Abstract
This article reviews the existing fertility preservation options for females diagnosed with Turner syndrome and provides practical guidelines for the practitioner. Turner syndrome is the most common sex chromosome abnormality in females, occurring in approximately one in 2500 live births. Women with Turner syndrome are at extremely high risk for primary ovarian insufficiency (POI) and infertility. Although about 70–80% have no spontaneous pubertal development and 90% experience primary amenorrhea, the remainder may possess a small residual of ovarian follicles at birth or early childhood. The present challenge is to identify these women as early in life as is possible, so as to allow them to benefit from a variety of existing fertility preservation options. To maximize the benefits of fertility preservation, all women with Turner syndrome should be evaluated by an expert as soon as possible in childhood as the vast majority will have their ovarian reserve depleted before adulthood. Cryopreservation of mature oocytes and embryos is a proven fertility preservation approach, while cryopreservation of ovarian tissue is a promising technique with a growing number of live births, but remain investigational. Oocyte cryopreservation has been performed in children with Turner syndrome as young as 13 and ovarian tissue cryopreservation in prepubertal children affected. However, current efficacy of these approaches is unknown in this cohort.. For those who have already lost their ovarian reserve, oocyte or embryo donation and adoption are strategies that allow fulfillment of desire for parenting. For those with Turner syndrome related cardiac contraindications to pregnancy, utilization of gestational surrogacy allows the possibility of biological parenting by using their own oocytes. Alternatively, gestational surrogacy can serve to carry pregnancy resulting from the use of donor oocytes or embryos, if needed.
Keywords: Fertility preservation, Turner syndrome, Oocyte cryopreservation, Ovarian Tissue Cryopreservation, Embryo cryopreservation, Adoption
Introduction
Sixty-five years have passed since Ford1 reported the association of a 45, X cell line with the phenotypic findings that had been described 21 years earlier by Henry Turner2. Turner syndrome (TS) is one of the most common chromosomal disorders, occurring in approximately one in 2500 newborn female infants3. Under normal circumstances a female infant is born with approximately two million oocytes within her ovaries, yet this is not the case in females with TS, who are born with markedly fewer3. How do oocytes develop during embryonic life prior to birth, and how is this process altered in the absence of two intact X chromosomes? The progenitors of a female’s oocytes arise outside the embryonic tissue that will become the ovary, migrate along the dorsal mesentery from the region of the junction of the allantois and yolk sac, populate the indifferent gonad, and undergo rapid amplification4. Human primordial germ cells have been detected in the yolk sac wall from three to four weeks post conception, in the hind gut epithelium from week four and in the genital area from week five. Evidence suggests that chemotactic molecules may guide these primordial germ cells to the gonad and adhesion molecules present on their surface appear to play a role in their migration5. Newer estimates of the number of primordial follicles at birth have been provided by Mamsen et al.6, who studied 53 human ovaries obtained at the time of elective pregnancy termination. The number of germ cells increased from a mean of 7,200 at 7 weeks gestation to 4,933,000 at 21 weeks gestation, as determined by stereological techniques using an optical dissector. This quantity then falls to approximately 600,000 at birth and 400,000 at puberty7. The mechanism of this loss appears to be primarily by apoptosis8.
Embryos bearing a 45, X karyotype have a high incidence of in utero mortality and studies by Singh and Carr of 45, X fetuses revealed the presence of germ cells in the early genital ridges in normal numbers9. However, by mid-gestation, their numbers were diminished significantly. A high incidence of follicle apoptosis, as determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, has been reported, when compared with that of a normal karyotype. Modi et al.10 studied 16 normal fetal ovaries and 4 from 45, X fetuses at 15 – 20 weeks gestation. Semi-quantitative measurements revealed 3–7% TUNEL positive cells in normal ovaries versus 50–70% in 45, X ovaries. These results are consistent with accelerated germ cell apoptosis in individuals with a 45, X karyotype.
There is considerable variability between individuals regarding the size of this pool of primordial follicles. This may be due, in part, to variation between females during the process that leads to follicular atresia, but may also reflect the presence of sex chromosome aneuploidy at the level of the ovaries, that may not be apparent in a leukocyte karyotype. If the ovary is populated by two populations of oogonia, one containing two X chromosomes (46, XX) and one missing an X chromosome (45, X), i.e. a mosaic karyotype, the number of oocytes at birth will be substantially greater than seen in ovaries of a single line of 45, X. The pool of primordial follicles in 46, XX/45, X mosaics might then be large enough for a young woman to undergo menarche and normal pubertal development11. Invariably, however, these women will exhaust their supply of primordial follicles at a faster rate than age comparable women with normal 46, XX genotype, and are destined to undergo premature ovarian insufficiency (POI)3.
Integrity of the long arm of the X chromosome is required for maintaining fertility. Quilter et al.12 analyzed the X chromosome by array comparative genomic hybridization (aCGH), documenting copy number variants in 15 of 42 women with POI. The majority was found in Xq. Mercer et al.13 studied 20 women missing a part of the long arm of the X chromosome (Xq) using aCGH. Clinical features of TS were only seen in a minority of women in their study, and they commonly presented with abnormalities of menstruation and fertility. The larger terminal deletions were associated with a higher incidence of POI occurring at a younger age. Recently, Hook and Warburton14 reanalyzed the data on the frequency of karyotypes at various stages of life associated with TS, finding evidence of a high incidence of cryptic mosaicism in 45, X individuals with TS. Both the method of ascertainment of the cases, as well as the varying sensitivity of the markers used and number of cells analyzed were important determinants in detecting cryptic sex chromosome mosaicism. The classic TS then appears to be one end of a spectrum of ovarian dysfunctions associated with deletions and duplications of regions of the X chromosome and occult mosaicism associated with loss of primordial germ cells leading to POI.
Although its physiologic role is not fully elucidated, anti-Mullerian hormone (AMH) is the best endocrine marker of the size of the population of the dynamic reserve pool of small antral follicles within the ovaries15. AMH is a member of the TGF-Beta family of growth factors and is secreted by granulosa cells of primary and small antral follicles, but neither primordial nor larger growing follicles16. AMH concentrations in the small antral follicles of human ovaries have been found to be nearly three orders of magnitude higher than in the follicular fluid of preovulatory follicles17. In female mice, evidence has demonstrated that AMH functions as an inhibitor of primordial follicle recruitment18.
Visser et al.19 studied AMH levels in girls and adolescents with TS. They found detectable AMH levels in 21.9% of these girls, the results correlating with karyotypes. AMH was detected in 77% of girls with 45, X/46, XX karyotypes, but in only 10% of those with 45, X karyotypes. Hagen et al. performed longitudinal studies of serum AMH levels in 926 healthy females and 172 with TS, finding evidence that AMH is a promising marker of ovarian function in healthy girls and TS patients20. It is apparent that a subset of girls with TS possesses a small residual of ovarian follicles at birth or early childhood. These would be further lost in late childhood and early puberty, and the majority of whom will exhibit a mosaic karyotype. The present challenge is to identify these girls as early in life as is possible, so as to allow them to benefit from a variety of existing fertility preservation options.
Fertility Preservation Strategies
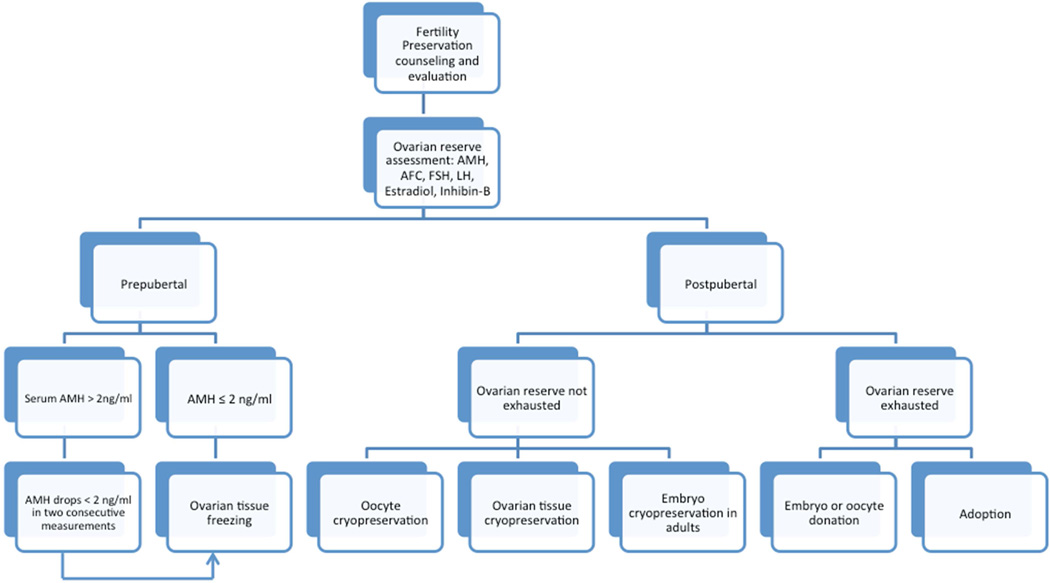
Spontaneous conceptions are rarely described in women with TS and infertility attributable to lacking or exhausted ovarian reserve is the norm21. The reproductive phenotype of TS is variable for a given genotype19,20. Ovarian dysgenesis is commonly encountered in the setting of full 45, X genotype and use of donated oocytes or embryos can allow a chance for a successful conception22. In contrast, varying degrees of ovarian function can be retained for varying time intervals in those with the 45, X/46, XX mosaic genotype, although POI is unfortunately inevitable. It is in this latter subgroup of young women with TS that salvage of existing viable oocytes through the application of assisted reproductive technologies is plausible. Below, we review the currently available and experimental techniques to preserve fertility in females diagnosed with TS (Figure 1).
Figure 1.
A proposed algorhythmic approach to decision making for fertility preservation in females diagnosed with Turner Syndrome. For prepubertal girls with sufficient ovarian reserve, expert experience dictates utility of serial serum AMH assessments to delay intervention to a postpubertal age so that oocyte cryopreservation can be considered. Serum AMH level of <2 ng/mL corresponds to levels in the lower quartile for girls aged 5–1345. In postpubertal girls, since the risk of follicle loss is extremely high and can proceed at a fast pace, we recommend fertility preservation regardless of the initial AMH.
Oocyte Cryopreservation
While ovarian primordial follicle reserve may be depleted well before puberty in nearly all TS girls with non-mosaic karyotype, in mosaic cases and depending on the degree of mosaicism, ovarian reserve may persist for a variable period post menarche23. However, because the reserve would be low and the depletion of this reserve would still occur at an accelerated rate, the majority may still not reach adulthood with sufficient ovarian reserve. It is therefore highly important to preserve oocytes as early as possible after menarche in these girls.
While ovarian stimulation followed by oocyte retrieval and cryopreservation have been reported in adults with TS24, the utility of this technology for the peri-pubescent population had not been studied until recently. Oktay et al have reported on successful oocyte cryopreservation in post-pubertal children with mosaic TS25,26. In these reports, three girls aged 13–15 with mosaic TS were identified to have decreased ovarian reserve by serum AMH hormone measurements and antral follicle counts on ultrasound examinations. Ovarian stimulation was achieved with the use of recombinant FSH and later during stimulation with luteinizing hormone supplementation in the form of human menopausal gonadotropins or recombinant LH, in an antagonist cycle. Because of the relative immaturity of the function of hypothalamus-pituitary-ovarian axis, LH levels may be oversuppressed after the initiation of GnRH antagonist use. For this reason Oktay & Bedoschi supplemented the stimulation with LH to achieve adequate steroidogenesis. Daily gonadotropin doses ranged from 225 to 300 IU. Ovarian response to stimulation was monitored using a combination of serial transabdominal ultrasound exams and serum estradiol measurements during ovarian stimulation. Oocyte maturation was triggered either with recombinant human chorionic gonadotropin, highly purified human chorionic gonadotropin or Leuprolide acetate 34 hours prior to the retrieval. Oocyte retrievals were performed transvaginally under general anesthesia. On average 7–19 oocytes were retrieved, of which 4–10 were mature and cryopreserved. In one case, two cycles of oocyte cryopreservation were performed in a 14 year old girl one year apart; notably, mature oocyte yield dropped from 8 to 4 in the second cycle, possibly due to the rapid decline in ovarian reserve26.
These young girls, as well as their parents, were closely evaluated both for physical and psychosocial suitability for the procedure prior to being accepted to the ovarian stimulation and oocyte freezing program. This included a physical exam, preoperatory evaluation by the anesthesiologist as well as counseling of parents and the child by the physician.
Others have also reported cryopreservation of in vitro matured oocytes; immature oocytes were obtained during an ovarian cryopreservation procedure27. As will be discussed later, this creates another opportunity for oocyte cryopreservation without ovarian stimulation, especially in those girls undergoing ovarian tissue harvesting for cryopreservation. However, the pregnancy potential of in vitro matured oocytes from harvested ovarian tissue has not been demonstrated in either women with or without TS, hence the potential benefit of this approach is unknown.
The first birth attributable to use of cryopreserved and thawed oocyte in humans was reported in 198628. Considered “experimental” for nearly three decades, refinements of the vitrification freezing protocols over the past decade have significantly advanced the field. Oocyte cryopreservation is now considered an established procedure in adults and is increasingly being offered to and utilized by reproductive age women prior to initiation of gonadotoxic therapy for the management of both cancerous and non-cancerous conditions29,30. However the success rates of oocyte cryopreservation in TS girls has not been demonstrated. Depending on the level of mosaicism, not all oocytes will be suitable for fertilization or will develop with a normal karyotype as a fraction will be missing the X chromosome. However, technologies such as preimplantation genetic screening have entered into routine use and embryos originating from the stored oocytes can be screened for numerical chromosomal abnormalities. In addition, similar genetic assessment can be accomplished by polar body biopsy in oocytes as well31. Given these technological advances in oocyte and embryo screening, it is anticipated that at least a fraction of oocytes frozen from TS girls should lead to successful pregnancies.
However, despite the existing technology and potential for applicability and utilization of oocyte freezing early in reproductive life as a fertility preserving strategy for TS patients, one cannot disregard possible oocyte aneuploidy and risk therein to the progeny. Experience relating to quantitative ovarian response to exogenous gonadotropins is also limited, and data on qualitative aspects of oocytes retrieved in this population do not exist. Financial and emotional costs attributable to the possible freezing of genetically abnormal oocytes harvested from ovaries of women with TS cannot be trivialized and until the safety of autologous oocytes is established, oocyte cryopreservation by women with TS should be offered only under careful oversight with an understanding that resulting embryos should be offered pre implantation genetic diagnosis to test for the genetic normalcy of the progeny. However, the latter may not always be feasible due to cost, religious or ethical issues.
Maternal oocyte donation and cryopreservation for subsequent use by a TS daughter is a plausible strategy for girls with evidence of POI32. Such an approach however creates potential ethical concerns given that the egg donor would be the biological parent of her grandchild. Sibling-to-sibling oocyte donation is a less tenuous and a more acceptable alternative. Careful psychosocial evaluation of all participants and of the family dynamics is warranted when considering a mother-child or sibling-sibling egg donation approach for preservation of procreative potential.
In summary, oocyte cryopreservation appears to be the most viable option for girls with TS who have experienced menarche and are endocrinologically and psychologically mature enough to undertake the processes involved. We recommend that all postmenarchal girls with TS be evaluated for ovarian reserve assessment and both patients and families counseled regarding oocyte cryopreservation as a viable fertility preservation option.
Ovarian Tissue Cryopreservation
Ovarian tissue cryopreservation is a fertility preservation technique, which is still considered experimental in adults33. The process involves surgical removal of a part or all of an ovary, typically undertaken laparoscopically in an outpatient setting; pieces of the outer layer (cortex) of the removed ovarian tissue (which harbors the primordial follicles that make up the ovarian reserve) are then cryopreserved for future use. Because ovarian reserve is already low in girls with TS, it is recommended to remove as much tissue as possible, typically an entire ovary. When the patient is ready to attempt fertility, the cryopreserved pieces of ovarian tissues are then thawed followed by auto-transplantation back into the patient. A variety of anatomical sites have been tried for autotransplantation including the ovarian fossae in the pelvis (orthotopic) and under the skin of an easily accessible area such as the forearm34,35. The first successful ovarian transplantation with cryopreserved and thawed tissue was reported in 2000 by Oktay and Karlikaya36 resulting in restoration of hormonal function in a previously menopausal woman. The subsequent decade witnessed pregnancies resulting from these procedures with the current total of reported pregnancies standing above 4037. It is important to appreciate that thus far, all these pregnancies with the exception of a recent case report38, resulted from use of ovarian tissue that was harvested from and subsequently transferred back to adult women choosing ovarian tissue cryopreservation as a fertility preserving strategy in the setting of medical diagnoses such as cancer or other medical conditions. Data on efficiency of this approach in girls or women with TS are lacking. Demeestre et al38 recently reported a live-birth when ovarian tissue, cryopreserved at the age of 13, was auto transplanted during adulthood. The latter establishes that ovarian tissue will be functional regardless of the age that the tissue was cryopreserved. As such, the full biological development of ovaries are complete during the third trimester of pregnancy and potentially even a newborn ovary can produce follicles and ovulate when proper hormonal signals are received39. A good clinical example of this is central precocious puberty in girls with ovulation occurring at very young ages40.
The foregoing information offers hope for girls with TS who cannot wait until menarche to undergo ovarian stimulation for the purpose of egg harvesting and subsequent cryopreservation. Because the ovarian reserve can be lost even during the very first few years of life in full TS cases, ovarian tissue cryopreservation may be the only, albeit experimental, option for this population. While ovarian reserve evaluation may be difficult in children under the age of 520, a combination of serum FSH, Inhibin-B, AMH and antral follicle counts on ultrasound may provide useful information23,25.
Hreinsson et al. reported that primordial follicles can be found in ovarian tissue collected for cryopreservation from both mosaic and non-mosaic cases of TS in girls up to age 1741. In a larger study, the same group later reported that ovarian biopsy was feasible in 47 of the 57 girls and that in 15 of the 57 cases (26%), there were follicles in the tissue pieces analyzed histologically. Furthermore, follicle yield varied by genotype, being evident in 6/7 (86%) cases of 46, XX/45, X mosaicism, in 6/22 (27%) with structural chromosomal abnormalities, and in 3/ 28 (10.7%) with 45, X karyotype. Follicles were evident in biopsied ovarian tissue in 8/13 (62%) girls with spontaneous menarche, and 11/19 (58%) of those exhibiting signs of spontaneous puberty. The 12–16 years old age group had the highest proportion of girls with evidence of ovarian follicles on biopsied tissue. Normal FSH and AMH concentrations for age and pubertal stage were more frequent in girls with tissue evidence of follicles42.
However, the presence of follicles on ovarian biopsy does not guarantee that ovarian cryopreservation will be successful. Because the number of primordial follicles will be much reduced in tissues cryopreserved from girls with TS and the success of ovarian cryopreservation and transplantation will depend on the number of oocytes present in the transplanted tissue. Unless ovarian tissue is cryopreserved at very early ages, success of this technology for girls with TS may be limited.
In summary, ovarian tissue cryopreservation can be offered to girls with TS who are found to have adequate ovarian reserve but who cannot wait until sufficient maturity to undergo oocyte cryopreservation. The promise of this technology for girls with TS is at present hypothetical, given that no girl with TS who has undertaken this approach thus far has returned for autotransplantation of the previously cryopreserved ovarian tissue. The probability of success of this approach is unknown and this option remains experimental at the current time.
Embryo Cryopreservation
The promise of procreative success attributable to use of cryopreserved embryos far exceeds that offered by frozen oocytes43. Indeed, in some centers, live birth rates following transfer of cryopreserved embryos are comparable to fresh embryo transfer cycles19. Protocols and processes involved up to the step of oocyte collection are identical for oocyte and embryo freeze cycles. However, beyond the step of oocyte retrieval, an immediate access to sperm (either from a partner or a sperm donor) is a requisite, thus limiting applicability of an efficacious option for fertility preservation to the post pubertal girls and un-partnered young women diagnosed with TS. As with oocyte freezing, medical and ethical concerns regarding genetic normality of embryos resulting from fertilization of oocytes retrieved from TS patients’ remains. Thus, for TS patients who have identified a sperm source, elective embryo cryopreservation as a fertility preservation strategy should be offered under oversight with available pre implantation genetic diagnosis44 of the embryos prior to transfer to test the genetic normalcy of the progeny.
Practical Issues and Patient Counseling for Fertility Preservation in Turner syndrome
One of the key determinants of success in fertility preservation for girls with TS is early referral to a fertility specialist. Because of the rapid and accelerated decline in ovarian reserve unique to this population, these girls should be evaluated as early in postnatal life as possible. For the non-mosaic cases, where ovarian reserve can be depleted within the first few years of life, this referral should occur as soon as a diagnosis is made, even in infancy. For the mosaic cases, an expedient approach is also recommended but it may be possible to defer fertility preservation until post-menarchal ages.
Karyotypic diagnosis must be established as soon as possible. While interpretability of ovarian reserve markers in the TS population, particularly in the prepubescent girls, can be challenging, ovarian reserve assessment should be undertaken utilizing well-validated biomarkers (FSH, LH, estradiol, inhibin-B, AMH and the antral follicle count). Serum AMH norms have been established for children and adults, and these levels are reliable after the age of 520. In addition, ovarian volume and antral follicle assessment may be helpful in older children. Elevated FSH levels in the presence of undetectable AMH levels, the absence of visible antral follicles and evidence of streak ovaries indicate that ovarian failure has already occurred, making the probability of yield for oocyte or ovarian tissue cryopreservation highly unlikely
Recommendations
Discussion of fertility preservation options is advised at the earliest for any girl with TS; this may be at the time of diagnosis, even when the diagnosis is arrived at during infancy or childhood. Serial assessment of ovarian reserve markers can be undertaken. In prepubertal girls with mosaic TS who have a low percentage of cells with 45, X karyotype relative to 46, XX and when AMH levels are not inappropriately low for age (levels in the lower quartile for age45), one of the authors (KO) utilizes frequent (every two to three months) AMH monitoring to determine the time of intervention. When AMH levels begin to show a decline on two consecutive measurements, fertility preservation may be considered. This approach may enable TS girls to further mature both physically and psychosocially, and become more compliant with procedures relating to oocyte cryopreservation instead of being limited to the currently experimental ovarian tissue cryopreservation option.
If the ovarian reserve assessment indicates an age-inappropriate decline in ovarian reserve in girls with mosaic TS, and regardless of ovarian reserve in the non-mosaic TS population, we recommend consideration of fertility preservation at the earliest age possible.
For those who are sexually and or psychosocially immature or unable to tolerate ovarian stimulation procedures, ovarian cryopreservation may be recommended under experimental, IRB-approved protocols. In addition to obtaining an IRB-approved consent from the parents, verbal assent for the procedure must additionally be obtained from children older than nine years of age. The consent form should adequately explain that currently the success of ovarian cryopreservation and transplantation cannot be quantified in girls with TS.
In post-menarchal girls, typically 13 years of age or older and developmentally mature enough to tolerate ovarian stimulation, oocyte cryopreservation should be offered. Although the procedure is no longer considered experimental in adults, a Human Subjects and Institutional Review Board approved consent is still strongly encouraged for this procedure to be undertaken in the pediatric population. At minimum, parents and children should be provided with a detailed written consent form explaining the potential limitations including the lack of TS specific success rates from this procedure. Embryo cryopreservation can be considered under similar circumstances for those rare females with TS who have reached adulthood with sufficient ovarian reserve remaining and have a committed partner or those who are willing to use donor sperm.
Pregnancy in Turner Syndrome
Pregnancy with Autologous Oocytes
Between 2–5% of TS women are able to conceive spontaneously, although these pregnancies are more likely to occur in women with mosaic karyotypes, rather than full monosomy X46,47. They are reported to have higher than normal rates of chromosomal abnormalities and rates of miscarriage as high as 50%48,49. Congenital and chromosomal abnormalities have been identified in approximately 50% of babies born to women with TS and this is thought to be due to an inherited imbalance in genetic regulation50. For this reason, cycling mosaic TS women may consider prenatal testing or even in vitro fertilization (IVF) with pre-implantation genetic testing to avoid aneuploidy prior to embryo transfer. Additionally, girls and women with TS are at an increased risk for POI, thus shortening the available time for natural conception for the affected population. Women considering fertility should be offered referral to reproductive endocrinologists/infertility specialists for consultations about the family building options available to them that include use of donor oocytes or embryos and of IVF with autologous oocytes.
IVF and pregnancy with donor oocytes
For most patients with TS, the preferred option for childbearing that offers the highest likelihood of success is pregnancy with donor oocytes. Despite the use of donor oocytes, miscarriage rates in resulting pregnancies amongst TS recipients are slightly higher compared to the general donor oocyte recipient population21. While the exact cause of this increase in miscarriage risk is unclear, and may even reflect a surveillance bias, reduced uterine receptivity from prolonged hypoestrogenism and relatively hypoplastic uteri have been suggested as plausible mechanisms48,49,51.
Pregnancy Risks and Screening in Turner syndrome
Obstetrical and Maternal Cardiovascular Risks
Pregnancies in TS women are more likely to be complicated by thyroid dysfunction, obesity, diabetes and hypertensive disorders, including pre-eclampsia (up to 40%)49,51. Low birth weight, intrauterine growth restriction, preterm labor and preterm delivery are also more likely in pregnancies in women with TS22,50.
The well-known cardiovascular strains of pregnancy, including a 50% greater cardiac output, can significantly exacerbate underling abnormalities such as congenital heart disease, mostly left-sided disorders, which is recognized in up to 50% of women with TS52–54. In addition to the pre-existing cardiac anomalies, women with TS have connective tissue defects, which predispose them to aortic root dissection, a catastrophic complication that is well recognized to complicate pregnancies in women with TS and carries a higher mortality rate. Overall, maternal mortality in TS women has been reported to be as high as 1–2%, which is 100–200 times greater than in the general population47,52,55.
Pre-conception cardiac evaluation to include measurement of the aortic size index (or ASI, which adjusts aortic size dimensions for body surface area) is strongly recommended for any woman with TS contemplating pregnancy; an ASI > 2 cm/m2 is considered a contraindication for pregnancy due to the well-recognized higher risk of maternal mortality secondary to aortic dissection in this population56. Aortic dissections may also occur postpartum (20%)56, and hence close surveillance should continue after delivery.
Beyond the risk of cardiovascular detriment, pregnancy in women with TS poses additional challenges. Renal anomalies are present in up to 30–40% of women with TS with predisposition to development of hydronephrosis or obstructive nephropathy in pregnancy47,57. Hypothyroidism, diabetes, and celiac disease- all seen more frequently encountered in women with TS compared to genetically normal age comparable women, and may first manifest or worsen during pregnancy44.
In conclusion, the occurrence of pregnancy in women with TS poses unique challenges with attending risks that include maternal death. Careful pre-pregnancy planning is strongly endorsed. Pre-pregnancy management should include cardiac evaluation and pre-pregnancy counseling by a multidisciplinary team of specialists that includes cardiologists, infertility specialists, genetic counselors, and experts in maternal-fetal medicine. With careful intra- and post-pregnancy vigilance and monitoring under the care of a dedicated multidisciplinary team, the risks of maternal morbidity and mortality can be mitigated and render the attainment of healthy pregnancy a real possibility for women with TS.
Gestational surrogacy for women with Turner syndrome
Gestational surrogacy (GS) entails the planned pregnancy of a woman carried on behalf of another woman. Given the known potential cardiac and medical complications of TS, GS is both a reasonable and advisable alternative to pregnancy in those countries and states in the U.S. that legally allow it. Gestational surrogacy may also provide an opportunity for women with TS to be biological parents of their own children. Even those patients with a normal cardiac evaluation may be at risk for cardiovascular complications that arise during third trimester of pregnancy or postpartum. Therefore, the American Society of Reproductive Medicine (ASRM) recommends that all patients with TS should be counseled about GS and adoption as alternatives to pregnancy56. Gametes can be autologous or those donated, especially in the case of POI.
In the U.S., GS utilizing fresh nondonor embryos comprised approximately 1% of all ART cycles in 201258. ASRM recommends GS for indications including those of medical conditions that either prevent a woman from carrying a pregnancy or those that impart significant risk of morbidity or mortality to the woman or fetus59. In women with TS, indications include major cardiovascular risks and those due to uterine abnormalities as a result of decreased endometrial receptivity or long-standing hypoestrogenism (as previously mentioned)48,51,60. In all cases it is advised that the indications be well documented in the patient’s medical record59. Recommendations for specific medical tests have also been published and both psychosocial evaluation and education are advised for the genetic parents and gestational surrogate59. Both genetic parents and GS must be of legal age and medically screened for presence or risk of infectious diseases and carcinoma. Gestational surrogacy should also be counseled about the risks of pregnancy.
Although limited data are available for pregnancy and live birth rates for women with TS21,46, published data for pregnancy outcomes of GS (whether autologous or donor oocytes or embryos) are largely lacking. Thus, studies that address pregnancy outcomes in this specific patient population are needed so that women with TS will be better informed about their reproductive options.
Adoption as a Family Building Option for Women with Turner syndrome
In 2000, there were a total of 2,058,915 adopted children living in the United States, corresponding to 2.5% of all children61,62. There are three kinds of domestic adoption in the United States: state licensed public agency adoptions, private agency adoptions, and independent adoptions, which involve the direct placement of a child through a mediating agency or attorney61. Adoption agencies provide connections between children in need of home, pregnant women who wish to place a child with an adoptive family, and prospective adoptive parents. Agencies typically assess the parental capabilities of prospective adoptive families and assist in the legal process of the adoption including applications to the court for legal adoptions. All adoption agencies have requirements mandating that prospective parents meet certain criteria to be eligible to adopt a child63. The initial step in the eligibility process is typically a home study, and most states require additional background checks and probationary placement periods along with age and health requirements thereafter. Just as adoption agency structure is varied, preferences, policies, and requirements regarding prospective parents differ considerably across agency lines. Although some prospective families have had difficulties with adoption due to health requirements (e.g., cancer survivors), there are no documented cases of a woman with TS being restricted from adoption.
International adoptions may be available to individuals pursuing adoption in the U.S., however recent restrictions and regulatory issues have caused a sharp decline in international adoptions in the U.S. At its peak in 2004, there were 23,000 children from other countries adopted by U.S. families64. The most recent figures for international adoption in 2012 show a little over 10,000 adoptions. The top five countries from which children were adopted in 2012 include China, Ukraine, Ethiopia, Russia, and South Korea.
For women with TS who desire to be parents, adoption is a viable option and one that has been pursued by many women with TS. The need to weigh multiple factors such as the low rates of spontaneous pregnancy; potential health risks of pregnancy to the mother and genetic outcomes of the fetus; coupled with highly personal desires such as the wish to carry a pregnancy; and or the want for a biological child; and future parenting goals can result in complex decisions. Such decisions are best made with input from a personal physician, a reproductive endocrinologist, a partner and family.
Summary and Conclusions
In summary, women with TS are at extremely high risk for POI and infertility. There are existing techniques, albeit some still in the experimental realm, that can offer preservation of fertility potential for some girls with TS, provided timely consideration is entertained. To maximize the benefits of fertility preservation, all girls with TS should be evaluated by an expert as soon as possible in childhood as the vast majority will have their ovarian reserve depleted before adulthood. For those who have already lost their ovarian reserve, donor oocytes, donated embryos and adoption are strategies that can allow fulfillment of desire for parenting. For those with TS-related cardiac contraindications to pregnancy, utility of GS can allow the possibility of biological parenting through use of their own oocytes; alternatively, GS can serve to carry pregnancy resulting from the use of donor oocytes or embryos.
Acknowledgments
Kutluk Oktay, M.D., Ph.D., was the chair of the “Fertility Preservation Guidelines for Girls with Turner Syndrome Committee” organized by the Turner Syndrome Foundation (TSF). The other members of the committee are Karen Berkowitz, M.D., Richard Bronson, M.D., Peter McGovern, M.D., Lubna Pal, M.D., Gwendolyn Quinn, Ph.D., and Karen Rubin, M.D. In addition, Giuliano Bedoschi, M.D., and Banafsheh Kashani, M.D., participated in the manuscript writing as fellows. We thank the Turner Syndrome Foundation and especially Laavanya Pasupuleti, D.O., Rosemary Scales, R.N., M.S. and Laura Fasciano at TSF for spearheading this initiative. We also thank Richard Reindollar, M.D., Allan J Fisher, M.D. and Ann Gardner, M.D. for their helpful comments during the revision stage.
The goal of the TSF, a non-proft patient advocacy organization, is to support research initiatives and facilitate education programs to increase professional awareness and enhance medical care of those affected by Turner Syndrome. Patients and providers are encouraged to see the TSF website (www.TurnerSyndromefoundation.org) for further resources related to this topic. Though TSF commissioned this committee to create this document, it does not maintain an official position on its content.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford CE, Jones KW, Polani PE, De Almeida JC, Briggs JH. A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner's syndrome) Lancet. 1959 Apr 4;1(7075):711–713. doi: 10.1016/s0140-6736(59)91893-8. [DOI] [PubMed] [Google Scholar]
- 2.Classic pages in obstetrics and gynecology by Henry H. Turner. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. American journal of obstetrics and gynecology. 1972 May 15;113(2):279. Endocrinology, vol. 23, pp. 566-574, 1938. [PubMed] [Google Scholar]
- 3.Reindollar RH. Turner syndrome: contemporary thoughts and reproductive issues. Seminars in reproductive medicine. 2011 Jul;29(4):342–352. doi: 10.1055/s-0031-1280919. [DOI] [PubMed] [Google Scholar]
- 4.Witschi E. Migration of the germ cells of human embryos from the york sac to the primitive gonadal folds. Contrib Embryol. 1948;209:67–80. [Google Scholar]
- 5.Mamsen LS, Brochner CB, Byskov AG, Mollgard K. The migration and loss of human primordial germ stem cells from the hind gut epithelium towards the gonadal ridge. The International journal of developmental biology. 2012;56(10–12):771–778. doi: 10.1387/ijdb.120202lm. [DOI] [PubMed] [Google Scholar]
- 6.Mamsen LS, Lutterodt MC, Andersen EW, Byskov AG, Andersen CY. Germ cell numbers in human embryonic and fetal gonads during the first two trimesters of pregnancy: analysis of six published studies. Human reproduction. 2011 Aug;26(8):2140–2145. doi: 10.1093/humrep/der149. [DOI] [PubMed] [Google Scholar]
- 7.Pelosi E, Simonsick E, Forabosco A, Garcia-Ortiz JE, Schlessinger D. Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biology of reproduction. 2015 May;92(5):130. doi: 10.1095/biolreprod.114.127381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poljicanin A, Vukusic Pusic T, Vukojevic K, et al. The expression patterns of pro-apoptotic and anti-apoptotic factors in human fetal and adult ovary. Acta histochemica. 2013 Jul;115(6):533–540. doi: 10.1016/j.acthis.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Singh RP, Carr DH. The anatomy and histology of XO human embryos and fetuses. The Anatomical record. 1966 Jul;155(3):369–383. doi: 10.1002/ar.1091550309. [DOI] [PubMed] [Google Scholar]
- 10.Modi DN, Sane S, Bhartiya D. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Molecular human reproduction. 2003 Apr;9(4):219–225. doi: 10.1093/molehr/gag031. [DOI] [PubMed] [Google Scholar]
- 11.Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in Turner's syndrome. Italian Study Group for Turner's Syndrome. The Journal of clinical endocrinology and metabolism. 1997 Jun;82(6):1810–1813. doi: 10.1210/jcem.82.6.3970. [DOI] [PubMed] [Google Scholar]
- 12.Quilter CR, Karcanias AC, Bagga MR, et al. Analysis of X chromosome genomic DNA sequence copy number variation associated with premature ovarian failure (POF) Human reproduction. 2010 Aug;25(8):2139–2150. doi: 10.1093/humrep/deq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercer CL, Lachlan K, Karcanias A, et al. Detailed clinical and molecular study of 20 females with Xq deletions with special reference to menstruation and fertility. European journal of medical genetics. 2013 Jan;56(1):1–6. doi: 10.1016/j.ejmg.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Hook EB, Warburton D. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45, X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Human genetics. 2014 Apr;133(4):417–424. doi: 10.1007/s00439-014-1420-x. [DOI] [PubMed] [Google Scholar]
- 15.Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Human reproduction update. 2014 Sep-Oct;20(5):688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 16.Weenen C, Laven JS, Von Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Molecular human reproduction. 2004 Feb;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 17.Andersen CY, Byskov AG. Estradiol and regulation of anti-Mullerian hormone, inhibin-A, and inhibin-B secretion: analysis of small antral and preovulatory human follicles' fluid. The Journal of clinical endocrinology and metabolism. 2006 Oct;91(10):4064–4069. doi: 10.1210/jc.2006-1066. [DOI] [PubMed] [Google Scholar]
- 18.Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002 Mar;143(3):1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 19.Visser JA, Hokken-Koelega AC, Zandwijken GR, Limacher A, Ranke MB, Fluck CE. Anti-Mullerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Human reproduction. 2013 Jul;28(7):1899–1907. doi: 10.1093/humrep/det089. [DOI] [PubMed] [Google Scholar]
- 20.Hagen CP, Aksglaede L, Sorensen K, et al. Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. The Journal of clinical endocrinology and metabolism. 2010 Nov;95(11):5003–5010. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- 21.Bryman I, Sylven L, Berntorp K, et al. Pregnancy rate and outcome in Swedish women with Turner syndrome. Fertility and sterility. 2011 Jun 30;95(8):2507–2510. doi: 10.1016/j.fertnstert.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Foudila T, Soderstrom-Anttila V, Hovatta O. Turner's syndrome and pregnancies after oocyte donation. Human reproduction. 1999 Feb;14(2):532–535. doi: 10.1093/humrep/14.2.532. [DOI] [PubMed] [Google Scholar]
- 23.Purushothaman R, Lazareva O, Oktay K, Ten S. Markers of ovarian reserve in young girls with Turner's syndrome. Fertility and sterility. 2010 Sep;94(4):1557–1559. doi: 10.1016/j.fertnstert.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Kavoussi SK, Fisseha S, Smith YR, Smith GD, Christman GM, Gago LA. Oocyte cryopreservation in a woman with mosaic Turner syndrome: a case report. The Journal of reproductive medicine. 2008 Mar;53(3):223–226. [PubMed] [Google Scholar]
- 25.Oktay K, Bedoschi G. Oocyte cryopreservation for fertility preservation in postpubertal female children at risk for premature ovarian failure due to accelerated follicle loss in Turner syndrome or cancer treatments. Journal of pediatric and adolescent gynecology. 2014 Dec;27(6):342–346. doi: 10.1016/j.jpag.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oktay K, Rodriguez-Wallberg KA, Sahin G. Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with Turner syndrome mosaicism and impending premature ovarian failure. Fertility and sterility. 2010 Jul;94(2):753 e715–753 e759. doi: 10.1016/j.fertnstert.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 27.Huang JY, Tulandi T, Holzer H, et al. Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic Turner syndrome: Case Report. Human reproduction. 2008 Feb;23(2):336–339. doi: 10.1093/humrep/dem307. [DOI] [PubMed] [Google Scholar]
- 28.Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986 Apr 19;1(8486):884–886. doi: 10.1016/s0140-6736(86)90989-x. [DOI] [PubMed] [Google Scholar]
- 29.Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertility and sterility. 2013 Aug;100(2):492–499. e493. doi: 10.1016/j.fertnstert.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Practice Committees of American Society for Reproductive M, Society for Assisted Reproductive T. Mature oocyte cryopreservation: a guideline. Fertility and sterility. 2013 Jan;99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Montag M, Koster M, Strowitzki T, Toth B. Polar body biopsy. Fertility and sterility. 2013 Sep;100(3):603–607. doi: 10.1016/j.fertnstert.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 32.Gidoni YS, Takefman J, Holzer HE, et al. Cryopreservation of a mother's oocytes for possible future use by her daughter with Turner syndrome: case report. Fertility and sterility. 2008 Nov;90(5):2008 e2009–2008 e2012. doi: 10.1016/j.fertnstert.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 33.Practice Committee of American Society for Reproductive M. Ovarian tissue cryopreservation: a committee opinion. Fertility and sterility. 2014 May;101(5):1237–1243. doi: 10.1016/j.fertnstert.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 34.Oktay K, Buyuk E, Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004 Mar 13;363(9412):837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 35.Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. Jama. 2001 Sep 26;286(12):1490–1493. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]
- 36.Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. The New England journal of medicine. 2000 Jun 22;342(25):1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- 37.Oktay K, Bedoschi G, Pacheco F, Turan V. First Pregnancies, Livebirth and In Vitro Fertilization Outcomes After Transplantation of Frozen-Banked Ovarian Tissue with a Human Extracellular Matrix Scaffold using Robot-Assisted Minimally Invasive Surgery. Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.10.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demeestere I, Simon P, Dedeken L, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Human reproduction. 2015 Jun 9; doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 39.Peters H, Byskov AG, Grinsted J. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clinics in endocrinology and metabolism. 1978 Nov;7(3):469–485. doi: 10.1016/s0300-595x(78)80005-x. [DOI] [PubMed] [Google Scholar]
- 40.Jay N, Mansfield MJ, Blizzard RM, et al. Ovulation and menstrual function of adolescent girls with central precocious puberty after therapy with gonadotropin-releasing hormone agonists. The Journal of clinical endocrinology and metabolism. 1992 Sep;75(3):890–894. doi: 10.1210/jcem.75.3.1517382. [DOI] [PubMed] [Google Scholar]
- 41.Hreinsson JG, Otala M, Fridstrom M, et al. Follicles are found in the ovaries of adolescent girls with Turner's syndrome. The Journal of clinical endocrinology and metabolism. 2002 Aug;87(8):3618–3623. doi: 10.1210/jcem.87.8.8753. [DOI] [PubMed] [Google Scholar]
- 42.Borgstrom B, Hreinsson J, Rasmussen C, et al. Fertility preservation in girls with turner syndrome: prognostic signs of the presence of ovarian follicles. The Journal of clinical endocrinology and metabolism. 2009 Jan;94(1):74–80. doi: 10.1210/jc.2008-0708. [DOI] [PubMed] [Google Scholar]
- 43.Bedoschi G, Oktay K. Current approach to fertility preservation by embryo cryopreservation. Fertility and sterility. 2013 May;99(6):1496–1502. doi: 10.1016/j.fertnstert.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabanes L, Chalas C, Christin-Maitre S, et al. Turner syndrome and pregnancy: clinical practice. Recommendations for the management of patients with Turner syndrome before and during pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 2010 Sep;152(1):18–24. doi: 10.1016/j.ejogrb.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Lie Fong S, Visser JA, Welt CK, et al. Serum anti-mullerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. The Journal of clinical endocrinology and metabolism. 2012 Dec;97(12):4650–4655. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hadnott TN, Gould HN, Gharib AM, Bondy CA. Outcomes of spontaneous and assisted pregnancies in Turner syndrome: the U.S. National Institutes of Health experience. Fertility and sterility. 2011 Jun;95(7):2251–2256. doi: 10.1016/j.fertnstert.2011.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucaccioni L, Wong SC, Smyth A, et al. Turner syndrome--issues to consider for transition to adulthood. British medical bulletin. 2015 Mar;113(1):45–58. doi: 10.1093/bmb/ldu038. [DOI] [PubMed] [Google Scholar]
- 48.Abir R, Fisch B, Nahum R, Orvieto R, Nitke S, Ben Rafael Z. Turner's syndrome and fertility: current status and possible putative prospects. Hum Reprod Update. 2001 Nov-Dec;7(6):603–610. doi: 10.1093/humupd/7.6.603. [DOI] [PubMed] [Google Scholar]
- 49.Hewitt JK, Jayasinghe Y, Amor DJ, et al. Fertility in Turner syndrome. Clinical endocrinology. 2013 Nov;79(5):606–614. doi: 10.1111/cen.12288. [DOI] [PubMed] [Google Scholar]
- 50.Tarani L, Lampariello S, Raguso G, et al. Pregnancy in patients with Turner's syndrome: six new cases and review of literature. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 1998 Apr;12(2):83–87. doi: 10.3109/09513599809024955. [DOI] [PubMed] [Google Scholar]
- 51.Yaron Y, Ochshorn Y, Amit A, Yovel I, Kogosowki A, Lessing JB. Patients with Turner's syndrome may have an inherent endometrial abnormality affecting receptivity in oocyte donation. Fertil Steril. 1996 Jun;65(6):1249–1252. [PubMed] [Google Scholar]
- 52.Wong SC, Cheung M, Zacharin M. Aortic dilatation and dissection in Turner syndrome: what we know, what we are unclear about and what we should do in clinical practice? International journal of adolescent medicine and health. 2014;26(4):469–488. doi: 10.1515/ijamh-2013-0336. [DOI] [PubMed] [Google Scholar]
- 53.Boissonnas CC, Davy C, Bornes M, et al. Careful cardiovascular screening and follow-up of women with Turner syndrome before and during pregnancy is necessary to prevent maternal mortality. Fertility and sterility. 2009 Mar;91(3):929 e925–929 e927. doi: 10.1016/j.fertnstert.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 54.Matura LA, Ho VB, Rosing DR, Bondy CA. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007 Oct 9;116(15):1663–1670. doi: 10.1161/CIRCULATIONAHA.106.685487. [DOI] [PubMed] [Google Scholar]
- 55.Karnis MF, Zimon AE, Lalwani SI, Timmreck LS, Klipstein S, Reindollar RH. Risk of death in pregnancy achieved through oocyte donation in patients with Turner syndrome: a national survey. Fertility and sterility. 2003 Sep;80(3):498–501. doi: 10.1016/s0015-0282(03)00974-9. [DOI] [PubMed] [Google Scholar]
- 56.Practice Committee of American Society for Reproductive Medicine. Increased maternal cardiovascular mortality associated with pregnancy in women with Turner syndrome. Fertility and sterility. 2012 Feb;97(2):282–284. doi: 10.1016/j.fertnstert.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 57.Hagman A, Loft A, Wennerholm UB, et al. Obstetric and neonatal outcome after oocyte donation in 106 women with Turner syndrome: a Nordic cohort study. Human reproduction. 2013 Jun;28(6):1598–1609. doi: 10.1093/humrep/det082. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2012 Assisted Reproductive Technology National Summary Report. Atlanta (GA): US Dept of Health and Human Services; 2014. [Google Scholar]
- 59.Practice Committee of the American Society for Reproductive M, Practice Committee of the Society for Assisted Reproductive T. Recommendations for practices utilizing gestational carriers: a committee opinion. Fertil Steril. 2015 Jan;103(1):e1–e8. doi: 10.1016/j.fertnstert.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 60.Bakalov VK, Shawker T, Ceniceros I, Bondy CA. Uterine development in Turner syndrome. J Pediatr. 2007 Nov;151(5):528–531. 531 e521. doi: 10.1016/j.jpeds.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleinman EL. Caring for our own: why American adoption law and policy must change. Columbia J Law Soc Probl. 1997;30:327. [Google Scholar]
- 62.Kreider RM. Adopted Children and Stepchildren: 2000 CSR, CENSR- 6RV. [Accessed June 26, 2015]; http://www.census.gov/prod/2003pubs/censr-6.pdf.
- 63.Gardino SL, Russell AE, Woodruff TK. Adoption after cancer: adoption agency attitudes and perspectives on the potential to parent post-cancer. Cancer treatment and research. 2010;156:153–170. doi: 10.1007/978-1-4419-6518-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.U.S. Department of State. FY 2014 Annual Report on Intercountry Adoption. [Accessed June 27, 2015]; http://travel.state.gov/content/adoptionsabroad/en/about-us/statistics.html.