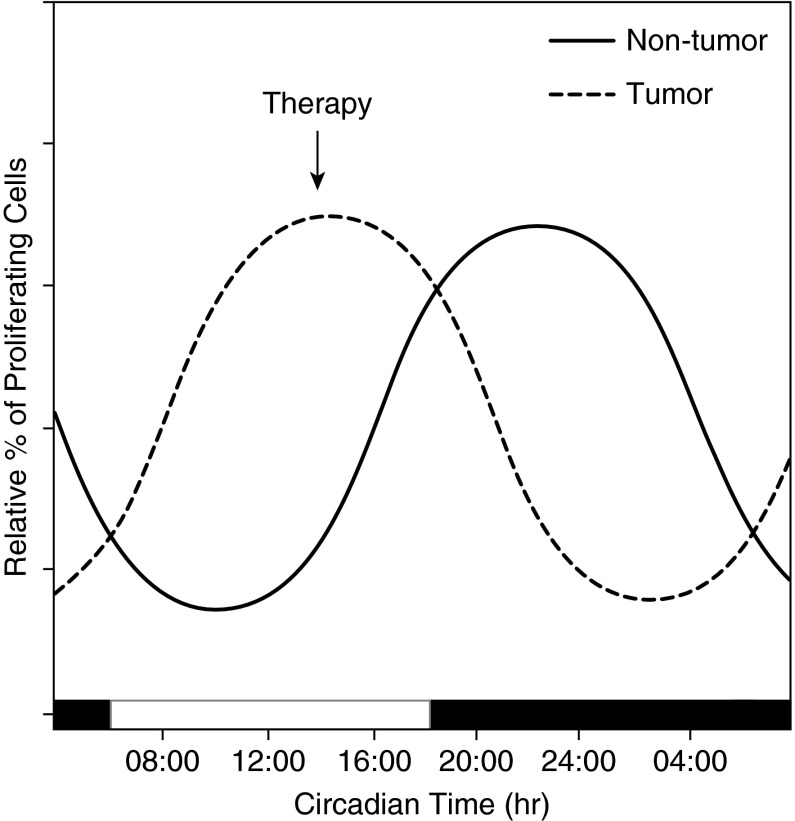
Because most chemotherapy agents target a certain stage in the cell cycle, properly timed chemotherapy delivery may reduce treatment toxicities and maximize efficacy (Figure 4). Several clinical trials looking at various chemotherapy agents showed promising results in reducing toxicity and improving treatment efficacy (Table 2). Two phase III trials showed chronomodulated chemotherapy reduced the incidence of severe mucositis by 80% and halved the incidence of peripheral neuropathy (110, 111). In breast cancer, leukopenia was significantly less common if chemotherapy was delivered at 5:00 p.m. compared with other times of day (112). Studies of patients with rectal cancer, pancreatic cancer, endometrial cancer, and lymphoblastic leukemia also demonstrate the safety of chronomodulated chemotherapy and support the benefits of minimized toxicity and positive response rate (113–117). One phase III study found that chronomodulated chemotherapy for patients with metastatic colon cancer had a stark sex difference: risk of death increased by 38% in women and decreased by 25% in men (118). Rigorous studies of chronomodulated therapy will help identify patients who could receive maximal benefit from therapy.
Figure 4.
Concept of chronotherapy in cancer therapy. The timing of proliferation is different between tumor and nontumor cells (95). Chemotherapy with time-specific delivery (arrow) maximizes therapeutic potential while minimizing effect on normal cells.
Table 2.
Evidence for chronomodulated chemotherapy by cancer type
| Cancer Type | Trial Design (No. of Patients) | Intervention | Endpoint | Results | References |
|---|---|---|---|---|---|
| Breast | Randomized, multicenter (90) | Time of delivery of vinorelbine | Toxicity | Leukopenia significantly less if maximum delivery at 5:00 p.m. | 112 |
| Colorectal | Randomized, multicenter (92) | 5-FU + LV 4:00 a.m., oxaliplatin 4:00 p.m. vs. continuous infusion | Toxicity | Severe stomatitis decreased by fivefold with chronotherapy schedule (18 vs. 89%, P < 0.001) | 110 |
| Randomized, multicenter (186) | Severe stomatitis decreased by fivefold with chronotherapy schedule (14% vs 76%, P < 0.0001) | 111 | |||
| Randomized, multicenter (92) | Tumor response rate | Response rate significantly higher in chronotherapy schedule (53 vs. 32%, P < 0.05) | 110 | ||
| Randomized, multicenter (186) | Response rate higher in chronotherapy schedule (51 vs. 29%, P = 0.003) | 111 | |||
| Phase III (564) | Survival | Risk of death in men decreased by 25% with chronotherapy (18 vs. 21 mo, P = 0.02) | 118 | ||
| Risk of death in women increased by 38% with chronotherapy (19 vs. 16 mo, P = 0.03) | |||||
| Endometrial | Phase II (33) | Doxorubicin 6:00 a.m., cisplatin 6:00 p.m. | Toxicity | Well tolerated with 60% response rate | 117 |
| Phase III (342) | Efficacy | No difference in response rate (46% in standard group vs. 49% in chronotherapy group, P = 0.26) | 113 | ||
| Decreased leukopenia (75% in standard group, 64% in chronotherapy group; P < 0.05), and granulocytopenia (81% in standard group, 74% in chronotherapy group; P < 0.05) | |||||
| Lymphoblastic leukemia | Retrospective cohort (118) | 6-MP and MTX before 10:00 a.m. vs. after 5:00 p.m. | Disease-free survival | Increased long-term survival for evening group; risk of relapse 2.6 times greater for morning group | 114 |
| Pancreas | Phase I (16) | 5-FU 4:00 a.m. vs. continuous infusion | Toxicity | Acceptable toxicity at 4:00 a.m. | 115 |
| Rectal | Single center, nonrandomized (28) | 5-FU administered at 9:00 p.m. × 5 d, with radiation, followed by surgery | Toxicity, efficacy | High response rate with minimal toxicity; 7% with grade III toxicities, 52.6% with significant downstaging | 116 |
Definition of abbreviations: 5-FU = fluorouracil; 6-MP = 6-mercaptopurine; LV = leucovorin; MTX = methotrexate.
As more is learned about chronotherapy for oncologic patients, personalized chronotherapy may become a central goal in the field of oncology. After decades of chronotherapy research, technologic advances now allow chronomodulated drug delivery through programmable pumps and oral multiunit preparations (119, 120).
Conclusions
Remarkable progress has been made in the understanding of circadian rhythms in common but debilitating medical conditions, including sepsis, obstructive lung disease, cancer, and OSA (Table 3). Circadian rhythms affect these conditions, misalignment of circadian rhythms results in adverse consequences, and direction of therapy toward entrainment of healthy circadian rhythms results in increased positive outcomes. Specifically, chronotherapy is a promising approach.
Table 3.
Summary
| Many physiology and pathophysiology of disease processes exhibit circadian patterns, involving behavioral or functional variations that cycle every 24 h. |
| Immune responses due to sepsis vary at different times of the day. Circadian disruption, demonstrated by genetics models and sleep deprivation in humans, affects the severity of immune response. |
| Airflow obstruction tends to worsen in the early morning, corresponding to circadian changes of pulmonary function and abundance of immune cells in the airway. |
| Upper airway collapsibility and apnea length follow an endogenous circadian pattern in obstructive sleep apnea and are worse in early morning. |
| Many properties of tumorigenesis, such as cell proliferation and DNA repair, are regulated in a circadian fashion. Chronic circadian disruption in shift workers increases cancer risk. |
| Chronotherapy, the practice of giving treatment at specific times, has been shown to be beneficial, with reduced toxicity in treatment of asthma and chemotherapy for various malignancies. |
Future Research
Circadian rhythm disruption is common, and the health consequences due to circadian disruption cannot be ignored. The mechanisms of circadian disruption leading to health consequences in humans are not fully understood, and there is a paucity of therapeutic options for correcting misalignment or deentrainment of patients’ circadian rhythms. Future translational and clinical research in circadian rhythm disorders on modalities to restore or prevent circadian disruption is indispensable to optimizing and personalizing medical treatments.
Footnotes
Supported in part by National Heart, Lung, and Blood Institute of the National Institutes of Health grants R01HL085188 (A.M.), K24HL132105 (A.M.), and K23HL110216 (M.A.G.) and Southern California Institute for Research and Education (C.S.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kramer A, Merrow M, editors. Berlin; Springer-Verlag: 2013. Circadian clocks. [Google Scholar]
- 2.Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schibler U. The daily timing of gene expression and physiology in mammals. Dialogues Clin Neurosci. 2007;9:257–272. doi: 10.31887/DCNS.2007.9.3/uschibler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 6.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 8.Argentino C, Toni D, Rasura M, Violi F, Sacchetti ML, Allegretta A, Balsano F, Fieschi C. Circadian variation in the frequency of ischemic stroke. Stroke. 1990;21:387–389. doi: 10.1161/01.str.21.3.387. [DOI] [PubMed] [Google Scholar]
- 9.Lampert R, Rosenfeld L, Batsford W, Lee F, McPherson C. Circadian variation of sustained ventricular tachycardia in patients with coronary artery disease and implantable cardioverter-defibrillators. Circulation. 1994;90:241–247. doi: 10.1161/01.cir.90.1.241. [DOI] [PubMed] [Google Scholar]
- 10.Litinski M, Scheer FA, Shea SA. Influence of the circadian system on disease severity. Sleep Med Clin. 2009;4:143–163. doi: 10.1016/j.jsmc.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudreau P, Dumont GA, Boivin DB. Circadian adaptation to night shift work influences sleep, performance, mood and the autonomic modulation of the heart. Plos One. 2013;8:e70813. doi: 10.1371/journal.pone.0070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vetter C, Juda M, Roenneberg T. The influence of internal time, time awake, and sleep duration on cognitive performance in shiftworkers. Chronobiol Int. 2012;29:1127–1138. doi: 10.3109/07420528.2012.707999. [DOI] [PubMed] [Google Scholar]
- 13.Walia HK, Hayes AL, Przepyszny KA, Karumanchi P, Patel SR. Clinical presentation of shift workers to a sleep clinic. Sleep Breath. 2012;16:543–547. doi: 10.1007/s11325-011-0540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Liu Y, Huang X, Rong Y, He M, Wang Y, Yuan J, Wu T, Chen W. The effects of shift work on sleeping quality, hypertension and diabetes in retired workers. Plos One. 2013;8:e71107. doi: 10.1371/journal.pone.0071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archer SN, Oster H. How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res. 2015;24:476–493. doi: 10.1111/jsr.12307. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 18.Hennig J, Kieferdorf P, Moritz C, Huwe S, Netter P. Changes in cortisol secretion during shiftwork: implications for tolerance to shiftwork? Ergonomics. 1998;41:610–621. doi: 10.1080/001401398186784. [DOI] [PubMed] [Google Scholar]
- 19.Grundy A, Richardson H, Burstyn I, Lohrisch C, SenGupta SK, Lai AS, Lee D, Spinelli JJ, Aronson KJ. Increased risk of breast cancer associated with long-term shift work in Canada. Occup Environ Med. 2013;70:831–838. doi: 10.1136/oemed-2013-101482. [DOI] [PubMed] [Google Scholar]
- 20.Lin X, Chen W, Wei F, Ying M, Wei W, Xie X. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16:1381–1387. doi: 10.1016/j.sleep.2015.02.543. [DOI] [PubMed] [Google Scholar]
- 21.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009;35:781–795. doi: 10.1007/s00134-009-1397-4. [DOI] [PubMed] [Google Scholar]
- 23.Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subjects exposed to ICU noise. Sleep Med. 2005;6:423–428. doi: 10.1016/j.sleep.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–4145. doi: 10.1182/blood-2003-03-0779. [DOI] [PubMed] [Google Scholar]
- 25.Obermann HL, Bauer S. Toll-like receptor 9, what o’clock is it? Immunity. 2012;36:159–161. doi: 10.1016/j.immuni.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 31.Halberg F, Johnson EA, Brown BW, Bittner JJ. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc Soc Exp Biol Med. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- 32.Kleber A, Altmeyer S, Wolf B, Wolf A, Volk T, Fink T, Kubulus D. Impact of melatonin receptor deletion on intracellular signaling in spleen cells of mice after polymicrobial sepsis. Inflamm Res. 2014;63:1023–1033. doi: 10.1007/s00011-014-0779-4. [DOI] [PubMed] [Google Scholar]
- 33.Fink T, Glas M, Wolf A, Kleber A, Reus E, Wolff M, Kiefer D, Wolf B, Rensing H, Volk T, et al. Melatonin receptors mediate improvements of survival in a model of polymicrobial sepsis. Crit Care Med. 2014;42:e22–e31. doi: 10.1097/CCM.0b013e3182a63e2b. [DOI] [PubMed] [Google Scholar]
- 34.Haimovich B, Calvano J, Haimovich AD, Calvano SE, Coyle SM, Lowry SF. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med. 2010;38:751–758. doi: 10.1097/CCM.0b013e3181cd131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, Ferti L, Siostrzonek P. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30:536–540. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Mullington J, Korth C, Hermann DM, Orth A, Galanos C, Holsboer F, Pollmächer T. Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- 37.Haspel JA, Chettimada S, Shaik RS, Chu J-H, Raby BA, Cernadas M, Carey V, Process V, Hunninghake GM, Ifedigbo E, et al. Circadian rhythm reprogramming during lung inflammation. Nat Commun. 2014;5:4753. doi: 10.1038/ncomms5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock. 2008;29:127–132. doi: 10.1097/shk.0b013e318142c5a2. [DOI] [PubMed] [Google Scholar]
- 39.Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int. 2009;26:1430–1442. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–E261. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 41.Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 43.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831–835. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 44.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 45.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. doi: 10.1186/cc6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gitto E, Karbownik M, Reiter RJ, Tan DX, Cuzzocrea S, Chiurazzi P, Cordaro S, Corona G, Trimarchi G, Barberi I. Effects of melatonin treatment in septic newborns. Pediatr Res. 2001;50:756–760. doi: 10.1203/00006450-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Gitto E, Reiter RJ, Cordaro SP, La Rosa M, Chiurazzi P, Trimarchi G, Gitto P, Calabrò MP, Barberi I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: beneficial effects of melatonin. Am J Perinatol. 2004;21:209–216. doi: 10.1055/s-2004-828610. [DOI] [PubMed] [Google Scholar]
- 48.Seabra ML, Bignotto M, Pinto LR, Jr, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res. 2000;29:193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 49.Galley HF, Lowes DA, Allen L, Cameron G, Aucott LS, Webster NR. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res. 2014;56:427–438. doi: 10.1111/jpi.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wunsch H, Gershengorn H, Mayer SA, Claassen J. The effect of window rooms on critically ill patients with subarachnoid hemorrhage admitted to intensive care. Crit Care. 2011;15:R81. doi: 10.1186/cc10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verceles AC, Silhan L, Terrin M, Netzer G, Shanholtz C, Scharf SM. Circadian rhythm disruption in severe sepsis: the effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med. 2012;38:804–810. doi: 10.1007/s00134-012-2494-3. [DOI] [PubMed] [Google Scholar]
- 52.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, Johnston SH, Allen R, Kelly KA, Souetre E, Schultz PM, Starz KE. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13:354–361. [PubMed] [Google Scholar]
- 53.Cole RJ, Smith JS, Alcalá YC, Elliott JA, Kripke DF. Bright-light mask treatment of delayed sleep phase syndrome. J Biol Rhythms. 2002;17:89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- 54.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 55.Sarton G, Drabkin IE, Drabkin IE. Caelius Aurelianus: on acute diseases and on chronic diseases. Isis. 1951;42:148–150. [Google Scholar]
- 56.Turner-Warwick M. Epidemiology of nocturnal asthma. Am J Med. 1988;85:6–8. doi: 10.1016/0002-9343(88)90231-8. [DOI] [PubMed] [Google Scholar]
- 57.Cochrane GM, Clark JH. A survey of asthma mortality in patients between ages 35 and 64 in the Greater London hospitals in 1971. Thorax. 1975;30:300–305. doi: 10.1136/thx.30.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hetzel MR, Clark TJ, Branthwaite MA. Asthma: analysis of sudden deaths and ventilatory arrests in hospital. BMJ. 1977;1:808–811. doi: 10.1136/bmj.1.6064.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hetzel MR, Clark TJ. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax. 1980;35:732–738. doi: 10.1136/thx.35.10.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonnet R, Jörres R, Heitmann U, Magnussen H. Circadian rhythm in airway responsiveness and airway tone in patients with mild asthma. J Appl Physiol (1985) 1991;71:1598–1605. doi: 10.1152/jappl.1991.71.4.1598. [DOI] [PubMed] [Google Scholar]
- 61.Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996;154:1505–1510. doi: 10.1164/ajrccm.154.5.8912772. [DOI] [PubMed] [Google Scholar]
- 62.Kelly EA, Houtman JJ, Jarjour NN. Inflammatory changes associated with circadian variation in pulmonary function in subjects with mild asthma. Clin Exp Allergy. 2004;34:227–233. doi: 10.1111/j.1365-2222.2004.01866.x. [DOI] [PubMed] [Google Scholar]
- 63.Kraft M, Martin RJ, Wilson S, Djukanovic R, Holgate ST. Lymphocyte and eosinophil influx into alveolar tissue in nocturnal asthma. Am J Respir Crit Care Med. 1999;159:228–234. doi: 10.1164/ajrccm.159.1.9804033. [DOI] [PubMed] [Google Scholar]
- 64.Spengler CM, Shea SA. Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med. 2000;162:1038–1046. doi: 10.1164/ajrccm.162.3.9911107. [DOI] [PubMed] [Google Scholar]
- 65.Soutar CA, Carruthers M, Pickering CA. Nocturnal asthma and urinary adrenaline and noradrenaline excretion. Thorax. 1977;32:677–683. doi: 10.1136/thx.32.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soutar CA, Costello J, Ijaduola O, Turner-Warwick M. Nocturnal and morning asthma: relationship to plasma corticosteroids and response to cortisol infusion. Thorax. 1975;30:436–440. doi: 10.1136/thx.30.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kraft M, Hamid Q, Chrousos GP, Martin RJ, Leung DY. Decreased steroid responsiveness at night in nocturnal asthma: is the macrophage responsible? Am J Respir Crit Care Med. 2001;163:1219–1225. doi: 10.1164/ajrccm.163.5.2002058. [DOI] [PubMed] [Google Scholar]
- 68.Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, White DP. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol (1985) 1990;68:2034–2041. doi: 10.1152/jappl.1990.68.5.2034. [DOI] [PubMed] [Google Scholar]
- 69.Mohiuddin AA, Martin RJ. Circadian basis of the late asthmatic response. Am Rev Respir Dis. 1990;142:1153–1157. doi: 10.1164/ajrccm/142.5.1153. [DOI] [PubMed] [Google Scholar]
- 70.Yigla M, Tov N, Solomonov A, Rubin AHE, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma. 2003;40:865–871. doi: 10.1081/jas-120023577. [DOI] [PubMed] [Google Scholar]
- 71.Beam WR, Weiner DE, Martin RJ. Timing of prednisone and alterations of airways inflammation in nocturnal asthma. Am Rev Respir Dis. 1992;146:1524–1530. doi: 10.1164/ajrccm/146.6.1524. [DOI] [PubMed] [Google Scholar]
- 72.Reinberg A, Halberg F, Falliers CJ. Circadian timing of methylprednisolone effects in asthmatic boys. Chronobiologia. 1974;1:333–347. [PubMed] [Google Scholar]
- 73.Pincus DJ, Szefler SJ, Ackerson LM, Martin RJ. Chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol. 1995;95:1172–1178. doi: 10.1016/s0091-6749(95)70073-0. [DOI] [PubMed] [Google Scholar]
- 74.Pincus DJ, Humeston TR, Martin RJ. Further studies on the chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol. 1997;100:771–774. doi: 10.1016/s0091-6749(97)70272-0. [DOI] [PubMed] [Google Scholar]
- 75.Gagnon M, Côte J, Milot J, Turcotte H, Boulet LP. Comparative safety and efficacy of single or twice daily administration of inhaled beclomethasone in moderate asthma. Chest. 1994;105:1732–1737. doi: 10.1378/chest.105.6.1732. [DOI] [PubMed] [Google Scholar]
- 76.Noonan M, Karpel JP, Bensch GW, Ramsdell JW, Webb DR, Nolop KB, Lutsky BN. Comparison of once-daily to twice-daily treatment with mometasone furoate dry powder inhaler. Ann Allergy Asthma Immunol. 2001;86:36–43. doi: 10.1016/S1081-1206(10)62353-8. [DOI] [PubMed] [Google Scholar]
- 77.D’Urzo A, Karpel JP, Busse WW, Boulet L-P, Monahan ME, Lutsky B, Staudinger H. Efficacy and safety of mometasone furoate administered once-daily in the evening in patients with persistent asthma dependent on inhaled corticosteroids. Curr Med Res Opin. 2005;21:1281–1289. doi: 10.1185/030079905X56402. [DOI] [PubMed] [Google Scholar]
- 78.Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy. 1994;49:827–832. doi: 10.1111/j.1398-9995.1994.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 79.Noonan MJ, Chervinsky P, Brandon M, Zhang J, Kundu S, McBurney J, Reiss TF Montelukast Asthma Study Group. Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Eur Respir J. 1998;11:1232–1239. doi: 10.1183/09031936.98.11061232. [DOI] [PubMed] [Google Scholar]
- 80.D’Alonzo GE, Smolensky MH, Feldman S, Gianotti LA, Emerson MB, Staudinger H, Steinijans VW. Twenty-four hour lung function in adult patients with asthma: chronoptimized theophylline therapy once-daily dosing in the evening versus conventional twice-daily dosing. Am Rev Respir Dis. 1990;142:84–90. doi: 10.1164/ajrccm/142.1.84. [DOI] [PubMed] [Google Scholar]
- 81.Calverley PM, Lee A, Towse L, van Noord J, Witek TJ, Kelsen S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax. 2003;58:855–860. doi: 10.1136/thorax.58.10.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsai CL, Brenner BE, Camargo CA., Jr Circadian-rhythm differences among emergency department patients with chronic obstructive pulmonary disease exacerbation. Chronobiol Int. 2007;24:699–713. doi: 10.1080/07420520701535753. [DOI] [PubMed] [Google Scholar]
- 83.Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014;28:176–194. doi: 10.1096/fj.13-232629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao H, Sundar IK, Huang Y, Gerloff J, Sellix MT, Sime PJ, Rahman I. Disruption of Sirtuin 1-mediated control of circadian molecular clock and inflammation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2015;53:782–792. doi: 10.1165/rcmb.2014-0474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, Depner CM, Elmquist J, Franken P, Grandner MA, et al. Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. Sleep. 2015;38:1849–1860. doi: 10.5665/sleep.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Chami M, Shaheen D, Ivers B, Syed Z, Badr MS, Lin HS, Mateika JH. Time of day affects the frequency and duration of breathing events and the critical closing pressure during NREM sleep in participants with sleep apnea. J Appl Physiol (1985) 2015;119:617–626. doi: 10.1152/japplphysiol.00346.2015. [DOI] [PubMed] [Google Scholar]
- 87.Butler MP, Smales C, Wu H, Hussain MV, Mohamed YA, Morimoto M, Shea SA. The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep. 2015;38:1793–1801. doi: 10.5665/sleep.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 89.Yoshida D, Aoki N, Tanaka M, Aoyama S, Shibata S. The circadian clock controls fluctuations of colonic cell proliferation during the light/dark cycle via feeding behavior in mice. Chronobiol Int. 2015;32:1145–1155. doi: 10.3109/07420528.2015.1065415. [DOI] [PubMed] [Google Scholar]
- 90.Idda ML, Kage E, Lopez-Olmeda JF, Mracek P, Foulkes NS, Vallone D. Circadian timing of injury-induced cell proliferation in zebrafish. Plos One. 2012;7:e34203. doi: 10.1371/journal.pone.0034203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790–18795. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 93.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 94.Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, Cam E, Millar SE, Smyth P, Ihler A, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci USA. 2012;109:11758–11763. doi: 10.1073/pnas.1209592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klevecz RR, Shymko RM, Blumenfeld D, Braly PS. Circadian gating of S phase in human ovarian cancer. Cancer Res. 1987;47:6267–6271. [PubMed] [Google Scholar]
- 96.Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. 2012;18:1249–1260. doi: 10.2119/molmed.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. Plos One. 2010;5:e10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 99.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gu X, Xing L, Shi G, Liu Z, Wang X, Qu Z, Wu X, Dong Z, Gao X, Liu G, et al. The circadian mutation PER2(S662G) is linked to cell cycle progression and tumorigenesis. Cell Death Differ. 2012;19:397–405. doi: 10.1038/cdd.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Filipski E, King VM, Li X, Granda TG, Mormont MC, Liu X, Claustrat B, Hastings MH, Lévi F. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 102.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 103.Kwon P, Lundin J, Li W, Ray R, Littell C, Gao D, Thomas DB, Checkoway H. Night shift work and lung cancer risk among female textile workers in Shanghai, China. J Occup Environ Hyg. 2015;12:334–341. doi: 10.1080/15459624.2014.993472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17:108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 105.Benabu JC, Stoll F, Gonzalez M, Mathelin C. Night work, shift work: Breast cancer risk factor [in French] Gynecol Obstet Fertil. 2015;43:791–799. doi: 10.1016/j.gyobfe.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 106.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 107.Wise J. Danish night shift workers with breast cancer awarded compensation. BMJ. 2009;338:b1152. doi: 10.1136/bmj.b1152. [DOI] [PubMed] [Google Scholar]
- 108.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Painting, firefighting, and shiftwork. IARC Monogr Eval Carcinog Risks Hum 2010;98:9–764. [PMC free article] [PubMed]
- 109.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Adverse health effects of nighttime lighting: comments on American Medical Association policy statement. Am J Prev Med. 2013;45:343–346. doi: 10.1016/j.amepre.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 110.Lévi FA, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, Chollet P, Garufi C, Itzhaki M, Dogliotti L, et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst. 1994;86:1608–1617. doi: 10.1093/jnci/86.21.1608. [DOI] [PubMed] [Google Scholar]
- 111.Lévi F, Zidani R, Misset JL International Organization for Cancer Chronotherapy. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet. 1997;350:681–686. doi: 10.1016/s0140-6736(97)03358-8. [DOI] [PubMed] [Google Scholar]
- 112.Coudert B, Focan C, Genet D, Giacchetti S, Cvickovic F, Zambelli A, Fillet G, Chollet P, Amoroso D, Van Der Auwera J, et al. A randomized multicenter study of optimal circadian time of vinorelbine combined with chronomodulated 5-fluorouracil in pretreated metastatic breast cancer patients: EORTC trial 05971. Chronobiol Int. 2008;25:680–696. doi: 10.1080/07420520802384036. [DOI] [PubMed] [Google Scholar]
- 113.Gallion HH, Brunetto VL, Cibull M, Lentz SS, Reid G, Soper JT, Burger RA, Andersen W Gynecologic Oncology Group Study. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2003;21:3808–3813. doi: 10.1200/JCO.2003.10.083. [DOI] [PubMed] [Google Scholar]
- 114.Rivard GE, Infante-Rivard C, Dresse M-F, Leclerc J-M, Champagne J. Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival. Chronobiol Int. 1993;10:201–204. doi: 10.3109/07420529309073888. [DOI] [PubMed] [Google Scholar]
- 115.Bertheault-Cvitkovic F, Lévi F, Soussan S, Brienza S, Adam R, Itzhaki M, Misset JL, Bismuth H. Circadian rhythm-modulated chemotherapy with high-dose 5-fluorouracil: a pilot study in patients with pancreatic adenocarcinoma. Eur J Cancer. 1993;29A:1851–1854. doi: 10.1016/0959-8049(93)90536-o. [DOI] [PubMed] [Google Scholar]
- 116.Asao T, Sakurai H, Harashima K, Yamaguchi S, Tsutsumi S, Nonaka T, Shioya M, Nakano T, Kuwano H. The synchronization of chemotherapy to circadian rhythms and irradiation in pre-operative chemoradiation therapy with hyperthermia for local advanced rectal cancer. Int J Hyperthermia. 2006;22:399–406. doi: 10.1080/02656730600799873. [DOI] [PubMed] [Google Scholar]
- 117.Barrett RJ, Blessing JA, Homesley HD, Twiggs L, Webster KD. Circadian-timed combination doxorubicin-cisplatin chemotherapy for advanced endometrial carcinoma: a phase II study of the Gynecologic Oncology Group. Am J Clin Oncol. 1993;16:494–496. doi: 10.1097/00000421-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 118.Giacchetti S, Bjarnason G, Garufi C, Genet D, Iacobelli S, Tampellini M, Smaaland R, Focan C, Coudert B, Humblet Y, et al. European Organisation for Research and Treatment of Cancer Chronotherapy Group. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006;24:3562–3569. doi: 10.1200/JCO.2006.06.1440. [DOI] [PubMed] [Google Scholar]
- 119.Roy P, Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: current perspectives. J Control Release. 2009;134:74–80. doi: 10.1016/j.jconrel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 120.Alvarez-Lorenzo C, Concheiro A. Intelligent drug delivery systems: polymeric micelles and hydrogels. Mini Rev Med Chem. 2008;8:1065–1074. doi: 10.2174/138955708785909952. [DOI] [PubMed] [Google Scholar]