Abstract
A large body of evidence in animals and humans implicates the amygdala in promoting memory for arousing experiences. Although the amygdala can trigger threat-related noradrenergic-sympathetic arousal, in humans amygdala activation and noradrenergic-sympathetic arousal do not always concur. This raises the question how these two processes play a role in enhancing emotional declarative memory. This study was designed to disentangle these processes in a combined subsequent-memory/fear-conditioning paradigm with neutral items belonging to two conceptual categories as conditioned stimuli. Functional MRI, skin conductance (index of sympathetic activity), and pupil dilation (indirect index of central noradrenergic activity) were acquired throughout procedures. Recognition memory for individual items was tested 24 h later. We found that pupil dilation and skin conductance responses were higher on CS+ (associated with a shock) compared with CS− trials, irrespective of later memory for those items. By contrast, amygdala activity was only higher for CS+ items that were later confidently remembered compared with CS+ items that were later forgotten. Thus, amygdala activity and not noradrenergic-sympathetic arousal, predicted enhanced declarative item memory. This dissociation is in line with animal models stating that the amygdala integrates arousal-related neuromodulatory changes to alter mnemonic processes elsewhere in the brain.
Keywords: memory encoding, fear conditioning, arousal, amygdala, functional MRI
Introduction
The amygdala has been shown to be critically involved in promoting memory in both animals (McGaugh, 2004; Roozendaal and McGaugh, 2011) and humans (LaBar and Cabeza, 2006; Murty et al., 2011). The increased retention found for emotional declarative memory is thought to be driven by arousal (Cahill and McGaugh, 1995). Indeed, efferent pathways from the (central nucleus of the) amygdala are involved in regulating arousal-related autonomic, endocrine, neuromodulatory, and behavioral responses to threat (LeDoux et al., 1988; Reyes et al., 2011). In human fear-conditioning experiments, however, amygdala activity is often absent even though a sympathetic arousal response (e.g. skin conductance) is robustly measured (see Mechias et al., 2010; Bach et al., 2011; Fullana et al., 2015). This indicates that amygdala activity and arousal-related sympathetic activity do not always coincide. Therefore, these findings raise the question what exact roles these two processes play in enhancing emotional declarative memory.
Early studies investigating declarative memory have shown that arousal at the time of encoding is associated with enhanced memory. For example, stimuli that are perceived as more arousing (Bradley et al., 1992) or stimuli that elicit a sympathetic arousal response, as measured using skin conductance responses (SCRs; (Kleinsmith and Kaplan, 1963; Buchanan et al., 2006), are typically well remembered. Functional neuroimaging work on the amygdala, which normally lacks the resolution to dissociate amygdala subregions, has revealed activation of this structure related to processing of arousing material, such as threatening or salient stimuli and faces (Morris et al., 1997; Whalen et al., 1998; Vuilleumier et al., 2001; Hariri et al., 2002). Amygdala activity during encoding furthermore predicts later memory for such stimuli (Hamann, 2001; Erk et al., 2003; Dolcos et al., 2004; Richardson et al., 2004; LaBar and Cabeza, 2006; Murty et al., 2011). Indeed, subsequent memory-related amygdala activation at the time of encoding seems to correspond with subjective arousal (Canli et al., 2000).
These findings are in line with a role for the amygdala in activating arousal-related autonomic responses to threat (Chapman et al., 1954; Kaada et al., 1954; Reis and LeDoux, 1987; Gläscher and Adolphs, 2003; Reyes et al., 2011). Even though this could be a potential pathway through which mnemonic processes are altered, there is also data demonstrating that noradrenergic manipulations are ineffective in modulating memory in the absence of a functional amygdala (Liang et al., 1982; Cahill and McGaugh, 1991). Such findings indicate that amygdala activation during encoding of arousing material observed in humans may alternatively be a consequence of arousal-related noradrenergic-sympathetic activation, and reflect a modulation of mnemonic processing of the arousing material elsewhere in the brain (Van Stegeren et al., 1998; Ferry and McGaugh, 1999; McGaugh, 2004; Strange and Dolan, 2004; McIntyre et al., 2005; Roozendaal et al., 2008; Roozendaal and McGaugh, 2011). Existing human neuroimaging studies on emotional declarative memory, however, are inconclusive about these interpretations. In these paradigms, amygdala activity and noradrenergic-sympathetic arousal cannot be disentangled because the to-be-remembered stimuli are arousing by themselves (i.e. the arousing items later remembered might be more arousing than arousing items later forgotten). It is therefore unclear whether the amygdala activity found for items later remembered reflects neural activity associated with the initiation of a noradrenergic-sympathetic arousal response or an enhancement of mnemonic processing induced by this response.
There are also human neuroimaging findings that challenge the view of a tight coupling between amygdala activity and noradrenergic-sympathetic arousal responses. Dissociations between these two responses are often seen in neuroimaging experiments using classical fear conditioning, a widely used model for fear learning in which a neutral stimulus is associated with an unconditioned stimulus (UCS) such as an electrical shock. After acquisition of the fear association, participants exhibit robust and persistent noradrenergic-sympathetic arousal responses to the previously neutral stimulus (LaBar et al., 1998; Maren, 2001). Although lesion studies in humans indicate that the amygdala is necessary to acquire conditioned fear (Bechara et al., 1995; LaBar et al., 1995; Klumpers et al., 2014), a persistent amygdala response during the expression of conditioned fear is usually not observed (see Mechias et al., 2010; Bach et al., 2011; Fullana et al., 2015). This latter finding is in line with data from nonhuman primates showing that the amygdala is not necessary for the expression of conditioned fear (Antoniadis et al., 2009). These studies show that a noradrenergic-sympathetic arousal response to conditioned stimuli does not require activation of the amygdala. Thus, existing data from human fear conditioning experiments reveal a clear dissociation between amygdala activation and noradrenergic-sympathetic arousal responses, but cannot establish what the roles of these two processes are in enhancing declarative memory.
We therefore designed a functional MRI study to disentangle the roles of these two processes by orthogonalizing arousal and item memory. Participants took part in a combined subsequent-memory/fear-conditioning paradigm with neutral items belonging to two conceptual categories as stimuli (Dunsmoor et al., 2012). During encoding, items of one of the two categories (CS+; counterbalanced across participants) were paired with an aversive electrical shock in 50% of the presentations, while items of the other category (CS−) were never reinforced. In contrast to typical emotional memory paradigms, the specific item itself therefore does not trigger noradrenergic-sympathetic arousal responses. Participants returned to the lab 24 h later for a recognition test in which they were shown the items seen during encoding and new items they had not seen before. Subsequent memory effects during encoding were tested by separating confidently remembered items from misses and unsure hits (i.e. forgotten items). Physiological responses to CS+ and CS− items were measured using skin conductance (an index of sympathetic activation; Lang et al., 1993) and pupil dilation (an indirect index of locus coeruleus-noradrenergic activity; Aston-Jones and Cohen, 2005; Bradley et al., 2008; Gilzenrat et al., 2010). We reasoned that if the role of the amygdala in emotional enhancement of declarative memory is to modulate mnemonic processing of the to-be-remembered material rather than to generate the noradrenergic-sympathetic arousal response, then (i) amygdala activation should predict subsequent memory for items belonging to the CS+ category, but not show a differential conditioning effect (CS + > CS−); and (ii) noradrenergic-sympathetic activation should show a robust differential conditioning effect, but should not be directly associated with subsequent item memory.
Methods
Participants
Twenty-four right-handed healthy volunteers [12 female, 12 male; 19–32 years (mean = 23.25)] took part in the study. An additional seven participants did not complete the entire experiment due to apparatus failure or non-compliance with instructions. Exclusion criteria were: current or lifetime history of psychiatric, neurological or endocrine illness, abnormal hearing or (uncorrected) vision, average use of more than 3 alcoholic beverages daily, current treatment with any medication that affects central nervous system or endocrine systems, average use of recreational drugs weekly or more, habitual smoking, predominant left-handedness, intense daily physical exercise and any contraindications for MRI. All participants gave written informed consent and were paid for their participation. This study was approved by the local ethical review board (CMO region Arnhem-Nijmegen).
Design and procedure
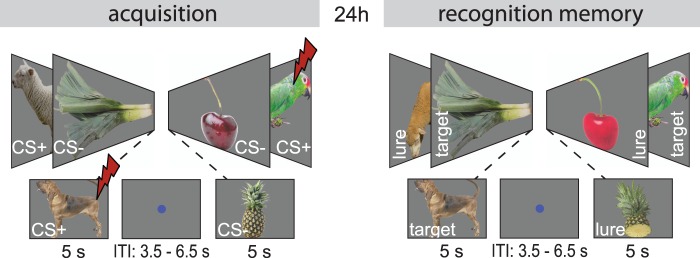
Participants were tested in a subsequent-memory/fear-conditioning paradigm (see Figure 1) including neutral items belonging to two distinct conceptual categories. In 50% of the trials, one category was paired to an electrical shock (i.e. UCS). On day 1, first, the intensity of electrical shock was adjusted individually using a standardized procedure (see below). Following this procedure, participants underwent the subsequent-memory/fear-conditioning paradigm. Twenty-four hours later, recognition memory was tested for the individual items presented during encoding. This test also included the same amount of unseen lures. Which items served a targets and which ones as lures was randomized across subjects. Additionally, the experimental procedure included a category representation localizer paradigm and resting-state blocks. Analyses on these data are reported elsewhere (De Voogd et al., 2016). All experiments were programmed using Presentation software (Version 0.70, www.neurobs.com).
Fig. 1.
Overview of the experimental design. The experiment took place on two consecutive days. During acquisition, items from one of the two categories (CS+) were associated with an electrical shock. CS+ and CS− items were shown in pseudo-random order during the conditioning blocks (32 CSs per block). CS+s co-terminated with shock on 50% of the acquisition trials. During recognition, pictures from acquisition (64) were mixed with lures (64). Participants had to indicate whether it was an old or a new picture. Responses included three confidence bins (very sure, sure, unsure). ITI, inter trial interval.
Stimuli
Stimuli consisted of 128 items which were either animals or fruits/vegetables. We excluded items with a higher threat value (such as lions and snakes) to avoid additional arousal and facilitated conditioning (Öhman and Mineka, 2001). The pictures were selected from the Hemera Photo-Objects set (http://hemera-technologies-inc.software.informer.com) and publicly available resources on the internet. Luminance of all pictures, including the grey background, was equalized.
Subsequent-memory/fear-conditioning paradigm
The encoding paradigm included 32 CS+ items (50% reinforcement rate) and 32 CS− items (Dunsmoor et al., 2012). Which of the two categories (animals or fruit/vegetables) served as CS+ was randomly counterbalanced across participants. The paradigm included two acquisition blocks and each block comprised 16 CS+ and 16 CS− items presentations, each with a 5 s duration. The intertrial interval (ITI) varied randomly between 3.5 and 6.5 s. Items were presented in a pseudorandom order with no more than three repetitions of the same category. Participants were instructed to figure out the relationship between the categories and the UCS, but did not do any other task when viewing the items. Sympathetic arousal and amygdala activity was measured in response to the individual items.
Item recognition memory test
The recognition test contained all 64 items presented during encoding (targets) with an additional 64 new items (lures), each with 5 s duration. The ITI varied randomly between 3.5 and 6.5 s. The lures were similar to the targets to prevent ceiling effects, which would make it impossible to test for subsequent memory effects. For example, if during encoding a dog was presented, then one of the lures was also a dog, but a different one. Participants were instructed to indicate whether they had seen the picture before, or whether it was a new picture. Response options consisted of three confidence bins (very sure, sure, unsure). Items were presented in a consecutive order. The presentation order of targets and lures was random.
For the subsequent memory analyses, we only included the very sure and sure hits in the remembered category to restrict this category to confident memory and not guesses (see Murray and Ranganath, 2007; Takashima et al., 2006; Turk-Browne et al., 2006; Wagner et al., 1998). Instead of omitting the unsure hits, we collapsed these with the misses to accommodate the low number of misses (i.e. too low to reliably estimate subsequent memory effects). Memory accuracy increased with confidence [F(2,46)=78.85, P = 1.37E-15, Pη2=0.77] and was higher for the very sure [F(1,23)=179.216, P = 2.42E-12, Pη2 = 0.89] as well as the sure [F(1,23)=17.951, P = 3.12E-4, Pη2 = 0.44] bins compared with unsure bins. Although at the group level, there was still above-chance level performance in the unsure bin [F(1,21)=15.721, P = 0.001, Pη2 = 0.43], at the individual level, there were on average only 1.6 unsure hit trials more than unsure false alarm trials per participant. The vast majority of the unsure hit trials is therefore likely to reflect forgotten items that were correctly guessed. We therefore define forgotten items as a combination of unsure hits and all misses.
Measurements of sympathetic arousal
Electrodermal activity was assessed using two Ag/AgCl electrodes attached to the distal phalanges of the first and second finger of the left hand using a BrainAmp MR system and recorded using BrainVision Recorder software (Brain Products GmbH, Munich, Germany). SCRs were analyzed using in-house software implemented in Matlab 7.14 (MathWorks). SCR amplitudes were determined for each trial within a latency window from 1 to 5 s after stimulus onset, where the peak could only occur 500 ms after baseline. Responses were square root-transformed prior to statistical analysis. Pupil dilation was measured using an MR-compatible eye tracking system (MEye Track-LR camera unit, SMI, SensoMotoric Instruments). Data were analyzed using in-house software (Hermans et al., 2013) implemented in Matlab 7.14 (MathWorks), which was based on methods described previously by others (Siegle et al., 2003). Eyeblink artifacts were identified by differentiating the signal to detect eye pupil changes occurring too rapidly (<60 ms) to represent actual dilation. Blinks were removed from the signal using linear interpolation. Pupil diameter for each trial was normalized by dividing the signal with the average of 1 s pre-stimulus onset baseline. The averaged baseline-corrected pupil diameter within a 1 to 5 s window during picture presentation was used as response measure. Statistical analyses on SCR and pupil dilation were done by comparing later remembered (confident hits) and later forgotten (misses and unsure hits) items for both CS types.
Physiological noise correction
Finger pulse was recorded using a pulse oximeter affixed to the third finger of the left hand. Respiration was measured using a respiration belt placed around the participant’s abdomen. Pulse and respiration measures were used for retrospective image-based correction (RETROICOR) of physiological noise artifacts in BOLD-fMRI data (Glover et al., 2000). Raw pulse and respiratory data were processed offline using in-house software for interactive visual artifact correction and peak detection, and were used to specify fifth-order Fourier models of the cardiac and respiratory phase-related modulation of the BOLD signal (Van Buuren et al., 2009), yielding 10 nuisance regressors for cardiac noise and 10 for respiratory noise. Additional regressors were calculated for heart rate frequency, heart rate variability, (raw) abdominal circumference, respiratory frequency, respiratory amplitude, and respiration volume per unit time (Birn et al., 2006), yielding a total of 26 RETROICOR regressors.
Peripheral stimulation
Electrical shocks were delivered via two Ag/AgCl electrodes attached to the distal phalanges of the second and third finger of the right hand using a MAXTENS 2000 (Bio-Protech) device. Shock duration was 200 ms, and intensity varied in 10 intensity steps between 0 and 40 V/0 and 80 mA. During the standardized shock intensity adjustment procedure, each participant received and subjectively rated five shocks, allowing shock intensity to converge to a level experienced as uncomfortable, but not painful. The resulting average intensity step was 5.5 (SD: 2.0) on a scale from 1 to 10 intensity steps.
MRI data acquisition and multi-echo weighting
MRI scans were acquired using a Siemens (Erlangen, Germany) MAGNETOM Skyra 3.0T MR scanner. T2*-weighted blood oxygenation level-dependent (BOLD) images were recorded using a customized multi-echo EPI sequence with ascending slice acquisition (37 axial slices; TR, 2.38 s; TE, 15 and 36 ms; Generalized Autocalibrating Partially Parallel Acquisitions (Griswold et al., 2002) acceleration factor 4; flip angle, 90°; slice matrix size, 106 × 106; slice thickness, 2.0 mm; slice gap, 0.26 mm; field of view (FOV), 212 × 212 mm; bandwidth: 1748 Hz/px; echo spacing: 0.7 ms). The functional scans only had partial brain coverage, which was aligned to the temporal pole and included the amygdala and (partially) the dorsal anterior cingulate cortex. To allow for correction of distortions due to magnetic field inhomogeneity, we acquired field maps using a dual echo 2D gradient-echo sequence (64 axial slices; TR, 1020 ms; TE, 10 ms and 12.46 ms; flip angle, 90°; slice matrix size, 64 × 64, slice thickness, 2 mm; FOV, 224 × 224 mm). A high-resolution structural image (1 mm isotropic) was acquired using a T1-weighted 3D magnetization-prepared rapid gradient-echo sequence (MP-RAGE; TR, 2.3 s; TE, 3.03 ms; flip angle, 8°; FOV, 256 × 256 × 192 mm).
To correct EPI images for head motion, geometric distortions due to magnetic field inhomogeneity, and interactions between these, we used an integrated fieldmap-based unwarp/realign method (Hutton et al., 2002). Unwarping and realignment parameters were estimated from the first echo and applied to both echoes. Next, to account for regional variation in susceptibility-induced signal dropout, voxel-wise weighted sums of both echoes were calculated based on local contrast-to-noise ratio (Poser et al., 2006).
MRI data preprocessing and analyses
MRI data for the subsequent-memory/fear-conditioning paradigm were pre-processed in standard stereotactic (MNI152) space (using SPM8; http://www.fil.ion.ucl.ac.uk/spm; Wellcome Department of Imaging Neuroscience, London, UK). Mutual information maximization based rigid body registration was used to register structural and (motion and geometric distortion-corrected) functional images. Structural images were segmented into grey matter, white matter and cerebrospinal fluid (CSF) images using a unified probabilistic template registration and tissue classification method (Ashburner and Friston, 2005). Tissue images were then registered with site-specific tissue templates (created from 384 T1-weighted scans) using DARTEL (Ashburner, 2007), and registered (using an affine transformation) with the MNI152 template included in SPM8. Identical transformations were applied to all functional images, which were resliced into 2 mm isotropic voxels and smoothed with a 6 mm FWHM Gaussian kernel.
For statistical analyses, responses to CS+ remembered items (confident hits), CS+ forgotten items (misses and unsure hits), CS− remembered items (confident hits), CS− forgotten items (misses and unsure hits), and shocks were estimated using a finite impulse response (FIR) model which included the two runs. This first-level model makes no assumptions regarding the haemodynamic response function (HRF) shape, and yields independent response estimates for all 6 TR bins within the peri-stimulus time histogram. The last bin before CS offset, but still before the shock onset was used to provide the best possible estimate of the peak of the BOLD response to CSs. With this procedure, responses to CS+ trials can be fully separated from those to shocks. To verify this, we performed a separate FIR model in which everything was the same except for the regressors of interest. We included CS+ reinforced, CS+ unreinforced and CS− trial as regressors and compared the CS+ reinforced and the CS+ unreinforced trials in a direct contrast for the same bin. This did not yield any whole brain differences in CS response estimates [family-wise error (FWE) P < 0.05, whole-brain cluster corrected, or within the amygdala after small volume correction (SVC)]. The first-level models additionally included six movement parameter regressors (three translations, three rotations) derived from rigid body motion correction, 26 RETROICOR physiological noise regressors (see above), high pass filtering (1/128 Hz cut-off), and AR(1) serial correlations correction. Single-subject contrast maps obtained from first-level analyses for the four conditions were entered into a second-level factorial ANOVA to test for the interaction and a second-level random effects analyses (one sample t-test) for additional simple effect analyses. We used a cluster-forming voxel-level threshold of P < 0.005 (uncorrected). Alpha was set at .05, whole-brain FWE corrected at the cluster level using Gaussian Random Field Theory based methods (Friston et al., 1996). Based on a priori hypotheses, results for amygdala were corrected for a reduced search volume using SVCs based on an anatomical mask of the amygdala (Automated Anatomical Labeling atlas; Tzourio-Mazoyer et al., 2002).
Results
Item recognition memory test
Memory accuracy in the item recognition test was assessed by comparing the hit rates and false alarm rates for the CS+ and CS− items. Overall performance was above chance level [overall hit rate > false alarm rate; F(1,22)=153.65, P = 2.13E-11, Pη2 = 0.88]. There was no accuracy difference between the CS+ and CS− items [F(1,22) = 0.07, P = 0.80, Pη2 = 0.003]. We found a non-significant trend towards a more liberal response bias (i.e. tendency to say ‘old’) for the CS+ items [F(1,22) = 2.857, P = 0.11, Pη2 = 0.12]. See Tables 1 and 2 for descriptive statistics.
Table 1.
Grouping of number of trials based on subsequent memory performance
| Item recognition memory test |
||||
|---|---|---|---|---|
| Misses | Hits |
|||
| Unsure | Sure | Very sure | ||
| CS+r | 4.83 (2.76) | 2.25 (1.65) | 3.63 (2.28) | 4.96 (2.44) |
| CS+ur | 5.42 (2.83) | 2.58 (2.24) | 3.46 (1.67) | 4.29 (2.87) |
| Total | 10.25 (4.95) | 4.83 (3.24) | 7.07 (3.32) | 9.25 (4.95) |
| CS− | 12.08 (4.28) | 4.50 (3.08) | 6.88 (3.29) | 8.08 (5.29) |
Notes: Very sure and sure were grouped as later remembered and misses and unsure hits were grouped as later forgotten. r, reinforced; ur, unreinforced.
Table 2.
Proportion of memory performance based on confidence interval
| Hit rate |
False alarm rate |
|||||
|---|---|---|---|---|---|---|
| Unsure | Sure | Very sure | Unsure | Sure | Very sure | |
| CS+r | 0.56 (0.33) | 0.63 (0.27) | 0.82 (0.19) | 0.41 (0.32) | 0.25 (0.23) | 0.23 (0.30) |
| CS+ur | 0.52 (0.27) | 0.62 (0.22) | 0.81 (0.30) | 0.46 (0.27) | 0.23 (0.22) | 0.16 (0.24) |
| Total | 0.56 (0.28) | 0.62 (0.19) | 0.80 (0.21) | 0.45 (0.24) | 0.25 (0.18) | 0.18 (0.23) |
| CS− | 0.53 (0.25) | 0.56 (0.20) | 0.77 (0.19) | 0.36 (0.20) | 0.25 (0.17) | 0.12 (0.11) |
Physiological measures
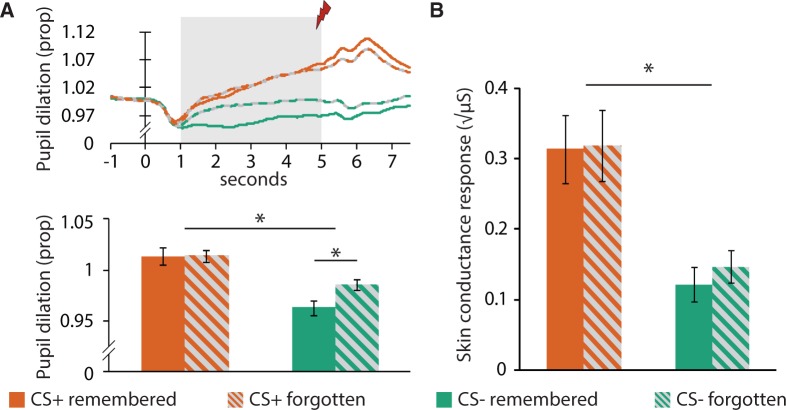
First, our sympathetic arousal measures revealed robust differential conditioning effects. SCRs [F(1,23) = 19.975, P = 1.75E-4, Pη2 = 0.47] as well as pupil dilation responses [F(1,23) = 27.58, P = 2.50E-5, Pη2 = 0.55] were higher for CS+ items compared with CS− items. There was no difference in SCRs between items later remembered (confident hits) and items later forgotten (misses and unsure hits) [F(1,23) = 0.1.681, P = 0.21, Pη2 = 0.07], and no interaction between CS type (CS+, CS−) and memory (Remembered, Forgotten) [F(1,23) = 0.562, P = 0.46, Pη2 = 0.02] in SCRs. For pupil dilation responses, we did find an interaction between CS type (CS+, CS−) and later memory (Remembered, Forgotten) [F(1,23) = 5.49, P = 0.03, Pη2 = 0.21]. An unexpected finding, however, was that this interaction was driven by an increased pupil dilation for CS− items that were later forgotten compared with CS− items that were later remembered [t(23) = 3.098, P = 0.005, D = 1.29]. Pupil dilation was similar for CS+ items that were later remembered vs later forgotten [t(23) = 0.82, P = 0.94, D = 0.34]. In conclusion, we found a differential conditioning effect in the sympathetic arousal measures, however, sympathetic arousal to CS+ items did not predict item memory. See Figure 2.
Fig. 2.
Behavioral and physiological results. (A) Pupil dilation responses related to memory formation for both CS types and averaged across encoding trials that were later forgotten and those that were later remembered. The top graph shows the baseline corrected time course and the bottom graph the average of the 1–5 s latency window. (B) SCRs related to memory formation for both CS types and encoding trials that were later forgotten and those that were later remembered. Rem, trials later remembered; For, trials later forgotten.
Functional MRI
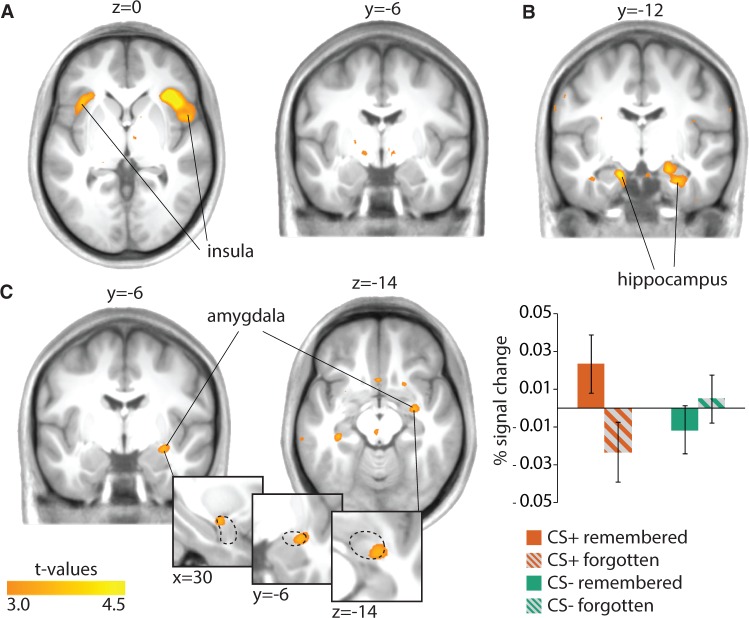
We then tested whether there was a differential conditioning response in the amygdala, however this was not the case (no voxels exceeding the clustering threshold of P < 0.005, uncorrected). Whole-brain analyses showed activation in the anterior insula, left [cluster size = 3376 mm3, cluster P = 0.002, whole-brain corrected] and right [cluster size = 9480 mm3, cluster P = 4.843E-08, whole-brain corrected] in response to the CS+ items vs CS− items. Deactivations were found in the ventral medial prefrontal cortex [cluster size = 3784 mm3, cluster P = 0.001, whole-brain corrected]. See Table 3 and Figure 3A.
Table 3.
Peak voxel coordinates and cluster statistics and size for the subsequent-memory/fear-conditioning paradigm
| Region | Side | x(mm) | y(mm) | z(mm) | Z-score | Cluster p | Size (mm3) |
|---|---|---|---|---|---|---|---|
| CS + > CS − | |||||||
| Anterior insula | R | 36 | 26 | 2 | 6.32 | 4.843E-08 | 9480 |
| Anterior insula | L | −30 | 24 | −4 | 4.53 | 0.002 | 3376 |
| Supramarginal gyrus | R | 60 | −46 | 26 | 4.31 | 0.007 | 2696 |
| CS− > CS + | |||||||
| Ventromedial prefrontal cortex | R/L | 0 | 58 | −6 | 4.30 | 0.001 | 3784 |
| Remembered > forgotten | |||||||
| Superior occipital gyrus/Cuneus/Precuneus | R | 26 | −68 | 42 | 4.86 | 9.281E-08 | 9040 |
| Fusiform gyrus/Inferior occipital gyrus | L | −30 | −60 | −14 | 4.74 | 1.110E-16 | 26 360 |
| Inferior frontal gyrus | R | 48 | 34 | 12 | 4.74 | 8.064E-06 | 6216 |
| Parahippocampal gyrus/hippocampus | L | −16 | −12 | −22 | 4.65 | 0.018 | 2256 |
| Inferior orbital frontal cortex | L | −36 | 32 | −14 | 4.55 | 0.001 | 3776 |
| Fusiform gyrus/Inferior occipital gyrus | R | 52 | −62 | −12 | 4.53 | 1.464E-07 | 8736 |
| Parahippocampal gyrus/hippocampus | R | 26 | −12 | −28 | 3.82 | 0.013 | 2400 |
| Supramarginal gyrus | L | −58 | −22 | 36 | 3.46 | 0.038 | 1944 |
| Interaction | |||||||
| Amygdala | R | 30 | −6 | −14 | 3.43 | 0.048 (SVC) | 168 |
| CS+ remembered > CS− forgotten | |||||||
| Amygdala | R | 28 | −8 | −14 | 3.97 | 0.044 (SVC) | 184 |
Notes: All coordinates are defined in MNI152 space. All reported statistics are significant at P < 0.05, cluster-level corrected for the whole brain unless indicated otherwise (SVC).
Fig. 3.
Subsequent memory analyses of the categorical fear conditioning paradigm. (A) Brain activation for the contrast CS + > CS − (bilateral insula). (B) Brain activation for the contrast Remembered > Forgotten (bilateral hippocampus). (C) Brain activation for the interaction CS type and later memory (right amygdala). The dashed line illustrates the outline of the Automated Anatomical Labeling amygdala mask. Post hoc test shows increased amygdala activity for CS+ remembered vs CS+ forgotten (P = 0.04, Small Volume Corrected). For visualization purposes a threshold of P < 0.005 uncorrected was used. For corrected inferential statistics based on cluster size see Table 3. Extracted average from the contrast estimates from the significant cluster from the interaction contrast in the amygdala is plotted in a bar graph for illustration purposes.
Next, we tested whether there was an interaction between CS type (CS+, CS−) and subsequent memory (Remembered, Forgotten). We found a significant cluster in the right amygdala (cluster P = 0.048, SVC). As expected, amygdala activity was higher for CS+ items later remembered than for CS+ items later forgotten (cluster P = 0.044, SVC). There was no difference between the CS− items later remembered and CS− items later forgotten (no voxels exceeding the clustering threshold of P < 0.005, uncorrected). As can be seen from Figure 3C, the interaction cluster seems to lie toward the edge of the dorsal part of the AAL amygdala mask. We therefore performed additional analyses to ensure we can attribute the activation cluster to the amygdala. These analyses show that 64% of all voxels of that cluster are within the mask, including the peak voxel (t = 3.55, P = 0.021 FWE-SVC voxel level). The activation cluster is not part of another, bigger cluster and the remaining voxels outside of the mask fall within white matter and not within another structure. Furthermore, when we increase the whole-brain cluster-defining threshold from P < 0.005 to < 0.001 we see that the percentage of voxels that fall within the mask increases to 82% (P = 0.017, FWE-SVC cluster level). Thus, the central part of the cluster (including the peak voxel) is within the AAL amygdala mask and we therefore attribute the cluster to the amygdala.
Last, for the main effect of subsequent memory (Remembered, Forgotten) we found activations in the hippocampus extending into the parahippocampal gyrus, left [cluster size = 2256 mm3, cluster P = 0.018, whole-brain corrected] and right [cluster size = 2400 mm3, cluster P = 0.013, whole-brain corrected] and fusiform gyrus, left [cluster size = 26 360 mm3, cluster P = 1.11E-16, whole-brain corrected] and right [cluster size = 8736 mm3, cluster P = 1.46E-07, whole-brain corrected], among others (see Table 3). There were no significant deactivations. In conclusion, amygdala activity is not enhanced for CS+ items overall, but does predict memory for CS+ items. See Figure 3B.
Discussion
The aim of this study was to disentangle the roles of noradrenergic-sympathetic arousal and the amygdala in emotional declarative memory by using an experimental design in which we were able to orthogonalize arousal (CS+ vs CS−) and subsequent item memory (remembered vs forgotten items). We found that skin conductance and pupil dilation showed a robust differential conditioning effect, but did not predict subsequent item memory. In contrast, amygdala activity did not show a differential conditioning effect, but predicted subsequent item memory specifically for CS+ trials. Thus, we demonstrate a dissociation between the roles of amygdala activation and noradrenergic-sympathetic arousal in emotional declarative memory.
We found robust differential conditioning effects in our noradrenergic-sympathetic arousal measures, but not in the amygdala. This finding seems to contradict findings from the rodent literature showing that the (central nucleus of the) amygdala is involved in regulating autonomic (LeDoux et al., 1988) and noradrenergic responses (Reyes et al., 2011). Indeed, stimulation of the amygdala leads to changes in autonomic responses in both humans and animals (Chapman et al., 1954; Kaada et al., 1954; Reis and LeDoux, 1987). Our null finding, however, is consistent with the human neuroimaging literature on fear conditioning (see Mechias et al., 2010; Bach et al., 2011; Fullana et al., 2015). In humans, differential conditioning effects in the amygdala are often only seen during the first few trials, when fear learning takes place (LaBar et al., 1998; Büchel and Dolan, 2000). Furthermore, using a Pavlovian reversal learning paradigm, it has been shown that BOLD signal in the amygdala tracks an associability signal rather than a reinforcement prediction error signal (Li et al., 2011), meaning that amygdala responsivity is related to the extent to which a cue has previously been accompanied by an unexpected event. Thus, our findings fit with the existing human neuroimaging literature and suggest that, rather than fear expression, activation of the amygdala primarily reflects enhanced encoding of relevant information in ambiguous situations or when the predictive value of information is uncertain (see Whalen et al., 1998; Davis and Whalen, 2001).
Although functional neuroimaging data can only provide correlational evidence, this interpretation is also in line with data from studies on amygdala lesions. Although the rodent literature has indicated that the amygdala plays a crucial role in both fear acquisition and expression (LeDoux, 2003), in primates, the amygdala does not seem to be crucially involved in the expression of conditioned fear (Antoniadis et al., 2009). Human patients with selective bilateral amygdala damage typically show deficits in conditioned fear responses (Bechara et al., 1995; Klumpers et al., 2014). However, this is not causal evidence for a role of the amygdala in the expression of conditioned fear in humans, since the amygdala lesion is also present when the fear association is learned. Nevertheless, responses to unconditioned stimuli are usually intact (Bechara et al., 1995; LaBar et al., 1995; Klumpers et al., 2014) meaning that noradrenergic-sympathetic arousal responses can be present in the absence of a functional amygdala. Recent data on one patient with amygdala damage, furthermore, indicated that this patient is able to experience subjective feelings of fear and panic after CO2 inhalation (Feinstein et al., 2013; but see Feinstein et al., 2011). Finally, humans with amygdala damage typically do not show an emotional enhancement effect of episodic memory (Cahill et al., 1995; LaBar and Phelps, 1998). This indeed suggest that noradrenergic-sympathetic arousal is ineffective in modulating memory in the absence of a functional amygdala indicated by rodent data (Cahill and McGaugh, 1991; Liang et al., 1982). Thus, our findings are in line with amygdala lesion data showing that the amygdala is crucially involved in fear acquisition and modulating memory processes rather than expressing fear.
Second, we found that amygdala activity was increased on CS+ items that were later remembered compared with CS+ items that were later forgotten, even though both evoked noradrenergic-sympathetic arousal responses. This subsequent memory effect in the amygdala is in line with previous literature (Hamann, 2001; Erk et al., 2003; Dolcos et al., 2004; Richardson et al., 2004; LaBar and Cabeza, 2006; Murty et al., 2011). However, these previous studies could not disentangle the separate roles of noradrenergic-sympathetic arousal and amygdala activation in enhancing declarative memory. Amygdala responses found in these paradigms could therefore reflect a response to arousing material (Morris et al., 1997; Whalen et al., 1998; Vuilleumier et al., 2001; Hariri et al., 2002) as well as perceptual-mnemonic processes. We therefore extend these findings by showing that noradrenergic-sympathetic arousal only predicts declarative memory for arousing stimuli when coinciding with amygdala activation.
Our data are furthermore in line with findings in rodents showing that the amygdala, in particular the basolateral amygdala (BLA), is necessary for arousal-related neuromodulators to have an effect on memory processes elsewhere in the brain (McGaugh, 2004; Roozendaal and McGaugh, 2011). Indeed, direct infusion of neuromodulatory agents affecting the noradrenergic system into the BLA after learning, have been shown to enhance memory (Ferry and McGaugh, 1999; McIntyre et al., 2005). This is even the case for learning events that are low in arousal (Roozendaal et al., 2008), meaning that in absence of noradrenergic-sympathetic arousal induced by the stimulus, noradrenergic activation in the amygdala can influence memory. Moreover, the effects of these post-training manipulations of noradrenergic activity in the BLA influence memory types that are dependent on other brain regions such as the hippocampus, caudate nucleus, and insular cortex (Packard et al., 1994; Hatfield and McGaugh, 1999; Beldjoud et al., 2015). Additionally, these effects are blocked when the amygdala is lesioned (Liang et al., 1982; Cahill and McGaugh, 1991). In humans it was found that β-adrenergic antagonist (i.e. propranolol) administration blocks the emotional enhancement effect for arousing material (Cahill et al., 1994; Van Stegeren et al., 1998) and abolishes the subsequent memory effect in the amygdala (Strange and Dolan, 2004). Importantly, the emotional enhancement effects are driven by central and not per se peripheral noradrenergic activation (Van Stegeren et al., 1998). These findings align closely with the present study in showing the importance of noradrenergic activation of the amygdala, but do not directly demonstrate a dissociation between the noradrenergic-sympathetic response and amygdala activation.
Rodent data showing functional specificity within amygdala subregions raise the question whether we can attribute the BOLD activation found in the present study to any subregion of the amygdala. Although we observed that the activation lies more toward the central nucleus of the amygdala rather than the BLA, it is questionable whether we can draw inferences at this level of spatial specificity with BOLD-fMRI at this resolution. A comparison between subregions of the amygdala using BOLD-fMRI is inherently difficult because signal loss and distortion due to magnetic field inhomogeneity increases towards the ventral part of the brain, where the BLA is located (Merboldt et al., 2001; Sladky et al., 2013). Moreover, we applied spatial smoothing to improve signal-to-noise ratio and accommodate the anatomical and functional variability between subjects, but this further reduces the spatial accuracy. Thus, whether the effect we observed can be attributed to a specific subregion of the amygdala remains an open question.
Our behavioral data did not show enhanced item memory recognition for CS+ items compared with CS− items, even though previous studies using a similar paradigm did find a memory enhancement for CS+ items (Dunsmoor et al., 2012, 2015). A plausible explanation for this null finding is that the lures in our paradigm were more similar to the targets (i.e. if the target was a dog, the lure was a different dog). We included a relatively small number of trials (i.e. 64 encoding trials) due to the fear conditioning procedure. This made the task more difficult in order to prevent ceiling effects and to be able to reliably investigate subsequent memory effects. Another crucial difference is that our task did not include expectancy ratings for the UCS (Dunsmoor et al., 2012, 2015). These expectancy ratings might have had similar effects on encoding as do judgments tasks (e.g. living/non-living judgments in response to objects or animals) in memory paradigms, which are used to ensure more elaborate encoding (Gabrieli et al., 1997; Takashima et al., 2006; Turk-Browne et al., 2006). A lack of an overall emotional enhancement effect is not uncommon, nevertheless, in studies using recognition memory tests (Richardson et al., 2004; Windmann and Kutas, 2001), even when amygdala activity predicts recognition of individual emotional items (Richardson et al., 2004). Indeed, the effect of emotion on memory is thought to be reduced (or not present) when assessing memory via recognition instead of recollection (Yonelinas and Ritchey, 2015). When we compared all items later remembered versus all items later forgotten we did find a strong subsequent memory effect in the hippocampus and parahippocampal gyrus. These findings are consistent with a crucial role for these regions in non-emotional declarative memory (Wagner et al., 1998). Thus, hippocampal and parahippocampal activations predict overall memory, while the amygdala specifically predicts memory for CS+ items.
To summarize, we demonstrate that noradrenergic-sympathetic activation is not sufficient to enhance emotional declarative memory, but requires additional activation of the amygdala. Our data show that these two processes do not play a uniform role in memory. These findings support animal models stating that the amygdala integrates arousal-related neuromodulatory changes to modulate mnemonic processes elsewhere in the brain and thereby strengthens declarative memory.
Conflict of interest. None declared.
References
- Antoniadis E. a., Winslow J.T., Davis M., Amaral D.G. (2009). The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biological Psychiatry, 65(3), 241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38, 95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. (2005). Unified segmentation. NeuroImage, 26, 839–51. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 28(1), 403–50. [DOI] [PubMed] [Google Scholar]
- Bach D.R., Weiskopf N., Dolan R.J. (2011). A stable sparse fear memory trace in human amygdala. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(25), 9383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H., Adolphs R., Rockland C., Damasio A.R. (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science (New York, N.Y.), 269, 1115–8. doi:10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Beldjoud H., Barsegyan A., Roozendaal B. (2015). Noradrenergic activation of the basolateral amygdala enhances object recognition memory and induces chromatin remodeling in the insular cortex. Frontiers in Behavioral Neuroscience, 9, 108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Diamond J.B., Smith M.A., Bandettini P.A. (2006). Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31, 1536–48. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Greenwald M.K., Petry M.C., Lang P.J. (1992). Remembering pictures: pleasure and arousal in memory. Journal of Experimental Psychology. Learning, Memory, and Cognition, 18(2), 379–90. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Miccoli L., Escrig M. a., Lang P.J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45(4), 602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T.W., Etzel J.A., Adolphs R., Tranel D. (2006). The influence of autonomic arousal and semantic relatedness on memory for emotional words. International Journal of Psychophysiology, 61(1), 26–33. [DOI] [PubMed] [Google Scholar]
- Büchel C., Dolan R.J. (2000). Classical fear conditioning in functional neuroimaging. Current Opinion in Neurobiology, 10, 219–23. [DOI] [PubMed] [Google Scholar]
- Cahill L., Prins B., Weber M., McGaugh J.L. (1994). Beta-adrenergic activation and memory for emotional events. Nature, 371, 702–4. [DOI] [PubMed] [Google Scholar]
- Cahill L., Babinsky R., Markowitsch H.J., McGaugh J.L. (1995). The amygdala and emotional memory. Nature, 377, 295.. [DOI] [PubMed] [Google Scholar]
- Cahill L., McGaugh J.L. (1991). NMDA-induced lesions of the amygdaloid complex block the retention-enhancing effect of posttraining epinephrine. Psychobiology, 19(3), 206–10. [Google Scholar]
- Cahill L., McGaugh J.L. (1995). A novel demonstration of enhanced memory associated with emotional arousal. Consciousness and Cognition, 4, 410–21. [DOI] [PubMed] [Google Scholar]
- Canli T., Zhao Z., Brewer J., Gabrieli J.D., Cahill L. (2000). Event-related activation in the human amygdala associates with later memory for individual emotional experience. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 20, RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman W.P., Schroeder H.R., Geyer G., et al. (1954). Physiological evidence concerning importance of the amygdaloid nuclear region in the integration of circulatory function and emotion in man. Science (New York, N.Y.), 120(1), 949–50. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- De Voogd L.D., Fernández G., Hermans E.J. (2016). Awake reactivation of emotional memory traces through hippocampalneocortical interactions. NeuroImage 134, 563–72. [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K.S., Cabeza R. (2004). Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 42, 855–63. [DOI] [PubMed] [Google Scholar]
- Dunsmoor J.E., Martin A., LaBar K.S. (2012). Role of conceptual knowledge in learning and retention of conditioned fear. Biological Psychology, 89(2), 300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor J.E., Murty V.P., Davachi L., Phelps E.A. (2015). Emotional learning selectively and retroactively strengthens memories for related events. Nature, 520, 345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S., Kiefer M., Grothe J., Wunderlich A.P., Spitzer M., Walter H. (2003). Emotional context modulates subsequent memory effect. NeuroImage, 18, 439–47. [DOI] [PubMed] [Google Scholar]
- Feinstein J.S., Adolphs R., Damasio A., Tranel D. (2011). The human amygdala and the induction and experience of fear. Current Biology, 21(1), 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein J.S., Buzza C., Hurlemann R., et al. (2013). Fear and panic in humans with bilateral amygdala damage. Nature Neuroscience, 16(3), 270–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B., McGaugh J.L. (1999). Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiology of Learning and Memory, 72(1), 8–12. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A., Poline J.B., Price C.J., Frith C.D. (1996). Detecting activations in PET and fMRI: levels of inference and power. NeuroImage 4, 223–35. [DOI] [PubMed] [Google Scholar]
- Fullana M.A., Harrison B.J., Soriano-Mas C., Vervliet B., Cardoner N., Àvila-Parcet A., Radua J. (2015). Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Molecular Psychiatry, 21(4), 500–8. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D.E., Brewer J.B., Desmond J.E., Glover G.H. (1997). Separate neural bases of two fundamental memory processes in the human medial temporal lobe. 264, 264–6. [DOI] [PubMed] [Google Scholar]
- Gilzenrat M.S., Nieuwenhuis S., Cohen J.D. (2010). Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. 10(2), 252–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J., Adolphs R. (2003). Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 23(32), 10274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G.H., Li T.Q., Ress D. (2000). Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine, 44, 162–7. [DOI] [PubMed] [Google Scholar]
- Griswold M.A., Jakob P.M., Heidemann R.M., et al. (2002). Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine, 47, 1202–10. [DOI] [PubMed] [Google Scholar]
- Hamann S. (2001). Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences, 5(9), 394–400. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Tessitore A., Mattay V.S., Fera F., Weinberger D.R. (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage, 17(1), 317–23. [DOI] [PubMed] [Google Scholar]
- Hatfield T., McGaugh J.L. (1999). Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiology of Learning and Memory, 71(2), 232–9. [DOI] [PubMed] [Google Scholar]
- Hermans E.J., Henckens M. J a G., Roelofs K., Fernández G. (2013). Fear bradycardia and activation of the human periaqueductal grey. NeuroImage, 66, 278–87. [DOI] [PubMed] [Google Scholar]
- Hutton C., Bork A., Josephs O., Deichmann R., Ashburner J., Turner R. (2002). Image distortion correction in fMRI: A quantitative evaluation. NeuroImage, 16, 217–40. [DOI] [PubMed] [Google Scholar]
- Kaada B.R., Andersen P., Jansen J. (1954). Stimulation of the amygdaloid nuclear complex in unanesthetized cats. Neurology, 4(1),48–64. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L.J., Kaplan S. (1963). Paired-associate learning as a function of arousal and interpolated interval. Journal of Experimental Psychology, 65(2),190–3. [DOI] [PubMed] [Google Scholar]
- Klumpers F., Morgan B., Terburg D., Stein D.J., van Honk J. (2015). Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Social Cognitive and Affective Neuroscience, 10(9),1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K.S., Cabeza R. (2006). Cognitive neuroscience of emotional memory. Nature Reviews. Neuroscience, 7(1), 54–64. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., Gatenby J.C., Gore J.C., LeDoux J.E., Phelps E.A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron, 20, 937–45. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., LeDoux J.E., Spencer D.D., Phelps E. a. (1995). Impaired fear conditioning following unilateral temporal lobectomy in humans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 15, 6846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K.S., Phelps E.A. (1998). Arousal-mediated memory consolidation: role of the medial temporal lobe in humans. Psychological Science, 9(6), 490–3. [Google Scholar]
- Lang P.J., Greenwald M.K., Bradley M.M., Hamm A.O. (1993). Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology, 30(3), 261–73. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (2003). The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology, 23(4–5), 727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E., Iwata J., Cicchetti P., Reis D.J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 8, 2517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Schiller D., Schoenbaum G., Phelps E.A., Daw N.D. (2011). Differential roles of human striatum and amygdala in associative learning. Nature Neuroscience, 14(10), 1250–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K.C., McGaugh J.L., Martinez J.L., Jensen R. a., Vasquez B.J., Messing R.B. (1982). Post-training amygdaloid lesions impair retention of an inhibitory avoidance response. Behavioural Brain Research 4(3),237–49. doi:10.1016/0166-4328(82)90002-X [DOI] [PubMed] [Google Scholar]
- Maren S. (2001). Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience, 24, 897–931. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience, 27, 1–28. [DOI] [PubMed] [Google Scholar]
- McIntyre C.K., Miyashita T., Setlow B., et al. (2005). Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 102, 10718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias M.L., Etkin A., Kalisch R. (2010). A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. NeuroImage, 49(2), 1760–8. [DOI] [PubMed] [Google Scholar]
- Merboldt K.D., Fransson P., Bruhn H., Frahm J. (2001). Functional MRI of the human amygdala? NeuroImage, 14(2), 253–7. [DOI] [PubMed] [Google Scholar]
- Morris J.S., Friston K.J., Dolan R.J. (1997). Neural responses to salient visual stimuli. Proceedings. Biological Sciences/the Royal Society, 264(1382),769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L.J., Ranganath C. (2007). The dorsolateral prefrontal cortex contributes to successful relational memory encoding. Journal of Neuroscience, 27(20), 5515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V.P., Ritchey M., Adcock R.A., LaBar K.S. (2011). Reprint of: fMRI studies of successful emotional memory encoding: a quantitative meta-analysis. Neuropsychologia, 49(12), 695–705. doi:10.1016/j.neuropsychologia.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Öhman A., Mineka S. (2001). Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review, 108, 483–522. [DOI] [PubMed] [Google Scholar]
- Packard M.G., Cahill L., McGaugh J.L. (1994). Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proceedings of the National Academy of Sciences of the United States of America, 91(18), 8477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser B.A., Versluis M.J., Hoogduin J.M., Norris D.G. (2006). BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: Parallel-acquired inhomogeneity-desensitized fMRI. Magnetic Resonance in Medicine, 55, 1227–35. [DOI] [PubMed] [Google Scholar]
- Reis D.J., LeDoux J.E. (1987). Some central neural mechanisms governing resting and behaviorally coupled control of blood pressure. Circulation, 76(1 Pt 2), I2–9. [PubMed] [Google Scholar]
- Reyes B. a S., Carvalho a. F., Vakharia K., Van Bockstaele E.J. (2011). Amygdalar peptidergic circuits regulating noradrenergic locus coeruleus neurons: linking limbic and arousal centers. Experimental Neurology, 230(1), 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M.P., Strange B. a., Dolan R.J. (2004). Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neuroscience, 7(3), 278–85. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Castello N.A., Vedana G., Barsegyan A., Mcgaugh J.L. (2008). Neurobiology of Learning and Memory Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology Learning Memory, 90, 576–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. (2011). Memory modulation. Behavioral Neuroscience, 125(6),797–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Stenger V.A., Konecky R., Carter C.S. (2003). Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. NeuroImage, 20, 114–24. [DOI] [PubMed] [Google Scholar]
- Sladky R., Baldinger P., Kranz G.S., et al. , (2013). High-resolution functional MRI of the human amygdala at 7 T. European Journal of Radiology, 82(5), 728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B. a., Dolan R.J. (2004). Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences of the United States of America, 101(31), 11454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A., Petersson K.M., Rutters F., et al. (2006). Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proceedings of the National Academy of Sciences of the United States of America, 103(3), 756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne N.B., Yi D.J., Chun M.M. (2006). Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron, 49(6), 917–27. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Van Buuren M., Gladwin T.E., Zandbelt B.B., et al. (2009). Cardiorespiratory effects on default-mode network activity as measured with fMRI. Human Brain Mapping, 30, 3031–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stegeren A.H., Everaerd W., Cahill L., McGaugh J.L., Gooren L.J.G. (1998). Memory for emotional events: Differential effects of centrally versus peripherally acting??-blocking agents. Psychopharmacology, 138, 305–10. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron, 30(3), 829–41. [DOI] [PubMed] [Google Scholar]
- Wagner A.D., Schacter D.L., Rotte M., et al. (1998). Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science (New York, N.Y.), 281(5380), 1188–91. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Rauch S.L., Etcoff N.L., McInerney S.C., Lee M.B., Jenike M. a. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 18(1), 411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmann S., Kutas M. (2001). Electrophysiological correlates of emotion-induced recognition bias. Journal of Cognitive Neuroscience, 13(5), 577–92. [DOI] [PubMed] [Google Scholar]
- Yonelinas A.P., Ritchey M. (2015). The slow forgetting of emotional episodic memories: an emotional binding account. Trends in Cognitive Sciences, 19(5), 259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]