Abstract
Reward feedback following visual search causes the visual characteristics of targets to become salient and attention-drawing, but little is known about the mechanisms underlying this value-driven capture effect. Here, we use transcranial random noise stimulation (tRNS) to demonstrate that such reward potentiation involves induced plasticity in visual cortex. Human participants completed a feature-search reward-learning task involving the selection of a red or green colored target presented among distractors of various color. Each correct trial garnered reward and the magnitude of reward was determined by the color of the target. Three groups completed this task: two groups received tRNS over either occipital or frontal cortex, and the third group received sham stimulation as a control. In a subsequent test phase of the experiment participants searched for a unique shape presented among colored distractors. During the test phase, no tRNS was applied and no reward was available. However, in some trials a single distractor had color matching that associated with reward during training. Search for the target was impacted by the presence of such reward-associated distractors in the occipital stimulation group, demonstrating that plasticity in visual cortex contributes to value-driven attentional capture.
Keywords: transcranial random noise stimulation, value-driven attentional capture, reward, plasticity, attention
Introduction
Theories of animal learning suggest that reward-related dopamine can prime sensation and perception of environmental stimuli that might serve as predictors of good outcome (Berridge and Robinson, 1998; Ikemoto and Panksepp, 1999). In line with this, a number of recent studies in humans have established that reward-associated visual stimuli will draw selective attention during visual search even when they are task-irrelevant (Anderson et al., 2011a; Anderson, 2013; see also Della Libera and Chelazzi, 2009; Hickey et al., 2010; Hickey and Peelen, 2015; Chelazzi et al., 2013 for review). This value-driven attentional capture has been commonly investigated using experimental paradigms in which reward is initially conditioned to specific visual features before objects characterized by these features act as task-irrelevant distractors. Results show that such distractors draw attention and slow search for targets, even when participants know that reward-conditioned features (like specific colors) are irrelevant and that the target will be defined within some other feature dimension (like shape; Anderson et al., 2011; Anderson, 2013; see also Theeuwes and Belopolsky, 2012; Wang et al., 2013; Qui et al., 2013; Roper et al., 2014; Infanti et al., 2015).
Two broad accounts for this phenomenon have been offered. On one hand, value-driven capture might reflect residual influence of a high-level task strategy—instantiated in frontal cortex—that was reinforced during training (Chelazzi et al., 2013; Anderson, 2013). In the paradigm used to elicit value-driven capture, participants must switch strategies between training and test phases, discarding the previously rewarded set for color-defined targets in order to adopt a strategy suitable for the new search task. If reward has made it hard to discard the reinforced set, value-driven capture could reflect the perseverated use of an outdated strategy caused by a failure in this task-switching process. The instantiation and maintenance of such strategic attentional control settings in working memory has been associated with activity in lateral frontal cortex (Hopfinger et al., 2000; de Fockert et al., 2001).
On the other hand is the idea that attentional biases to reward-associated stimuli might reflect an enhanced response to reinforced objects in sensory cortex. This could be caused by perceptual learning of target features, a reinforcement of attentional templates instantiated in sensory cortex, or some combination of these mechanisms (Hickey et al., 2010; Anderson, 2013). For example, recent theoretical work on perceptual learning has suggested that reward signals in sensory cortex may act to boost subsequent responses to the characteristics of stimuli attended at the time the reward signal arrives (Roelfsema et al., 2010; Noudoost and Moore, 2011). According to this perspective, value-driven attentional capture could reflect induced plasticity in visual cortex that potentiates responses to reward-associated stimuli, effectively increasing their ability to draw attention. Importantly, these two possibilities are not mutually exclusive. Value-driven capture could reflect the compound influence of both perseveration of task sets instantiated in frontal cortex and increased perceptual sensitivity for entrained stimuli in sensory cortex.
Here we use transcranial random noise stimulation (tRNS) to independently test two ideas: that value-driven capture reflects changes in representations in frontal cortex, and that value-driven capture involves changes in representations in visual cortex. In tRNS, alternating current is applied at the scalp surface with a randomly varying frequency (e.g. 100–640 Hz). Although the technique is relatively new, several studies have demonstrated its efficacy in the modulation of neuroplasticity and cognition. Two findings are particularly relevant to the current paper. First, tRNS over occipital cortex impacts perceptual learning, creating both a baseline benefit to perceptual sensitivity and an improvement in practice effects (Fertonani et al., 2011; Pirulli et al., 2013; Camilleri et al., 2014). Second, tRNS over lateral frontal cortex creates costs in the categorization of visual stimuli, consistent with a degradation of category templates in working memory (Ambrus et al., 2011). If perceptual sensitivity plays a role in value-driven capture, tRNS over the occipital lobe during training may accentuate the phenomenon. If value-driven attentional capture additionally or alternatively reflects perseverated use of a task set, tRNS over lateral frontal cortex may disrupt the representation of category templates and task goals, reducing the degree to which these subsequently influence search.
Method
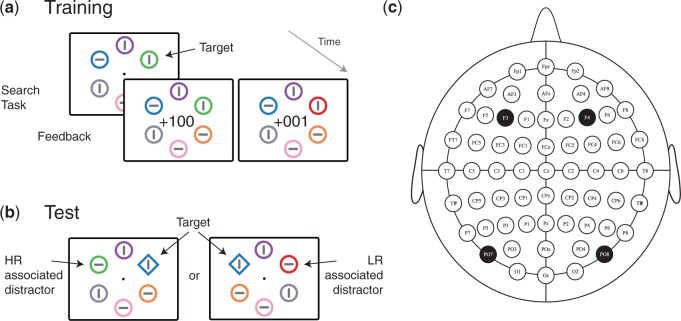
To test these predictions, we asked three groups of participants to complete a visual search task based on that introduced by Anderson et al. (2011; see Figure 1). During a single block of training (192 trials) participants were asked to search for targets defined by either red or green color. The specific color that defined the target was randomly determined for each trial, targets were presented among distractor circles of various color, and all stimuli, including the target, contained oriented line elements that could be vertical or horizontal. Participants were instructed to respond based on the orientation of the line contained in the target and they received reward feedback immediately following correct response. For each participant, correct response to targets characterized by one of the two target colors garnered high-magnitude reward (100 points) whereas correct response to targets characterized by the other color garnered low-magnitude reward (1 point). The mapping of color to reward outcome was counterbalanced across participants and each participant received a cash bonus based on the number of points received. Immediately following the training session, participants completed a test phase of three blocks of 192 trials where no reward feedback was available. In the test phase, search was for a uniquely shaped element—a diamond among circles or vice versa. Critically, in some trials one of the distractors had a color that had characterized a target during the preceding training session. Of the three groups that completed this task, two underwent tRNS stimulation at frontal or occipital locations during training. The third acted as control with sham stimulation.
Fig. 1.
Experimental paradigm during (A) training and (B) test sessions. During training, the color of the target predicted the magnitude of reward received following correct performance. At test objects with these colors could appear as task-irrelevant distractors. (C) Electrode stimulation sites. Note that pdf version of article includes colors, but in black and white print version colors are represented by dash size.
Prior work has found that reaction time (RT) in the test phase of this task increases when the search array contains either a high- or low-magnitude reward-conditioned distractor. However, it is uncommon to find that this RT effect reliably differs as a function of reward magnitude. For example, in Anderson et al. (2011a; Exp 1), RT was 16 ms slower when search displays contained a high-reward distractor and 8 ms slower when search displays contained a low-reward distractor, but this difference was not reliable (see also Anderson et al., 2011b). The absence of a difference between high- and low-magnitude conditions complicates interpretation of the value-driven capture effect: because the targets were always associated with reward during training, it is possible that the targets’ ability to subsequently interfere with search reflects their prior status as targets rather than any association to reward.
Recent results from Infanti et al. (2015) are important in this context. These authors examined congruency effects derived from RT and accuracy in a value-driven attentional capture task. As in this study, the test phase of Infanti et al. (2015) involved search for a target that contained a vertically or horizontally oriented line and was presented among distractors that also contained oriented line segments. Prior research has shown that attentional capture by a physically salient distractor under these circumstances will cause (i) a performance benefit when the line element in the distractor is of the same orientation as the line element in the target and (ii) a cost when the line element in the distractor is of a different orientation (e.g. Theeuwes and Burger, 1998). This pattern can be used to derive a summated congruency effect that is larger in magnitude than raw effects on RT or accuracy. Infanti et al. (2015) found such a congruency effect when search arrays contained a reward-associated distractor, suggesting that attention had been deployed to this object. Moreover, the effect of congruency was reliably larger when the distractor had been associated with high- rather than low-magnitude reward, suggesting that attention had been captured either more strongly or more often. Given the sensitivity to value-driven capture evident in results from Infanti et al. (2015), this study was designed to allow for the isolation of this measure.
Participants
Sixty participants (23.8 ± 3.6 years, mean ± standard deviation; 40 women; all right handed) were recruited online and passed a safety-screening questionnaire (Keel et al., 2001) before providing informed consent. Forty participants received tRNS stimulation, 20 over lateral occipital locations (PO7/8 in the 10/10 electrode location system), and twenty over lateral frontal locations (F3/4; see Figure 1C). An additional 20 participants received sham stimulation with inactive electrodes placed at occipital sites.
Eight participants were replaced due to low accuracy during the post-stimulation test phase of the experiment (i.e. <55% when reward-associated distractors were absent; 4 from occipital group, 2 from frontal group and 2 from sham group). One additional participant in the frontal stimulation group was replaced due to stimulator malfunction. The experimental session lasted 1.5–2 h and participants received between €26 and €32 for their participation. The study was approved by the Institutional Review Board of the University of Trento.
Experimental design
Stimuli were presented on an LCD monitor (120 Hz) located 57 cm from the eyes using OpenSesame (Mathôt et al., 2012). Trials in the training phase (Figure 1A) began with the presentation of a black central fixation point (0.4° visual angle) on a light grey background for 600 ms. A visual search array subsequently appeared for 750 ms.
This consisted of six coloured empty circles (2° radius; 0.3° line thickness), each containing a horizontal or vertical black line (1° × 0.3°; at least 2 horizontal and 2 vertical lines were present in each trial). Array items were equally spaced along an imaginary circle (5° radius) and were presented for 750 ms. A target, defined by red or green color, was present in each search array. Subjects reported the orientation of the line inside the target by pressing the ‘z' key of a standard keyboard with their left index finger if the line segment was horizontal or the ‘m’ key with their right index finger if it was vertical. The colours of non-target array items were chosen randomly without replacement from a set of six alternatives (blue, cyan, pink, orange, yellow and black).
Feedback indicating the number of points earned in each trial was overlaid at fixation for 1000 ms following response. Subjects received no points (‘000’) when response was incorrect or not made within a 750 ms interval following stimulus onset. Low-magnitude reward (‘+001’) followed correct trials with a low-magnitude reward-associated color, and high-magnitude reward (‘+100’) followed trials with a high-magnitude reward-associated color. The colors associated with high- and low-magnitude reward did not change throughout the training phase of the experiment. Subjects were not informed of the reward contingency prior to beginning the experiment but were told that they would be paid based on the number of points they accumulated. A short break was given every 30 trials and the total number of points earned was presented in this interval.
In the test phase (Fig 1b), trials again began with a fixation point (600 ms) followed by a search array containing six objects (1500 ms). However, at test the search target was either a diamond (2° × 2°) presented among five circles or a circle presented among five diamonds. Again, each object contained a horizontal or vertical line and response was based on the orientation of the line in the target. In half of trials, the colors of all six objects were randomly chosen without replacement from the set of six non-target colors employed at training. In the remaining trials, the target and four distractors had colors taken from this list, but one of the non-targets had a color that had defined targets during training. This could be the color associated with high- or low-magnitude reward with equal probability. Maximum response time was 1.5 s., after which the next trial began. No feedback was provided during the test phase of the experiment and participants completed three blocks of 192 trials.
tRNS stimulation
Subjects were fitted with a tight silicon swimming cap with saline soaked sponge electrodes (5 × 7 cm) placed under the cap at marked stimulation sites. After application the electrodes were allowed to settle for about 3 min while participants practiced the task. Subjects in the occipital and frontal stimulation groups subsequently received 20 min of high frequency tRNS (100–640 Hz) at 1000 μA (zero offset) delivered using a battery-driven stimulator (neurConn) while they continued the training task. Stimulation started and ended with a fade in/out ramp of 15 s and sham stimulation consisted of the fade in/out ramp but only 5 s of stimulation. Stimulation intensity was well below the threshold of cutaneous perception (Ambrus et al., 2010; Fertonani et al., 2011).
Results
Trials with RT more than 3 standard deviations from the per-subject mean were removed from analysis. The criterion value for this exclusion parameter was calculated separately for each of the training and test tasks. Analysis began with a repeated measures analyses of variance (RANOVA) of RT in the training task with factors for target reward association (high vs. low) and stimulation location (frontal, occipital and sham). This revealed a significant effect of reward association (high-magnitude: 575 ms, low-magnitude: 581 ms, F(1,57) = 7.177, P = 0.010, ηp2 = 0.112), and a marginal effect of stimulation location (frontal: 579 ms, occipital: 586 ms, sham: 568 ms, F(2,57) = 2.625, P = 0.081, ηp2 = 0.084), but no interaction between these factors (F < 1). A corresponding analysis of accuracy revealed a similar pattern (reward association: 84.5% vs 81.6%, F(1,57) = 9.024, P = 0.004, ηp2 = 0.137; stimulation group: frontal 82%, occipital 81%, sham 86%, F(2,57) = 1.492, P = 0.234, ηp2 = 0.234; interaction: F < 1). Participants in the sham condition tended to respond slightly quicker than those in the stimulation conditions, resulting in fewer trials in which the 750 ms response deadline was exceeded, but these types of error did not differ as a function of reward outcome.
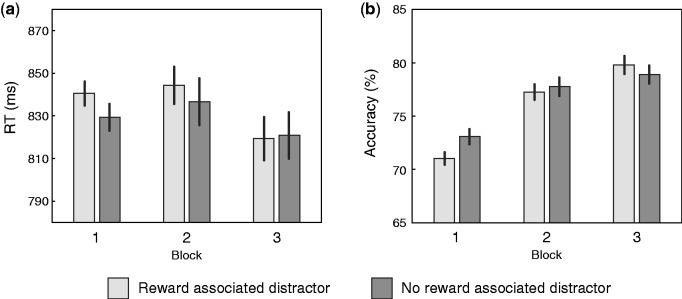
Results from the test session are illustrated in Figure 2. Participants were slower and less accurate when the search array contained a distractor that had been associated with either high-magnitude or low-magnitude reward during training. Statistical assessment of this pattern began with an omnibus RANOVA of RT with within-subject factors for distractor reward-association (high-magnitude, low-magnitude, or reward-neutral) and block (1, 2 or 3), and a between-subject factor for stimulation location (frontal, occipital or sham). This revealed a significant difference between stimulation location groups (sham: 787 ms, occipital: 880 ms; frontal: 839 ms; F(2,57) = 4.449, P = 0.016, ηp2 = 0.135) but no within-subject effects (reward: F(2,114) = 2.680, P = 0.074; block: F(2,114) = 2.439, P = 0.092; reward × block: F(4,228) = 1.764, p = 0.137; all other ps > 0.2). Corresponding analysis of accuracy revealed a main effect of block (block 1: 72%, block 2: 78%, block 3: 79%, F(2,114) = 43.825, P < 0.001, ηp2 = 0.435; reward x block: F(4, 228) = 2.196, P = 0.076, ηp2 = 0.037; all other ps > 0.2). Follow up t-test contrasts demonstrated that the occipital stimulation group was reliably slower than the sham stimulation group (t(38) = 2.726, P = 0.010), but that there was no reliable difference between occipital and frontal stimulation groups (t(38) = 1.034, P = 0.308) or between the frontal stimulation group and the sham group (t(38) = 1.543, P = 0.131).
Fig. 2.
(A) RT and (B) accuracy during the test phase of the experiment collapsed across high-magnitude and low-magnitude reward conditions and across stimulation groups. Error bars here and in Figure 3 reflect within-subject SE (Cousineau, 2005).
As noted above, value-driven capture is commonly indexed in a comparison of results from conditions where a distractor had either low- or high-magnitude reward association to results when no reward-associated distractor was present in the display. We accordingly conducted a second set of RANOVAs with the same factors for block and stimulation-location, but where the factor for distractor reward-association collapsed across high- and low-magnitude conditions (see Figure 2). In the case of RT, this analysis detected an effect of reward-associated distractor presence (present vs absent; F(1,57) = 5.936, P = 0.018, ηp2 = 0.094) and a significant interaction between block and reward-associated distractor presence (F(2,114) = 3.335, P = 0.042, ηp2 = 0.055) but no other effects in addition to those identified above. A corresponding analysis of accuracy identified an interaction of reward-associated distractor presence and block (F(2,114) = 3.614, P = 0.033, ηp2 = 0.060) but no other new effects. Distractors associated with high- or low-magnitude reward thus disrupted search for the target, with this effect extinguishing over the three trial blocks of the test phase, but analysis of raw RT and accuracy failed to detect any reliable difference across the low- and high-magnitude reward-association conditions or any interaction with stimulation group.
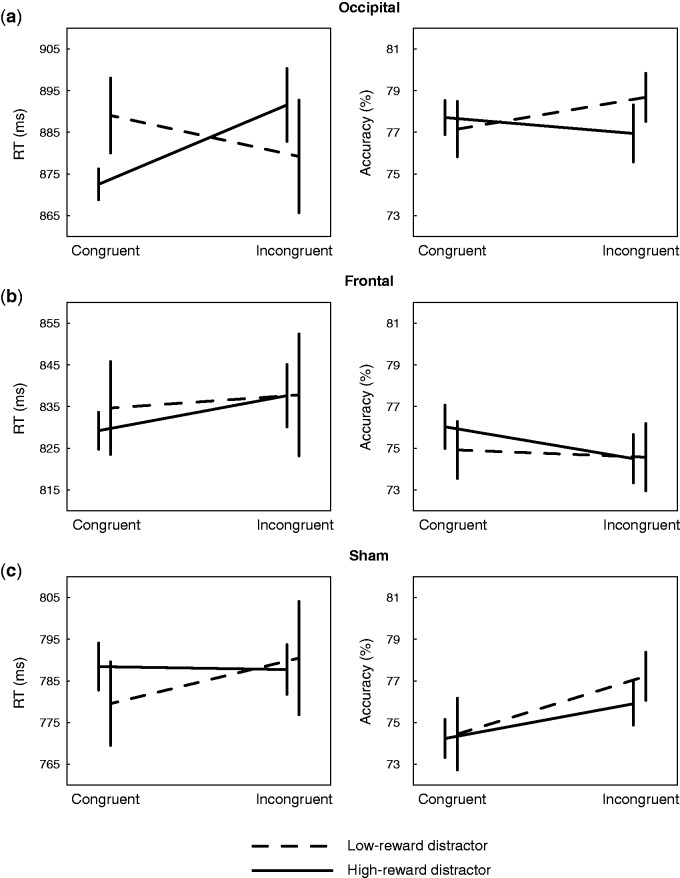
Results from congruency analysis are illustrated in Figure 3. In the occipital stimulation group (Figure 3A), there is an apparent interaction between factors for distractor reward-association (high-magnitude vs low-magnitude) and target-distractor congruency (congruent vs incongruent). No corresponding pattern is evident following frontal (Figure 3B) or sham stimulation (Figure 3C), and there is no speed-accuracy trade-off evident in accuracy results. Statistical assessment of this RT pattern began with an omnibus RANOVA with within-subject factors for block, distractor reward-association, and target-distractor congruency, and a between-subject factor for stimulation location. This revealed a main effect of stimulation location (F(2,57) = 4.431, P = 0.016, ηp2 = 0.135), a significant interaction of congruency and block (F(2,57) = 6.038, P = 0.004, ηp2 = 0.096), and a marginal main effect of block (F(2,57) = 3.283, P = 0.055, ηp2 = 0.054), alongside a critical interaction between distractor reward-association, congruency, and stimulation location (F(2,57) = 3.851, P = 0.027, ηp2 = 0.119; all other ps > 0.2). This three-way interaction indicates that the relationship between distractor reward-association and congruency evident in the occipital stimulation group (Figure 3A) reliably varies as a function of tRNS stimulation during training. Corresponding omnibus analysis of accuracy revealed a main effect of block, reflecting an improvement in performance over the course of the test session (F(2,114) = 37.584, P < 0.001, ηp2 = 0.397) but no other effects (congruency × group: F(2,57) = 2.246, P = 0.115, ηp2 = 0.073; all other ps > 0.2).
Fig. 3.
Results from congruency analysis of RT (left column) and accuracy (right column) in (A) occipital, (B) frontal and (C) sham stimulation groups.
The critical three-way interaction detected in the omnibus RANOVA of RT remained reliable when analysis was limited to the sham and occipital stimulation conditions (F(1,38) = 8.526, P = 0.006, ηp2 = 0.183), demonstrating that the interactive pattern evident following occipital stimulation (Figure 3A) was reliably different from results observed following sham stimulation (Figure 3C). When frontal and occipital stimulation conditions were contrasted (Figure 3A vs. Figure 3B), this interaction trended to reliability (F(2,57) = 2.683, P = 0.110, ηp2 = 0.066), but when frontal and sham stimulation groups were compared (Figure 3B vs. Figure 3C) it was not reliable (F(1,38) = 1.166, P = 0.287, ηp2 = 0.030).
A follow-up RANOVA with factors for distractor reward association and congruency limited to results from the occipital stimulation group (Figure 3A) revealed a reliable interaction (F(1,19) = 10.715, P = 0.004, ηp2 = 0.361) but no main effects (congruency: F(1,19) = 1.065, P = 0.315, ηp2 = 0.053; reward: F < 1). The same analysis applied to results from frontal and sham stimulation groups detected no reliable effects (frontal, congruency: F(1,19) = 1.150, P = 0.297, ηp2 = 0.57, all other Fs < 1; sham, congruency × reward: F(1,19) = 1.181, P = 0.291, ηp2 = 0.059, all other Fs < 1). Finally, t-test contrasts of RT in the occipital stimulation group (Figure 3A) identified a reliable effect of distractor reward-association when the target and distractor contained congruent line elements (t(19) = 2.538, P = 0.020). The effect of distractor reward-association did not reach significance when line elements were incongruent (t(19) = 1.284, P = 0.215), and corresponding contrasts applied to accuracy identified no effects (ps > 0.35). Participants were thus faster to respond to the target when its line element was congruent with that contained in a high-magnitude reward-associated distractor.
Discussion
The current results demonstrate that tRNS stimulation over occipital cortex has a discrete effect on value-driven attentional capture, causing high-magnitude reward-associated distractors to draw attention during search for a uniquely-shaped target. This is evident in the influence these distractors have on the target response: RT is faster when the target contains a response-relevant line orientation that is congruent with the irrelevant orientation of the line contained within the reward-associated distractor.
This finding is consistent with a developing literature investigating the impact of tRNS stimulation on cortical excitability and perceptual learning. In a seminal report, Terney et al. (2008) found that 10 minutes of high frequency tRNS reliably increased the excitability of primary motor cortex and suggested this might reflect a facilitation of motor learning. This was proposed to occur via an influence of tRNS on sodium ion channels, wherein stimulation acted to depolarize cell membranes and increase the probability of action potentials. Subsequent studies from Fertonani et al. (2011) and Pirulli et al. (2013) applied tRNS over occipital cortex, finding that stimulation both increased baseline perceptual sensitivity for orientation change and improved the effect of practice. Largely in line with Terney et al. (2008), these authors suggest that the benefit to perceptual learning might reflect increased sensitivity to ongoing depolarizing post-synaptic potentials. This would increase the likelihood that a cell responds to external stimulation and strengthen the relationship between neurons, with the ultimate effect of increasing perceptual sensitivity to the entrained stimulus.
Our results show that while occipital tRNS during training accentuates value-driven capture, it also generally slows visual search performance at test. This pattern—increased sensitivity to reward-associated stimuli but decreased overall response speeds—may be in line with recent accounts of stimulation effects that rely on the concept of stochastic resonance. Counter-intuitively, the introduction of random noise can act to benefit the detection of weak input signals in nonlinear systems like the human brain (Benzi et al., 1981). This principle is thought to underlie otherwise puzzling psychophysical phenomenon such as increased perceptual sensitivity for weak visual stimuli when their contrast randomly varies by a small amount (Kitajo et al., 2003; see Ward et al., 2006, for review). At the same time, the introduction of noise has a better-known ability to degrade the quality of relatively strong neurophysiological signals. Broadly, transcranial electrical stimulation has a pattern of benefits and detriments that may mirror that associated with stochastic resonance, leading to the suggestion that this principle underlies stimulation effects (Fertonani and Miniussi, 2016; Miniussi et al., 2013). In this context, the introduction of noise via tRNS in the current experiment may have benefitted subtle, near-threshold learning effects underlying value-driven capture, at the same time degrading supra-threshold signals linked to task completion more generally.
An interpretation of our results is thus that tRNS increased the influence of reinforcement on perceptual learning of the high-magnitude reward-associated color. This could result in an increase of the raw visual salience of this specific feature, as suggested by studies that investigate the effect of reward on vision while minimizing the involvement of top-down attention (Hickey et al., 2010, 2015). However, alternative accounts are possible. One is that tRNS acts not to boost the effect of reward on perceptual learning, but rather on the resilience of attentional templates instantiated in occipital cortex. These templates are thought to be strategically established in sensory cortex by higher-order areas like lateral frontal cortex, causing treatment of task-relevant stimuli to be prioritized from early in the processing stream (Carlisle et al., 2011). If occipital tRNS caused reward to more strongly ‘stamp in’ these templates, this could underlie residual effects on selection even when a change in task made these templates irrelevant and unnecessary.
As noted in the introduction, these are not mutually exclusive possibilities. Perceptual learning and the instantiation of attentional set are very likely complementary and integrated mechanisms in vision, and the impact of tRNS observed here could reflect a combined influence on both processes. This would be in line with recent models of the relationship between attention and perceptual learning, such as the attention-gated reinforcement learning (AGREL) model of Roelfsema and van Ooyen (2005) and Roelfsema et al. (2010). This suggests that perceptual learning occurs when the application of attention to a stimulus co-occurs with the receipt in visual cortex of reward signals indicating unexpectedly good outcome.
Finally, it is worth noting that the current results are suggestive of an impact of tRNS and reward on the binding of stimuli features. If tRNS were to boost the integration of object features caused by reinforcement, increased binding of object features could cause subsequent selection of a stimulus with reward-associated color to more strongly activate the response associated with the line contained in this object. This would be beneficial when orientation of the line in the distractor was consistent with that of the target, perhaps generating the congruency effect we observe in the current results.
In contrast to results observed following lateral occipital stimulation, stimulation of lateral frontal cortex did not lead to any change in value-driven capture in our results. We approached the study with the idea that frontal stimulation might degrade the maintenance of task set in working memory, in line with existing tRNS results (Ambrus et al., 2011) and a broader literature implicating lateral frontal cortex in strategic attentional control (de Fockert et al., 2001; Carlisle et al., 2011). The absence of this effect is of course ambiguous: it may be that value-driven capture relies in part on mnemonic representations of attentional templates instantiated in frontal cortex but the type or location of stimulation we employed failed to impact these representations. However, our results do unambiguously show that if frontal representations of attentional strategy do play a role in value-driven capture, this occurs in conjunction with effects instantiated in sensory cortex.
The idea that reward and reward-related dopamine might impact approach behaviour by priming perception has been broadly influential (Berridge and Robinson, 1998; Ikemoto and Panksepp, 1999). For example, the incentive sensitization theory of addiction (Robinson and Berridge, 1993) is centered around the idea that drug craving is triggered by the presence of drug-associated environmental stimuli, with drug stimulation of the dopamine system causing these objects to become salient and attention drawing. Similar accounts have been proposed for abnormal eating behaviour (Berridge, 2009). The current results suggest that the maladaptive attribution of incentive salience to visual stimuli involves induced plasticity in sensory cortex, and thus that complete understanding of phenomenon like addiction may require insight on selective bias in perception and attention.
Acknowledgements
Our thanks to John Assad for insightful comments and discussion regarding this work. This project was supported by the Autonomous Province of Trento, Italy (call 'Grandi Progetti 2012,' project 'Characterizing and improving brain mechanisms of attention—ATTEND').
Conflict of interest. None declared.
References
- Ambrus G.G., Paulus W., Antal A. (2010). Cutaneous perception thresholds of electrical stimulation methods: comparison of tDCS and tRNS. Clinical Neurophysiology, 121,1908–14. [DOI] [PubMed] [Google Scholar]
- Ambrus G.G., Zimmer M., Kincses Z.T., et al. (2011). The enhancement of cortical excitability over the DLPFC before and during training impairs categorization in the prototype distortion task. Neuropsychologia, 49, 1974–80. [DOI] [PubMed] [Google Scholar]
- Anderson B.A. (2013). A value-driven mechanism of attentional selection. Journal of Vision, 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.A., Laurent P.A., Yantis S. (2011a). Value-driven attentional capture. Proceedings of the National Academy of Sciences USA, 108, 10367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.A., Laurent P.A., Yantis S. (2011b). Learned value magnifies salience-based attentional capture. PLoS One, 6, e27926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi R., Sutera A., Vulpiani A. (1981). The mechanism of stochastic resonance. Journal of Physics a: Mathematical and General, 14, L453. [Google Scholar]
- Berridge K.C. (2009). ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiology & Behavior, 97, 537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28, 309–69. [DOI] [PubMed] [Google Scholar]
- Camilleri R., Pavan A., Ghin F., Battaglini L., Campana G. (2014). Improvement of uncorrected visual acuity and contrast sensitivity with perceptual learning and transcranial random noise stimulation in individuals with mild myopia. Frontiers in Psychology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle N.B., Arita J.T., Pardo D., Woodman G.F. (2011). Attentional templates in visual working memory. The Journal of Neuroscience, 31, 9315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L., Perlato A., Santandrea E., Della Libera C. (2013). Rewards teach visual selective attention. Vision Research, 85, 58–72. [DOI] [PubMed] [Google Scholar]
- Della Libera C., Chelazzi L. (2009). Learning to attend and to ignore is a matter of gains and losses. Psychological Science, 20, 778–84. [DOI] [PubMed] [Google Scholar]
- Fertonani A., Pirulli C., Miniussi C. (2011). Random noise stimulation improves neuroplasticity in perceptual learning. The Journal of Neuroscience, 31, 15416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertonani A., Miniussi C. (2016). Transcranial electrical stimulation: What we know and do not know about mechanisms. The Neuroscientist, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C., Peelen M.V. (2015). Neural Mechanisms of Incentive Salience in Naturalistic Human Vision. Neuron, 85, 512–8. [DOI] [PubMed] [Google Scholar]
- Hickey C., Chelazzi L., Theeuwes J. (2010). Reward guides attention via anterior cingulate cortex. Journal of Neuroscience, 30, 11096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger J.B., Buonocore M.H., Mangun G.R. (2000). The neural mechanisms of top-down attentional control. Nature Neuroscience, 3, 284–91. [DOI] [PubMed] [Google Scholar]
- Ikemoto S., Panksepp J. (1999). The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews, 31, 6–41. [DOI] [PubMed] [Google Scholar]
- Infanti E., Hickey C., Turatto M. (2015). Reward associations impact both iconic and visual working memory. Vision Research, 107, 22–9. [DOI] [PubMed] [Google Scholar]
- Keel J.C., Smith M.J., Wassermann E.M. (2001). A safety screening questionnaire for transcranial magnetic stimulation. Clinical Neurophysiology, 112, 720. [DOI] [PubMed] [Google Scholar]
- Kitajo K., Nozaki D., Ward L.M., Yamamoto Y. (2003). Behavioral stochastic resonance within the human brain. Physical Review Letters, 90, 218103. [DOI] [PubMed] [Google Scholar]
- Mathôt S., Schreij D., Theeuwes J. (2012). OpenSesame: An open-source, graphical experiment builder for the social sciences. Behavior Research Methods, 44, 314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi C., Harris J.A., Ruzzoli M. (2013). Modelling non-invasive brain stimulation in cognitive neuroscience. Neuroscience & Biobehavioral Reviews, 37, 1702–12. [DOI] [PubMed] [Google Scholar]
- Noudoost B., Moore T. (2011). The role of neuromodulators in selective attention. Trends in Cognitive Sciences, 15, 585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirulli C., Fertonani A., Miniussi C. (2013). The role of timing in the induction of neuromodulation in perceptual learning by transcranial electric stimulation. Brain Stimulation, 6, 683–9. [DOI] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews, 18, 247–91. [DOI] [PubMed] [Google Scholar]
- Roelfsema P.R., van Ooyen A. (2005). Attention-gated reinforcement learning of internal representations for classification. Neural Computation, 17, 2176–214. [DOI] [PubMed] [Google Scholar]
- Roelfsema P.R., van Ooyen A., Watanabe T. (2010). Perceptual learning rules based on reinforcers and attention. Trends in Cognitive Sciences, 14, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper Z.J., Vecera S.P., Vaidya J.G. (2014). Value-driven attentional capture in adolescence. Psychological Science, 0956797614545654. [DOI] [PubMed] [Google Scholar]
- Theeuwes J., Belopolsky A.V. (2012). Reward grabs the eye: oculomotor capture by rewarding stimuli. Vision Research, 74, 80–5. [DOI] [PubMed] [Google Scholar]
- Theeuwes J., Burger R. (1998). Attentional control during visual search: the effect of irrelevant singletons. Journal of Experimental Psychology: Human Perception and Performance, 24, 1342. [DOI] [PubMed] [Google Scholar]
- Wang L., Yu H., Zhou X. (2013). Interaction between value and perceptual salience in value-driven attentional capture. Journal of Vision, 13, 5. [DOI] [PubMed] [Google Scholar]
- Ward L.M., Doesburg S.M., Kitajo K., MacLean S.E., Roggeveen A.B. (2006). Neural synchrony in stochastic resonance, attention, and consciousness. Canadian Journal of Experimental Psychology, 60, 319. [DOI] [PubMed] [Google Scholar]