Abstract
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that modulates cortical excitability and influences motor behavior. There is limited information available regarding the effects of anodal tDCS on lower limb reaction time. In this study, we aimed to investigate the effects of anodal tDCS on lower limb simple reaction time (SRT) and choice reaction time (CRT). We probed this question further by examining the effects of anodal tDCS of the lower limb M1 on an upper limb RT task and a cognitive measure. Fourteen healthy young adults received anodal tDCS and sham tDCS to the lower limb M1 on two separate testing days in a counter balanced order. After stimulation we assessed the effects of tDCS on ankle dorsiflexion SRT and CRT, ankle plantarflexion SRT and CRT, wrist extension SRT and CRT and the Symbol Digits Modality Test (SDMT). Anodal tDCS significantly improved response times from baseline for ankle CRT but not for ankle SRT or wrist SRT or CRT. A significant decrement (i.e. longer response time) was noted for the sham tDCS conditions. There was a significant difference between anodal and sham conditions for all RT tasks, suggesting that anodal tDCS improved RT compared to sham. No change in SDMT scores was observed for both conditions. Anodal tDCS appeared to differentially modulate ankle SRT and CRT, suggesting an influence of anodal tDCS on complex motor processes and/or the supplementary motor area (SMA). Absence of effects on wrist CRT or SDMT suggest a spatial specificity of the influence of tDCS. Anodal tDCS also appears to potentially negate the effects of fatigue or task-switching that was detrimental to RT in the sham condition.
Keywords: tDCS, lower limb motor cortex, reaction time, motor processing, ankle
Introduction
In order to safely perform basic activities of daily living, humans rely on the ability to rapidly react to their environment. Reaction time (RT) is defined as the delay between the presentation of a stimulus (perceptual phase) and the response by the human (motoric phase) (Magill 2011). There are two kinds of RT tasks: simple and choice. Simple RT involves one response corresponding to one stimulus. Choice RT involves a distinct response to each type of stimulus, typically presented in a random sequence. Simple RT is typically due to input-output delays of single neurons and of neural chains, and thus exhibit faster responses than choice RT tasks. Choice RT is considered to indicate a more complex processing requirement of the central nervous system (Nijhawan 2008).
A growing body of literature has demonstrated that anodal (facilitatory) transcranial direct current stimulation (tDCS) of the upper limb motor cortex (M1) has the ability to improve performance of complex functional motor tasks in healthy and patient populations (Nitsche et al. 2008). Anodal tDCS of the upper limb M1 has also shown to improve simple motor tasks of the upper limb such as reaction time performance (Hummel et al. 2006; Kang and Paik 2011; Leite et al. 2011). This finding has also been supported in individuals with neurological disorders (Fregni et al. 2006; Stagg et al. 2012). In the lower limb, anodal tDCS has shown to improve performance of complex tasks such as skilled visuomotor tracking, muscle strength and walking. (Tanaka et al. 2009; Madhavan et al. 2011; Tanaka et al. 2011; Sriraman et al. 2014; Park et al. 2015). However, reports on the effects of anodal tDCS of the leg M1 on simple lower limb motor tasks (such as RT) are limited to one study to the best of our knowledge. Tanaka et al. (2009) instructed healthy participants to respond to a simple visual GO signal by pressing a pedal with the left leg as quickly as possible. No significant improvements in lower limb simple RT was reported after anodal tDCS of the leg M1. Given the increasing investigation of tDCS as a motor priming tool for walking, it is important that we try to understand the effects of tDCS on lower limb RT. Fast goal directed lower limb movement is an important component of locomotion and is necessary for accurate execution of daily activities. Also, understanding the effects on simple motor tasks may enable tDCS to be beneficial to individuals with higher level of impairments who are unable to perform complex functional movements and may benefit from improvements in simple motor tasks.
The primary aim of the present study was to examine the effects of anodal tDCS of the leg M1 on lower limb (ankle) simple RT (SRT) and choice RT (CRT). Despite the many reported benefits of tDCS on motor function, little is known about the mechanisms by which tDCS produces these improvements. We attempted to probe further into these mechanisms by examining if tDCS of the lower limb M1 enables global changes in performance. Specifically we examined the effects of anodal tDCS of the leg M1 on other cognitive and motor processes that are not directly related to changes in neuromotor networks of the leg M1. Hence, we examined the effects of anodal tDCS of the leg M1 on the upper limb M1 using an upper limb (wrist) simple and choice RT task. Also we examined the effects of anodal tDCS of the leg M1 on cognitive processing using the Symbol Digit Modality Test (SDMT), which is highly dependent on the dorsolateral prefrontal cortex (DLPFC). We hypothesized that anodal tDCS of the lower limb M1 would improve ankle simple and choice RT, but will not influence wrist RT or SDMT.
Methods
Participants
Fourteen young healthy participants (6 males, 8 females, age range 20–32 years) with no neurological, musculoskeletal or cardiovascular disorders were recruited to participate in this study. A description of the study was provided and written consent approved by the Institutional Review Board was obtained from each participant. Before inclusion in the study, all participants were screened for contraindications to non-invasive brain stimulation procedures which included unexplained headaches, pregnancy, metal implants, cardiac pacemakers, history of seizures or epilepsy and medications likely to alter cortical excitability, such as antidepressants. All participants reported to be right leg dominant (preferred leg used to kick a ball).
Experimental Design
We used a single blinded, sham controlled, repeated measures study design where each individual participated in two experimental sessions (anodal tDCS and sham stimulation) which were counter balanced to avoid order effects. The two experimental sessions were separated by a period of 7 – 9 days. At the beginning of each session, participants were administered the Symbol Digit Modality Test (SDMT). Next, they were provided instructions on how to perform the RT tasks, and five practice trials for each task was given to ensure that the participant understood the instructions. Then, baseline RTs for the following were collected in a random order: ankle dorsiflexion (DF) SRT, ankle plantarflexion (PF) SRT, wrist SRT, ankle DF CRT, ankle PF CRT and wrist CRT. Participants were then administered anodal tDCS or sham stimulation for 15 minutes while they performed a motor tracking task with the ankle. Post RT measures (in random order) were collected 5 minutes after the end of stimulation. Post SDMT was collected after the RT trials, typically within 20–25 minutes the end of stimulation.
Reaction Time Task
Subjects were seated comfortably in a chair in front of a computer with the feet on the ground, hip and knee at 90 degrees, and forearm resting comfortably on a table by the side of the chair. All reaction time trials were performed with the non-dominant foot and non-dominant wrist. Spike 2 software (Cambridge Electronic design, Cambridge, UK) was used to generate the visual stimulus for the RT tasks. The visual stimulus (GO signal) was a red rectangular analog pulse (5V, 100 ms). The participants were seated in front of a monitor placed at eye level. When the experimenter hit ‘start’, participants were presented with a moving red flat line which became the rising/falling GO signal. The inter-trial intervals were varied with a mean of 10 seconds (random sequence, range 5–15 seconds). The participants were instructed to provide the appropriate response according to the task (described below) as soon as they saw the GO signal. Participants were allowed to practice for 5 trials for each task (simple RT and choice RT) prior to testing in order to familiarize themselves with the procedure. No further instructions or feedback was provided after the practice trials. All participants quickly understood the task and did not need any further practice or instruction.
Ankle Simple reaction time
Subjects were instructed to respond as quickly as possible to a GO signal presented at random intervals. During the ankle DF RT trials, the GO signal was a rising event and subjects were asked to respond by lifting the forefoot as quickly as possible. For the ankle PF RT trials, the GO signal was a falling event and subjects were asked to respond by lifting the heel as quickly as possible. Fifteen ankle DF SRT and fifteen PF SRTs were collected.
Ankle Choice Reaction time
For the CRT trials, the GO signal was randomly selected by the computer program to be a rising or falling event. Subjects were instructed to provide the appropriate response (dorsiflexion or plantarflexion) that corresponded to the rising or falling GO signal respectively. Fifteen dorsiflexion CRTs and fifteen plantarflexion CRTs were collected.
Wrist reaction time
The wrist RT task was similar to the ankle task except that the subjects were instructed to perform wrist extension and wrist flexion in response to the visual stimuli. As post-tDCS effects are time sensitive, only wrist extension simple RTs were performed to reduce the number of trials after stimulation. The choice RT task consisted of wrist extension and flexion but only the data for wrist extension are reported.
Electromyography
The onset of muscle activity was used to calculate reaction time. Surface Ag/AgCl electrodes were placed over the muscle belly of the non-dominant tibialis anterior, medial gastrocnemius, and extensor carpi radialis muscles. Before placement of electrodes, the skin was cleaned with alcohol and shaved if necessary. The reference electrode was placed over the non-dominant olecranon process. A Delsys EMG system (Bagnoli 8, MA, USA) was used to sample EMG data at 2000 Hz, with amplification of *1000 and passed through a filter band at 10–500 Hz. Spike2 software was used to collect all EMG data.
Transcranial direct current stimulation
A simple form of constant current stimulator (Chatanooga Ionto, TN, USA) was used to deliver the stimulation. An oblong rectangular saline soaked sponge electrode (5 cm × 2.5 cm) was placed over the non-dominant leg motor representation. Transcranial magnetic stimulation was used to locate the ‘hotspot’ of the non-dominant tibialis anterior muscle representation. The midpoint of the active electrode was then placed over this location which was on average 1 cm lateral and 1 cm posterior to Cz on the 10–20 international electroencephalogram system. The active electrode was positioned in a direction parallel to the midline (invisible line connecting the nasion and the inion). The reference electrode (7cm × 5 cm) was placed over the contralateral supraorbital region. During anodal stimulation, 1 mA of current was delivered for 15 minutes. The current density at the stimulation electrode was 0.08 mA/cm2 and the total charge was 0.072 C/cm2. All participants performed a visuomotor task using ankle dorsiflexion and plantarflexion during anodal/sham tDCS. This motor task has been explained previously (Madhavan and Stinear 2010; Shah et al. 2013; Sriraman et al. 2014). In brief, participants were seated comfortably with their non-dominant foot attached to a custom made ankle tracker device. They were required to track as accurately as possible a computer-generated sinusoidal waveform with continuous ankle dorsiflexion and plantarflexion movements with their non-dominant ankle for 15 minutes (which included 1 minute of rest after every 4 minutes of tracking). We chose to deliver tDCS during a motor task than at rest as the effects of tDCS have shown to be task dependent and more effective when performing a skilled motor task (Sriraman et al. 2014). For sham stimulation, the electrodes were placed in the same position as the anodal stimulation and the same ankle visuomotor task was performed. However the DC stimulator was turned off in 30 seconds after the initial ramping up of current.
Cognitive Function
The Symbol Digit Modality Test was used to test cognitive function before and after tDCS. The cognitive demands of the SDMT include attention, visual scanning, and motor and psychomotor speed (Smith 1968; Smith 1982). In this paper and pencil test, the participant was required to pair digits to assigned symbols using a reference key. The score is the total number of correct written responses in 90 seconds. Higher scores indicate better cognitive performance. At the start of the test, participants were given a brief practice set to familiarize with the instructions.
Data Analysis
For error free trials, RT was calculated as the time interval between the onset of the visual stimulus and onset of activity in the muscle of interest. Onset latencies for the muscle of interest were determined using a semi-automatic computer algorithm that selected the first instant at which the mean EMG activity exceeded a threshold of 2 standard deviations (SD) above the mean background activity, calculated over a 200 ms period just prior to the GO signal. Onsets were first selected by the computer algorithm, then visually approved and (when necessary) corrected.
Errors in reaction time were defined as either incorrect movement, or correct movement with unconventional response times (early or delayed) generated by extraneous factors (noted during data collection) or skipped movement, and were not included in the RT analyses. The mean error averaged for 14 participants was less than 1 (0.6 SD). Because of the small amount of errors, we did not do any further analyses on the error data. The mean RT for each individual for the pre and post sessions were calculated. A percentage change in RT was also calculated.
For the SDMT, the number of correct responses (out of 110 total) were calculated as a raw score for the pre and post trials.
Statistical Analysis
Statistical analysis was performed using SPSS software v22 (SPSS Inc. Chicago, IL). The data (mean response times) from each task (ankle DF SRT, ankle PF SRT, wrist SRT, ankle DF CRT, ankle PF CRT, and wrist CRT) were analyzed separately. All scores were normally distributed (p>0.05), as assessed by Shapiro-Wilk's test of normality on the studentized residuals. Paired t-tests were used to compare the baseline RT (Pre) between anodal and sham conditions for all RT tasks. To examine the effects of stimulation, a 2-way repeated measures ANOVA with Condition (Anodal, Sham) and Time (Pre, Post) as the repeated factors was performed for each dependent variable. A Greenhouse-Geisser correction was applied when assumptions of Sphericity were not met. Post hoc tests using Bonferroni corrections for multiple comparisons were performed for significant main or interaction effects. Paired t-tests were used to examine the effect of intervention (i.e. anodal stimulation) by comparing the normalized RT (percentage change from Pre to Post) between Anodal and Sham conditions. Critical p values were set at 0.05 for all tests. All data are expressed as means ± standard error.
Results
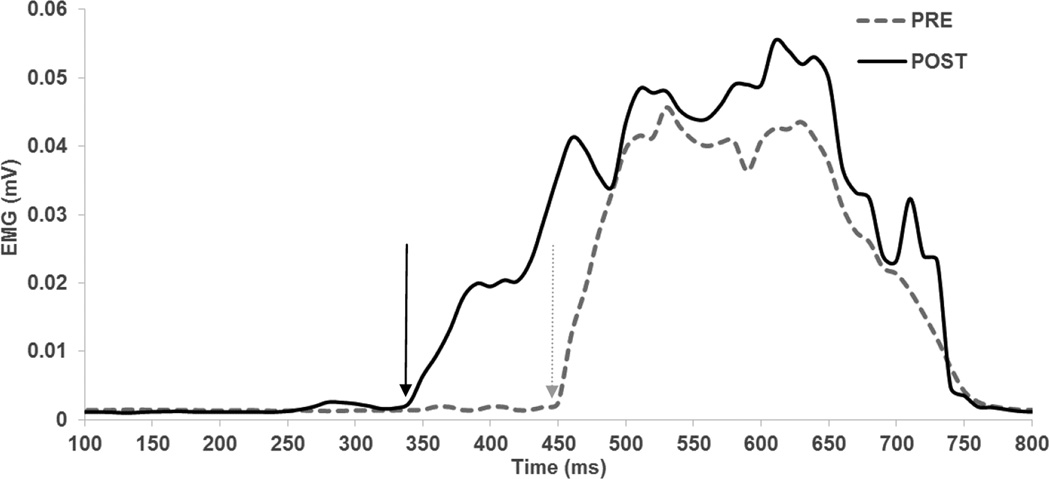
No participant reported any adverse events during the experiments. No participant noticed a difference between the real and sham stimulation conditions. A few participants reported initial mild discomfort (verbalized as “itching” beneath the reference electrode) for both the anodal and sham conditions which typically disappeared within a minute of stimulation. The EMG traces showing the reaction time trials from a representative subject are shown in Figure 1.
Figure 1. EMG signal from a representative subject showing the shorter reaction time induced by anodal tDCS.
Grey dotted lines represent trials before anodal tDCS (PRE) and solid black lines represent the trials after anodal tDCS (POST). Rectified and smoothed muscle activity of the tibialis anterior muscle during a choice reaction time task is depicted. Mean of three CRT trials is shown. Arrows indicate reaction time.
Baseline RT
No differences in the baseline RT scores between the anodal and sham stimulation conditions were noticed: ankle DF SRT (t13 = 1.717, p=0.110), ankle PF SRT (t13 = − 1.112, p=0.286), wrist extension SRT (t13 = 0.378, p = 0.711), ankle DF CRT (t13 = 1.457, p=0.169), ankle PF CRT (t13 = 0.94, p=0.36) and wrist extension CRT (t13 = 0.578, p = 0.57). Baseline RT (mean ± SEM, average of both conditions) for each task was as follows: ankle DF SRT 314 ± 8 ms, ankle PF SRT 320 ± 9 ms, ankle DF CRT 385 ± 13 ms, ankle PF CRT 416 ± 14 ms, wrist SRT 310 ± 8 ms, and wrist CRT 372 ± 10 ms.
Simple Reaction Time (SRT)
Ankle DF SRT
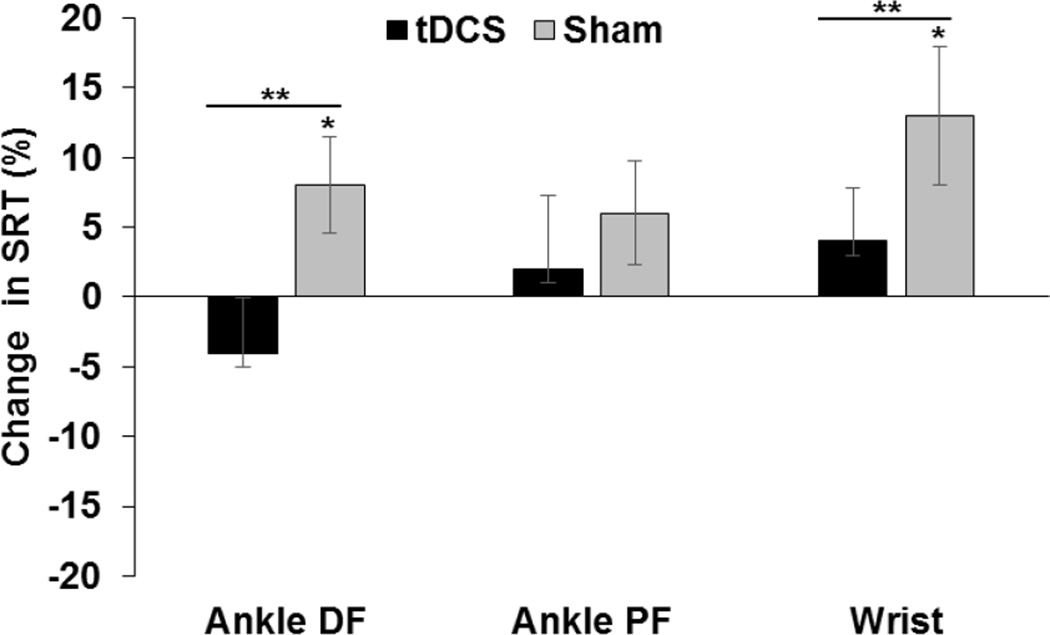
Ankle DF SRT revealed a statistically significant two-way interaction between Condition and Time (F1, 13 = 6.06, p = 0.029). The mean SRT for the Anodal condition was 15 ms (4%) lower at Post compared to Pre, a difference that was not statistically significant (p = 0.160). There was a statistical difference between the Pre and Post time points for the Sham condition (F1, 13 = 7.98, p = 0.014). Ankle DF SRT was 20 ms (8%) higher at Post than Pre for Sham. Paired t-tests comparing normalized ankle DF SRT between conditions revealed a significant difference (t=−2.32, p =0.01) between Anodal (−4.13%) and Sham (7.5%) conditions.
Ankle PF SRT
No significant interaction or main effects was noticed (p>0.05) for ankle PF SRT. No significant difference in normalized ankle PF SRT between conditions was also noticed (p>0.05).
Wrist SRT
A statistically significant two-way interaction between condition and time (F1, 13 = 5.91, p = 0.03) was noticed for wrist SRT. Similar to ankle DF SRT, there was a statistical difference between the Pre and Post time points only for the Sham condition (F1, 13 = 13.99, p = 0.002). Wrist SRT was 40 ms (13%) higher at Post than Pre for Sham. Paired t-tests comparing normalized wrist SRT revealed a significant difference (t=−2.41, p =0.01) between Anodal (4.4%) and Sham (13.4%) conditions. Percentage changes in SRT are shown in Figure 1.
Choice Reaction Time (CRT)
Ankle DF CRT
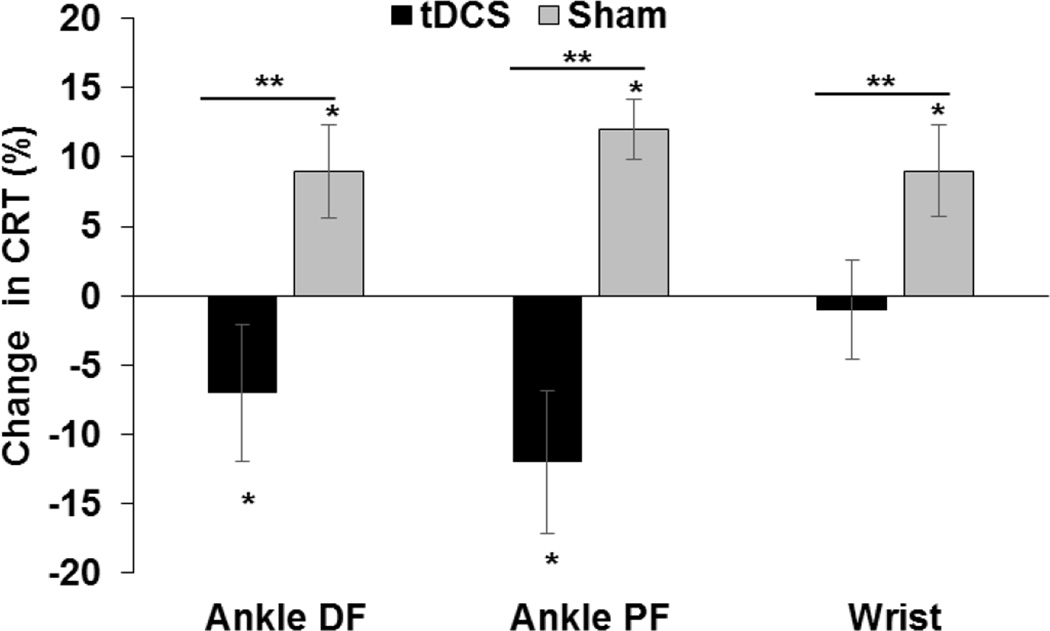
The ANOVA revealed a statistically significant two-way interaction between Condition and Time (F1, 13 = 10.71, p = 0.006) for ankle DF CRT. There was a statistical difference between the Pre and Post time points for the Anodal condition (F1, 13 = 4.44, p = 0.05). Ankle DF CRT was 31 ms (7%) lower at Post than Pre. There was also a statistical difference between the Pre and Post time points for the Sham condition (F1, 13 = 9.162, p = 0.01). Ankle DF CRT was 28 ms (9%) higher at Post than Pre. Paired t-tests comparing normalized ankle DF CRT between conditions revealed a significant difference (t=3.12, p =0.004) between Anodal (−7.4%) and Sham (8.7%) conditions.
Ankle PF CRT
The results for the ankle PF CRT were similar to DF CRT. There was a statistically significant two-way interaction between condition and time (F1, 13 = 20.979, p = 0.001). Post hoc analyses revealed a significant difference between the Pre and Post time points for the Anodal condition, (F1, 13 = 48.85, p = 0.011). Ankle PF CRT was 53 ms (12%) lower at Post than Pre during Anodal stimulation. There was also a statistical difference between the Pre and Post time points for the Sham condition (F1, 13 = 27.2, p = 0.001). Ankle PF CRT was 45 ms (12%) higher at Post than Pre during Sham. Paired t-tests comparing normalized ankle PF CRT between conditions revealed a significant difference (t=−4.72, p =0.001) between Anodal (−12.1%) and Sham (12.2%) conditions.
Wrist CRT
There was a statistically significant two-way interaction between condition and time (F1, 13 = 4.82, p = 0.047) for wrist CRT. Post hoc analyses revealed a statistical difference between the Pre and Post time points for the Sham condition (F1, 13 = 10.54, p = 0.006). Wrist CRT for Sham was 30 ms (9%) higher at Post than Pre. No significant change between Pre and Post was noted for the Anodal condition. Paired t-tests comparing normalized wrist CRT between conditions revealed a significant difference (t=−2.1, p =0.02) between Anodal (−1%) and Sham (9.2%) conditions. Percentage changes in CRT are shown in Figure 2.
Figure 2. Simple Reaction Time.
Mean percentage changes in simple reaction time (SRT) induced by anodal (solids black bars) and sham (grey bars) tDCS for ankle dorsiflexion (DF), ankle plantarflexion (PF) and wrist extension are shown. * indicates a significant difference (p<0.05) between Pre and Post time points within each stimulation condition (post hoc effect). ** indicates a significant difference (p<0.05) between Anodal and Sham conditions for normalized RT.
Symbol Digit Modality Test (SDMT)
The 2-way ANOVA for SDMT scores revealed no significant effects of interaction of Condition and Time (F1, 13 =0.879, p=0.366), or main effect of Condition (F1, 13 =0.089, p=0.77). But a significant effect of Time was seen (F1, 13 =42.843, p=0.000). Both conditions revealed a significant increase (11%, Pre 72.3 ± 4 to Post 79.3 ± 4) in SDMT scores after anodal/sham stimulation.
Discussion
In the present study, we examined the effects of anodal tDCS of the lower limb M1 on lower limb reaction time in healthy young adults. To our knowledge, this is the first study to examine the influence of anodal tDCS on ankle reaction time using a comprehensive experimental paradigm that included simple and choice motor reaction time tasks for the stimulated area of interest, a remote reaction time task and a cognitive measure, which has not been not accounted for in previous lower limb studies. The results of this study suggest that a 15-minute application of anodal tDCS to the lower limb M1 during a visuomotor ankle task improves ankle choice reaction time (CRT), as denoted by faster CRT responses after anodal stimulation compared to baseline. We did not observe an effect of anodal stimulation (i.e. change from baseline) for SRT, remote motor (wrist) RT or cognitive task (SDMT). Interestingly, we noted that the normalized RT was significantly faster for the anodal condition when compared to the sham condition for ankle DF SRT, wrist SRT and all CRT tasks.
Differential effects of anodal stimulation on ankle SRT vs. CRT
One of the important findings of this study was the differential effects of anodal tDCS on ankle simple and choice RT. Based on findings from previous tDCS studies of the upper limb M1, we expected anodal tDCS of the lower limb M1 to improve both simple and choice ankle RT. However this hypothesis was only partly supported. A RT is a measure of speed and efficiency of central processing, with a CRT reflecting more complex brain processing than a SRT. During RT, three basic process unfold: sensory coding (perceiving the stimulus), decision formation (determining the required action to the stimulus) and motor execution (issuing a motor command and performing the desired action) (Sternberg 1969). A SRT task, where there is only one stimulus and one response, represents the most basic pathway of translating the external input into a speeded motor output. Whereas in a CRT task, where different stimuli require different responses, more executive level of processing is required as it involves additional steps of stimulus identification and selection of responses. During CRT tasks, neurons in cortical and subcortical areas have been shown to alter their firing rates during preparatory delay (Hanes and Schall 1996; Kilavik et al. 2014). The mechanisms of action of anodal tDCS has been attributed to short term depolarization of neuronal membranes and modulation of neuronal firing with resulting long term changes in intracortical neuronal excitability within the cortex (Stagg and Nitsche 2011). The increased neuronal modulation in the stimulated M1 could have made it more receptive to commands from other brain regions to allow complex tasks to be performed more efficiently. Anodal tDCS may have enabled better integration between different sets of nuclei necessary for the execution of a complex cognitively demanding task, thereby enhancing CRT and not SRT.
A second possible explanation could be attributed to the electrode montage used in the present study. The supplementary motor area (SMA) is situated in front of the lower limb M1, and is important for establishing motor programs especially in complex task situations following visual cues (Cunnington et al. 2002; Grezes and Decety 2002). The SMA is deeply involved in movement preparation as supported by changes in SMA neuronal involvement before movement execution (Tanji and Mushiake 1996). A recent study showed that anodal tDCS of the SMA selectively interferes with movement preparation without affecting voluntary movement, suggesting that these two components of movement execution have separate pathways (Bolzoni et al. 2015). It is possible that the electrode location or the direction of current flow in the present study may have influenced cortical networks in the SMA, and thus enhancing motor planning and not movement execution, thereby causing a selective improvement in CRT.
Alternatively, it is possible that young healthy subjects display a ceiling effect in SRT thereby affecting the ability to show improvements in SRT or that our study was underpowered for SRT measurements. It is important to note here that we measured RT as muscle activity onset and not movement onset. It is possible that we may be underestimating the effects of anodal tDCS on SRT as we did not measure its effect on movement execution.
Slowing of response times during sham stimulation
Another important finding of this study was the slower RT during sham stimulation which was not observed during anodal stimulation. We found that the RT was slower (as evidence by the longer response times) for the sham condition for both the simple and choice RT tasks. In addition, we noticed that the amount of change in RT during the anodal stimulation was significantly different (faster) than sham stimulation. A similar finding of slower reaction times during sham stimulation was observed by Hummel et al. (2006) in their study on stroke survivors. The slower reaction time could be due to increased peripheral and/or central fatigue accompanying the demanding motor tracking task. Previous studies have shown that short periods of rhythmic contractions performed at submaximal rates are followed by a period of reduced corticomotor excitability and increased intracortical inhibition (Benwell et al. 2006; Teo et al. 2012). It is possible that the fatigue-related effect that was observed during the sham condition but may have been negated by facilitation of neuronal activity during anodal stimulation.
An alternative explanation for decrease in RT performance during the sham condition could be attributed to task-switching. Subjects asked to perform a task as quickly as possible will perform poorly if they have recently completed a different task than if they performed the same task (Gilbert and Shallice 2002). Neuroimaging studies have reported that functionally identified regions of interest in the brain (motor, prefrontal, parietal and/or temporal cortices depending on the task performed) are more active during task-switching, suggesting that this activity may simply reflect the energy required to prepare neural components for the next action (Rogers et al. 1998; Kimberg et al. 2000). Studies have also reported that task-switching could activate a common frontal parietal network, which may interfere with working memory (Sternberg 1969; Dreher and Grafman 2003). In the current study, participants were performing a skilled visuomotor task during the anodal/sham stimulation prior to the post stimulation RT trials. The sham condition may have decreased performance because the new motor pattern interfered with their awareness and ability to recall and perform the reaction time task. It is possible that the detrimental effect of task-switching was decreased due to the enhanced cortical excitability induced by anodal tDCS that may have given the neural networks the necessary energy to prepare for the new task. This is an important finding requiring further experiments to examine the effects of anodal tDCS on task-switching.
Effects on wrist RT
We included wrist RT trials as a control condition to understand the specificity of anodal tDCS. As modeling studies have shown that the effects of tDCS are typically highest over the area directly beneath the electrode with decreasing intensity in the surrounding areas (Miranda et al. 2006; Miranda et al. 2009), we anticipated the effects of anodal tDCS to be focal over the leg motor area, which is anatomically distant from the upper limb M1. We did not observe faster wrist CRT responses during anodal stimulation, perhaps partly invalidating our SMA hypothesis mentioned above. If the SMA was solely responsible for changes in ankle CRT, we would expect to observe an improvement in wrist CRT as well. We argue that a concurrent increase in neuronal facilitation of the M1 is necessary to demonstrate improvements in CRT. The absence of change in wrist CRT during the anodal condition supports specificity of anodal stimulation which has been observed in a previous study (Tanaka et al. 2009).
The slowing of responses during the sham condition was also apparent in wrist SRT and CRT tasks. Previous studies have shown that fatigue due to exercising one limb has global consequences affecting unexercised and distant muscles. Haleperin et al. (2014) demonstrated that knee extension fatiguing exercise affected force production in the unexercised elbow flexor muscle. Similarly Aboordarda et al. (2015) reported that elbow flexor fatigue affects knee extensor muscle activity and force. Hence, it is plausible to suppose that fatigue caused by the ankle visuomotor task affected wrist RT in the sham condition.
Absence of change in cognitive function
In the present study, anodal tDCS over the leg M1 did not significantly affect SDMT. The SDMT is a brief test of information processing and attention, sensitive to cognitive impairment in patients with neurological disorders (Benedict et al. 2008; Charvet et al. 2014).We included this cognitive outcome measure to provide further behavioral evidence about the selectivity of tDCS on specific neuronal populations stimulated by the electrode, and to rule out the possibility that the change in RT may be due to changes in homologous distant neuronal networks, due to a general increase in awareness, or due to a placebo effect of stimulation. If the anodal stimulation had a global or placebo effect, we would expect to see corresponding changes in SDMT scores. The shortening of ankle CRT responses in the anodal condition was not accompanied by changes in SDMT scores. Improvements in information processing and executive function, as assessed by SDMT, have been typically correlated with activation of the DLPFC (Rorie and Newsome 2005), suggesting that anodal stimulation of the lower limb M1 did not have an effect on DLPFC in the current study. We were surprised to note that the fatigue and/or task-switching effects causing slowing of motor responses in the sham condition did not impair performance of the SDMT. The relationship between exercise-induced fatigue or mental fatigue and cognitive function is still unclear. One study has indicated that short periods of physical exercise improved cognitive functioning in adults (Hancock and McNaughton 1986), others either did not find any benefits (Cote et al. 1992) or even reported deterioration of cognitive function (Cian et al. 2001). Also the relationship between tDCS and cognitive function is not yet clear. Pope et al. (2015) showed that tDCS over prefrontal cortex may have differential effects depending on the complexity of the task. In their study, after anodal tDCS they observed an enhancement of a cognitive subtraction task but not of a less challenging addition task (Pope et al. 2015). It is possible that the SDMT is not sensitive or challenging enough to be affected by fatigue. Alternatively, as the SDMT was administered 20 minutes post stimulation, the effects of stimulation may have diminished and thus not apparent in SDMT performance. The SDMT has also been shown to have a practice effect in young healthy adults, which was revealed in our study as a main effect of Time, and may have diminished any differences between the two stimulation conditions (Pereira et al. 2015). More streamlined experiments are needed to examine the effects of tDCS of motor areas on cognitive and executive function.
Conclusions
In summary, our data showed that anodal tDCS over the leg motor area during an ankle motor tracking task enhances ankle CRT. These effects were not evident for ankle SRT, wrist SRT, wrist CRT and cognition (SDMT). The effect of anodal stimulation on ankle CRT and not ankle SRT is attributed to an effect of anodal tDCS on complex motor processing and/or its influence on the SMA. Interestingly, we noted that there was a general slowing of RT in the sham condition which was suppressed during anodal stimulation, suggesting perhaps a suppression of fatigue-related processes or task-switching effects due to neuronal facilitation in the anodal condition. These results support the role of tDCS for lower limb motor neurorehabilitation, especially for patients with higher impairments who many benefit from increased performance of simple motor skills. The effects of anodal tDCS on complex motor processing and its possible influence on fatigue and task-switching requires further exploration to better understand the mechanisms of tDCS.
Figure 3. Choice Reaction Time.
Mean percentage changes in choice reaction time (CRT) induced by anodal (solids black bars) and sham (grey bars) tDCS for ankle dorsiflexion (DF), ankle plantarflexion (PF) and wrist extension are shown. * indicates a significant difference (p<0.05) between Pre and Post time points within each stimulation condition (post hoc effect). ** indicates a significant difference (p<0.05) between Anodal and Sham conditions for normalized RT.
References
- Aboodarda SJ, Copithorne DB, Power KE, Drinkwater E, Behm DG. Elbow flexor fatigue modulates central excitability of the knee extensors. Appl Physiol Nutr Metab. 2015;40:924–930. doi: 10.1139/apnm-2015-0088. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Duquin JA, Jurgensen S, et al. Repeated assessment of neuropsychological deficits in multiple sclerosis using the Symbol Digit Modalities Test and the MS Neuropsychological Screening Questionnaire. Mult Scler. 2008;14:940–946. doi: 10.1177/1352458508090923. [DOI] [PubMed] [Google Scholar]
- Benwell NM, Sacco P, Hammond GR, Byrnes ML, Mastaglia FL, Thickbroom GW. Short-interval cortical inhibition and corticomotor excitability with fatiguing hand exercise: a central adaptation to fatigue? Exp Brain Res. 2006;170:191–198. doi: 10.1007/s00221-005-0195-7. [DOI] [PubMed] [Google Scholar]
- Bolzoni F, Bruttini C, Esposti R, Castellani C, Cavallari P. Transcranial direct current stimulation of SMA modulates anticipatory postural adjustments without affecting the primary movement. Behav Brain Res. 2015;291:407–413. doi: 10.1016/j.bbr.2015.05.044. [DOI] [PubMed] [Google Scholar]
- Charvet LE, Beekman R, Amadiume N, Belman AL, Krupp LB. The Symbol Digit Modalities Test is an effective cognitive screen in pediatric onset multiple sclerosis (MS) J Neurol Sci. 2014;341:79–84. doi: 10.1016/j.jns.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol. 2001;42:243–251. doi: 10.1016/s0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- Cote J, Salmela J, Papathanasopoulu KP. Effects of progressive exercise on attentional focus. Percept Mot Skills. 1992;75:351–354. doi: 10.2466/pms.1992.75.2.351. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15:373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Santos MC, et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson's disease. Mov Disord. 2006;21:1693–1702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Shallice T. Task switching: a PDP model. Cogn Psychol. 2002;44:297–337. doi: 10.1006/cogp.2001.0770. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Does visual perception of object afford action? Evidence from a neuroimaging study. Neuropsychologia. 2002;40:212–222. doi: 10.1016/s0028-3932(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Halperin I, Aboodarda SJ, Behm DG. Knee extension fatigue attenuates repeated force production of the elbow flexors. Eur J Sport Sci. 2014;14:823–829. doi: 10.1080/17461391.2014.911355. [DOI] [PubMed] [Google Scholar]
- Hancock S, McNaughton L. Effects of fatigue on ability to process visual information by experienced orienteers. Percept Mot Skills. 1986;62:491–498. doi: 10.2466/pms.1986.62.2.491. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Voller B, Celnik P, Floel A, Giraux P, Gerloff C, Cohen LG. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EK, Paik NJ. Effect of a tDCS electrode montage on implicit motor sequence learning in healthy subjects. Exp Transl Stroke Med. 2011;3:4. doi: 10.1186/2040-7378-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik BE, Confais J, Riehle A. Signs of timing in motor cortex during movement preparation and cue anticipation. Adv Exp Med Biol. 2014;829:121–142. doi: 10.1007/978-1-4939-1782-2_7. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M. Modulation of task-related neural activity in task-switching: an fMRI study. Brain Res Cogn Brain Res. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Fregni F, Goncalves OF. Task-specific effects of tDCS-induced cortical excitability changes on cognitive and motor sequence set shifting performance. PLoS One. 2011;6:e24140. doi: 10.1371/journal.pone.0024140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan S, Stinear JW. Focal and bidirectional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimulation. 2010;3:42–50. doi: 10.1016/j.brs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan S, Weber KA, 2nd, Stinear JW. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Exp Brain Res. 2011;209:9–17. doi: 10.1007/s00221-010-2511-0. [DOI] [PubMed] [Google Scholar]
- Magill R. Motor Learning and Control: Concepts and Applications. New York: McGraw-Hill Education; 2011. [Google Scholar]
- Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clin Neurophysiol. 2009;120:1183–1187. doi: 10.1016/j.clinph.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117:1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Nijhawan R. Visual prediction: psychophysics and neurophysiology of compensation for time delays. Behav Brain Sci. 2008;31:179–198. doi: 10.1017/S0140525X08003804. discussion 198–239. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Park SD, Kim JY, Song HS. Effect of application of transcranial direct current stimulation during task-related training on gait ability of patients with stroke. J Phys Ther Sci. 2015;27:623–625. doi: 10.1589/jpts.27.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DR, Costa P, Cerqueira JJ. Repeated Assessment and Practice Effects of the Written Symbol Digit Modalities Test Using a Short Inter-Test Interval. Arch Clin Neuropsychol. 2015;30:424–434. doi: 10.1093/arclin/acv028. [DOI] [PubMed] [Google Scholar]
- Pope PA, Brenton JW, Miall RC. Task-Specific Facilitation of Cognition by Anodal Transcranial Direct Current Stimulation of the Prefrontal Cortex. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson's disease. Brain. 1998;121(Pt 5):815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- Rorie AE, Newsome WT. A general mechanism for decision-making in the human brain? Trends Cogn Sci. 2005;9:41–43. doi: 10.1016/j.tics.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Shah B, Nguyen TT, Madhavan S. Polarity independent effects of cerebellar tDCS on short term ankle visuomotor learning. Brain Stimul. 2013;6:966–968. doi: 10.1016/j.brs.2013.04.008. S1935-861X(13)00127-7 [pii] [DOI] [PubMed] [Google Scholar]
- Smith A. Learning Disroders. In: Helmuth J, editor. The symbol-digit modalities test: a neuropsychologic test of learning and other cerebral disorders. Seattle: Special Child Publications; 1968. [Google Scholar]
- Smith A. Symbol Digits Modalities Test. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Sriraman A, Oishi T, Madhavan S. Timing-dependent priming effects of tDCS on ankle motor skill learning. Brain Res. 2014 doi: 10.1016/j.brainres.2014.07.021. doi: S0006-8993(14)00950-0 [pii]10.1016/j.brainres.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, O'Shea J, et al. Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke. Brain. 2012;135:276–284. doi: 10.1093/brain/awr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. doi: 17/1/37 [pii]10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Memory-scanning: mental processes revealed by reaction-time experiments. Am Sci. 1969;57:421–457. [PubMed] [Google Scholar]
- Tanaka S, Hanakawa T, Honda M, Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res. 2009;196:459–465. doi: 10.1007/s00221-009-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Takeda K, Otaka Y, et al. Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabil Neural Repair. 2011;25:565–569. doi: 10.1177/1545968311402091. doi: 1545968311402091 [pii]10.1177/1545968311402091. [DOI] [PubMed] [Google Scholar]
- Tanji J, Mushiake H. Comparison of neuronal activity in the supplementary motor area and primary motor cortex. Brain Res Cogn Brain Res. 1996;3:143–150. doi: 10.1016/0926-6410(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Teo WP, Rodrigues JP, Mastaglia FL, Thickbroom GW. Post-exercise depression in corticomotor excitability after dynamic movement: a general property of fatiguing and non-fatiguing exercise. Exp Brain Res. 2012;216:41–49. doi: 10.1007/s00221-011-2906-6. [DOI] [PubMed] [Google Scholar]