Abstract
Although social situations regularly involve multiple persons acting together, research on the mirror neuron system has focused on situations in which a single agent is observed. Therefore, the goal of the current study was to explore the role of the mirror mechanism in situations involving multiple agents. Specifically, we used transcranial magnetic stimulation (TMS) to investigate whether mirror activation is modulated by the number of observed agents. Based on group contagion research, we hypothesized that multiple agents would provide a stronger trigger to the motor system and would therefore produce a stronger mirror response than a single agent. Participants observed movements performed by a single hand or by two hands while TMS was applied to the primary motor cortex. The results confirmed that activation in the motor system was stronger for two hands. This suggests that input to the motor system increases as the number of agents grows. Relating back to group contagion, our study suggests that groups may be more contagious simply because their actions resonate louder. Given that the mirror mechanism has been linked to a variety of social skills, our findings additionally have important implications for the understanding of social interaction at the group level.
Keywords: TMS, action, observation, imtation, mirror neuron
Since their discovery in the monkey brain (Gallese et al., 1996; Rizzolatti et al., 1996), mirror neurons have been studied extensively in the literature. As a result of this research, it is now well established that a shared system for perception and action does not only exist in monkeys but can be found in humans as well (Molenberghs et al., 2012; Rizzolatti and Sinigaglia, 2010). A useful technique to study the human mirror neuron system is transcranial magnetic stimulation (TMS). Numerous studies have now shown that the application of TMS to the primary motor cortex increases corticospinal excitability of the muscles involved in executing the observed movement (Fadiga et al., 1995, 2005; Maeda et al., 2002; Naish et al., 2014). Furthermore, it has been shown that these effects rely on input from regions within the frontoparietal mirror neuron network such as the premotor and intraparietal cortex (Avenanti et al., 2007, 2013; Koch et al., 2010; Catmur et al., 2011; Enticott et al., 2012). Interestingly, it has been argued that this mirror mechanism facilitates social interaction because it allows individuals to obtain first-person knowledge on the actions of others (Rizzolatti and Fabbri-Destro, 2008). In line with this argument, studies have shown that motor resonance does not only reflect the kinematics (Maeda et al., 2002), but also the intention (Cattaneo et al., 2007; Tidoni et al., 2013), the goal (Cattaneo et al., 2009) and the outcome (Aglioti et al., 2008) of an observed action. This is further supported by evidence suggesting that motor activation is facilitated when an observed action is produced by another person but suppressed when it is produced by oneself (Schütz-Bosbach et al., 2006).
However, research on the mirror neuron system has so far mainly focused on situations in which a single agent is observed. It is therefore largely unknown how this system behaves in situations that include multiple agents. If the mirror system is involved in multi-agent interactions, activation in this system should be sensitive to the number of observed agents. In support of this idea, research in social psychology has shown that the behavior of groups is more contagious than that of a singleton. For example, Milgram et al. (1969) monitored the behavior of pedestrians as they passed by one or multiple confederates looking at a sixth floor window. It was shown that the tendency of passers-by to copy this behavior was stronger when the confederates formed a group (see also: Knowles and Bassett, 1976; Gallup et al., 2012). In other work, similar effects were also obtained in the context of applause contagion (Freedman and Birsky, 1980), queue formation (Mann, 1977), helping behavior (Latané and Darley, 1968), and action imitation (Herrmann et al., 2013). However, these findings have mainly been explained in terms of high-level interpretive processes. In the study of Milgram et al. (1969), for instance, it was argued that the gaze of a group is followed more often because groups are more likely to be attending something of interest. In contrast to this idea, we have recently shown that imitative tendencies increase for multiple agents in a simple movement paradigm where interpretive processes are unlikely to contribute (Cracco et al., 2015). Given that motor resonance is considered to be at the basis of automatic imitation (Bien et al., 2009; Catmur et al., 2009; Heyes, 2011), this suggests that activation in the mirror neuron system might be sensitive to the number of observed agents. Specifically, multiple agents may provide a stronger trigger to the mirror system and hence produce a stronger motor response. As a result, groups could be more contagious simply because their actions resonate louder (Raafat et al., 2009).
To test the hypothesis that multiple agents evoke a stronger mirror response, the current study measured corticospinal excitability by means of TMS while participants passively observed two agents of whom a single agent or both agents performed a movement. To eliminate the influence of interpretive processes, the social context was minimized by reducing the agents to two hands making an index or little finger abduction movement (Cracco et al., 2015). Based on previous TMS research, we expected that action observation would enhance corticospinal excitability of the action relevant muscles but not of the action irrelevant muscles (Fadiga et al., 2005; Naish et al., 2014). In addition, we expected this effect to be stronger when the observed action was performed by two agents instead of a single agent.
Materials and methods
Participants
Thirty-six right-handed males (Mage = 22.25, SDage = 3.06) participated in the study in exchange for 25 euros. However, as described below, two participants were excluded from analysis. This resulted in a sample of 34 participants (Mage = 22.27, SDage = 3.14). All subjects had good or corrected vision, had no history of neurological or psychiatric disorder, and complied to the TMS safety precautions (Rossi et al., 2009). Written informed consent was given before the start of the experiment. The study was approved by the Medical Ethic Review Board of the Ghent University Hospital and was conducted in accordance with the 1964 Helsinki Declaration.
Stimuli and apparatus
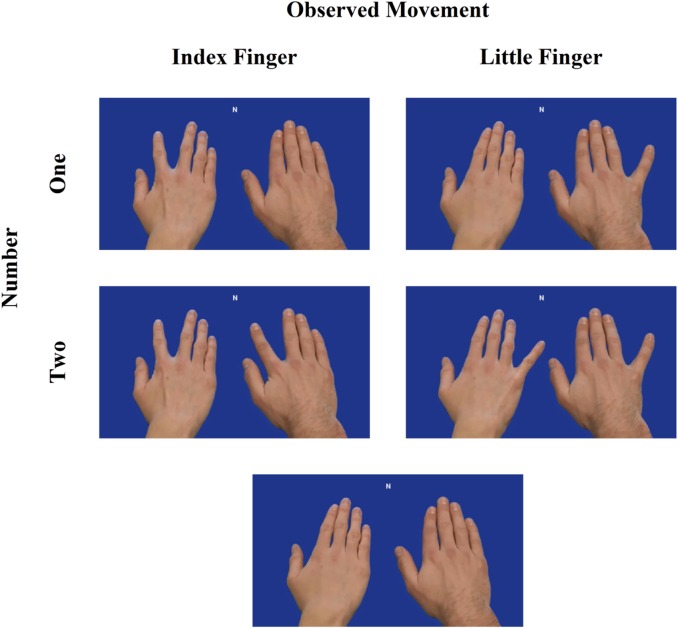
The experiment was programmed with Tscope (Stevens et al., 2006). Stimuli consisted of frames that were extracted from video clips (Figure 1). The stimuli (1010 × 568 pixels) depicted two different male right hands. The hands were presented next to each other on a blue background from a first person perspective. The position (left/right) of the hands on the screen was counterbalanced. To produce an illusion of movement, a picture of the hands in their end posture was superimposed on a picture of the hands in their starting posture (see also: Catmur et al., 2007, 2011). The hands could either not move or abduct the index or little finger. Importantly, when both hands made a movement they always performed the same movement. As a result, the experiment included seven possible end postures: Static-Static, IndexFinger-Static, Static-IndexFinger, LittleFinger-Static, Static-LittleFinger, IndexFinger-IndexFinger and LittleFinger-LittleFinger.
Fig. 1.
Design of the experiment. TMS was applied over the left primary motor cortex while participants observed two hands. Either a single hand made a movement or both hands made an identical movement. Two static hands were used as a baseline condition.
Task and procedure
The experiment took about 45 min and consisted of four blocks of 105 trials each. All end postures were presented an equal number of times in each block in a random order. The experimental task required participants to monitor a cue (N, W or P) appearing at the top of the screen simultaneously with the presentation of the end posture. Participants were instructed to abduct the index finger when W (10%) was presented and to abduct the little finger when P (10%) was presented. When N (80%) was presented, no action was required. The movement (W or P) and no-movement (N) trials were distributed equally among the seven possible end postures. On the movement trials, the cue was chosen randomly so that W and P appeared an equal number of times. This resulted in 14% neutral movement trials, 44% congruent movement trials and 42% incongruent movement trials. The rationale behind the task was twofold. First, we wanted to maintain the attention of the participants. Second, we wanted to ensure that the relevant motor representations remained active throughout the experiment. Note that analyses were restricted to the N trials. As a result, motor execution processes could not influence the results.
Each trial started with a picture of the hands in their starting posture and a fixation cross at the top of the screen for 500 ms. The hands were then presented in their end posture for a duration of 1000 ms together with the cue. A TMS pulse was delivered on every trial. The pulse was delivered randomly at 300, 400 or 500 ms after the presentation of the end posture. The pulses were distributed equally among the three stimulation moments. The trial ended with the presentation of a black screen for a jittered duration of 4000, 5000 or 6000 ms.
TMS and electromyography
Single pulse TMS stimulation was applied with a biphasic magnetic stimulator (Rapid2 Magstim, Whitland, UK) that was connected to a polyurethane-coated figure-of-eight coil (5.4-cm inner diameter windings). The coil was positioned tangentially over the hand area of the left primary motor cortex. The handle of the coil pointed backwards and formed an angle of 45° with respect to the sagittal plane. Electromyographical (EMG) activity was recorded from the first dorsal interosseous (FDI) and the abductor digiti minimi (ADM) of the right hand with the ActiveTwo system (BioSemi, Amsterdam, The Netherlands) using sintered 11 × 17-mm active Ag–AgCl electrodes. The FDI is involved in abducting the index finger and the ADM is involved in abducting the little finger.
Before the start of the experiment, the hotspot within the left primary motor cortex hand area was determined as the stimulation site that produced the largest motor-evoked potentials (MEP) in both the FDI and ADM. When the hotspot was found, the motor threshold was determined as the minimal stimulation intensity that produced a peak-to-peak MEP of 50 μV or more in both muscles in 50% of the pulses. The stimulation intensity was set at 110% of the motor threshold during the experiment.
Data analysis
The MEP peak-to-peak amplitude was computed in MATLAB. All statistical analyses were performed in R (R Development Core Team, 2013). Two participants were excluded because their accuracy rate on the movement trials (75 and 80%) was more than 2.5 median absolute deviations (MADs) away from the median (Leys et al., 2013). Note, however, that including these two participants did not have an influence on the results.
For the remaining participants, only trials in which no movement was required were included in the analysis. Trials in which a movement was nonetheless produced were excluded (1.48%). To account for noise, we additionally removed trials in which the root mean square of the EMG signal was above 50 μV in the 100 ms before the pulse (0.49%) and trials in which the MEP was below 50 μV (4.13%). Finally, we excluded trials in which the MEP was >2.5 MADs away from the median (6.56%) to remove outliers (Leys et al., 2013). In total, 12.17% of the no-movement trials were excluded.
Data analysis was performed on the percentage of change in the MEPs with respect to the Static-Static baseline condition. The obtained change scores were subjected to a 2 (muscle: action relevant or action irrelevant) × 2 (number: one or two) × 3 (pulse moment: 300, 400 or 500 ms) repeated measures MANOVA. On each trial, the action relevant muscle was defined as the muscle involved in executing the observed movement and the action irrelevant muscle as the muscle not involved in executing the observed movement. For example, the FDI was defined as action relevant when an index finger abduction movement was observed but as action irrelevant when a little finger abduction movement was observed (and vice versa for the ADM).
Results
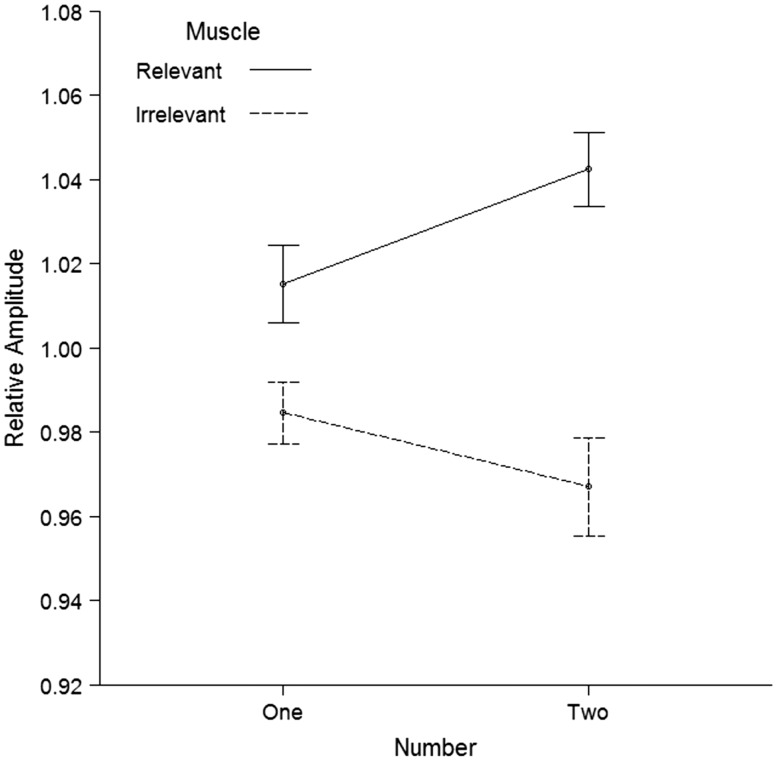
The behavioral data revealed a mean response time of 632 ms (SD = 127 ms) and an accuracy rate of 95% (SD = 4%) on the movement trials. As can be seen in Figure 2, the TMS results on the no-movement trials confirmed that MEPs in the action relevant muscle were stronger than MEPs in the action irrelevant muscle, F(1, 33) = 22.80, P < 0.001, ηp2 = 0.41, d = 0.82. As expected, this effect was modulated by the number of observed movements, F(1, 33) = 8.25, P = 0.007, ηp2 = 0.20, d = 0.49, with a larger difference between the action relevant and action irrelevant muscle when two movements were observed, t(33) = 4.87, P < 0.001, d = 0.84, compared with when a single movement was observed, t(33) = 2.70, P = 0.011, d = 0.46. Importantly, follow-up two-tailed t tests revealed that seeing two identical movements compared with a single movement increased MEPs in the action relevant muscle, t(33) = 2.50, P = 0.018, d = 0.43, but did not modulate MEPs in the action irrelevant muscle t(33) = −1.37, P = 0.181, d = −0.24. The analysis additionally revealed a significant Muscle x Pulse interaction, F(2, 32) = 3.37, P = 0.047, ηp2 = 0.17, indicating that the difference between the action relevant and action irrelevant muscle was stronger when the pulse was delivered at 400 ms, t(33) = 5.18, P < 0.001, d = 0.89, than at 300 ms, t(33) = 2.53, P = 0.016, d = 0.44, or at 500 ms, t(33) = 2.15, P = 0.039, d = 0.37 (Supplementary Table S1). None of the other main or interaction effects reached significance, all F < 0.70, all P ≥ 0.503.
Fig. 2.
TMS results of the experiment. The two lines depict the MEP amplitude relative to the static hands condition in the action relevant muscle and in the action irrelevant muscle. The action relevant muscle was defined as the muscle involved in executing the observed movement and the action irrelevant muscle as the muscle not involved in executing the observed movement. Error bars represent standard errors of the mean corrected for within-subject designs according to Morey (2008). The TMS results for the two separate muscles are available in supplementary material (Supplementary Figure S1).
Discussion
The goal of this study was to study the role of the mirror neuron system in situations that involve multiple agents acting at the same time. Based on group contagion research in social psychology (e.g. Milgram et al., 1969; Knowles and Bassett, 1976; Gallup et al., 2012), it was hypothesized that multiple agents would provide a stronger trigger to the mirror system and would therefore produce a stronger mirror response. To test this hypothesis, we conducted a TMS study in which participants passively observed two hands of which a single hand made a movement or both hands made an identical movement. As predicted, a stronger mirror response was found when two identical movements were observed. This finding extends previous TMS research on the mirror neuron system (Fadiga et al., 1995, 2005; Naish et al., 2014) by showing that the mirror mechanism does not only play a role in dyadic interactions but also in multi-agent interactions (Raafat et al., 2009).
Importantly, the obtained results cannot easily be explained in terms of attentional facilitation or motor inhibition. With regard to attentional facilitation, it could be argued that two simultaneous movements attracted more attention and therefore produced a stronger mirror response. However, orienting responses are known to be fast and transient. That is, studies on inhibition of return have consistently shown that attention does not remain at the location where it has previously been drawn by a salient stimulus (Klein, 2000). In particular, these studies have demonstrated that the facilitatory influence of attention on both sensory and motor processes (Tian et al., 2008, 2011) disappears around 250–300 ms (Klein, 2000; Samuel and Kat, 2003). Because TMS was applied at 300, 400 or 500 ms following movement observation, attentional influences were likely to have tapered off at the time of stimulation. Instead, research suggests that TMS at these time points is optimal to study muscle-specific modulations of motor resonance (Naish et al., 2014).
With regard to motor inhibition, it could be argued that the static hand triggered an inhibition response when there was only one hand making a movement. According to this account, the results of the current study should not be interpreted as an increase in MEPs in the two movement condition but as a decrease in MEPs in the one movement condition. However, previous work has shown that the observation of passive body parts results in excitation rather than inhibition of corticospinal excitability (Schütz-Bosbach et al., 2006; Borgomaneri et al., 2012; Mattiassi et al., 2014). Moreover, because a static hand is not linked to a particular movement, inhibition of this hand should target both the FDI and ADM. As a result, the motor inhibition account predicts that MEPs in the one movement condition should be smaller than MEPs in the two movement condition both for the action relevant and action irrelevant muscle. Because the number of observed movements only influenced MEPs in the action relevant muscle, it is unlikely that inhibition of the static hand can explain the obtained results.
By showing that the mirror neuron system is sensitive to the number of observed agents, the current study sheds light on the neurocognitive mechanisms underlying multi-agent interactions. Although it is broadly accepted that the mirror system supports social interaction (Keysers and Gazzola, 2006; Knoblich and Sebanz, 2006; Rizzolatti and Fabbri-Destro, 2008), previous work has mainly focused on situations in which a single agent is observed. The finding that motor resonance is sensitive to the number of observed agents opens up the possibility that the mirror system is also involved in social situations involving multiple agents. In particular, it provides an alternative explanation for the phenomenon that groups are more contagious than individuals (e.g. Milgram et al., 1969; Knowles and Bassett, 1976; Gallup et al., 2012). Although previous studies on this phenomenon have mostly explained their findings in terms of high-level interpretive processes, our results indicate that groups may instead be more contagious simply because they trigger the motor system more strongly. This sensorimotor interpretation fits well with recent work in which we showed that imitative tendencies are stronger for multiple agents even when the influence of interpretive processes is minimized (Cracco et al., 2015). Adding to this work, this study identifies the mirror mechanism as a possible neural mechanism behind these effects.
Nevertheless, it should be noted that the account outlined above does not necessarily exclude an influence of interpretive processes at later stages of processing. When motor resonance produces the urge to imitate, interpretive processes could for instance be recruited to decide if the evoked behavior is reasonable given the context. Such an evaluative process could then determine whether the prepared action is eventually executed or inhibited. However, in this view, interpretive processes are not the antecedent but the consequence of imitative tendencies. In support of this proposition, research has shown that the conscious decision to imitate is driven by the gating of mirror activation (Bien et al., 2009). Similarly, interpretive processes could serve as a gating mechanism to regulate imitative tendencies in social group situations (Freedman and Birsky, 1980; Gallup et al., 2012; Herrmann et al., 2013; Knowles and Bassett, 1976; Latané and Darley, 1968; Mann, 1977; Milgram et al., 1969).
To conclude, the finding that motor resonance is modulated by the number of observed agents suggests that the mirror system is involved in social interactions that go beyond a dyadic structure. In particular, it opens up the possibility that groups are more contagious not because of interpretive processes (Latané and Darley, 1968; Milgram et al., 1969; Knowles and Bassett, 1976; Mann, 1977; Freedman and Birsky, 1980; Gallup et al., 2012; Herrmann et al., 2013) but because they produce a stronger mirror response. Given that the mirror mechanism has been linked to a variety of social skills, among which theory of mind (Keysers and Gazzola, 2006) and empathy (Carr et al., 2003; Gazzola et al., 2006), our findings may additionally have important implications for the understanding of social interaction at the group level.
Supplementary data
Supplementary data are available at SCAN online.
Funding
This research was supported by the Research Foundation Flanders Grant FWO14/ASP/050 awarded to the first author.
Conflict of interest. None declared.
Supplementary Material
References
- Aglioti S.M., Cesari P., Romani M., Urgesi C. (2008). Action anticipation and motor resonance in elite basketball players. Nature Neuroscience, 11(9), 1109–16. [DOI] [PubMed] [Google Scholar]
- Avenanti A., Annella L., Candidi M., Urgesi C., Aglioti S.M. (2013). Compensatory plasticity in the action observation network: virtual lesions of STS enhance anticipatory simulation of seen actions. Cereb Cortex, 23(3),570–80. [DOI] [PubMed] [Google Scholar]
- Avenanti A., Bolognini N., Maravita A., Aglioti S.M. (2007). Somatic and motor components of action simulation. Current Biology, 17(24), 2129–35. [DOI] [PubMed] [Google Scholar]
- Bien N., Roebroeck A., Goebel R., Sack A.T. (2009). The brain’s Intention to Imitate: The Neurobiology of Intentional versus Automatic Imitation. Cerebral Cortex, 19(10), 2338–51. [DOI] [PubMed] [Google Scholar]
- Borgomaneri S., Gazzola V., Avenanti A. (2012). Motor mapping of implied actions during perception of emotional body language. Brain Stimulation, 5(2), 70–6. [DOI] [PubMed] [Google Scholar]
- Carr L., Iacoboni M., Dubeau M.C., Mazziotta J.C., Lenzi G.L. (2003). Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences of the United States of America, 100(9), 5497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catmur C., Mars R.B., Rushworth M.F., Heyes C. (2011). Making mirrors: premotor cortex stimulation enhances mirror and counter-mirror motor facilitation. Journal of Cognitive Neuroscience, 23(9), 2352–62. [DOI] [PubMed] [Google Scholar]
- Catmur C., Walsh V., Heyes C. (2007). Sensorimotor learning configures the human mirror system. Current Biology, 17/(17), 1527–31. [DOI] [PubMed] [Google Scholar]
- Catmur C., Walsh V., Heyes C. (2009). Associative sequence learning: the role of experience in the development of imitation and the mirror system. Philosophical Transactions of the Royal Society B-Biological Sciences, 364(1528), 2369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L., Caruana F., Jezzini A., Rizzolatti G. (2009). Representation of goal and movements without overt motor behavior in the human motor cortex: A transcranial magnetic stimulation study. The Journal of Neuroscience, 29(36), 11134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L., Fabbri-Destro M., Boria S., et al. , (2007). Impairment of actions chains in autism and its possible role in intention understanding. Proceedings of the National Academy of Sciences of the United States of America, 104(45), 17825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco E., De Coster L., Andres M., Brass M. (2015). Motor simulation beyond the dyad: Automatic imitation of multiple actors. Journal of Experimental Psychology: Human Perception and Performance, 41(6), 1488–501. [DOI] [PubMed] [Google Scholar]
- Enticott P.G., Arnold S.L., Fitzgibbon B.M., Hoy K.E., Susilo D.A., Fitzgerald P.B. (2012). Transcranial direct current stimulation (tDCS) of the inferior frontal gyrus disrupts interpersonal motor resonance. Neuropsychologia, 50(7), 1628–31. [DOI] [PubMed] [Google Scholar]
- Fadiga L., Craighero L., Olivier E. (2005). Human motor cortex excitability during the perception of others’ action. Current Opinion in Neurobiology, 15(2), 213–8. [DOI] [PubMed] [Google Scholar]
- Fadiga L., Fogassi L., Pavesi G., Rizzolatti G. (1995). Motor facilitation during action observation: A magnetic stimulation study. Journal of Neurophysiology, 73, 2608–11. [DOI] [PubMed] [Google Scholar]
- Freedman J.L., Birsky J. (1980). Environmental determinants of behavioral contagion: Density and number. Basic and Applied Social Psychology, 1(2), 155–61. [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. (1996). Action recognition in the premotor cortex. Brain, 119(2), 593–609. [DOI] [PubMed] [Google Scholar]
- Gallup A.C., Hale J.J., Sumpter D.J.T., et al. , (2012). Visual attention and the acquisition of information in human crowds. Proceedings of the National Academy of Sciences of the United States of America, 109(19), 7245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V., Aziz-Zadeh L., Keysers C. (2006). Empathy and the somatotopic auditory mirror system in humans. Current Biology, 16(18), 1824–9. [DOI] [PubMed] [Google Scholar]
- Herrmann P.A., Legare C.H., Harris P.L., Whitehouse H. (2013). Stick to the script: The effect of witnessing multiple actors on children’s imitation. Cognition, 129(3), 536–43. [DOI] [PubMed] [Google Scholar]
- Heyes C. (2011). Automatic imitation. Psychological Bulletin, 137(3), 463–83. [DOI] [PubMed] [Google Scholar]
- Keysers C., Gazzola V. (2006). Towards a unifying neural theory of social cognition. Progress in Brain Research, 156, 379–401. [DOI] [PubMed] [Google Scholar]
- Klein R.M. (2000). Inhibition of return. Trends in Cognitive Sciences, 4(4), 138–47. [DOI] [PubMed] [Google Scholar]
- Knoblich G., Sebanz N. (2006). The social nature of perception and action. Current Directions in Psychological Science, 15, 99–104. [Google Scholar]
- Knowles E.S., Bassett R.L. (1976). Groups and crowds as social entities: Effects of activity, size, and member similarity on nonmembers. Journal of Personality and Social Psychology, 34(5), 837–45. [Google Scholar]
- Koch G., Versace V., Bonnì S., et al. , (2010). Resonance of cortico-cortical connections of the motor system with the observation of goal directed grasping movements. Neuropsychologia, 48(12), 3513–20. [DOI] [PubMed] [Google Scholar]
- Latané B., Darley J.M. (1968). Group inhibition of bystander intervention in emergencies. Journal of Personality and Social Psychology, 10(3), 215–21. [DOI] [PubMed] [Google Scholar]
- Leys C., Ley C., Klein O., Bernard P., Licata L. (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology, 49(4), 764–6. [Google Scholar]
- Maeda F., Kleiner-Fisman G., Pascual-Leone A. (2002). Motor facilitation while observing hand actions: Specificity of the effect and role of observer’s orientation. Journal of Neurophysiology, 87(3), 1329–35. [DOI] [PubMed] [Google Scholar]
- Mann L. (1977). The effect of stimulus queues on queue-joining behavior. Journal of Personality and Social Psychology, 35(6), 437–42. [Google Scholar]
- Mattiassi A.D., Mele S., Ticini L.F., Urgesi C. (2014). Conscious and unconscious representations of observed actions in the human motor system. Journal of Cognitive Neuroscience, 26(9), 2028–41. [DOI] [PubMed] [Google Scholar]
- Milgram S., Bickman L., Berkowitz L. (1969). Note on the drawing power of crowds of different size. Journal of Personality and Social Psychology, 13(2), 79–82. [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. (2012). Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews, 36(1), 341–9. [DOI] [PubMed] [Google Scholar]
- Morey R.D. (2008). Confidence intervals from normalized data: a correction to Cousineau (2005). Tutorials in Quantitative Methods for Psychology, 4(2), 61–4. [Google Scholar]
- Naish K.R., Houston-Price C., Bremner A.J., Holmes N.P. (2014). Effects of action observation on corticospinal excitability: Muscle specificity, direction, and timing of the mirror response. Neuropsychologia, 64, 331–48. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2013). R: A language and environment for statistical computing R Foundation for Statistical Computing . Vienna, Austria: R Development Core Team. [Google Scholar]
- Raafat R.M., Chater N., Frith C. (2009). Herding in humans. Trends in Cognitive Sciences, 13(10), 420–8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fabbri-Destro M. (2008). The mirror system and its role in social cognition. Current Opinion in Neurobiology, 18(2), 179–84. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fadiga L., Gallese V., Fogassi L. (1996). Premotor cortex and the recognition of motor actions. Cognitive Brain Research, 3, 131–41. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. (2010). The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nature Reviews Neuroscience, 11(4), 264–74. [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., et al. , (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel A.G., Kat D. (2003). Inhibition of return: a graphical meta-analysis of its time course and an empirical test of its temporal and spatial properties. Psychonomic Bulletin and Review, 10(4), 897–906. [DOI] [PubMed] [Google Scholar]
- Schütz-Bosbach S., Mancini B., Aglioti S.M., Haggard P. (2006). Self and other in the human motor system. Current Biology, 16(18), 1830–4. [DOI] [PubMed] [Google Scholar]
- Stevens M., Lammertyn J., Verbruggen F., Vandierendonck A. (2006). Tscope: a C library for programming cognitive experiments on the MS Windows platform. Behavior Research Methods, 38(2), 280–6. [DOI] [PubMed] [Google Scholar]
- Tian Y., Klein R.M., Satel J., Xu P., Yao D. (2011). Electrophysiological explorations of the cause and effect of inhibition of return in a cue-target paradigm. Brain Topography, 24(2), 164–82. [DOI] [PubMed] [Google Scholar]
- Tian Y., Yao D. (2008). A study on the neural mechanism of inhibition of return by the event-related potential in the Go/Nogo task. Biological Psychology, 79(2), 171–8. [DOI] [PubMed] [Google Scholar]
- Tidoni E., Borgomaneri S., di Pellegrino G., Avenanti A. (2013). Action simulation plays a critical role in deceptive action recognition. The Journal of Neuroscience, 33(2), 611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.