Abstract
Hundreds of studies have found that humans’ decisions are strongly influenced by the opinions of others, even when making simple perceptual decisions. In this study, we aimed to clarify whether this effect can be explained by social influence biasing (early) perceptual processes. We employed stimulus evoked potentials, lateralized readiness potentials (LRPs) and a diffusion model analysis of reaction time data to uncover the neurocognitive processes underlying social conformity in perceptual decision-making. The diffusion model analysis showed that social conformity was due to a biased uptake of stimulus information and accompanied by more careful stimulus processing. As indicated by larger N1-amplitudes, social influence increased early attentional resources for stimulus identification and discrimination. Furthermore, LRP analyses revealed that stimulus processing was biased even in cases of non-conformity. In conclusion, our results suggest that the opinion of others can cause individuals to selectively process stimulus information supporting this opinion, thereby inducing social conformity. This effect is present even when individuals do not blindly follow the majority but rather carefully process stimulus information.
Keywords: : social conformity, perceptual decision-making, diffusion model analysis, event-related potentials, lateralized readiness potentials
Introduction
Starting with the seminal experiments of Sherif (1935) and Asch (1956), hundreds of social psychological studies have found that humans’ decisions are strongly influenced by the opinions of others, even when making simple perceptual decisions. Strikingly, however, research has yet to clarify whether this can be explained by social influence biasing perceptual processes. To address this issue, this study aimed to uncover the neurocognitive processes underlying social conformity in perceptual decision-making.
Recent neuroscientific research has mainly focused on social conformity in stimulus evaluation and found that social influence can modulate activity in brain areas associated with reward processing and subjective value of stimuli (e.g. Zaki et al. 2011; for a review, see Schnuerch and Gibbons 2014). However, few studies have directly investigated whether social influence can bias processing of sensory stimulus information.
Schnuerch et al. (2015) employed a task in which participants had to evaluate the attractiveness of faces and measured the N170 as an indicator of the general strength of face encoding. Prior to the presentation of each face, an alleged group judgment was presented. The authors found that the N170-amplitude was inversely related to the degree with which participants agreed to the group judgments. Hence, they hypothesized that conformity is necessarily associated with superficial encoding of stimulus information. However, as acknowledged by Schnuerch et al., since there was no control condition without social influence, these correlational results could also be due to the fact (i) that superficial stimulus encoding moderated the degree of conformity induced by the group judgments or (ii) that superficial encoding and conformity were associated because of a third unknown variable. For example, it is possible that participants with low task motivation paid less attention to the faces and coincidentally followed the group judgments more often. Additionally, it remained unclear whether superficial encoding was the only precursor of social conformity, because Schnuerch et al. did not test whether social influence biased processing of faces according to the content of group judgments (e.g. whether a negative group judgment led participants to selectively focus on unattractive aspects of a face).
Berns et al. (2005) found that social conformity in a mental rotation task was associated with increased activity in task specific brain areas (i.e. the occipital–parietal network). The authors concluded that social influence can bias early perceptual processing. However, this conclusion is premature, since the activity in this network cannot solely be related to early perceptual changes but could also be due to later decisional processes (Gold and Shadlen, 2007; Mojzisch and Krug, 2008).
Trautmann-Lengsfeld and Herrmann (2013, 2014) observed higher P1- and lower N1- as well as higher P3-amplitudes when participants were confronted with alleged correct (vs incorrect) group judgments during a two stimuli discrimination task. These results suggest that social influence can alter early stimulus processing (P1, N1) as well as later processes associated with stimulus discrimination (P3). However, group judgments were presented as formalized faces ipsi- vs contralateral to the upcoming target. Thus, the observed effects may have been due to the fact that the faces operated as exogenous or endogenous spatial cues (Bindemann et al., 2005, 2007) and not due to social influence. In line with this interpretation, the observed effects did not predict behavioral conformity. Additionally, as only the effects of correct vs incorrect group judgments were compared, it remains unclear how social influence per se (irrespective of its validity) alters perceptual decision-making.
Germar et al. (2014) employed a control condition to address these critical points. In each experimental session, four participants were asked to complete a perceptual decision-making task on separate computers. To induce social influence, the alleged decisions of the other three participants formed a unanimous majority response, which was presented before the stimulus was shown. To control for mere priming effects, participants in the control condition were informed that, during a given trial, each participant would respond to a different stimulus. Hence, majority responses would be irrelevant for their own responses. In contrast, in the experimental condition, participants were informed that, during each trial, they were all presented with the same stimulus and, hence, majority responses would be relevant for them. Thus, any differences between the experimental and control condition could be attributed to social influence per se. To disentangle the cognitive processes underlying social influence, a diffusion model analysis of the reaction time data (Ratcliff, 1978) was conducted. This analysis showed that, although participants in the experimental condition processed stimuli more carefully than those in the control condition, they also showed more conformity because they were biased toward stimulus information supporting the given majority response. However, the neurocognitive processes underlying the results of the diffusion model analysis as well as their time course are still to be uncovered.
In sum, past research has neither clarified whether social influence can alter (early) perceptual processes nor which neurocognitive mechanisms can explain social conformity in perceptual decision-making. To address this issue, we employed three complementary methods: stimulus evoked potentials (SEPs), lateralized readiness potentials (LRPs) and a diffusion model analysis of reaction time data. Moreover, we adapted the paradigm described above (Germar et al., 2014), which allowed us to investigate the effects of social influence per se.
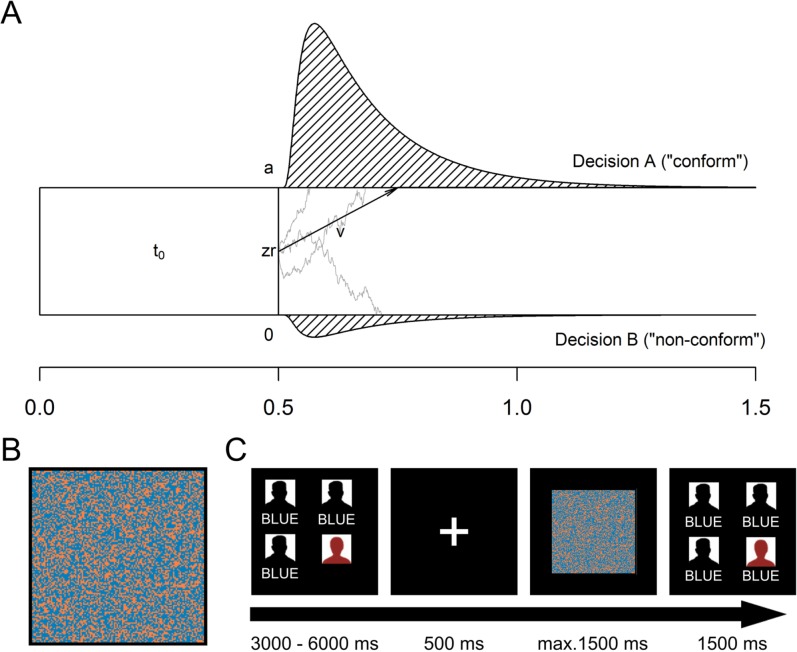
On the behavioral level, we applied the diffusion decision model (Voss et al., 2013), which is a widely used method to examine cognitive processes in speeded binary decisions (Ratcliff and McKoon, 2008). Based on the assumptions illustrated in Figure 1, the model extracts parameters from the response time distributions of the two choice options. Three parameters were relevant for our research question. The drift rate (ν) represents the speed and direction of information accumulation. Positive (negative) values indicate that information is accumulated faster for decision ‘A’ (‘B’). In our study, ‘A’ corresponds to the alleged majority response participants were presented with. Thus, positive (negative) values indicate that stimulus information that supports (contradicts) the majority response is accumulated faster. The distance between thresholds (a) describes how much information is needed to reach a decision (i.e. how carefully stimuli are processed). The relative starting point (zr) defines the point between thresholds (relative to a) at which the accumulation process starts. When zr deviates from the midpoint (0.5), less information is needed to reach the closer threshold, indicating a bias in decision criteria unrelated to perceptual processes (Voss et al., 2004). In our study, zr values above 0.5 would indicate a shift in decision criteria toward the majority response. Based on Germar et al. (2014), we predicted that social influence would lead to biased (i.e. higher values of ν) and more careful (i.e. lager values of a) stimulus processing but not to a shift in decision criteria (i.e. changes in zr).
Fig. 1.
Diffusion model, stimulus and paradigm. (A) Graphic representation of the diffusion decision model. The model assumes that stimulus information is sequentially accumulated over time. This process is described by a noisy stochastic diffusion process, which starts at position zr and moves over time (horizontal axis) with the average slope given by drift rate (ν) until a lower threshold (0) or an upper threshold (a) is reached. Then, the corresponding decision (‘A’ or ‘B’, ‘conform’ or ‘non-conform’) is executed. The duration of non-decisional processes (e.g. response execution) is given by t0. Note that processes contributing to t0 might occur before or after the decision phase. Outside the thresholds, predicted response time distributions for both decisional outcomes are presented. (B) Example of a stimulus. (C) Trial sequence (see Methods).
On the electrophysiological level, we measured SEPs, which allowed us to uncover whether social influence affects early or later processing stages. Following Trautmann-Lengsfeld and Herrmann (2013, 2014), we analysed early perceptual components (P1, N1) and later central components (P3).
Extending previous research, we computed stimulus-locked LRPs. The LRP is a negative potential, which is observed over central electrodes (C3, C4) contralateral to the responding hand. The LRP onset marks the time point at which the brain begins to prepare a response (Smulders and Miller, 2012). The period between stimulus and LRP onset (i.e. the LRP onset latency) can be used to measure how fast information is extracted from a stimulus before the brain begins to prepare the response, that is, how fast the brain selects a decision based on the accumulated stimulus information (Miller et al., 1999; Smulders and Miller, 2012). Hence, comparing LRP onset latencies of responses that conform vs non-conform to the majority response represents another approach to investigate whether social influence biased the rate of information uptake. In addition, we investigated the LRP-amplitude to explore whether social influence alters response preparation (e.g. the LRP-amplitude can reveal preliminary activation of a response that is never actually executed; Mattler et al., 2006; Smulders and Miller, 2012).
Materials and methods
Participants
Forty female students participated. All participants had normal or corrected-to-normal vision and gave written informed consent to the study. We did not test for color blindness, but no participant reported having difficulties discriminating the colors during the task. No participant voiced suspicions concerning the cover story used in our task (see below; see also Supplementary material for a detailed analysis). One participant had to be excluded due to technical problems. Hence, 39 participants (Mage = 24, s.d. = 4.4) were included in further analyses. The study procedure was designed and performed according to the Helsinki Declaration (1964) and was approved by the local ethics committee.
Task, stimuli and design
Participants’ task was to indicate the dominant color of squares consisting of 128 × 128 orange and blue pixels. On the basis of a pretest (Germar et al., 2014), we chose two types of stimuli for this study: In one-half of the trials, stimuli were presented whose dominant color had been identified well above chance level (i.e. 80% correct responses on average) in the pretest. These stimuli were either dominated by the color blue (52% blue pixels) or orange (48% blue pixels). In the other half of the trials, ambiguous stimuli were presented (i.e. proportions of both colors were 50%) which had resulted in a random performance in the pretest. Stimuli spanned a visual angle of 8.84°. The assignment of colors to the response keys was counterbalanced between participants. The experiment was run with PsychoPy-2 (Peirce, 2007, 2009).
In order to induce social influence, the alleged responses of three fellow group members were presented before stimulus onset on each trial. These responses were manipulated to form a unanimous majority response, which was either ‘orange’ in one-half of the trials or ‘blue’ in the other half.
Type of stimulus (unambiguous vs ambiguous) and majority response (blue vs orange) composed the within-subjects factor congruency which varied across three levels: (i) the majority response was congruent to the stimulus (CON, e.g. the majority response was blue and the dominant color in the stimulus was blue), (ii) the congruency between majority response and stimulus was ambiguous, because the stimulus had no dominant color (i.e. proportions of both colors were 50%; AMB) and (iii) the majority response was incongruent to the stimulus (INC, e.g. the stimulus was dominated by the color blue and the majority response was orange).
Since the majority response was presented before stimulus onset, effects on stimulus processing could be due to both social influence and priming. In order to disentangle these processes, participants were randomly assigned to one of two between-subjects conditions (Germar et al., 2014): Participants in the experimental condition were told that their fellow group members would consecutively respond to the same stimulus per trial; therefore, the majority response would be relevant for the participants’ own responses. In contrast, participants in the control condition were instructed that their fellow group members’ responses were not relevant to them because all participants would respond to different stimuli on each trial. Thus, all stimuli including the majority response were the same in both conditions but the majority response was only socially relevant in the experimental condition. Therefore, if we observe more conformity in the experimental than in the control condition, this is due to genuine effects of social influence and not due to priming.
In sum, we employed a 2 × 3 design consisting of the between-subjects factor condition (control vs experimental) and the within-subjects factor congruency (CON vs AMB vs INC).
Procedure
We used a modified version of the procedure by Germar et al. (2014). On arrival, participants were told that they would take part in a study on group performance. To this end, groups allegedly consisting of four participants would complete a computer-based color discrimination task. All group members would be seated in individual rooms. Their computers would be connected via a server running the task. In reality, only one participant took part in each experimental session. During the perceptual task, the displayed responses of the other alleged group members were preprogrammed.
Participants were informed that the group performance would be measured by subtracting the sum of each member’s errors from the sum of each member’s correct responses. After the experimental session, all members would meet to learn the performance of the group and of each individual member. The five best groups would receive a bonus of 15€.
Participants were told that the group members would respond in a specific order in each trial. The server would randomly determine the order at the beginning of the task. Since the order would be visualized by portrait photos on the computer screens (Figure 1), photos of all members would be taken and uploaded before the task. To provide the group members with the opportunity to get to know each other, each member would introduce herself by stating her first name, field of study and age in a short video clip to be recorded, uploaded and presented prior to the task. In fact, the video clips and portrait photos of the remaining three group members were identical for all participants and showed three female confederates.
At the beginning of the task, participants were instructed that they had been randomly chosen to respond last in each trial. Thus, each trial began with the other group members’ alleged responses, which were presented consecutively and separated by random delays between 1000 and 2000 ms to simulate realistic response latencies. After the onset of the third response, all responses rested on the screen for 500 ms. Next, a fixation cross was shown for 500 ms. Then, the stimulus was presented for a maximum of 1500 ms. During this time, participants had to give their response, which terminated the stimulus presentation. Thereafter, all portraits and responses were presented for 1500 ms and the next trial began (Figure 1). Participants completed 240 experimental trials (i.e. 60 CON-, 120 AMB- and 60 INC-trials). Additionally, filler trials were added in which either the other group members gave non-unanimous responses (48 trials) or one of the participants failed to give an answer (6 trials). There were three experimental blocks and a practice block of 20 trials. Blocks were separated by breaks of 2 min. After the task, participants were probed for suspicion and debriefed.
Analysis of behavioral results
Prior to all analyses, trials were rejected when no response was given (overall 1.9% of trials) or the response represented a fast guess (< 300 ms, overall 0.9% of trials). Proportions of choices conform to the majority response were submitted to a 2 (condition) × 3 (congruency) analysis of variance (ANOVA) with repeated measures on the last factor.
Diffusion model analysis
Parameter estimation. We conducted the diffusion model analysis for each participant following Germar et al. (2014; see Supplementary material for a more detailed description). We calculated drift rates (ν) so that values directly indicate whether stimulus processing was biased toward the majority responses. Positive (negative) values indicate that stimulus information that supports (contradicts) the majority response was accumulated faster. Values were entered into a 2 (condition) × 3 (congruency) ANOVA with repeated measures on the last factor. The relative starting point (zr) was calculated so that values above (below) 0.5 indicate that the decision criterion was shifted toward (away from) the majority response. The distance between decision thresholds (a) was calculated to investigate how carefully participants processed the stimuli. T-tests for independent samples were employed to test whether zr and a values differed between control and experimental condition, respectively (see Supplementary material for the remaining parameters).
Model fit. As a measure for model fit, fast-dm provides P values of the Kolmogorov–Smirnov statistic. We determined critical values for poor model fit with a Monte Carlo simulation (Voss et al., 2013). Results indicate a poor fit at P < 0.018 for alpha = 0.01. Two of the empirical models had a poor fit according to this criterion. We excluded these two participants from the analysis (see Supplementary material for a more detailed description).
EEG recording and analysis
EEG data were recorded using a Biosemi Active-Two system with 64 electrodes (placed according to the enhanced 10–20 system). Two additional electrodes were placed at both mastoids. Horizontal eye movements were monitored by bipolar electrodes placed at the outer canthi of both eyes. Vertical eye movements and blinks were monitored by an electrode below the left eye. Data were sampled with a frequency of 512 Hz, electrode offsets were kept below ±25 mV. Data were re-referenced offline to the linked mastoids. All data analyses were performed using EEGLAB (Delorme and Makeig, 2004) and ERPLAB (Lopez-Calderon and Luck, 2014) toolboxes.
For the analysis of SEPs, the continuous EEG signal was bandpass filtered at 0.53–30 Hz (IIR butterworth filter, 12 dB/octave) and segmented into epochs from 200 ms before to 800 ms after target onset and baseline corrected over the pre-target period. Epochs containing eye blinks or movements (VEOG or HEOG exceeding ±70 µV) around target onset (±200 ms), voltages exceeding ±150 µV or gradients exceeding ±50 µV were rejected. Remaining ocular artifacts were removed using Independent Component Analysis (Jung et al., 2000). After this procedure, six participants had to be excluded, because they had fewer than 40 trials per within condition. Overall 6.4% of trials had to be rejected for the remaining 34 participants.
After visual inspection of grand average waveforms at occipital und parietooccipital electrodes, the P1 and N1 were determined as components peaking around 120 and 165 ms, respectively. Mean amplitudes were extracted for the time windows of 110–130 ms and 150–180 ms, respectively, and pooled over left (P7, PO7, PO3), central (O1, Oz, O2) and right (P8, PO8, PO4) electrodes. Statistical analyses were performed by entering individual mean amplitudes into a 2 (condition) × 3 (congruency) × 3 (cluster of electrodes) ANOVA with repeated measures on the last two factors.
A P3-like late positivity (LP) was identified over central/parietal electrodes (Cz, CPz, Pz) peaking at 450 and 550 ms, respectively. Mean amplitudes were extracted for the time windows of 400–500 ms and 500–600 ms, respectively, and then entered in a 2 (condition) × 3 (congruency) ANOVA with repeated measures on the last two factors.
For the analysis of LRPs, trials were collapsed across the within-subjects factor congruency. EEG data were processed identically to SEP analysis with the exception that data were bandpass filtered at 0.01–4 Hz. The filtered signal was segmented into epochs from −100 ms to 1200 ms around target onset and baseline corrected over the pre-target period. LRPs were calculated separately for trials where participants conformed to the majority response and trials where participants did not conform to the majority response. Because several participants did not conform to the majority response on too few trials, LRPs were analysed only for a subsample of 31 participants (with 5.8% trials excluded due to artifacts). LRPs were extracted using the double-subtraction and averaging method (Eimer, 1998) according to the formula: [(C4′−C3′)left hand + (C3′−C4′)right hand]/2. To analyse differences in LRP onset latencies, we then used the jackknife-based scoring method with a threshold of 0.25 µV (Miller et al., 1998; using a relative criterion of 30% of the maximum amplitude revealed identical results, Ulrich and Miller, 2001). Onset latencies were then entered in a 2 (condition) × 2 (response type: conform vs non-conform) ANOVA with repeated measures on the last factor.
Results
Conformity
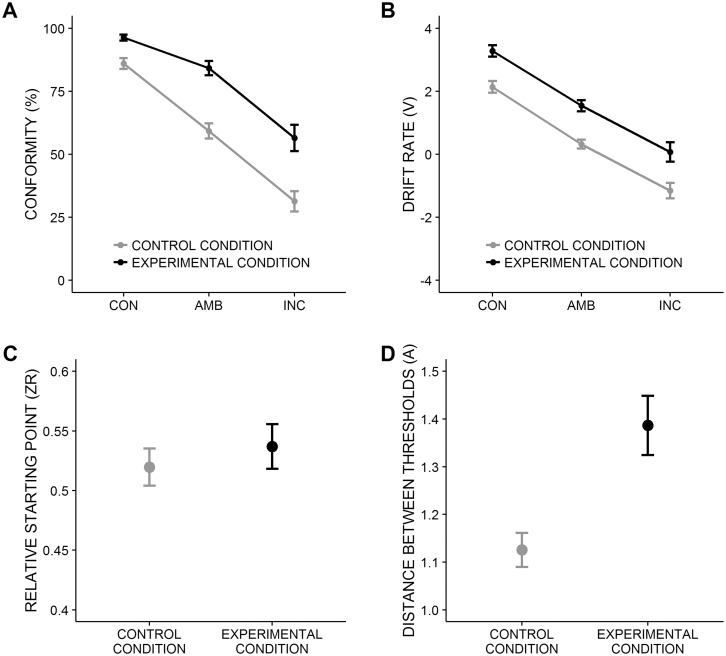
In both conditions, conformity decreased with decreasing congruency between majority response and stimulus, F(2, 74) = 183.23, P < 0.001, ηG2 = 0.65 (Figure 2A). More importantly, participants in the experimental condition conformed to the majority response more often (M = 79.04%, 95% CI [73.05, 85.02]) than in the control condition (M = 58.86%, 95% CI [52.01, 65.71]), F(1, 37) = 28.92, P < 0.001, ηG2 = 0.33. The interaction was also significant, F(2, 74) = 5.92, P = 0.004, ηG2 = 0.06, indicating that the difference between the control and experimental condition depended on the level of congruency. The difference was smallest for CON, t(37) = 4.21, P < 0.001, d = 1.33, MD = 10.31%, 95% CI [5.30, 15.32]; intermediate for INC, t(37) = 3.80, P = 0.001, d = 1.23, MD = 25.19%, 95% CI [11.74, 38.64]; and largest for AMB, t(37) = 6.00, P < 0.001, d = 1.92, MD = 25.04%, 95% CI [16.60, 33.48] (all Bonferroni-corrected). These results show that majority responses affected participants’ choices in the experimental condition beyond mere priming effects and led to high levels of conformity irrespective of the validity of the majority response.
Fig. 2.
Conformity and results from diffusion model analysis. Mean values and standard errors. CON, the majority response is congruent to the stimulus; AMB , the congruency between majority response and stimulus is ambiguous; INC, the majority response is incongruent to the stimulus. (A) Conformity as a function of congruency (x-axis) and condition (lines). Control and experimental condition differ significantly over all levels of congruency, all Ps < 0.001. (B) Drift rate as a function of congruency (x-axis) and condition (lines). Control and experimental condition differ significantly over all levels of congruency, all Ps < 0.001. Note the higher (lower) values indicate that stimulus information that supports (contradicts) the majority response is accumulated faster. (C) Relative starting point. Conditions did not differ significantly, P = 0.480. (D) Distance between thresholds. Conditions differ significantly, P < 0.001.
Diffusion model analysis
In both conditions, drift rates decreased with decreasing congruency between majority response and stimulus, F(2, 70) = 159.12, P < 0.001, ηG2 = 0.69, which is due to the fact that the less stimulus information supporting the majority response was physically available, the lower the rate at which this information was accumulated (Figure 2B). More importantly, and as predicted, drift rates were higher in the experimental (M = 1.65, 95% CI [1.19, 2.08]) than in the control condition (M = 0.44, 95% CI [0.01, 0.86]), F(1, 35) = 31.09, P < 0.001, ηG2 = 0.31. Hence, participants in the experimental condition accumulated more stimulus information supporting the majority response than participants in the control condition across all levels of congruency. The interaction was not significant, F(2, 70) = 0.03, P = 0.967, ηG2 = 0.00. Social influence did not bias the decision criterion, because there was no significant difference in the relative starting point between conditions, t(35) = 0.71, P = 0.480, d = 0.23 (Figure 2C). Moreover, as predicted, the distance between the decision thresholds was larger for the experimental (M = 1.39, 95% CI [1.27, 1.51]) than for the control condition (M = 1.13, 95% CI [1.01, 1.24]), t(35) = 3.69, P < 0.001, d = 1.22, indicating that participants under social influence processed stimuli more carefully (Figure 2D). In sum, we replicated the findings of Germar et al. (2014). Social conformity was (i) due to a biased uptake of stimulus information and (ii) accompanied by more careful stimulus processing.
EEG results
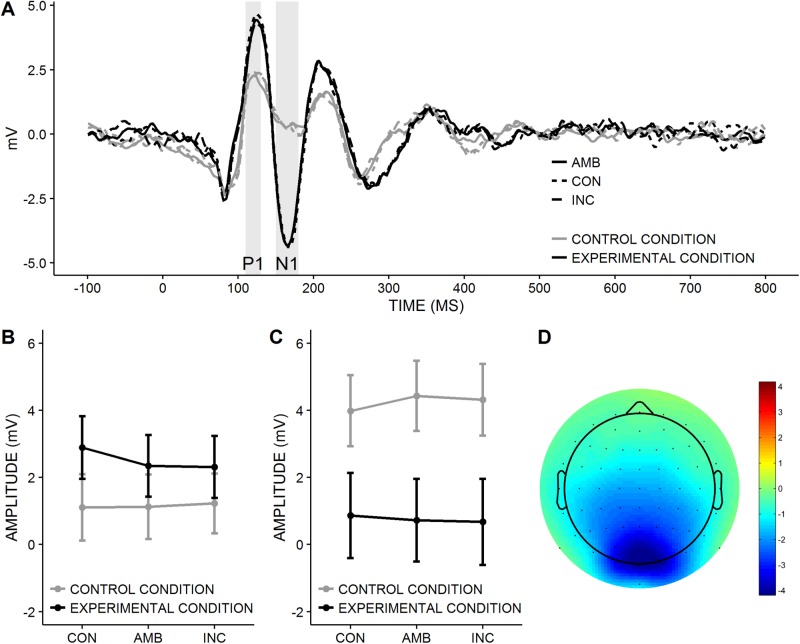
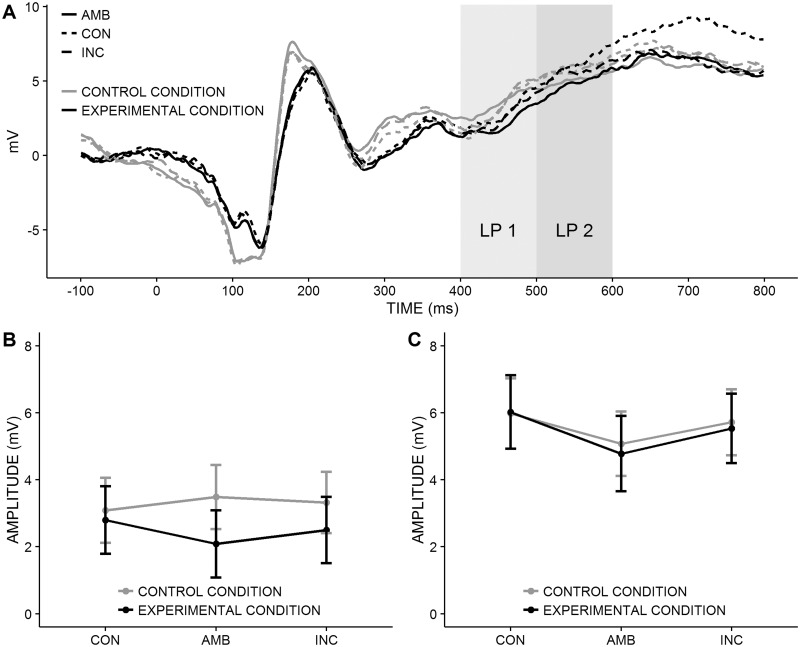
Stimulus evoked potentials. P1-amplitudes depended on the clusters of electrodes, F(2, 62) = 57.75, P < 0.001, ηG2 = 0.19. They were highest over central, intermediate over right, and lowest over left electrodes, all ts > 6.72, all Ps < 0.001 (Bonferroni-corrected). All other effects were not significant, all Fs < 1.78, all Ps > 0.138 (Figure 3B). N1-amplitudes also depended on the clusters of electrodes, F(2, 62) = 21.54, P < 0.001, ηG2 = 0.13. They were largest over central, intermediate over left and smallest over right electrodes, all ts > 6.80, all Ps < 0.001 (Bonferroni-corrected). More importantly, N1-amplitudes were significantly larger in the experimental than in the control condition, F(1, 31) = 4.64, P = 0.039, ηG2 = 0.10 (Figure 3C and D). The remaining effects were not significant, all Fs < 2.46, all Ps > 0.093. For the LP (Figure 4A–C), there were no significant effects in both time windows, all Fs < 2.49, all Ps > 0.091. Because the N1-amplitudes differed between conditions, we tested whether the N1-amplitudes could account for the observed differences in conformity, drift rate and distance between the decision thresholds. However, in both conditions, N1-amplitudes were not correlated with these variables, all rs < 0.20, all Ps > 0.48. Taken together, these results show that social influence per se altered early attentional processes. However, this effect does not explain conformity.
Fig. 3.
Early Stimulus evoked potentials. CON, the majority response is congruent to the stimulus; AMB, the congruency between majority response and stimulus is ambiguous; INC, the majority response is incongruent to the stimulus. (A) ERP-Waveforms at O1, Oz and O2 electrodes as a function of congruency relevance condition (lines) and time (x-axis). Time domains of P1 and N1 are labeled. (B) Mean P1-amplitudes and standard errors pooled over all clusters of electrodes as a function of congruency (x-axis) and condition (lines). Conditions did not differ significantly, P = 0.302. (C) Mean N1-amplitudes and standard errors pooled over all clusters of electrodes as a function of congruency (x-axis) and condition (lines). Conditions differ significantly, P = 0.039. (D) Topographic scalp map showing the difference in N1-amplitude between control and experimental condition.
Fig. 4.
Late positivity. CON, the majority response is congruent to the stimulus; AMB, the congruency between majority response and stimulus is ambiguous; INC, the majority response is incongruent to the stimulus. (A) ERP-Waveforms at Cz, CPz and Pz electrodes as a function of congruency (lines) condition (lines) and time (x-axis). LP 1 = first time window of the late positivity, LP 2 = second time window of the late positivity. (B) Mean LP-1-amplitudes and standard errors pooled over all clusters of electrodes as a function of congruency (x-axis) and condition (lines). Conditions did not differ significantly, P = 0.520. (C) Mean LP-2-amplitudes and standard errors pooled over all clusters of electrodes as a function of congruency (x-axis) and condition (lines). Conditions did not differ significantly, P = 0.916.
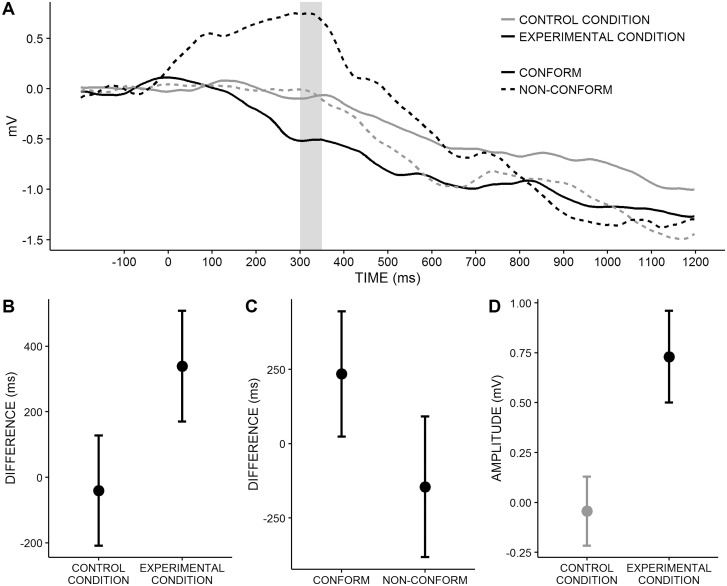
Lateralized readiness potentials. Onset latencies (Figure 5A and B) were investigated to test whether social influence altered the speed of stimulus processing. Conditions did not differ in onset latencies, F(1, 29) = 0.17, P = 0.681. However, there was a main effect for response type, F(1, 29) = 6.39, P = 0.017, which was qualified by an interaction between condition and response type, F(1, 29) = 10.33, P = 0.003. Pairwise comparisons revealed that, in the control condition, participants took the same time to process stimuli whether they conformed to the majority or not, t(18) = 0.45, P = 0.329, MD = −40.5 ms, 95% CI [−208.7, 127.7]. In contrast, in the experimental condition, participants processed stimuli faster before they conformed (vs not conformed) to the majority, t(11) = 7.18, P < 0.001, MD = 339.0 ms, 95% CI [170.1, 508.0]. Between-groups comparisons showed that when participants conformed to the majority, stimuli were processed faster in the experimental than in the control condition, t(29) = 2.28, P = 0.015, MD = 234.4 ms, 95% CI [24.0, 444.9]. In contrast, when they did not conform to the majority, processing speed did not differ between conditions; t(29) = 1.26, P = 0.109, MD = −145.1 ms, 95% CI [−381.8, 91.7].
Fig. 5.
LRPs. CONFORM, responses that conform to the majority response; NON-CONFORM, responses that do not conform to the majority response. (A) LRP-Waveforms as a function of response type (lines), condition (lines) and time (x-axis). Note that a negative deflection indicates activation toward the decision that is finally executed, whereas a positive deflection indicates activation toward the other decision. Time domain of LRP-amplitudes is colored gray. (B) Mean differences in LRP onset latencies and 95%CI between responses non-conform vs conform to the majority as a function of condition (x-axis). (C) Mean differences in LRP onset latencies and 95%CI between control vs experimental condition as a function of response type (x-axis). (D) LRP mean amplitudes and standard errors. Conditions differ significantly, P = 0.011.
In sum, participants in the experimental condition processed stimulus information faster before they adopted the majority response. Additional analyses showed that this effect could not be attributed to response activation prior to stimulus onset (see Supplementary material). Thus, this effect rather suggests that conformity was due to the fact that stimulus information supporting the majority response was accumulated at a higher rate, which in turn led to faster activation of responses in line with the majority. Supporting this notion, onset latencies for responses that conformed to the majority were negatively correlated to the drift rate in the experimental condition, r(10) = −0.60, P = 0.053, where conform responses were driven by social influence. In contrast, they were not correlated in the control condition, r(16) = 0.16, P = 0.531, where conform responses were not driven by social influence but rather by peripheral effects of the irrelevant majority response (see Stahl and Gibbons, 2004, for the use of correlations with jackknifed LRP onsets).
Next, we examined the idea that even when participants in the experimental condition did not conform to the majority, social influence biased stimulus processing. At first glance, the results of the LRP onset latencies speak against this idea because onset latencies of responses that did not conform to the majority responses did not differ between the experimental and the control condition (see above), suggesting that stimulus processing was not biased when participants in the experimental condition did not conform to the majority response. Interestingly, however, a visual inspection of the LRP time courses (Figure 5A and C) revealed that participants in the experimental condition initially activated a response that conformed to the majority response (as indicated by an initial positive deflection of the LRP), even when they finally did not conform. This effect was not present in the control condition. To test this observation in more detail, we examined whether LRP-amplitudes for responses that did not conform to the majority response were higher in the experimental than in the control condition. We calculated the mean amplitudes for 300–350 ms after stimulus onset, which was the time window directly prior to the LRP-onset in the control condition. Confirming our observation, amplitudes were significantly higher in the experimental than in the control condition, t(29) = 2.73, P = 0.011, d = 1.04. This finding provides support for the idea that even when participants in the experimental condition did not conform to the majority, social influence biased the information uptake at the beginning of stimulus processing, which led to a preliminary activation of a response that conformed to the majority.
Discussion
This study examined how social influence alters perceptual decision-making by combing three complementary methods: SEPs, LRPs and diffusion model analyses. Specifically, we employed a newly adapted paradigm (introduced by Germar et al., 2014) that allowed us to separate the pure effects of social influence from priming or cueing effects. We found that social influence affected perceptual decision-making in two ways. First, social influence altered early attentional processes (N1 effects) and led to more careful stimulus processing (increased threshold separation). Second, social influence induced conformity via biased stimulus processing (drift rate and LRP effects).
Social influence increases intensity of stimulus processing
N1-amplitudes were larger in the experimental than in the control condition suggesting that social influence per se increases early attentional resources for stimulus identification and discrimination (Vogel and Luck, 2000; Hopf et al., 2002). In addition, our diffusion analysis replicated that social influence leads to careful stimulus processing (i.e. a larger distance between decision thresholds, Germar et al., 2014). Both effects may be due to the fact that participants in the experimental condition increased their task effort compared to those in the control condition because majority members were able to track participants’ performance in the former but not in the latter condition. This interpretation is supported by two recent studies showing that the presence of peers during a task leads to increased effort (Gilman et al., 2015) and increased early attentional resources (Santamaria-Garcia et al., 2013).
Unlike Trautmann-Lengsfeld and Herrmann (2013, 2014), we did not find SEP effects depending on the congruency between majority responses and stimulus. This could be due to the differences in the employed paradigms. In contrast to the experimental paradigm employed in our study, in the Trautmann-Lengsfeld and Herrmann studies majority responses contained spatial information (position of target). Thus, their effects might be specifically due to the fact that participants (endogenously) attended to the side indicated by the majority responses (Vogel and Luck, 2000; Hopf et al., 2002).
Although we found that social influence per se altered early attentional processes, this effect was neither related to conformity nor to the drift rate (i.e. the bias in stimulus processing). This might seem surprising since our analyses of LRP onset latencies revealed that social conformity was characterized by biased stimulus processing, which was further supported by the result that this effect was related to the drift rate. However, we suppose that this divergence can be explained by the different nature of the two electrophysiological measures and the neurocognitive processes captured by them. On the one hand, the N1 is considered to specifically reflect the operation of a discrimination process applied to an attended stimulus. In contrast, the N1 does not depend on how difficult the discrimination of this stimulus is (i.e. how ambiguous stimulus information is, Vogel and Luck, 2000). Thus, in our study, the N1 could probably not capture whether social influence (i.e. the congruency between majority response and stimulus) altered the difficulty to discriminate what color dominated a given stimulus. As argued above, we think that the N1 rather reflects how many neuronal resources were generally mobilized for the discrimination task irrespective of stimulus difficulty. On the other hand, we used LRP onset latencies as an indirect measure of stimulus processing speed. Hence, as the drift rate and in contrast to the N1, this measure allowed us to more directly test the idea of whether social influence leads individuals to process stimulus information supporting the majority response faster than stimulus information contradicting the majority response.
Social conformity is due to biased stimulus processing
In line with Germar et al. (2014), the drift rate effects observed in this study showed that social influence led to conformity because participants predominantly accumulated stimulus information favoring the majority response. Our LRP analyses additionally support this interpretation. Participants in the experimental condition processed stimulus information faster before they adopted the majority response. Importantly, this could not be attributed to decreased effort in stimulus processing because participants under social influence actually processed stimuli more intensely as described above. Therefore, this effect can only be due to biased stimulus processing, that is, participants under social influence predominantly accumulated stimulus information favoring the majority response, which in turn led to faster activation of decisions in line with the majority.
Additionally, our LRP findings revealed the temporal dynamics of social influence, which could not be tracked by the diffusion model analysis and by other methods used before. Remarkably, participants under social influence initially activated choices in line with the majority even when they finally chose against the majority. This suggests that social influence always biased information uptake at the beginning of stimulus processing. Consequently, participants only made decisions against the majority when they had accumulated enough stimulus information contradicting the majority response after the initial bias in stimulus processing.
Theoretical implications
As participants in our study showed high levels of conformity as well as high processing effort, our results are at odds with those of Schnuerch et al. (2015) and question a wide range of theories that postulate that low processing effort is the main mediating mechanism for social conformity (e.g. conversion theory, elaboration likelihood model, and heuristic-systematic model; for an overview, see Erb and Bohner, 2007). We rather suggest that processing effort may operate as an important moderator of social conformity. To illustrate, suppose that participants in the experimental condition would have processed stimuli less carefully (i.e. invested less time). According to the diffusion model and our LRP analyses, this would have led to more decisions conforming to the majority when stimulus information actually supported decisions against the majority. This is because the probability would be decreased that, despite the initial processing bias caused by social influence, enough stimulus information could be accumulated favoring a decision against the majority. Moreover, our results decisively extend recent findings indicating that social influence can shape how the reward value of a stimulus is computed by the brain (Mason et al., 2009; Zaki et al., 2011), by showing that social influence can shape how sensory information is processed.
Limitations
Before we turn to the conclusion, two limitations of our study should be discussed. First, since we only tested female participants to increase homogeneity of the investigated sample, we cannot generalize our findings to males. However, Germar et al. (2014) did not find gender differences in behavioral conformity or in any of the diffusion model parameters. Additionally, to the best of our knowledge, previous studies or reviews neither reported nor discussed gender differences in the neurocognitive mechanism of social influence (e.g. Schnuerch and Gibbons, 2014; Trautmann-Lengsfeld and Herrmann, 2014). Thus, although research using the Asch paradigm consistently revealed higher levels of conformity for female than for male participants (Bond and Smith, 1996), there is insufficient evidence to suggest that this difference is due to qualitative gender differences in the mediating neurocognitive mechanisms. Consequently, we expect that our results also apply to male participants, which could be tested in further research.
Second, we used the same three levels of orange-to-blue-ratio for all participants to manipulate stimulus ambiguity. Thus, the (perceived) task difficulty varied between participants depending on their individual task ability, which could have influenced the way participants conformed to the majority (e.g. higher perceived task difficulty led to higher levels of conformity). One possibility to control for this source of inter-individual variance is to use stimuli which depend on participants’ individual perceptual thresholds (cf. Trautmann-Lengsfeld and Herrmann, 2013, 2014). Although we generally advocate using stimuli based on the participants’ individual perceptual threshold, we decided against it for three reasons. First, we designed our study to closely follow the experimental paradigm of Germar et al. (2014), who also did not use stimuli based on the participants’ individual perceptual threshold. Second, we followed the standard procedure of previous studies employing diffusion model analysis, which almost exclusively used constant stimuli across participants (Ratcliff and McKoon, 2008, for an exception see Mulder et al., 2012). Third, using stimuli based on the participants’ individual perceptual thresholds leads to inter-individual variability in physical characteristics of stimuli (e.g. individual orange-to-blue-ratios), which in turn could increase inter-individual variability in early exogenous components of SEPs (e.g. P1, N1). Since we tested for effects of social influence on early components in a between-subjects design (i.e. control vs experimental condition), this variability would have translated into error variance decreasing the chance to find existing effects. To overcome the mentioned issues and to decrease error variance from different sources, future studies could benefit from (i) employing within-subjects designs, in which social influence is separated from mere effects due to the presentation of social information and (ii) matching stimuli to participants’ task abilities by measuring their individual perceptual thresholds.
Conclusion
In a nutshell, our results clearly demonstrate that others’ decisions can cause individuals to selectively process stimulus information supporting these decisions, thereby inducing social conformity. This effect is present even when individuals do not blindly follow the majority but rather increase their attentional resources and carefully process stimulus information.
Supplementary data
Supplementary data are available at SCAN online.
Funding
The research reported in this article was supported by a grant from the Volkswagen Foundation for the project “Social conformity: Why do humans and monkeys make weak decisions under social influence?” (Az. 85 148) within the European Platform for Life Sciences, Mind Sciences, and the Humanities.
Supplementary Material
References
- Asch S.E. (1956). Studies of independence and conformity: I. A minority of one against a unanimous majority. Psychological Monographs: General and Applied, 70(9), 1–70. doi:10.1037/h0093718. [Google Scholar]
- Berns G.S., Chappelow J., Zink C.F., Pagnoni G., Martin-Skurski M.E., Richards J. (2005). Neurobiological correlates of social conformity and independence during mental rotation. Biological Psychiatry, 58(3),245–53. doi:10.1016/j.biopsych.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Bindemann M., Burton A.M., Hooge I.T.C., Jenkins R., de Haan E.H.F. (2005). Faces retain attention. Psychonomic Bulletin & Review, 12(6),1048–53. doi:10.3758/BF03206442. [DOI] [PubMed] [Google Scholar]
- Bindemann M., Burton A.M., Langton S.R.H., Schweinberger S.R., Doherty M.J. (2007). The control of attention to faces. Journal of Vision, 7(10),15.1–6. doi:10.1167/7.10.15. [DOI] [PubMed] [Google Scholar]
- Bond R., Smith P.B. (1996). Culture and conformity: a meta-analysis of studies using Asch's (1952b, 1956) line judgment task. Psychological Bulletin, 119(1), 111–37. doi:10.1037/0033-2909.119.1.111. [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. doi:10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Eimer M. (1998). The lateralized readiness potential as an on-line measure of central response activation processes. Behavior Research Methods, Instruments, & Computers, 30(1), 146–56. doi:10.3758/BF03209424. [Google Scholar]
- Erb H.P, Bohner G. (2007). Social influence and persuasion: recent theoretical developments and integrative attempts In: Fiedler K., editor. Frontiers of Social Psychology. Social Communication. New York: Psychology Press, 191–221. [Google Scholar]
- Germar M., Schlemmer A., Krug K., Voss A., Mojzisch A. (2014). Social influence and perceptual decision making: a diffusion model analysis. Personality and Social Psychology Bulletin, 40(2), 217–31. doi:10.1177/0146167213508985. [DOI] [PubMed] [Google Scholar]
- Gilman J.M., Treadway M.T., Curran M.T., Calderon V., Evins A.E. (2015). Effect of social influence on effort-allocation for monetary rewards. PLoS One, 10(5),e0126656. doi:10.1371/journal.pone.0126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.I., Shadlen M.N. (2007). The neural basis of decision making. Annual Review of Neuroscience, 30, 535–74. doi:10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Hopf J.M., Vogel E., Woodman G., Heinze H.J., Luck S.J. (2002). Localizing visual discrimination processes in time and space. Journal of Neurophysiology, 88(4), 2088–95. [DOI] [PubMed] [Google Scholar]
- Jung T.P., Makeig S., Humphries C., et al. (2000). Removing electroencephalographic artifacts by blind source separation. Psychophysiology, 37(2),163–78. doi:10.1111/1469-8986.3720163. [PubMed] [Google Scholar]
- Lopez-Calderon J., Luck S.J. (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. doi:10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M.F., Dyer R., Norton M.I. (2009). Neural mechanisms of social influence. Organizational Behavior and Human Decision Processes, 110(2), 152–9. doi:10.1016/j.obhdp.2009.04.001. [Google Scholar]
- Mattler U., van der Lugt A, Münte T.F. (2006). Combined expectancies: electrophysiological evidence for the adjustment of expectancy effects. BMC Neuroscience, 7, 37. doi:10.1186/1471-2202-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Patterson T., Ulrich R. (1998). Jackknife-based method for measuring LRP onset latency differences. Psychophysiology, 35(1),99–115. [PubMed] [Google Scholar]
- Miller J., Ulrich R., Rinkenauer G. (1999). Effects of stimulus intensity on the lateralized readiness potential. Journal of Experimental Psychology: Human Perception and Performance, 25(5), 1454–71. doi:10.1037/0096-1523.25.5.1454. [DOI] [PubMed] [Google Scholar]
- Mojzisch A., Krug K. (2008). Cells, circuits, and choices: social influences on perceptual decision making. Cognitive, Affective & Behavioral Neuroscience, 8(4), 498–508. doi:10.3758/CABN.8.4.498. [DOI] [PubMed] [Google Scholar]
- Mulder M.J., Wagenmakers E.J., Ratcliff R., Boekel W., Forstmann B.U. (2012). Bias in the brain: a diffusion model analysis of prior probability and potential payoff. The Journal of Neuroscience, 32(7), 2335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce J.W. (2007). PsychoPy—psychophysics software in Python. Journal of Neuroscience Methods, 162(1–2), 8–13. doi:10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce J.W. (2009). Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics, 2 Available: http://journal.frontiersin.org/article/10.3389/neuro.11.010.2008/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R. (1978). A theory of memory retrieval. Psychological Review, 85(2), 59–108. doi:10.1037/0033-295X.85.2.59. [Google Scholar]
- Ratcliff R., McKoon G. (2008). The diffusion decision model: theory and data for two-choice decision tasks. Neural Computation, 20(4), 873–922. doi:10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria-Garcia H., Pannunzi M., Ayneto A., Deco G., Sebastian-Galles N. (2013). ‘If you are good, I get better': the role of social hierarchy in perceptual decision-making. Social Cognitive and Affective Neuroscience, doi:10.1093/scan/nst133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnuerch R., Gibbons H. (2014). A review of neurocognitive mechanisms of social conformity. Social Psychology, 45(6), 466–78. doi:10.1027/1864-9335/a000213. [Google Scholar]
- Schnuerch R., Koppehele-Gossel J., Gibbons H. (2015). Weak encoding of faces predicts socially influenced judgments of facial attractiveness. Social Neuroscience, 10(6), 1–11. doi:10.1080/17470919.2015.1017113. [DOI] [PubMed] [Google Scholar]
- Sherif M. (1935). A study of some social factors in perception. Archives of Psychology (Columbia University), 187 Available: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1936-01332-001&site=ehost-live. Accessed 15 January 2015. [Google Scholar]
- Smulders F.T.Y, Miller J.O. (2012). The lateralized readiness potential In: Kappenman E.S., Luck S. J., editors. The Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press, 209–29. [Google Scholar]
- Stahl J., Gibbons H. (2004). The application of jackknife-based onset detection of lateralized readiness potential in correlative approaches. Psychophysiology, 41(6), 845–60. doi:10.1111/j.1469-8986.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- Trautmann-Lengsfeld S.A., Herrmann C.S. (2013). EEG reveals an early influence of social conformity on visual processing in group pressure situations. Social Neuroscience, 8(1), 75–89. doi:10.1080/17470919.2012.742927. [DOI] [PubMed] [Google Scholar]
- Trautmann-Lengsfeld S.A., Herrmann C.S. (2014). Virtually simulated social pressure influences early visual processing more in low compared to high autonomous participants. Psychophysiology, 51(2),124–35. doi:10.1111/psyp.12161. [DOI] [PubMed] [Google Scholar]
- Ulrich R., Miller J. (2001). Using the jackknife-based scoring method for measuring LRP onset effects in factorial designs. Psychophysiology, 38(5), 816–27. doi:10.1111/1469-8986.3850816. [PubMed] [Google Scholar]
- Vogel E.K., Luck S.J. (2000). The visual N1 component as an index of a discrimination process. Psychophysiology, 37(02),190–203. [PubMed] [Google Scholar]
- Voss A., Nagler M., Lerche V. (2013). Diffusion models in experimental psychology. Experimental Psychology (Formerly Zeitschrift Für Experimentelle Psychologie), 60(6), 385–402. doi:10.1027/1618-3169/a000218. [DOI] [PubMed] [Google Scholar]
- Voss A., Rothermund K., Voss J. (2004). Interpreting the parameters of the diffusion model: an empirical validation. Memory & Cognition, 32(7), 1206–20. [DOI] [PubMed] [Google Scholar]
- Zaki J., Schirmer J., Mitchell J.P. (2011). Social influence modulates the neural computation of value. Psychological Science, 22(7), 894–900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.