Abstract
Clinical observations and similarities to addiction suggest heightened reward sensitivity to food in patients with bulimic-type eating (BTE) disorders. Therefore, we investigated the expectation and receipt of food reward compared with monetary reward in patients with BTE. Fifty-six patients with BTE (27 patients with binge eating disorder and 29 with bulimia nervosa) and 55 matched healthy control participants underwent event-related functional magnetic resonance imaging while performing both food and monetary incentive delay tasks. BTE patients exhibited reduced brain activation in the posterior cingulate cortex during the expectation of food and increased activity in the medial orbitofrontal cortex, anterior medial prefrontal cortex and posterior cingulate cortex during the receipt of food reward. These findings were relevant to food because we found no significant group differences related to monetary reward. In the patients, higher brain activity in the medial orbitofrontal cortex during the receipt of food reward was related to higher levels of trait food craving and external eating. BTE patients exhibited increased hedonic processing during the receipt of food reward. These findings corroborate the notion that an altered responsiveness of the reward network to food stimuli is associated with BTE.
Keywords: bulimia nervosa, binge eating disorder, reward processing, functional MRI, medial orbitofrontal cortex, posterior cingulate cortex
Introduction
Binge eating behavior is characterized by recurrent episodes of eating an objectively large amount of food and is associated with feelings of loss of control. Binge eating behavior is clinically considered to be a subtype of overeating, although the neurobiological underpinnings of different subtypes of overeating remain unknown. Binge eating represents the core symptom of bulimic-type eating (BTE) disorders such as bulimia nervosa (BN) and binge eating disorder (BED). These are common mental disorders with heightened morbidities and all-cause mortalities (Arcelus et al., 2011). To counteract binge eating and prevent weight gain, BN patients exhibit compensatory behaviors such as self-induced vomiting or laxative misuse. BED patients exhibit no compensatory behaviors, overweight or obesity in the long term occurs in the majority of cases (Ágh et al., 2015).
A common theory that has been put forward to explain the phenomenon of overeating is the ‘food addiction model’, which suggests that an unbalanced neuronal reward system overrides the homeostatic regulation of food intake (Friederich et al., 2013; Smith and Robbins, 2013). There is evidence from experimental animal research that intermittent availability of sugar leads to deprivation-induced sugar binging that is associated with the sensitization of mesocorticolimbic reward pathways to energy-dense foods (Corwin et al., 2011).
Previous studies using functional magnetic resonance imaging (fMRI) in humans have found that, in response to visual food cues, patients with BTE display hyper-reactivity in reward regions including the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) (Uher et al., 2004; Schienle et al., 2009). Patients with BN also exhibit reduced anterior insula activity (Schienle et al., 2009; Bohon and Stice, 2011), which suggests the involvement of the primary gustatory cortex. However, not all studies found differences in neural processing of food cues in BTE (Van den Eynde et al., 2013).
Studies examining gustatory processing have found that the reactivity of the reward system is positively related to binge eating episodes in patients with BED (Wang et al., 2011), whereas in BN, both increases (Radeloff et al., 2014) and decreases (Frank et al., 2006; Bohon and Stice, 2011) in brain responses within the reward network have been observed. Furthermore, general reward processing (i.e. monetary reward) is not significantly altered in BTE patients compared with normal-weight controls (Wagner et al., 2010; Balodis et al., 2013). A previous study using repetitive transcranial magnetic stimulation (rTMS) found that stimulation of the dorsolateral prefrontal cortex reduces cue-induced food craving in patients with BTE (Van den Eynde et al., 2010), indicating a shared neural correlate of food craving across BTE disorders.
In summary, the available findings support the occurrence of altered responsivity of the reward system to both visual food cues and palatable food in BTE. However, most of the applied paradigms have not allowed for the differentiation between anticipation and receipt of food rewards nor have they directly compared food reward with general reward processing. Therefore, the aims of this study were to investigate whether BTE patients display dysfunctional activations in brain reward network during the anticipation and receipt of food reward and to examine whether these differences are specific to food reward or whether they result from a generalized impairment in reward processing. In line with the Research Domain Criteria framework (RDoC, NIMH, 2008), which proposes the investigation of specific neurobiological dimensions across mental disorders, we adopted a study design focusing on the investigation of the ‘positive valence system’ domain (Sanislow et al., 2010). This approach enables the identification of shared psychopathological mechanisms of the positive valence system across BTE disorders.
We hypothesized that BTE patients would exhibit heightened food reward sensitivity. Specifically, we expected increased activation in the mesocorticolimbic reward system, i.e. the ventral striatum (VS), during anticipatory food intake (O'Doherty et al., 2002; Simon et al., 2014b) and increased activation in the medial and lateral OFC during the receipt of food reward (Rolls, 2007; Simon et al., 2014b) but intact processing of monetary reward. Additionally, we expected to observe close associations between alterations in neural food reward processing and dysfunctional eating behavior.
Materials and Methods
Participants
Sixty patients with BTE (30 patients with BED and 30 patients with BN) and 61 age-, sex-, body mass index (BMI)- and education-matched healthy control participants (HC) were recruited. The control group consisted of two sub groups matched in age, sex, BMI and education to the BED or BN group, respectively (Table 1). All participants underwent a Structured Clinical Interview for the DSM-V (American Psychiatric Association, 2013) and filled out the Beck Depression Inventory (BDI, Hautzinger et al., 2006). All participants were right handed and over the age of 18 years. Exclusion criteria included claustrophobia, metallic implants and lifetime diagnoses of bipolar disorder, psychosis or alcohol or drug abuse. Participants who reported lifetime diagnoses of a borderline personality disorder were also excluded. Patients with BTE received no medication other than antidepressants (five participants in the BED and seven participants in the BN group were receiving antidepressant medications). Four of the BTE patients and five of the HCs were excluded (four participants exhibited head motion that exceeded 4 mm and another five were excluded due to other technical problems). Thus, data from 56 BTE patients and 55 HCs are reported. Patients were recruited from our wards and outpatient clinic, and HCs were recruited via advertisements. This study was approved by the local ethics committee of the Medical School of the University of Heidelberg. All participants provided written and oral informed consent.
Table 1.
Demographic and clinical characteristics of participants
|
Patients with BED (N
=
27) |
BED controls (N
=
28) |
P |
Patients with BN (N
=
29) |
BN controls (N
=
27) |
P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |||
| Age (years) | 38.26 | 13.75 | 38.00 | 10.85 | 0.94 | 27.45 | 10.55 | 25.74 | 5.25 | 0.45 |
| Body mass index | 32.61 | 4.55 | 34.02 | 4.50 | 0.25 | 21.33 | 2.99 | 21.85 | 1.85 | 0.44 |
| Education (years) | 12.07 | 1.69 | 12.18 | 2.06 | 0.84 | 12.66 | 1.40 | 12.70 | 0.87 | 0.88 |
| Beck depression inventory | 23.07 | 12.54 | 8.61 | 7.48 | <0.001 | 24.79 | 12.49 | 3.11 | 2.62 | <0.001 |
| Binge eating per week | 2.64 | 1.75 | — | — | 3.83 | 2.58 | — | — | ||
| Glucose (mg/dl) | 95.08 | 6.75 | 83.44 | 23.44 | 0.02 | 82.07 | 9.53 | 85.54 | 10.18 | 0.19 |
| DEBQ restrained eating | 19.37 | 6.93 | 15.39 | 6.97 | 0.04 | 28.83 | 7.63 | 10.19 | 7.19 | <0.001 |
| DEBQ emotional eating | 28.04 | 8.92 | 13.61 | 9.24 | <0.001 | 28.53 | 7.92 | 6.26 | 4.05 | <0.001 |
| DEBQ external eating | 26.04 | 6.99 | 20.54 | 6.37 | 0.004 | 25.17 | 7.18 | 18.33 | 6.92 | <0.001 |
| Food craving questionnaire—state | 41.30 | 14.64 | 33.21 | 13.49 | 0.05 | 39.55 | 13.49 | 33.00 | 10.06 | 0.04 |
| Food craving questionnaire—trait | 81.81 | 19.66 | 57.54 | 17.60 | <0.001 | 83.59 | 15.71 | 43.59 | 11.78 | <0.001 |
Procedure
All participants were asked to come to the clinic without having breakfast and to refrain from consuming alcoholic drinks for 24 h before the experiment. Participants then received a light standardized breakfast at 9:00 a.m. containing approximately 550 kcal. After completing the Structured Clinical Interview for the DSM-V, all participants were asked to complete the Dutch Eating Behavior Questionnaire (DEBQ; Grunert, 1989), which consists of subscales related to restrained eating, emotional eating and external eating and the State and Trait version of the General Food Craving Questionnaire (G-FCQ; Nijs et al., 2007). MRI scanning was performed at 12:00 p.m., which corresponded to lunchtime for most of the participants.
fMRI task
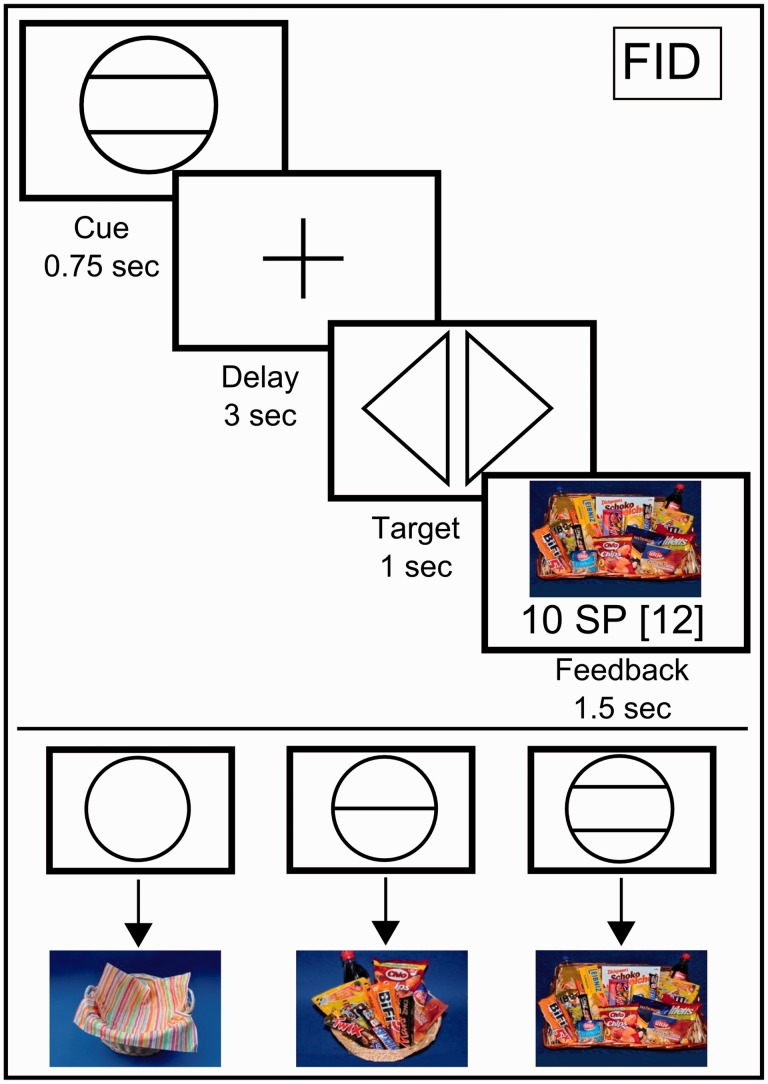
We used an adapted version of a well-established ‘monetary incentive delay’ task to study monetary and food reward processing [i.e. the ‘monetary and food incentive delay’ tasks (MID and FID), Figure 1]. Participants were able to win ‘money’ and ‘snack points’ (SPs), which they could then exchange for real money (MID task) or sweet and salty snacks as well as beverages and fruits (FID task), immediately after the MRI measurement. This task has been found to reliably induce activity in brain regions related to the anticipation and receipt of food reward (Simon et al., 2014a,b). Using abstract rather than palpable foods allowed us to directly compare food with monetary-related rewards as well as to avoid variance caused by interindividual differences in food preferences. Further details related to the task are given in Figure 1 and in the Supplementary Materials.
Fig. 1.
Graphical depiction of the FID task. Each trial began with a cue stipulating the amount of money or number of SPs the participants could win if they reacted correctly during the subsequent discrimination task (anticipation phase). Immediately after target presentation, participants were informed about the amount of money or number of SPs they had won during the trial and their cumulative total winnings thus far (i.e. the receipt of reward phase). The MID task used graphical depictions that showed a wallet filled with EUR 1, EUR 0.20 or EUR 0, which corresponded to the amount of money won during each trial. The FID task used pictures of a large basket filled with snacks, a small basket filled with snacks and an empty basket, depending on the number of SPs won.
fMRI acquisition and analysis
Images were collected using a Tim Trio 3-T whole-body MR scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a standard 32-channel head coil. To minimize susceptibility artifacts in the OFC, 30 oblique slices (interleaved acquisition) with a 10° angle relative to the AC-PC axis were acquired with a 1-mm interslice gap using a T2*-sensitive single-shot EPI sequence with following parameters: TR = 2000 ms, TE = 30 ms (which resulted in an in-plane resolution of 3 × 3 × 4 mm), flip angle = 80° and field of view = 192 × 192 mm. High-resolution T1 MPRAGE anatomical images were acquired (192 slices, voxel size 1 × 1 × 1 mm, TR = 1570 ms, TE = 2.63 ms, 9° flip angle) for anatomical reference.
The functional MRI data were pre-processed and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). To account for magnetic field equilibration, four volumes from the beginning of each functional run were excluded from the analyses. Functional images were checked manually for artifacts and corrected for differences in slice acquisition timing. All images were realigned, the allowed motion was limited to ±4 mm translation and ±3 degrees of rotation over the entire experiment and images were unwarped to correct for artifacts due to susceptibility-by-movement interactions. Individual T1 images were coregistered with the mean T2* images and subsequently segmented. Both structural and functional images were normalized to the standard anatomical Montreal Neurological Institute (MNI) space using the transformation parameters from the segmentation, which resulted in a voxel size of 3 mm3 for the functional images and a voxel size of 1 mm3 for the high-resolution anatomic images. Furthermore, functional images were smoothed with an 8-mm full-width half-maximum isotropic Gaussian kernel. A 128-s high-pass filter was used to remove low-frequency noise and signal drift.
At the first level of analysis, a general linear model was constructed by separately modeling regressors for the three different anticipation phases (i.e. anticipation of EUR 1, EUR 0.20 and EUR 0 outcomes in the MID task and anticipation of 10 SP, 2 SP and 0 SP outcomes in the FID task) and the five different outcome phases (i.e. receipt or omission of EUR 1, EUR 0.20 and EUR 0 and receipt or omission of 10 SP, 2 SP and 0 SP) as explanatory variables convolved with the gamma-variate function described by Cohen (1997). The targets and error trials were included as additional regressors of no interest. For the analysis of reward anticipation, we contrasted the anticipation of a high reward (EUR 1 or 10 SP) to the anticipation of no reward (EUR 0 or 0 SP). For the analysis of the effect of a rewarding outcome, we contrasted the receipt of a high reward (EUR 1 or 10 SP) to the receipt of no reward (EUR 0 or 0 SP) while controlling for the anticipation phase that preceded both of the outcome types. The rationale for choosing only the 1 Euro and 10 SP cues in our contrasts (1 Euro vs 0 Euros and 10 SP vs 0 SP) was to induce the strongest possible activation in the reward circuitry and to increase statistical power. All contrasts were modeled separately for each task.
At the second level of analysis, the individual contrast images of all participants were included in a random-effects analysis, allowing population inference (Holmes and Friston, 1998). Consistent with our a priori hypothesis and prior studies (Simon et al., 2014a,b), contrast images were calculated for the analysis of reward anticipation [anticipation of a high reward (EUR 1 or 10 SP) vs the anticipation of no reward (EUR 0 or 0 SP)] and reward receipt (receipt of a high reward (EUR 1 or 10 SP) vs the receipt of no reward (EUR 0 or 0 SP). We performed a region of interest (ROI) analysis of reward-sensitive brain regions (see below) by comparing brain activity between BTE patients and HCs using two-sample t-tests. We report the small-volume corrected results that were significant at a family-wise error extent threshold of P < 0.05. Furthermore, a whole-brain analysis using the specific contrasts of interests was performed. Between-group differences were analyzed using two-sample t-tests. We report the results that were significant at P < 0.05 cluster level corrected with a cluster-defining threshold P < 0.001 uncorrected and cluster size k > 10. Secondary group comparisons were conducted using two-sample t-tests to investigate whether brain activation of the reward network differ between subgroups of BTE (BN, BED) and between patient groups and their respective age- and BMI-matched control groups. We used the same significance levels for the ROI and whole brain analysis as mentioned above.
Regions of interest
Based on the literature (Knutson et al., 2001; Simon et al., 2014b), the following three anatomical ROIs were defined: the bilateral striatum, the medial OFC (mOFC) and the bilateral OFC (see Supplementary Figure S1). Furthermore, because of an observed activation in the posterior cingulate cortex (PCC) during the whole-brain analyses of the expectation and receipt of food reward (see Results), we also constructed an anatomical ROI for this region (see Supplementary Figure S1) to extract percent signal change for correlational analysis. The ROIs were created using the Wake Forest University PickAtlas (Maldjian et al., 2003), all regions were taken from the AAL atlas (Tzourio-Mazoyer et al., 2002). The mean percent signal change was extracted using MarsBaR (Brett et al., 2002). Correlation analyses between eating behavior and the mean percent signal changes extracted from the ROIs were performed using SPSS (version 20).
Results
The demographic and clinical characteristics of the participants are summarized in Table 1. The BTE group exhibited higher scores than HCs in the restrained, emotional and external eating (DEBQ) and the state and trait food craving (G-FCQ) scales. The BTE group reported higher scores of depression as assessed by the BDI. The diagnostic subgroups of BN and BED were different in terms of age (BN: mean 27.5 years, s.d. 10.6; BED: mean 38.3 years, s.d. 13.8, t = 3.3, P < 0.05) and BMI (BN: 21.3 kg/m2, s.d. 3.0; BED: 32.6 kg/m2, s.d. 4.6, t = 11, P < 0.001). The age range was 42 years for the BED group (Min = 19, Max = 61) and 35 years for the BN group (Min = 18, Max = 53). The BMI range was 15 for the BED group (Min = 27, Max = 42) and 10 for the BN group (Min = 17, Max = 28).
Behavioral performance
In both tasks, participants reacted significantly faster when expecting a high reward (EUR 1 or 10 SP, respectively) then when expecting no reward (FID: t = −1.73, P < 0.05, MID: t = −1.86, P < 0.05). We did not observe a significant difference of errors between tasks. Overall performance (reaction time and errors) during the FID and MID task are given in Supplementary Table S6. We observed no differences in reaction times, errors or amounts of money/SP won between groups (P > 0.29). In the BTE group, higher numbers of binge eating episodes per week were related to faster responses for 10 SPs compared with 0 SP in the FID task (r = −0.31, P < 0.05), but a corresponding correlation was not observed in the MID task (r = −0.15, P = 0.26). However, the differences in correlational findings with binge eating episodes per week reached no statistical significance (Z = −1, P = 0.32).
Imaging data
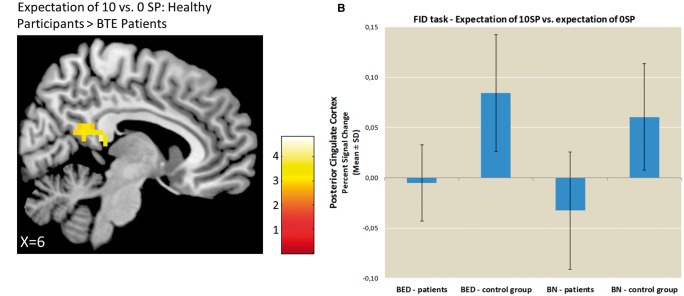
FID task. The findings during both the expectation and receipt of the high vs no reward contrast for both BTE patients and HCs are listed in Supplementary Tables S1 and S2. The results from the comparisons between BTE patients and HCs are displayed in Supplementary Table S5 and Figures 2–4. During the expectation of high vs no food reward, the ROI analysis did not reveal any significant group differences in the bilateral striatum. In the whole-brain analysis, HCs displayed higher activation in the PCC and there were no regions of greater reward-related activation in BTE patients (Figure 2).
Fig. 2.
Group comparison of brain activations during the expectation of food reward. Panel A depicts the differences in group activation during the expectation of 10 vs 0 SPs thresholded at P < 0.001, voxel-level uncorrected. Panel B depicts the percent signal changes extracted from the PCC during the expectation phase for the BTE patients and HCs. Panel C depicts the correlation between the percent signal changes extracted from the PCC during the expectation of 10 vs 0 SP and trait food craving in the patients with BTE.
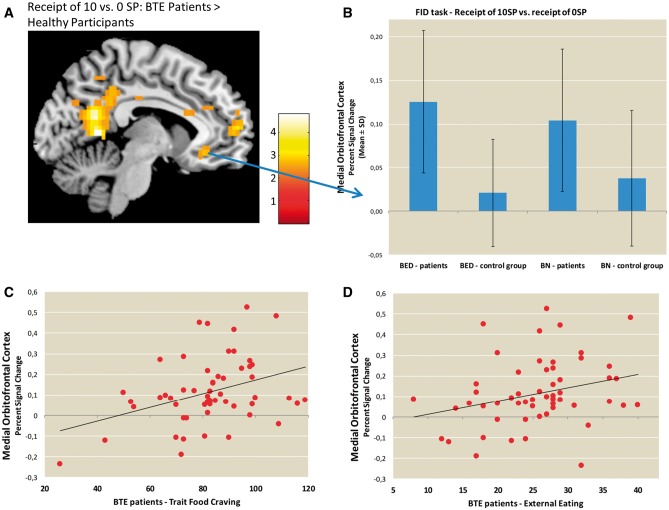
Fig. 3.
Group comparison of brain activations during the receipt of food reward. Panel A depicts the differences in group activation during the receipt of 10 vs 0 SPs thresholded at P < 0.001, voxel-level uncorrected. Panel B depicts the percent signal changes extracted from the medial OFC during the receipt phase for the BTE patients and the HCs. Panels C and D depict the correlations between the percent signal changes extracted from the medial OFC during the receipt of 10 vs 0 SP and external eating and trait food craving, respectively, in patients with BTE.
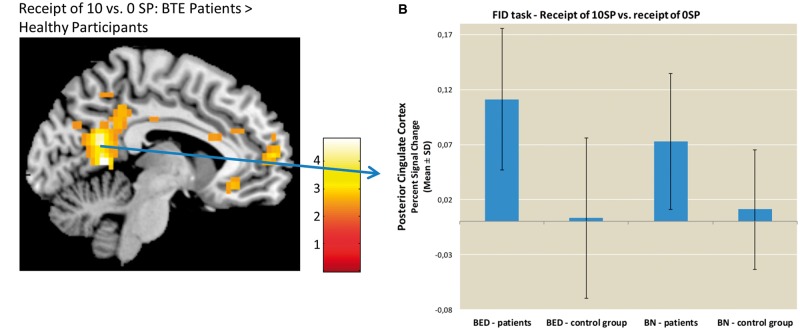
Fig. 4.
Comparison between groups of brain activations during the receipt of food reward. Panel A depicts the difference in group activation during the receipt of 10 vs 0 SPs thresholded at P < 0.001, voxel-level uncorrected. Panel B depicts the percent signal changes extracted from the PCC during the receipt phase for the BTE patients and the HCs.
During the receipt of high vs no food reward, the ROI analyses revealed stronger mOFC activation in BTE patients than HCs (MNI: −6,32,−10, t = 3.67, P < 0.05, cluster size = 15, Figure 3). In the whole-brain analyses, BTE patients exhibited greater activation in the PCC, anterior medial PFC (amPFC) and angular gyrus (see Supplementary Table S5) relative to controls. The HCs did not exhibit stronger activation in any region relative to the BTE patients. Including age and BMI as covariates in the ROI analysis did not change the results of the group comparison during the analysis of both the expectation and receipt of food-related reward.
MID task. The brain activations in the BTE and HC groups during both the expectation and receipt of high vs no reward are listed in Supplementary Tables S3 and S4. There were no group differences in monetary reward processing.
Secondary analyses of differences between subgroups
ROI analyses
There were no differences in brain activation between BTE subgroups using the above mentioned ROIs. Furthermore, we observed no differences in whole brain activation between the BN group and their respective control group. BED patients compared to BED controls displayed increased activation in the PCC (MNI: −6, −55, 6, t = 4.55, P < 0.05, cluster size = 229) and midcingulate cortex (MNI: 9, −22, 50, t = 4.47, P < 0.05, cluster size = 251).
Correlation analysis
In the BTE group, during the receipt of food reward (i.e. 10 SP compared to 0 SP), food craving as a trait (G-FCQ) as well as external eating (DEBQ) was positively correlated with mOFC activity (r = 0.32, P < 0.05, Figure 3C, r = 0.28, P < 0.05, Figure 3D, respectively). During the anticipation of food reward (i.e. 10 SP compared to 0 SP), no significant associations were observed between eating behavior (i.e. DEBQ, G-FCQ scores and binge eating frequency) and PCC activation in the BTE patients. We observed no correlations between scores of depression (BDI) and brain activation (P > 0.27).
Discussion
To our knowledge, this is the first study to compare the anticipation and receipt of both food and monetary rewards in patients with BTE. Compared with the HCs, the BTE patients exhibited decreased activation in the PCC during the anticipation of food reward and increased activation in the PCC, mOFC and amPFC during the receipt of food reward. These findings were relevant to food reward processing and we observed no group differences during general (i.e. monetary) reward processing. Furthermore, responses to the receipt of food reward in the mOFC were positively related to self-report measures of eating behavior in the BTE patients. Contrary to our first hypothesis, we did not observe greater activity in the bilateral striatum during the anticipation of food reward in BTE patients.
As hypothesized, BTE patients exhibited increased mOFC activity during the receipt of food reward. The mOFC plays an important role in reward-value coding, and our results suggest that BTE patients displayed increased hedonic processing (Kringelbach, 2005). The present results extend previous findings from BTE patients revealing altered functional neuroanatomy of the mOFC specifically during the receipt of food reward (Uher et al., 2004; Schienle et al., 2009; Schafer et al., 2010). However, taste reward processing and taste reward-related learning in BN have been related to decreased activation of the lateral OFC, a brain region including the secondary taste area (Bohon and Stice, 2011; Frank et al., 2011). These divergent findings may be explained by differences in the processing of abstract and palatable food rewards, which should be further investigated in future studies.
Reward valuation processing, operationalized as the level of mOFC activation, exhibited a close association with self-report measures of eating behavior (i.e. trait food craving and external eating). Although previous studies have often identified emotional eating as an essential aspect of BTE (Fischer et al., 2008; Leehr et al., 2015), we observed a positive association between external eating and mOFC activation. This finding suggests that in addition to internal satiety signals, external food-related cues constitute an important factor for recurrent binge eating in BTE patients. Contrary to our assumptions, the number of binge eating episodes was not related to brain activations. However, the frequency of binge eating was related to the performance in the FID task, but not in the MID task, albeit the differences in correlational findings reached no statistical significance.
Additionally, patients with BTE displayed increased activation in the amPFC. This region is responsive to both positive and negative motivationally salient events and is involved in decision making, emotional regulation and self-reflection [for a review, see Euston et al. (2012)]. Similar to the mOFC, the amPFC shows close connections with emotional and autonomic centers in the brain and is involved in assigning a subjective value to an event against the background of past experiences and personal values (Etkin et al., 2011).
Based on the present findings, the proneness to binge eating may be influenced by a hyper-responsivity of the brain regions involved in reward valuation, whereas the failure to observe greater activity in the bilateral striatum in BTE patients indicates that the ‘processing of incentive cues seems to be of less relevance. This finding is in line with those of previous studies (Bohon and Stice, 2011; Wang et al., 2011), albeit divergent results have been observed for taste reward-related learning (Frank et al., 2011). Increased hedonic processing displayed during the receipt of food reward by the BTE patients suggests greater involvement of dysfunctional opioidergic, rather than dopaminergic, neurotransmission (Berridge et al., 2010). Furthermore, animal research has found that opioid receptor stimulation or inhibition in the mPFC directly influences binge eating-type behaviors in rats, indicating that altered opioidergic neurotransmission in the mPFC may play a role in BTE (Blasio et al., 2014). Thus, the present findings in BTE differ from the current ‘food addiction model’ of overeating and obesity. The latter advocates a heightened anticipatory food reward processing and a concomitant blunted response to reward receipt as an underlying neurobiological phenotype for obesity (Smith and Robbins, 2013). Hyper-reactivity during reward valuation of food may serve as a marker for the differentiation of BTE and overeating without binge eating. These differences in BTE patients and obese patients without binge eating are supported by molecular research focusing on dopamine and opioid genetic markers (Davis et al., 2009).
Furthermore, the amPFC together with the PCC are key regions of the self-referential network, which allows for the distinction between self-related stimuli and those of no self-relevance (Northoff et al., 2006). In a previous study, we were able to show that, compared to monetary reward, food reward processing was characterized by increased activations in the amPFC and PCC (Simon et al., 2014b). The present results indicate an increased involvement of neural regions subserving self-referential processing in BTE patients compared to HCs. However, as we have not included an independent behavioral measure of self-control, this interpretation should be treated with caution.
The ventral portion of the PCC, which corresponds to our clusters of activation that were found both during anticipation and receipt of food-related reward, is thought to be involved in internally directed cognition, such as memory retrieval and planning (Leech et al., 2011; Leech and Sharp, 2014). Decreases in activation in the ventral PCC are observed during external focusing of attention primarily during demanding cognitive tasks (Leech et al., 2011). Studies using incentive delay tasks have consistently found that the ventral PCC is active during the anticipation of both monetary (Knutson et al., 2001) and food rewards (Simon et al., 2014b). Interestingly, greater activity in the ventral PCC has been related to the ability to resist cue-induced craving among smokers (Brody et al., 2007), and lower activity has been found to be related to poor self-control and self-reflection in response to cigarette cues in chronic smokers (Bourque et al., 2013). Furthermore, it has been found that hunger increases responsivity of the PCC to food-related stimuli (Siep et al., 2009), which indicates a greater need to control food cravings when physically hungry. Therefore, our finding of reduced activation in the PCC during the expectation of a food reward may indicate lower involvement of brain regions related to self-control in response to food-related cues in patients with BTE. However, the observed mismatch between greater PCC activation in BTE patients during the receipt but not the anticipation of food reward stresses the importance to differentiate between anticipation and receipt of food reward processing.
A main finding of this study is that increased reward valuation processing was food relevant, and no differences were observed in terms of responses to monetary reward in BTE patients. This finding is in accord with a previous fMRI study that assessed hedonic processing of general reward (i.e. monetary reward) in BED (Balodis et al., 2013). Thus, recurrent dieting and binge eating may lead to a sensitization of brain regions involved in hedonic valuation of food (i.e. mOFC and amPFC) in individuals with a certain biologically based vulnerability (Davis et al., 2009).
There are several limitations to our study. First, since we employed abstract food reward, we were not able to measure neural processing during actual food consumption. Although abstract rewards allow for analysis of food reward processing irrespective of individual food preferences, further studies should also include palpable stimuli. Second, BTE shows a high comorbidity for depression. As we have not included a clinical control group with depression, we cannot exclude that comorbid depression might have confounded the findings. However, previous studies in patients with depression showed decreased instead of increased brain activation in the prefrontal cortex during the processing of rewards (Kumar et al., 2008; Eshel and Roiser, 2010). Third, in contrast to our hypothesis BTE patients did not display heightened activity in the bilateral striatum during the anticipation of food-related reward. Further studies employing more naturalistic paradigms (i.e. employing palpable food stimuli) are needed to validate the present findings. Fourth, as opposed to previous studies from our group (Simon et al., 2014a,b), we did not find striatal activation in the control group during the expectation of monetary rewards, whereas striatal activation was observed as expected during the anticipation of food rewards. This could be due to habituation effects caused by the duration and low difficulty of the task, thereby reducing the incentive value of the monetary and food task differently. Finally, future studies should adopt a longitudinal approach to assess whether the observed activations were the cause or the result of eating pathology.
In summary, the findings provide novel insights in neural markers of motivation- and hedonic-related food processing in BTE. Our results indicate reduced involvement of regions related to self-control during the expectation of food reward, whereas the receipt of food reward was characterized by an increased neural hedonic response and higher involvement of regions related to self-related processing in BTE. Our correlational analyses indicate that these activations were relevant to disordered eating behavior. Furthermore, the present findings in the subgroup of overweight/obese patients with BED differ from alterations in the reward networks of overweight/obese controls and may suggest that overeating with binge eating compared to overeating without binge eating is differentially encoded in neural reward circuits.
Funding
This study was supported by a DFG grant (FR 2626/3-1).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Ágh T., Kovács G., Pawaskar M., Supina D., Inotai A., Vokó Z. (2015). Epidemiology, health-related quality of life and economic burden of binge eating disorder: a systematic literature review. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 20(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcelus J., Mitchell A.J., Wales J., Nielsen S. (2011). Mortality rates in patients with Anorexia Nervosa and other eating disorders. A meta-Analysis of 36 Studies', Archives of General Psychiatry, 68(7), 724–31. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013) Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Publishing. [Google Scholar]
- Balodis I.M., Kober H., Worhunsky P.D., et al. (2013). Monetary reward processing in obese individuals with and without binge eating disorder. Biological Psychiatry, 73(9), 877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Ho C.Y., Richard J.M., DiFeliceantonio A.G. (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Research, 1350, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A., Steardo L., Sabino V., Cottone P. (2014). Opioid system in the medial prefrontal cortex mediates binge-like eating. Addiction Biology, 19(4), 652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C., Stice E. (2011). Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. International Journal of Eating Disorders, 44(7), 585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque J., Mendrek A., Dinh-Williams L., Potvin S. (2013). Neural circuitry of impulsivity in a cigarette craving paradigm. Front Psychiatry, 4, 67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage, 16, S497. [Google Scholar]
- Brody A.L., Mandelkern M.A., Olmstead R.E., et al. (2007). Neural substrates of resisting craving during cigarette cue exposure. Biological Psychiatry, 62(6), 642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.S. (1997). Parametric analysis of fMRI data using linear systems methods. Neuroimage, 6(2), 93–103. [DOI] [PubMed] [Google Scholar]
- Corwin R.L., Avena N.M., Boggiano M.M. (2011). Feeding and reward: perspectives from three rat models of binge eating. Physiology and Behavior, 104(1), 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.A., Levitan R.D., Reid C., et al. (2009). Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity, 17(6), 1220–5. [DOI] [PubMed] [Google Scholar]
- Eshel N., Roiser J.P. (2010). Reward and punishment processing in depression. Biological Psychiatry, 68(2), 118–24. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston D.R., Gruber A.J., McNaughton B.L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76(6), 1057–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S., Smith G.T., Cyders M.A. (2008). Another look at impulsivity: a meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clinical Psychology Review, 28(8), 1413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G.K., Reynolds J.R., Shott M.E., O'Reilly R.C. (2011). Altered temporal difference learning in bulimia nervosa. Biological Psychiatry, 70(8), 728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G.K., Wagner A., Achenbach S., et al. (2006). Altered brain activity in women recovered from bulimic-type eating disorders after a glucose challenge: a pilot study. International Journal of Eating Disorders, 39(1), 76–9. [DOI] [PubMed] [Google Scholar]
- Friederich H.C., Wu M., Simon J.J., Herzog W. (2013). Neurocircuit function in eating disorders. International Journal of Eating Disorders, 46(5), 425–32. [DOI] [PubMed] [Google Scholar]
- Grunert S. (1989). Ein Inventar zur Erfassung von. Selbstaussagen zum Ernährungsverhalten. Diagnostica, 35, 167–79. [Google Scholar]
- Hautzinger M., Keller F, Kühner C. (2006) Beck Depressions-Inventar (BDI II). Frankfurt: Harcourt Test Services.
- Holmes A.P., Friston K.J. (1998). Generalisability, random effects & population inference. Neuroimage, 7, S754. [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L. (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews: Neuroscience, 6(9), 691–702. [DOI] [PubMed] [Google Scholar]
- Kumar P., Waiter G., Ahearn T., Milders M., Reid I., Steele J.D. (2008). Abnormal temporal difference reward-learning signals in major depression. Brain, 131(Pt 8), 2084–93. [DOI] [PubMed] [Google Scholar]
- Leech R., Kamourieh S., Beckmann C.F., Sharp D.J. (2011). Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. Journal of Neuroscience, 31(9), 3217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(Pt 1), 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehr E.J., Krohmer K., Schag K., Dresler T., Zipfel S., Giel K.E. (2015). Emotion regulation model in binge eating disorder and obesity–a systematic review. Neuroscience and Biobehavioral Reviews, 49C, 125–34. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Nijs I.M., Franken I.H., Muris P. (2007). The modified Trait and State Food-Cravings Questionnaires: development and validation of a general index of food craving. Appetite, 49(1), 38–46. [DOI] [PubMed] [Google Scholar]
- NIMH. (2008). The National Institute of Mental Health strategic plan. National Institute of Mental Health.
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage, 31(1), 440–57. [DOI] [PubMed] [Google Scholar]
- O'Doherty J.P., Deichmann R., Critchley H.D., Dolan R.J. (2002). Neural responses during anticipation of a primary taste reward. Neuron, 33(5), 815–26. [DOI] [PubMed] [Google Scholar]
- Radeloff D., Willmann K., Otto L., et al. (2014). High-fat taste challenge reveals altered striatal response in women recovered from bulimia nervosa: a pilot study. World Journal of Biological Psychiatry, 15(4), 307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. (2007). Sensory processing in the brain related to the control of food intake. Proceedings of the Nutrition Society, 66(1), 96–112. [DOI] [PubMed] [Google Scholar]
- Sanislow C.A., Pine D.S., Quinn K.J., et al. (2010). Developing constructs for psychopathology research: research domain criteria. Journal of Abnormal Psychology, 119(4), 631–9. [DOI] [PubMed] [Google Scholar]
- Schafer A., Vaitl D., Schienle A. (2010). Regional grey matter volume abnormalities in bulimia nervosa and binge-eating disorder. Neuroimage, 50(2), 639–43. [DOI] [PubMed] [Google Scholar]
- Schienle A., Schafer A., Hermann A., Vaitl D. (2009). Binge-eating disorder: reward sensitivity and brain activation to images of food. Biological Psychiatry, 65(8), 654–61. [DOI] [PubMed] [Google Scholar]
- Siep N., Roefs A., Roebroeck A., Havermans R., Bonte M.L., Jansen A. (2009). Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behavioural Brain Research, 198(1), 149–58. [DOI] [PubMed] [Google Scholar]
- Simon J.J., Skunde M., Hamze Sinno M., et al. (2014a). Impaired cross-talk between mesolimbic food reward processing and metabolic signaling predicts body mass index. Frontiers in Behavioral Neuroscience, 8, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J.J., Skunde M., Wu M., et al. (2014b). Neural dissociation of food and money related reward processing using an abstract incentive delay task. Social Cognitive and Affective Neuroscience, doi: 10.1093/scan/nsu162.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.G., Robbins T.W. (2013). The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biological Psychiatry, 73(9), 804–10. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- Uher R., Murphy T., Brammer M.J., et al. (2004). Medial prefrontal cortex activity associated with symptom provocation in eating disorders. American Journal of Psychiatry, 161(7), 1238–46. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F., Claudino A.M., Mogg A., et al. (2010). Repetitive transcranial magnetic stimulation reduces cue-induced food craving in bulimic disorders. Biological Psychiatry, 67(8), 793–5. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F., Giampietro V., Simmons A., et al. (2013). Brain responses to body image stimuli but not food are altered in women with bulimia nervosa. BMC Psychiatry, 13, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A., Aizenstein H., Venkatraman V.K., et al. (2010). Altered striatal response to reward in bulimia nervosa after recovery. International Journal of Eating Disorders, 43(4), 289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.J., Geliebter A., Volkow N.D., et al. (2011). Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity, 19(8), 1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.