Abstract
The aims of this study are to compare plasma levels of IL17A, A/F, and F biomarkers in RA patients versus controls, and to determine responsiveness to methotrexate (MTX), anti-TNFs, and abatacept. We selected plasma samples from RA cohorts consisting of a cross-sectional RA cohort (N=78) not receiving DMARDs at the time of sampling, as well as from longitudinal drug start cohorts (N=71 patients) with pre/post samples including anti-TNF, abatacept, and MTX-treated patients. We assayed IL-17A, IL-17F, and IL17-A/F using a highly sensitive immunoassay system. Plasma levels of IL-17A, IL-17A/F, and IL-17F were all significantly increased in RA versus controls. The difference was largest in IL-17F, with median IL-17F levels in RA patients being approximately 18-fold higher than controls (81 pg/mL in RA vs. 4.4 pg/mL in controls, p<0.001). Among the forms of IL-17, only IL-17F was decreased after therapy in the MTX cohort (p=0.006), abatacept cohort (p<0.001), and anti-TNF cohorts (p=0.02), whereas IL-17A and IL-17A/F were not significantly decreased for any of the three drug cohorts. Synovial fluid analysis demonstrated higher IL-17F levels in RA (p=0.016) than healthy controls. These results suggest a specific role for IL-17F in human RA pathogenesis and as a therapeutic target.
Keywords: interleukin-17, rheumatoid arthritis, biologics, biological markers
INTRODUCTION
Th17 cells and their cytokine products—the interleukin-17 (IL-17) family—have been recognized for involvement in the pathogenesis of autoimmune diseases, particularly rheumatoid arthritis (RA). Across preclinical studies, IL-17 has been specifically shown to induce inflammatory cytokine production (such as IL-1, TNF, and IL-6), and promote bone erosion and cartilage destruction by release of MMPs and RANKL [1].
IL-17 is comprised of a family of six distinct but homologous units (A-F). The two subunits are best characterized form homodimers IL-17AA (IL-17A) and IL-17FF (IL-17F), which share 50 % homology, and have many similar biologic properties [2]. A heterodimeric form of IL-17, IL-17 A/F, also exists. Traditionally, IL-17A has been considered a more potent inducer of inflammation, followed by IL-17A/F heterodimer, followed by IL-17F [3]. However, important pro-inflammatory roles for IL-17F have been recognized, particularly in the presence of TNF-a, both of which are known to be synergistic [4, 5]. The relevance of the IL-17A/F heterodimer in human disease is yet unknown.
Serum IL-17 levels measured by ELISA have been found to be both elevated in RA and correlate with disease activity [6], and also decrease in response to anti-TNF therapy [7]; however, conflicting reports exist in the literature [8, 9]. This may be in part due to the low naturally occurring abundance of IL-17 in serum and plasma. Of note, the prior studies have been limited to the assessment of IL-17A alone, not IL-17F. In this study, we sought to assess the combined biology of plasma IL-17A, A/F, and F concentrations with a highly sensitive immunoassay system across cohorts of RA patients. The RA cohorts included a cross-sectional cohort, as well as three drug cohorts with longitudinal (pre/posttreatment) assessments including methotrexate (MTX), anti-TNF biologics, and abatacept (CTLA-4Ig, a CD28/B7 co-stimulation modulator). In addition, IL17A, A/F, and F assays were performed on independent samples of synovial fluid specimens from patients with RA, OA, and healthy controls.
METHODS
We assayed plasma concentrations of the novel biomarkers IL-17A, IL-17F, IL17-A/F, as well as standard inflammatory cytokines (IL-1B, TNF-a, IL-6R alpha, IL-6, and IL-1RA) metallomatrix proteinase−2 (MMP-2) and hsC-reactive protein (hsCRP) using the highly sensitive Erenna® Immunoassay System (Singulex, Inc., Alameda, CA), which is based upon single molecule counting technology and has been described in detail previously [10]. The latter markers (hsCRP, IL-1B, IL6, and sTNFRII) were selected to reflect cohort and assay validity.
Briefly, the assay involved two steps: first, the immunoassay component involved the capture of the specific biomarker onto paramagnetic microparticles coated with a capture antibody and subsequent detection with an Alexafluor labeled secondary antibody. Second, the single molecule counting component involved the counting of fluor labeled detection antibodies in a 100-μm capillary flow system after the immune complex on the paramagnetic microparticles was disrupted. IL-17A and IL-17F assays used antibodies specific to the biomarker, while the IL-17A/F assay used an IL-17A capture and an IL-17F detection antibody. This highly sensitive technology has been previously used in numerous different biomarkers [11–13].
The analytic characteristics (limit of detection pg/mL, lower limit of quantification pg/mL, average low range CV, and average biomarker concentration in plasma obtained from healthy volunteers) for the IL-17 assays were determined to be IL-17A/F, 0.06, 0.3, 5 %, 0.61 pg/mL; IL-17A, 0.004, 0.05, 6 %, 0.35 pg/mL; and IL-17A/F, 0.4, 1.6, 7 %, 21.3 pg/mL. Specificities for the IL-17 biomarker assays were demonstrated by testing samples spiked with IL-17A, F, and A/F at concentrations 100-fold greater than the assay limit of quantification (see Supplementary Table S1).
Positive immunoassay interference from rheumatoid factor (RF) is a potential concern when testing plasma samples from rheumatoid arthritis patients. The assays were controlled for nonspecific reactivity to rheumatoid factor, heterophile antibodies, and human anti-mouse antibodies by inclusion of appropriate blockers (goat and mouse IgG as well as MAK-33; Roche) in the assay buffers. Of note, MAK-33 was specifically designed to block RF interferences.
During development, the assays were tested for RF interference using a panel of plasma from 16 RA patients with RF values ranging from 43 to 100. The idea being that if a specific sample caused assay interference, it would be noted across all assays. Such finding was not observed. In addition, the resulting biomarker values were correlated with RF values. If there was assay interference due to RF, one might expect biomarker results to correlate with the RF values. Out of all the assays, IL-6 was the only assay that provided a statistically significant Spearman correlation (r=0.52, p=0.38) with RF. To specifically test for potential RF interference on the IL-17F assay, 10 RF positive (values 70–270) plasma from non-RA patients and 25 plasma from healthy volunteers were tested in the assay. No differences were noted between the two groups. The RF positive plasma was also spiked with recombinant IL-17F to a final concentration of 50 pg/mL and then diluted serially (1:2) dilutions. When these preparations were tested for IL-17F, spike recovery of 100 % (range 92–110 %) and dilutional linearity of 99 % (range 92–108 %) were found. Taken together, these findings demonstrate that RF does not cause either positive or negative assay interference.
Plasma samples from previously recruited RA cohorts (N=148) as well as controls from a knee osteoarthritis (OA) cohort (N=128) were selected for analysis. The RA cohorts consisted of a cross-sectional RA cohort of 77 patients who were DMARD-naïve or not currently treated with DMARDs, as well as longitudinal drug start cohorts (N=71 patients) with pre/post samples including 33 anti-TNF starts, 27 abatacept starts, and 11 MTX starts. Clinical assessments across all cohorts at all study time points included tender and swollen 28-joint counts, patient global disease activity assessment (0–100), and ESR to enable calculation of the DAS28-ESR. Informed consent was obtained for all patients with approval from the New York University institutional review board.
Median biomarker values between RA versus OA cohorts were compared using the Wilcoxon rank sum test, and adjusted comparisons for age and gender were made using multivariate linear models. Correlations of markers were assessed using Spearman rank correlations. Change in marker value with treatment was assessed by the ratio of the post-drug value to the pre-drug value. Marker and ratio values were log-transformed. RA treatment cohorts were categorized into good/moderate versus nonresponders based on established EULAR response criteria using the DAS28 measure [14]. Synovial fluid analysis for IL-17 forms was carried out in a separate cohort of DMARD-naïve RA patients, OA patients, and healthy individuals (n=5 for each group). Analyses were performed using S-Plus 7.0 and StatXact-8.
RESULTS
Plasma Biomarker Levels RA Versus Controls
Baseline demographics and characteristics of RA patients can be seen in Table 1. Expectedly, patients receiving anti-TNF and abatacept had longer disease duration than both DMARD-naïve patients and those patients initiating methotrexate (MTX) therapy.
Table 1.
Baseline Characteristics of Rheumatoid Arthritis Cohorts by Treatment Group
| Cross-sectional RA cohort | Methotrexate initiators | Anti-TNFa initiators | Abatacept initiators | |
|---|---|---|---|---|
| Number of subjects | 77 | 11 | 33 | 27 |
| Age, mean (SD) | 46.8 (14.2) | 45.3 (12.4) | 54.1 (11.5) | 49.5 (13.4) |
| Gender female % | 83.1 | 63.6 | 90.9 | 85.2 |
| Caucasian (%) | 70.1 | 63.6 | 81.8 | 77.8 |
| Disease duration (median years, IQR) | 7 (6) | 3 (3) | 11.5 (16.5) | 12 (18) |
| Baseline tender joint count (median, IQR) | 12 (12.2) | 11 (15) | 13 (15) | 11 (14) |
| Baseline swollen joint count (median, IQR) | 6 (7) | 5 (6) | 6 (9) | 7 (8.5) |
| Baseline DAS28 (mean, SD) | 5.7 (1.5) | 6.2 (1.3) | 5.7 (1.5) | 5.6 (1.3) |
| Achieved DAS28 (mean, SD) | N/A | 4.3 (1.3) | 4.9 (1.5) | 5.2 (1.2) |
| Background MTX % | N/A | N/A | 50 | 65.4 |
N/A not available
Note: anti-TNF therapies included adalimumab, etanercept and infliximab
Of controls, 63 % were female, 68 % Caucasian, with a mean age of 65.3 years (SD 9.8). As expected, hsCRP, IL-6, and IL-6 R alpha were all significantly higher in the cross-sectional RA cohort than in OA controls (p<0.001 for each comparison) (Table S2).
Plasma levels of IL-17A, IL-17A/F, and IL-17F were all significantly increased in RA versus controls. The difference was largest in IL-17F, with median (interquartile range) IL-17F levels in RA patients (n=77) being approximately 18-fold higher than controls (n=128) (81 pg/mL [31–245 pg/mL] in RA vs. 4.4 pg/mL[4.4–29 pg/mL] in controls), (p<0.001 in both univariate and multivariate comparisons adjusted for age and gender). Results for IL-17F in RA versus control patients are shown in Fig. 2. For IL17A and IL17A/F, the difference between RA and control cohorts was smaller in magnitude (0.30 pg/mL[0.22–0.50 pg/mL] vs. 0.20 pg/mL[0.13–0.33 pg/mL], p<0.001 and 2.6 pg/mL[1.9–4.0 pg/mL] vs. 2.1 pg/mL[1.6–2.9 pg/mL], p=0.002, respectively) than IL17F comparisons. Differences in IL17-A/F did not retain significance in adjusted models.
Fig. 2.
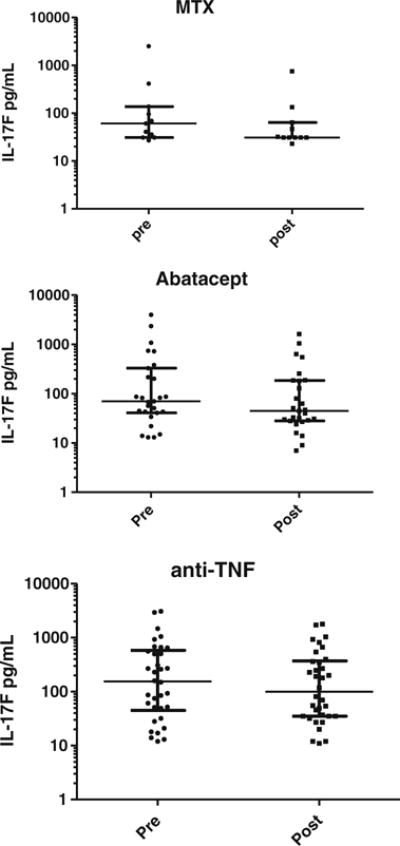
Effects on IL-17F levels for methotrexate, abatacept, and anti-TNF biologic drugs. MTX methotrexate data presented as median (longest horizontal bar) and interquartile range (smaller horizontal bars). Each dot is an individual patient.
A small but significant decrease in IL-17F with increasing age was seen in a multivariate model (p<0.001). No other significant relationship was detected between levels of IL-17A, A/F, or F with age, gender, or race/ethnicity. In the cross-sectional RA cohort, the correlation between DAS28-ESR and IL-17F (rho=0.32) was somewhat higher than DAS28-ESR and IL-17A (rho=0.11), although neither demonstrated strong correlations.
Plasma IL-17 Levels Pre- and Post-Therapy in RA
Inflammatory and cytokine/receptor markers measured both pre- and posttherapeutics across RA cohorts in shown in Table 2. Expectedly, inflammatory markers and pro-inflammatory cytokines/receptors such as hsCRP, IL-6, sTNF-RII, and IL-1b were variably decreased after MTX or biologic therapy. Among forms of IL-17, only IL-17F was consistently decreased after therapy across MTX (p=0.006), abatacept (p<0.001), and anti-TNF (p=0.02) treatment groups, whereas IL-17A and IL-17A/F were not significantly decreased. Change in IL-17F levels in individual patients is depicted in Figs. 1 and 2.
Table 2.
Median (IQR) Plasma Markers from RA Patients Pre- and Post-Drug Interventions
| Biomarker | Abatacept (n=27)
|
Anti-TNF (n=33)
|
Methotrexate (n=11)
|
|||
|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| IL-17 family | ||||||
| IL-17F (pg/mL) | 70 (42–273) | 45‡ (28–185) | 159 (51–560) | 118* (35–362) | 61 (32–117) | 23† (4–56) |
| IL-17A (pg/mL) | 0.54 (0.3–1.14) | 0.63 (0.29–1.14) | 0.92 (0.53–1.18) | 0.75 (0.45–1.09) | 0.31 (0.22–0.49) | 0.28 (0.16–0.44) |
| IL-17 A/F (pg/mL) | 1.2 (0.6–2) | 1.1 (0.8–2.5) | 1.3 (0.7–2.4) | 1.1 (0.6–2.4) | 2.4 (2.3–6.4) | 2.2 (2–4.6) |
| Other biomarkers | ||||||
| CRP (ng/mL) | 5786 (1228–14783) | 3528 (1188–8258) | 8381 (2407–26275) | 3173† (1803–9535) | 8493 (6128–17974) | 5590 (1744–8824) |
| IL-1 beta (pg/mL) | 0.5 (0.37–1.01) | 0.61 (0.32–1.55) | 0.56 (0.34–1.25) | 0.52 (0.32–1.27) | 0.32 (0.19–0.62) | 0.25* (0.08–0.38) |
| IL-6 (pg/mL) | 4.9 (1.5–12) | 4.4 (1.8–7.4) | 4.8 (2.5–15.5) | 3.8* (2–7) | 9.9 (6.7–12.9) | 3.8† (1.7–9) |
| sTNF RII (pg/mL) | 5.3 (4.3–9.1) | 4.7† (4–7) | 6.5 (5.3–9.4) | 10* (6.1–80.6) | 4.5 (3.1–4.7) | 3.6 (3.5–4.5) |
The log ratio of patients’ post- to pretreatment marker was different from 0 (the expected value if there were no effect) at the following significance levels:
0.01≤p<0.05;
0.001≤p<0.01;
p<0.001
Fig. 1.
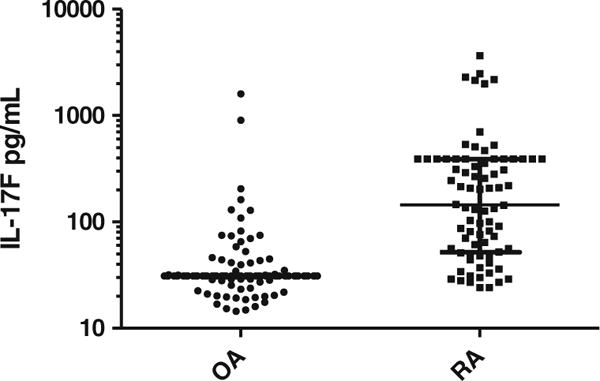
Plasma IL-17F concentrations in RA patients versus OA patients. Above depicts plasma IL-17F concentrations in OA patients (controls) and RA patients. Data presented as median (longest horizontal bar) and interquartile range (smaller horizontal bars) each dot is an individual patient. Of note, the interquartile range is quite small for OA patients. RA rheumatoid arthritis, OA osteoarthritis.
Synovial Fluid (SF) IL-17 Family Levels
As shown in Table 3, SF from both RA and OA patients had significantly elevated IL-17F levels compared to age-matched nonarthritic healthy individuals (p=0.016 and 0.048, respectively). No significant difference was seen in SF IL-17F levels between RA and OA patients. In contrast, SF IL-17A levels were not found to be different among any of the three groups.
Table 3.
IL-17 Median and IQR (pg/mL) in Synovial Fluid from RA and OA Patients and Healthy Controls
| Biomarker (units pg/mL) | RA (N=5) | OA (N=5) | Healthy controls (N=5) |
|---|---|---|---|
| IL-17A | 0.07 (0.07, 3.1) | 0.045 (0.043, 0.07) | 0.07 (0.07, 0.07) |
| IL-17F | 130* (120, 2000) | 95* (13, 450) | 1.6 (1.6, 2) |
| IL-17AF | 13 (8.8, 41) | 6 (1.5, 8.8) | 0.77 (0.77, 0.84) |
IL-17F was significantly higher in RA compared to healthy controls (p=0.016) and in OA compared to healthy controls (p=0.048). No other differences between groups were significant
DISCUSSION
T cells have long been suspected as playing a key role in RA pathogenesis, a disease characterized by synovial inflammation, pannus formation, cartilage destruction, and bone erosion. Th-17 cells have been added to the T cell repertoire, and along with their cytokine product—IL-17—have been found to promote RA across a series of experimental models [3]. Therapeutics targeting IL-17 across multiple autoimmune diseases including RA are under evaluation in randomized controlled trials [15].
As IL-17 cytokines are typically present in low concentrations in human plasma/sera, we used a highly sensitive and previously validated immunoassay system based upon novel single molecule counting for accurate quantification. As baseline concentrations for each of the IL-17 cytokine types in the RA cohorts were on average over 50-fold greater than assay sensitivity, we could optimally detect differences between cohorts and the effect of therapies, although caution is needed in result interpretation as the power of the study was limited particularly when different treatment groups were compared.
Subjects with knee OA were selected as controls for the study. As expected, the OA control cohort was older than RA patients. However, models adjusted for age and gender showed that the difference between RA patients and OA controls—particularly for IL-17F—still retained strongly significant differences. We thus believe that our results reflect a true difference relating to the RA disease process rather than confounding factors.
With respect to synovial fluid analyses, it is of interest that both RA and OA synovial fluid IL-17F levels were higher than healthy controls, suggesting that intraarticular cytokine-driven inflammatory processes are active in both diseases. Within the joint, it is important to note that IL-17 production may act to promote cartilage damage, since it exerts catabolic effects, increasing both nitric oxide and prostaglandin production [16, 17].
In adjusted models, IL-17A and IL-17F, and not IL-17A/F, were significantly higher in DMARD-naive RA patients than controls. Interestingly, the magnitude difference was more profound for IL-17F. We found only a modest degree of correlation between DAS and IL-17F and virtually no correlation with IL-17A. Furthermore, when pre/posttreatment patients were divided into clinical responders versus nonresponders, IL-17F levels were decreased across both subgroups of responders with high significance (p<0.001).
In the pre/posttreatment cohorts, IL-17A levels were not significantly decreased with MTX, anti-TNFs, or abatacept. In contrast, levels of IL-17F were markedly decreased across the three RA treatment groups. Our study is the first to our knowledge to demonstrate these contrasting effects between IL-17A and IL-17F with respect to responsiveness to drug interventions.
Abatacept exhibited statistically similar reduction in IL-17F to TNF-inhibitors. The downstream effects of abatacept (CTLA4-Ig) blockade of CD28/B7 co-stimulation are quite complex, and its effect on the Th-17 cell subset in humans is yet unknown. Of note, cells other than Th-17T cells are known to produce IL-17 [18], and one group has reported mast cells as the prominent producer of IL-17 in RA synovial tissue [19]. Thus, downstream effects of abatacept not specifically involving Th17 cells must be considered.
Our study results add to the literature on the relative importance of IL-17F versus IL-17A in RA patients, and the results may be relevant to the role of these isoforms in other autoimmune and chronic inflammatory conditions. Whereas IL-17A and IL-17F do share 50 % homology with similar biologic properties, IL-17A has been considered the more potent inducer of inflammation, when compared to IL-17F [2] [3]. Nevertheless, an important pro-inflammatory roles for IL-17F has been recognized, particularly in the presence of TNF-a, suggesting a synergistic role for IL-17F [4, 5]. This synergistic role for IL-17F in the presence of TNF-a may be relevant in other autoimmune and inflammatory diseases with upregulation of TNF-a and other pro-inflammatory cytokines.
CONCLUSION
Overall, the results of this study suggest that IL-17 isoforms, and in particular IL-17F levels, are higher in RA than controls. Synovial fluid IL-17F, but not IL-17A, is higher in RA patients than controls. IL-17F, but not IL17A or A/F, also decreases with multiple classes of DMARDs, including marked reductions in clinical responders. These results suggest a specific role for IL-17F in human RA pathogenesis and as a therapeutic target.
Supplementary Material
Acknowledgments
This work is supported in part by US National Institutes of Health grant R01-AR054817 (S.B.A.). Dr Greenberg received salary support from the NIH (K23AR054412).
Abbreviation
- hsCRP
High sensitivity C-reactive protein
- DMARD
Disease-modifying antirheumatic drug
- ELISA
Enzyme-linked immunosorbent assay
- ESR
Erythrocyte sedimentation rate
- IL-1 IL-1B
Interleukin-1
- IL-1B
Interleukin-1 beta
- IL-17
Interleukin-17
- IL-6
Interleukin-6
- IL-6R alpha
Interleukin 6 receptor alpha
- MMP
Matrix metalloproteinase
- MTX
Methotrexate
- OA
Osteoarthritis
- RA
Rheumatoid arthritis
- RANKL
Receptor activator of nuclear factor kappa-B ligand
- sTNFRII
Soluble tumor necrosis factor receptor 2
- TNF
Tumor necrosis factor
Footnotes
Contributions. MJ carried out data analysis, interpretation, and supervised manuscript drafting/revision.
MA supervised data acquisition, interpretation, and manuscript revision.
VF carried out data acquisition.
JT supervised data acquisition, interpretation, and manuscript drafting/revision.
ML carried out data analysis and manuscript drafting/revision.
QL carried out data analysis.
RR generated biomarker results and carried out data analysis.
SA supervised data analysis, interpretation, and manuscript revision.
JG supervised data acquisition, analysis, interpretation, and manuscript revision.
Conflict of Interest. JT, RR, and QL are employees of Singulex, the manufacturer of the assays used in the study. The other authors declare that they have no competing interests.
Electronic supplementary material The online version of this article (doi:10.1007/s10753-014-0020-1) contains supplementary material, which is available to authorized users.
References
- 1.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. The New England Journal of Medicine. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 2.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134:8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Current Rheumatology Reports. 2009;11:365–70. doi: 10.1007/s11926-009-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Annals of the Rheumatic Diseases. 2011;70:341–8. doi: 10.1136/ard.2010.132233. [DOI] [PubMed] [Google Scholar]
- 5.Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17 F-induced effects in human rheumatoid arthritis synoviocytes. Journal of Immunology. 2009;182:3112–20. doi: 10.4049/jimmunol.0801967. [DOI] [PubMed] [Google Scholar]
- 6.Metawi SA, Abbas D, Kamal MM, et al. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clinical Rheumatology. 2011;30:1201–7. doi: 10.1007/s10067-011-1737-y. [DOI] [PubMed] [Google Scholar]
- 7.Chen DY, Chen YM, Chen HH, et al. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-alpha therapy. Arthritis Research & Therapy. 2011;13:R126. doi: 10.1186/ar3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yue C, You X, Zhao L, et al. The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatology International. 2010;30:1553–7. doi: 10.1007/s00296-009-1179-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Li JM, Liu XG, et al. Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. Journal of Clinical Immunology. 2011;31:606–14. doi: 10.1007/s10875-011-9540-8. [DOI] [PubMed] [Google Scholar]
- 10.Todd J, Freese B, Lu A, et al. Ultrasensitive flow-based immunoassays using single-molecule counting. Clinical Chemistry. 2007;53:1990–5. doi: 10.1373/clinchem.2007.091181. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarawneh R, Lee JM, Ladenson JH, et al. CSF VILIP-1 predicts rates of cognitive decline in early Alzheimer disease. Neurology. 2012;78:709–19. doi: 10.1212/WNL.0b013e318248e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Ledger K, Agee SJ, Kasaian MT, et al. Analytical validation of a highly sensitive microparticle-based immunoassay for the quantitation of IL-13 in human serum using the Erenna immunoassay system. Journal of Immunological Methods. 2009;350:161–70. doi: 10.1016/j.jim.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 14.van Gestel AM, Prevoo ML, Hof MA van’t, et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis and Rheumatism. 1996;39:34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- 15.Buch MH, Emery P. New therapies in the management of rheumatoid arthritis. Current Opinion in Rheumatology. 2011;23:245–51. doi: 10.1097/BOR.0b013e3283454124. [DOI] [PubMed] [Google Scholar]
- 16.Attur MG, Patel RN, Abramson SB, et al. Interleukin-17 up-regulation of nitric oxide production in human osteoarthritis cartilage. Arthritis and Rheumatism. 1997;40:1050–3. doi: 10.1002/art.1780400609. [DOI] [PubMed] [Google Scholar]
- 17.LeGrand A, Fermor B, Fink C, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in ex-plants of human osteoarthritic knee menisci. Arthritis and Rheumatism. 2001;44:2078–83. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nature Reviews Immunology. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 19.Suurmond J, Dorjee AL, Boon MR, et al. Mast cells are the main interleukin 17-positive cells in anticitrullinated protein antibody-positive and -negative rheumatoid arthritis and osteoarthritis synovium. Arthritis Research & Therapy. 2011;13:R150. doi: 10.1186/ar3466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


