Abstract
Culture of Drosophila expressing the steroid-dependent GeneSwitch transcriptional activator under the control of the ubiquitous α-tubulin promoter was found to produce extensive pupal lethality, as well as a range of dysmorphic adult phenotypes, in the presence of high concentrations of the inducing drug RU486. Prominent among these was cleft thorax, seen previously in flies bearing mutant alleles of the nuclear receptor Ultraspiracle and many other mutants, as well as notched wings, leg malformations, and bristle abnormalities. Neither the α-tubulin-GeneSwitch driver nor the inducing drug on their own produced any of these effects. A second GeneSwitch driver, under the control of the daughterless promoter, which gave much lower and more tissue-restricted transgene expression, exhibited only mild bristle abnormalities in the presence of high levels of RU486. Coexpression of the alternative oxidase (AOX) from Ciona intestinalis produced a substantial shift in the developmental outcome toward a wild-type phenotype, which was dependent on the AOX expression level. Neither an enzymatically inactivated variant of AOX, nor GFP, or the alternative NADH dehydrogenase Ndi1 from yeast gave any such rescue. Users of the GeneSwitch system should be aware of the potential confounding effects of its application in developmental studies.
Keywords: inducible transgenes, nuclear receptor, Drosophila, cleft thorax, notched wings
The GeneSwitch (GS) system is commonly used to activate transgenes in Drosophila in a graded fashion. GS comprises a modified form of the yeast transcriptional activator Gal4, which is covalently linked to the hormone-binding fragment of the progesterone receptor, rendering its transcriptional activity dependent on an exogenously supplied progesterone analog, RU486 or mifepristone (Osterwalder et al. 2001). Any transgene governed by the UAS promoter element, rendering it Gal4-responsive, may be induced by the combination of GS and RU486 in a dose-dependent manner. Depending on the promoter to which GS is itself combined, plus its insertion site in the fly genome, drug-inducible transgene expression can be achieved in a wide variety of developmental patterns, cell-types, and overall strengths. Thus, the widely used α-tubulin-GS (tubGS) and actin5C-GS drivers confer ubiquitous, RU486-dependent transgene expression when crossed to lines bearing a UAS-governed transgene. Tissue-specific drivers such as the neuron-specific elav-GS enable transgene expression in just one tissue, but again at a level and timing that can be manipulated over a wide range. The use of this system is predicated on the assumption that the expression of GeneSwitch and exposure to RU486 do not themselves produce measurable effects on fly physiology and development, which is supported by controls in many studies.
Our laboratory has made use of this system, for example to express, in Drosophila, foreign transgenes coding for nonproton-motive alternative respiratory chain enzymes derived from simpler eukaryotes, such as the alternative oxidase (AOX) from Ciona intestinalis (Fernandez-Ayala et al. 2009; Kemppainen et al. 2014a). When supplied to adult Drosophila bearing both tubGS and a UAS-AOX transgene, RU486 produced dose-dependent transgene expression that saturated at drug concentrations (in fly food) of 100–200 μM (Kemppainen et al. 2014a). However, when supplied throughout development, RU486 concentrations two orders of magnitude lower were sufficient to induce maximal expression (Fernandez-Ayala et al. 2009). The precise reasons for this discrepancy in required dose are unclear, although early larvae, which are very rapidly growing (Church and Robertson 1966; Watts et al. 2006), must absorb larger amounts of drugs added to fly food than adults, which do not grow at all and even lose weight during early adult life (Fernandez-Ayala et al. 2009).
In this study, we addressed the issue of what happens to development when larvae expressing GeneSwitch drivers (but no other transgene) are exposed to RU486 concentrations in excess of those sufficient to produce maximal transgene expression. We detected a variety of developmental abnormalities dependent on driver expression and drug dose. Surprisingly, expression of AOX, but not other transgenes such as GFP or the yeast alternative NADH dehydrogenase Ndi1, mitigated these effects.
Materials and Methods
Drosophila stocks and maintenance
Wild-type (Oregon R), standard transgenic host strains w1118 and wDAH (Dahomey) and the UAS-GFP (Stinger) line (insertion on chromosome 2) were obtained from stock centers. The tubGS driver line with insertion on chromosome 3 (Sykiotis and Bohmann 2008) was a kind gift from Dr Scott Pletcher (University of Michigan). The daughterless-GS (daGS) line (Tricoire et al. 2009) was a kind gift from Dr Alberto Sanz (Newcastle University, UK). AOX and Ndi1 transgenic flies [lines UAS-AOXF6, UAS-AOXF24, tub-AOX7, tub-AOX35 tub-AOX50, UAS-AOX7.1 (targeted insertion on chromosome 3) UAS-AOXmut (denoted previously as UAS-AOX4.1, targeted insertion on chromosome 3), and UAS-Ndi1B20] were as described previously (Fernandez-Ayala et al. 2009; Sanz et al. 2010b; Kemppainen et al. 2014b; Andjelković et al. 2015). Flies were maintained in standard high-sugar medium (Fernandez-Ayala et al. 2009) at 25°, on a 12 hr light/dark cycle. Where indicated, medium was supplemented with RU486 (Mifepristone, Sigma) at the concentrations indicated in figures and legends.
Eclosion and phenotypic assays
Crosses were conducted in a minimum of three, usually four to five replicates, as described previously (Toivonen et al. 2001; Kemppainen et al. 2009). Either the number of flies eclosing or the percentage of pupae that successfully eclosed in individual vials were recorded in different experiments (see figures and legends). The proportion of the eclosed progeny falling into different phenotypic classes was scored by microscopy. Cleft thorax, where subclassified, was scored as mild or severe (heminota clearly separated), with the mildest abnormality, malformed scutellum, scored separately in some experiments. Wing phenotypes were scored as normal or notched, the latter ranging from single notches to grossly malformed wings that in some cases did not inflate properly. Flies showing any of the bristle abnormalities as described below were generally scored as a single category.
Microscopy
Light microscopy images of eclosed adult flies were taken with a Nikon Digital DS-Fi1 High-Definition Color Camera, using the Nikon stereoscopic zoom microscope SMZ 745T run by NIS-Elements D 4.20 software. Fluorescence microscopy of flies used a Zeiss Axio Imager 2 microscope (50 × magnification). Z projection images were generated using Carl Zeiss Zen 2012 software.
Protein analysis by western blotting
Total protein was extracted from batches of 20 pupae crushed in homogenization buffer, and processed as described previously (Andjelković et al. 2015). Primary antibodies used were customized rabbit anti-AOX (Fernandez-Ayala et al. 2009; 21st Centrury Biochemicals, 1:10,000), and mouse anti-ATP5A (Abcam, 1:100,000), with secondary antibodies as described previously (Andjelković et al. 2015).
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
tubGS plus high levels of RU486 produce developmental abnormalities
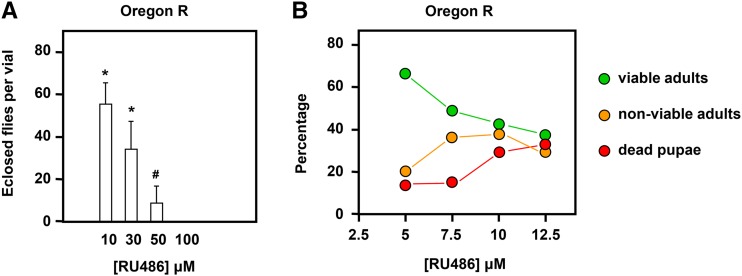
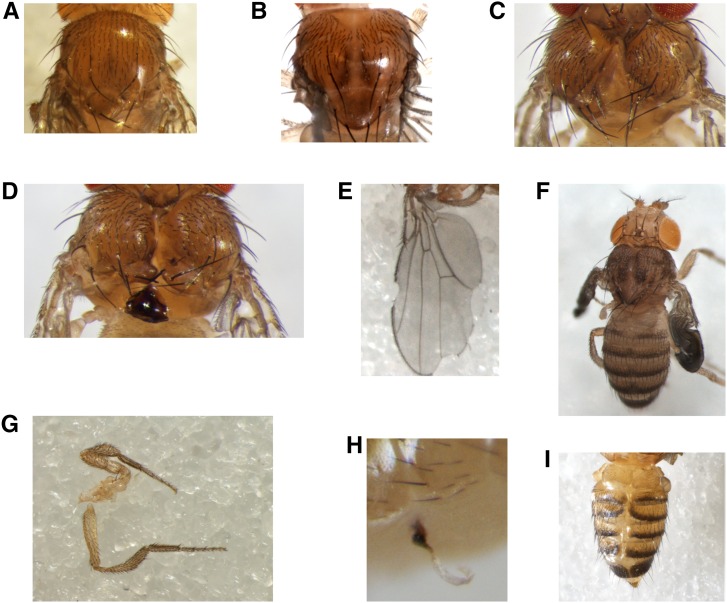
In initial trials, we noticed that doses of RU486 used routinely to induce UAS-dependent transgene expression in Drosophila, in combination with the tubGS driver in adult flies (200–500 μM; Kemppainen et al. 2014a), were lethal when present throughout development. In order to investigate possible mechanisms of this lethality, we reared flies at RU486 doses intermediate between this lethal level, and levels sufficient to induce full dose-dependent transgene expression, which in larvae was only 1–2 μM. In combination with tubGS, RU486 at 100 μM was still lethal (Figure 1A), whereas tubGS flies reared without drug, or wild-type flies reared at this concentration of RU486, developed normally. At intermediate drug concentrations (5–50 μM, Figure 1, A and B), we observed dose-dependent semilethality, although many of the eclosing flies were very weak and died within 1 d. In addition, even the viable flies displayed a range of dysmorphic phenotypes, illustrated in Figure 2 and Supplemental Material, Figure S1 and File S1, of which the commonest and most striking were cleft thorax (Figure 2, B–D) and notched wings (Figure 2E). The observed phenotypes were of varying severity. For example, some flies had single or multiple notches at the wing margin (Figure 2E), whereas others had wings that failed to inflate (Figure 2F). Cleft thorax ranged from severe, with the heminota completely separated (Figure 2, C and D), to very mild, showing only an abnormal, parted bristle pattern or just a reduced scutellum (Figure 2A). A minority of flies also showed necrotic tissue in the notum area (Figure 2D), leg abnormalities such as overgrown, reduced, and fused leg segments (Figure 2G), externalized trachea (Figure 2H), clefted abdomen (Figure 2I), or a variety of malformations of macrochaetae (supernumerary, missing, kinked, or short bristles, Figure S1). Clefting also extended along the abdomen in some cases (Figure 2I). tubGS flies reared without drug, or cultured in RU486 in the absence of tubGS, did not exhibit cleft thorax or other developmental abnormalities, indicating that these teratogenic effects require the combination of the modified transcription factor plus the inducing steroid.
Figure 1.
RU486 in combination with tubGS produces dose-dependent lethality. (A) Number of tubGS progeny eclosing at different doses of RU486 present throughout development, mean ± SD per vial, in OregonR background. Note that at 100 μM, no flies eclosed. * and # indicate significant differences from the next higher concentration tested in pairwise comparisons (Student’s t-test, P < 0.01 and 0.05, respectively). (B) Proportion (% of pupae formed) of tubGS progeny at different doses of RU486 present throughout development; combined data from sets of four vials at a given concentration, set up in parallel, in w1118 background. n = 205 (at 5 M), 193 (at 7.5 μM), 210 (at 10 μM), and 146 (at 12.5 μM). tubGS plus RU486 produced comparable amounts of pupal lethality also in the CantonS background. SD, standard deviation; tubGS; α-tubulin-GeneSwitch.
Figure 2.
Examples of dysmorphologies produced by the tubGS driver in the presence of 10 μM RU486. (A–D) Thoracic abnormalities: (A) missing scutellar part, (B) mild cleft, (C) severe cleft, and (D) necrotic tissue, always localized at the scutellum or notum. (E and F) Wing abnormalities: (E) notched wings, with notches localized on the marginal anterior or posterior side or both, (F) noninflated wings. (G) Leg abnormalities, including overgrown, reduced, and fused leg segments, sometimes present all together. (H) Externalized trachea, always in the ventral abdomen. (I) Abdominal clefting: strong midline splits between all dorsal tergite plates; laterotergites do not fuse at the dorsal midline and remain as hemitergites, with incomplete fusion of abdominal epidermis. These phenotypes were seen in all genetic backgrounds tested (OregonR, CantonS, w1118, and wDAH). tubGS; α-tubulin-GeneSwitch.
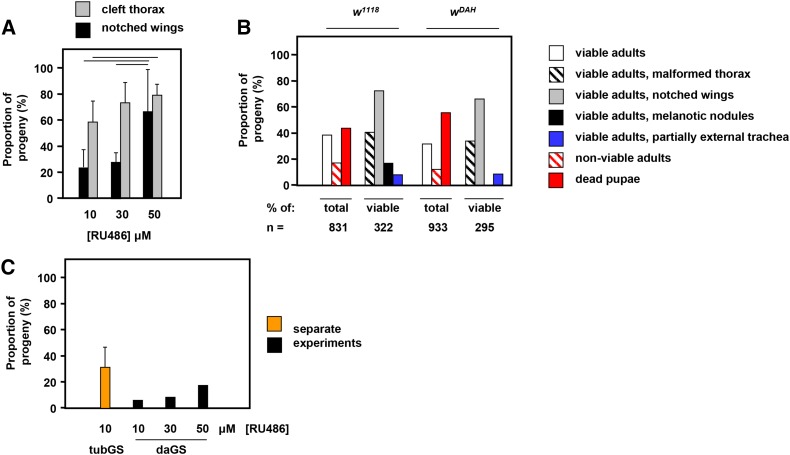
We quantified the main classes of abnormality and observed a dose-dependence on RU486 (Figure 3A). Although the proportion of progeny showing the two major dysmorphic phenotypes of cleft thorax or notched wings was already substantial at 10 μM RU486, increasing the dose to 30 μM resulted in a significant increase in the proportion exhibiting cleft thorax, whereas a further increase to 50 μM produced a significantly greater proportion with notched wings.
Figure 3.
Effects of drug concentration, driver, and genetic background on developmental abnormalities induced by GeneSwitch plus RU486. (A) Proportion of viable adult progeny exhibiting major phenotypic abnormalities as indicated, at different doses of RU486, in the Oregon R genetic background. Mean ± SD for sets of n ≥ 4 independent vials. Horizontal bars denote significant differences for a given phenotypic trait between the stated drug concentrations (Student’s t-test, P < 0.05). (B) Proportion of progeny in different phenotypic classes of tubGS flies in the w1118 and wDAH backgrounds grown at 10 μM RU486. Note that the adult phenotypes are scored as percentages of the viable adult flies that eclosed. Total numbers of pupae analyzed in each large-scale experiment (n) as indicated. (C) Proportion of adult progeny showing bristle abnormalities, as illustrated in Figure S1, in flies grown at the indicated doses of RU486, bearing the tubGS or daGS drivers as indicated. Large-scale experiment using the daGS driver analyzed n = 508 individual adult flies (10 μM), n = 758 (30 μM), and n = 246 (50 μM). The data for the tubGS driver at 10 μM is the mean ± SD for three independent experiments (n = 89, 284, and 157 adults analyzed). See also Figure S2. daGS, daughterless-GeneSwitch; SD, standard deviation; tubGS; α-tubulin-GeneSwitch.
In order to determine whether the induction of these developmental defects was a general property of GeneSwitch drivers, or a phenomenon specific to tubGS, we repeated the experiment using a second GeneSwitch driver under the control of the daughterless promoter. In contrast to tubGS, daGS in combination with 10 μM RU486 produced no clefting and no wing defects. The only developmental abnormality detected was in regard to bristle morphology and organization which, while less frequently observed than with the tubGS driver, did show a tendency to rise in frequency as the concentration of RU486 was increased (Figure 3C). However, neither cleft thorax nor notched wings were seen at these elevated drug concentrations, nor even at 100 μM. The difference in the findings between the two drivers is most likely attributable to the level and pattern of expression of the GeneSwitch transcription factor, as reflected in its ability to drive transgene expression, which we profiled quantitatively by western blotting using a UAS-AOX reporter (Figure S2A) and spatially using a UAS-GFP reporter (Figure S2B). Expression of UAS-AOX driven by daGS was quantitatively much less than when driven by tubGS, even at high RU486 concentrations (Figure S2A). Furthermore, unlike tubGS, which was able to drive expression ubiquitously in the developing larva, daGS produced transgene expression only in a minority of cells (Figure S2B), including salivary glands, parts of the trachea, some epithelial cells, and segmentally reiterated cell clusters.
Expression of AOX, but not Ndi1 or GFP, rescues cleft thorax caused by tubGS/RU486
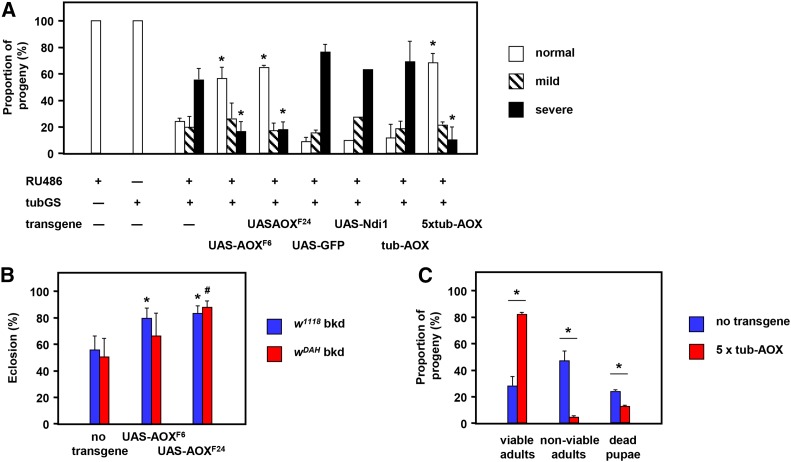
We tested whether concomitant expression of other transgenes driven by tubGS in the presence of RU486 was able to modify the developmental phenotypes resulting from the driver and drug alone (Figure 4). Once again, neither tubGS nor the drug on its own produced cleft thorax (Figure 4A) but, when combined, over 50% of the eclosing progeny manifested severe cleft thorax, and a further 20% showed mild clefting. Coexpression of Ciona AOX from either of two UAS-AOX transgenic lines (Fernandez-Ayala et al. 2009) produced a substantial rescue of the phenotype, with over 50% of the eclosing progeny now showing no cleft, and less than 20% having severe cleft. UAS-Ndi1 or UAS-GFP produced no rescue of the phenotype. Nor did a single copy of AOX, when constitutively expressed under the α-tubulin promoter at a much lower level than when driven by tubGS (Kemppainen et al. 2015). However, five copies of the tub-AOX transgene, when present simultaneously, did produce a rescue comparable with that of UAS-AOX. Coexpression of UAS-AOX with tubGS plus drug, in either of two backgrounds commonly used in transgenic studies (w1118 and wDAH) also increased the proportion of pupae eclosing (Figure 4B). The simultaneous presence of five tub-AOX transgenes (Figure 4C) also substantially rescued the eclosion frequency, as well as the survival of adults immediately after eclosion.
Figure 4.
AOX partially rescues cleft thorax and developmental lethality of tubGS/RU486. Proportion of adult progeny exhibiting the indicated phenotypes, with hemizygous transgenes as indicated, cultured with (+) or without (–) 10 μM RU486. n ≥ 3 replicate vials for each genotype studied (except UAS-Ndi, n = 2, hence no error bars shown). Transgenic lines containing tub-AOX transgenes (Kemppainen et al. 2014) had either a single hemizygous copy or else five copies (two homozygous, plus hemizygous copy on chromosome 3, combined with tubGS on the same chromosome). * denotes data classes significantly different from the equivalent class for control lacking any transgene additional to tubGS (Student’s t-test with Bonferroni correction, P < 0.01). (B) Proportion of pupae from two different genetic backgrounds (bkd), as shown, eclosing after culture in 10 μM RU486. All pupae carried the tubGS driver and either no other transgene, or either of two different UAS-AOX transgenes, as indicated. # and * denote data classes significantly different from nontransgenic flies in the same genetic background (Student’s t-test, P < 0.05 or 0.01, respectively). (C) Proportion of pupae eclosing as viable or nonviable adults after culture in 10 μM RU486. All pupae carried the tubGS driver and either no other transgene, or else five copies of tub-AOX transgenes (see above). Nonviable adults were those that died on the day of eclosion. * denotes phenotypic classes of transgenic flies significantly different from corresponding class of nontransgenic flies (Student’s t-test, P < 0.01). AOX, alternative oxidase; GFP, green fluorescent protein; tubGS; α-tubulin-GeneSwitch.
AOX rescues developmental abnormalities in a dose-dependent manner
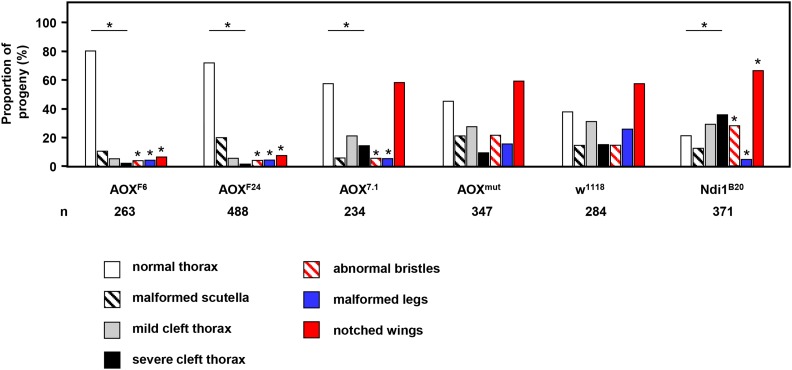
We next conducted a large-scale experiment, analyzing almost 2000 individual flies, for each of the major classes of developmental abnormality produced by tubGS in the presence of RU486, in the presence of different UAS-dependent transgenes (Figure 5). As negative control we used strain w1118, the background strain for all the transgenic lines that were crossed in the experiment. To determine whether the failure of a single copy of tub-AOX to rescue tubGS-induced cleft thorax was due to low expression, we made use of an additional UAS-AOX line, UAS-AOX7.1 (Andjelković et al. 2015), showing much lower expression than either of UAS-AOXF6 or UAS-AOXF24. Finally, to confirm that the enzymatic activity of AOX is required for the rescue, we also included a line (UAS-AOXmut) expressing a catalytically inactive variant of AOX (Andjelković et al. 2015). The proportion of abnormal phenotypes obtained using UAS-AOXmut was virtually indistinguishable from the background strain w1118, while the weakly expressing UAS-AOX7.1 transgene produced an intermediate spectrum of phenotypes, with cleft thorax, leg, and bristle abnormalities significantly improved over the background strain, but to a much lower extent than seen with the strongly expressing lines UAS-AOXF6 and UAS-AOXF24. UAS-AOX7.1 also produced no rescue of the notched wings phenotype, while UAS-Ndi1B20 significantly exacerbated all of the abnormal phenotypes compared with the background strain, with the exception of leg malformations, which were decreased in frequency.
Figure 5.
AOX rescues diverse developmental abnormalities produced by tubGS. Proportion of progeny hemizygous for both tubGS and the indicated transgenes, which exhibited the indicated developmental abnormalities, when reared on food containing 10 μM RU486. The total numbers of flies of each genotype analyzed, in a single large-scale experiment (n), is as shown. See supplemental material for a detailed description of phenotypic categories. Asterisks indicate significant differences (P < 0.001) from the w1118 background strain hemizygous for tubGS, based on chi-squared analysis for each phenotypic category or for the four thoracic phenotypes (normal thorax, malformed scutellum, mild cleft, and severe cleft) considered as a whole. AOX, alternative oxidase; tubGS; α-tubulin-GeneSwitch.
Discussion
In this study, we identified a range of developmental abnormalities associated with the use of the tubGS driver in combination with RU486. These were seen at concentrations only slightly above those commonly used to induce transgene expression in Drosophila during development. At concentrations of 2.5 μM or above, we observed substantial pupal lethality, while at 10 μM or above the majority of viable eclosed adults had visible dysmorphic features, commonly including notched wings and cleft thorax. Importantly, these phenotypes were dependent on both the driver and the drug: neither alone produced any evidence of developmental lethality or abnormality, and the effects did not appear to be background dependent, since they were seen in wild-type OregonR and Canton-S flies, as well as in two white-eyed lines commonly used in transgenic studies. A different GeneSwitch driver, with a much lower and more restricted expression pattern, based on its ability to drive GFP expression (Figure S2), produced only very subtle abnormalities in bristle organization.
Mechanism of developmental disturbance by tubGS/RU486
Previous authors have noted that RU486 treatment alone produces no detectable abnormal phenotypes, although expression of a small number of mRNAs is altered in adults treated with the drug (Etter et al. 2005). Given that we also saw no abnormalities from the use of tubGS or RU486 on their own, we can exclude the possibility that RU486 binds to or interferes with the activity of known nuclear receptors in Drosophila (Fahrbach et al. 2012), or that the GeneSwitch transcription factor is able to interact with any of their physiological ligands. However, ligand-bound GeneSwitch may be able to interact either with one or more of these receptors, its targets, or other regulatory factors involved in developmental patterning; for example, by the formation of nonphysiological heterodimers between ligand-bound GeneSwitch and bona fide nuclear receptors.
The major dysmorphologies we observed have been reported previously in a variety of mutants, often in combinations similar to those that we observed. Cleft thorax has been reported in mutants of Ultraspiracle (Henrich et al. 1994), a dimerization partner of the ecdysone receptor and thus one of the key nuclear receptors regulating development progression in the fly. It has also been reported in mutants of the GATA transcription factor pannier (Heitzler et al. 1996) and the zinc-finger pair-rule transcription factor gene odd (Tripura et al. 2011). Bristle abnormalities similar to those that we observed are also characteristic of mutants of the dimerization partner of pannier, u-shaped (Cubadda et al. 1997).
Mutants in the components of the AP-1 transcription factor, jun-related antigen (homolog of mammalian c-Jun) and kayak (homolog of mammalian c-Fos), as well as in the JNK signaling pathway that links AP-1 activity to various upstream developmental signals, cause cleft thorax (reviewed by Zeitlinger and Bohmann 1999; Kockel et al. 2001). Defects in JNK signaling also underlie wing defects and leg malformations (Kirchner et al. 2007), and have been implicated in midline closure defects in mammals (Chi et al. 2005; Zhu et al. 2016). Cleft-thorax can result both from downregulation of effectors of JNK signaling, such as the serine protease scarface (Srivastava and Dong 2015), or from mutations in receptor tyrosine kinase Pvr (Garlena et al. 2015), an upstream JNK pathway activator (Ishimaru et al. 2004; Igaki 2009). Thoracic closure also depends on downstream targets such as proteins implicated in cytokinesis and cell adhesion (Sfregola 2014), as well as intracellular protein trafficking (Thomas et al. 2009). Mutants of blistery, encoding tensin, result in blistered wings, and interact also with JNK signaling (Lee et al. 2003). Overexpression of the inhibitor of matrix metalloproteases (Timp) results in pupal lethality and cleft thorax (Srivastava et al. 2007). Finally, wing disc-specific knockdown of Tap42, a key regulator of protein phosphatases, gives rise to cleft thorax and to wing abnormalities similar to some that we observed (Wang et al. 2012).
Notched wings are another previously observed phenotype in many mutants, including those affecting the highly pleiotropic intercellular signaling factor Notch (originally discovered by Morgan; Welshons 1958), SNARE-dependent membrane trafficking (Stewart et al. 2001), protein phosphatase PP2A (Kunttas-Tatli et al. 2009), the RNA-binding fragile X protein FMR1 (Wan et al. 2000), and histone deacetylation (Pile et al. 2001).
The exact pattern of developmental abnormalities brought about by GeneSwitch together with its ligand appears to reflect the tissue specificity of its expression. Thus, whereas the widely expressed tubGS produces a plethora of abnormal phenotypes, daGS, with much more restricted larval expression (Figure S2), primarily in segmentally reiterated clusters of cells that might represent larval sense organs (Brewster and Bodmer 1995), has only a single visible phenotype in the adult, affecting the sensory bristles (Figure 3C). The use of other GeneSwitch drivers may help to further clarify how its level and pattern of expression affect the phenotypic outcome.
Finding a common thread through this rather bewildering array of phenotypes and genetic pathways may not be straightforward. However, transcriptional cascades are considered to be the main determinants of developmental processes, and the key system for regulating morphogenesis at pupal stage is the steroid hormone 20-hydroxyecdysone (Riddiford 1993). Thus, an interference with ecdysteroid-dependent transcription is the most parsimonious explanation for the pleiotropic effects we observed, even though molecular details remain to be filled in.
Mechanism of AOX rescue of developmental disturbance by tubGS/RU486
While the observation that GeneSwitch-plus-RU486 can produce a range of developmental abnormalities may be unexpected, their rescue by a mitochondrially localized electron-transfer protein from another phylum is even more surprising. It is important to note that, while the abnormal phenotypes were produced by using an engineered (and thus nonphysiological) transcription factor, and were rescued by a gene from a distant phylum, the effects were systematic in both cases, indicating meaningful underlying biological processes. Thus, the extent of AOX rescue of pupal lethality, cleft thorax, and other dysmorphologies was dependent on the AOX expression level, since strains expressing only at a low level (single-copy of constitutive tub-AOX, or low-expressor GAL4-dependent line UAS-AOX7.1) produced a less dramatic alleviation of the phenotypes studied than the corresponding high-expressors (5 × tub-AOX, UAS-AOXF24, and UAS-AOXF6). Rescue was dependent on the enzymatic activity of AOX and was not seen with an inert reporter protein (GFP) or a different mitochondrially localized electron-transfer protein, yeast Ndi, which appeared to exacerbate some phenotypes. AOX maintains ATP production, redox homeostasis, and metabolic flux under physiological conditions where respiratory complexes III and IV are limiting due to overload, toxins, or genetic damage, and concomitantly limits mitochondrial ROS production consequent upon overreduction of the quinone pool (El-Khoury et al. 2014). AOX also has an unexplained antioxidant effect, decreasing net mitochondrial ROS output even under conditions where the respiratory chain is functioning normally (Fernandez-Ayala et al. 2009; Sanz et al. 2010a).
How this links to a global alleviation of developmental perturbations brought about by interference with transcriptional cascades or cell signaling is far from clear. In a general sense, our findings hint at a common metabolic regulation of transcription, such as evidenced previously by AMPK sirtuins or PARP (Kraus and Lis 2003; Ghosh et al. 2010; Gut and Verdin 2013; Schiewer and Knudsen 2014; Salminen et al. 2016), although none of these is obviously implicated, so a novel pathway may be involved. In mice, nuclear receptors are responsive to a variety of metabolic effectors, which can also be microbiome-dependent (Montagner et al. 2016), while cross-talk between nutrient-based sensors and nuclear receptors is dependent on mitochondrial stress signals and influences mitochondrial gene expression (Kang et al. 2015).
Many transcription factors, including nuclear receptors such as LXRα (Serviddio et al. 2013) or NR4A1 (Shimizu et al. 2015) in mammals, are known to be activated in response to oxidative stress (Lavrovsky et al. 2000), and redox regulation of nuclear receptors such as the glucocorticoid receptor (Tanaka et al. 1999) is well established. AOX may therefore act by providing a general dampening of ROS, normalizing developmental outcomes dependent on such receptors, with which GeneSwitch plus RU486 interferes. An exhaustive study using different ROS scavengers may shed further light on this.
Another possibility is based on the observation that synthesis of 20-hydroxyecdysone requires mitochondrial Fe-S cluster-containing proteins dependent on frataxin (Palandri et al. 2015) and mitoferrin (Llorens et al. 2015). Because Fe-S proteins are highly susceptible to ROS damage, a general ROS dampening effect of AOX may counteract transcriptional interference from ligand-bound GeneSwitch, simply by boosting endogenous ecdysteroid synthesis.
Recommendations on use of GeneSwitch drivers
The GeneSwitch system was originally elaborated using other drivers than tubGS, i.e., those linked to the neuron- and muscle-specific elav and Mhc promoters, respectively (Osterwalder et al. 2001), or for specific expression in other tissues such as the fat body (Roman et al. 2001). Subsequently, the “ubiquitous” GS drivers (such as tubGS and Actin5C-GS) have been brought into use for inducing broad expression, both in adults and larvae (Ford et al. 2007; Waskar et al. 2009; Wigby et al. 2011; Paik et al. 2012; Kuo et al. 2012; Kemppainen et al. 2014a,b; Sun et al. 2014; Da-Rè et al. 2014).
Our work raises at least two concerns. First, the visible interference with developmental processes at saturating or near-saturating drug concentrations, using the tubGS driver, indicates the need for rigorous controls and cautious interpretation of all data obtained using this driver during development. Furthermore, we obviously cannot rule out subtler but also biologically significant effects that did not have visible manifestations, even at lower drug concentrations than those employed here. Second, other GeneSwitch drivers activated during development may also be vulnerable to such effects, since our data indicate that they depend on the drug and the transcription factor in combination, which applies wherever they are colocated. An example would be the recently published use of a GeneSwitch driver to overexpress malic enzyme (Kim et al. 2015). The driver in this example was originally reported to induce expression in the adult abdominal fat body (Hwangbo et al. 2004), although Kim et al. (2015) found that expression in larvae was instead driven in the salivary glands, Malpighian tubule, and part of the gut. In this particular paper, the appropriate controls without the transgene were indeed implemented for the adult (see Supplementary Table 1C of Kim et al. 2015), but some questions remain. The driver plus drug alone did not affect the body weight of L3 larvae (Figure 3A of Kim et al. 2015), but effects on stress resistance and lifespan in such controls were not documented. The concentrations of RU486 used by Kim et al. (2015), i.e., 2.5–10 μg/ml, corresponding with 5.8–23 μM, were within the range in which we saw major developmental effects using the tubGS driver. Similarly, in flies expressing GeneSwitch in specific endocrine cells during development, using a customized driver and RU486 at even higher concentrations from larval L2 stage onwards (Cho et al. 2014), clear developmental abnormalities were attributed to knockdown of a nuclear receptor, although driver-plus-drug controls were not included in all of the experiments reported. Some phenotypes observed (Figure 3 of Cho et al. 2014) resemble those that we report here (pupal lethality, uninflated wings, abdominal clefting, and leg malformations). While their interpretation that these are due to disrupted ecdysone signaling may be correct, an effect of GeneSwitch plus RU486 in the target cells cannot be excluded. Phenotypic rescue by injected ETH (Table 2 of Cho et al. 2014) confirmed the involvement of disrupted ecdysis, but not the underlying causes thereof. A further possible example already reported in the literature is the effect of the abdominal fat body-specific GeneSwitch driver on lifespan, when RU486-containing food was supplied in the adult to drive the supposedly inert GFP transgene (Ren and Hughes, 2014. RU486-dependent lethality in larvae containing the Elav-GeneSwitch driver (Shen et al. 2009), and embryonic lethality produced by either the Elav- or Actin5C-GeneSwitch drivers plus maternal RU486 (Landis et al. 2015), have been previously reported.
We would recommend that future users of all GeneSwitch drivers should routinely include otherwise nontransgenic controls bearing the drivers, plus and minus drug, in all experiments. Based on our findings (Figure S2B), the daGS driver is clearly not ubiquitous, despite the fact that the daughterless gene itself, as well as the “standard” daGAL4 drivers, do show widespread expression.
Supplementary Material
Acknowledgments
We thank Alberto Sanz and Scott Pletcher for kindly supplying Drosophila strains, Dmytro Gospodaryov for valuable discussions, and Annika Ketola, Ville Someri, and Tea Tuomela for technical assistance. This work was supported by funding from the European Research Council (advanced grant 232738 to H.T.J.), Academy of Finland; University of Tampere; Tampere University Hospital Medical Research Fund; the Sigrid Juselius Foundation, and the Finnish Cultural Foundation (grant to A.A.). The authors declare no conflict of interest.
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.030882/-/DC1
Communicating editor: C. Gonzalez
Literature Cited
- Andjelković A., Oliveira M. T., Cannino G., Yalgin C., Dhandapani P. K., et al. , 2015. Diiron centre mutations in Ciona intestinalis alternative oxidase abolish enzymatic activity and prevent rescue of cytochrome oxidase deficiency in flies. Sci. Rep. 5: 18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster R., Bodmer R., 1995. Origin and specification of type II sensory neurons in Drosophila. Development 121: 2923–2936. [DOI] [PubMed] [Google Scholar]
- Chi H., Sarkisian M. R., Rakic P., Flavell R. A., 2005. Loss of mitogen-activated protein kinase kinase kinase 4 (MEKK4) results in enhanced apoptosis and defective neural tube development. Proc. Natl. Acad. Sci. USA 102: 3846–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. H., Daubnerová I., Park Y., Zitnan D., Adams M. E., 2014. Secretory competence in a gateway endocrine cell conferred by the nuclear receptor βFTZ-F1 enables stage-specific ecdysone responses throughout development in Drosophila. Dev. Biol. 385: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church R. B., Robertson F. W., 1966. A biochemical study of the growth of Drosophila melanogaster. J. Exp. Zool. 162: 337–352. [Google Scholar]
- Cubadda Y., Heitzler P., Ray R. P., Bourouis M., Ramain P., et al. , 1997. u-shaped encodes a zinc finger protein that regulates the proneural genes achaete and scute during the formation of bristles in Drosophila. Genes Dev. 11: 3083–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da-Rè C., von Stockum S., Biscontin A., Millino C., Cisotto P., et al. , 2014. Leigh syndrome in Drosophila melanogaster: morphological and biochemical characterization of Surf1 post-transcriptional silencing. J. Biol. Chem. 289: 29235–29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khoury R., Kemppainen K. K., Dufour E., Szibor M., Jacobs H. T., et al. , 2014. Engineering the alternative oxidase gene to better understand and counteract mitochondrial defects: state of the art and perspectives. Br. J. Pharmacol. 171: 2243–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter P. D., Narayanan R., Navratilova Z., Patel C., Bohmann D., et al. , 2005. Synaptic and genomic responses to JNK and AP-1 signaling in Drosophila neurons. BMC Neurosci. 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach S. E., Smagghe G., Velarde R. A., 2012. Insect nuclear receptors. Annu. Rev. Entomol. 57: 83–106. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ayala D. J., Sanz A., Vartiainen S., Kemppainen K. K., Babusiak M., et al. , 2009. Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation. Cell Metab. 9: 449–460. [DOI] [PubMed] [Google Scholar]
- Ford D., Hoe N., Landis G. N., Tozer J., Luu A., et al. , 2007. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp. Gerontol. 42: 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlena R. A., Lennox A. L., Baker L. R., Parsons T. E., Weinberg S. M., et al. , 2015. The receptor tyrosine kinase Pvr promotes tissue closure by coordinating corpse removal and epidermal zippering. Development 142: 3403–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., George S., Roy U., Ramachandran D., Kolthur-Seetharam U., 2010. NAD: a master regulator of transcription. Biochim. Biophys. Acta 1799: 681–693. [DOI] [PubMed] [Google Scholar]
- Gut P., Verdin E., 2013. The nexus of chromatin regulation and intermediary metabolism. Nature 502: 489–498. [DOI] [PubMed] [Google Scholar]
- Heitzler P., Haenlin M., Ramain P., Calleja M., Simpson P., 1996. A genetic analysis of pannier, a gene necessary for viability of dorsal tissues and bristle positioning in Drosophila. Genetics 143: 1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich V. C., Szekely A. A., Kim S. J., Brown N. E., Antoniewski C., et al. , 1994. Expression and function of the ultraspiracle (usp) gene during development of Drosophila melanogaster. Dev. Biol. 165: 38–52. [DOI] [PubMed] [Google Scholar]
- Hwangbo D. S., Gershman B., Tu M. P., Palmer M., Tatar M., 2004. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429: 562–566. [DOI] [PubMed] [Google Scholar]
- Igaki T., 2009. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis 14: 1021–1028. [DOI] [PubMed] [Google Scholar]
- Ishimaru S., Ueda R., Hinohara Y., Ohtani M., Hanafusa H., 2004. PVR plays a critical role via JNK activation in thorax closure during Drosophila metamorphosis. EMBO J. 23: 3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. K., Putluri N., Maity S., Tsimelzon A., Ilkayeva O., et al. , 2015. CAPER is vital for energy and redox homeostasis by integrating glucose-induced mitochondrial functions via ERR-α-Gabpa and stress-induced adaptive responses via NF-κB-cMYC. PLoS Genet. 11: e1005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen E., Fernández-Ayala D. J., Galbraith L. C., O’Dell K. M. C., Jacobs H. T., 2009. Phenotypic suppression of the Drosophila mitochondrial disease-like mutant tko(25t) by duplication of the mutant gene in its natural chromosomal context. Mitochondrion 9: 353–363. [DOI] [PubMed] [Google Scholar]
- Kemppainen K. K., Kemppainen E., Jacobs H. T., 2014a The alternative oxidase AOX does not rescue the phenotype of tko25t mutant flies. G3 (Bethesda) 4: 2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen K. K., Rinne J., Sriram A., Lakanmaa M., Zeb A., et al. , 2014b Expression of alternative oxidase in Drosophila ameliorates diverse phenotypes due to cytochrome oxidase deficiency. Hum. Mol. Genet. 23: 2078–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. H., Lee Y. E., Lee G. H., Cho Y. H., Lee Y. N., et al. , 2015. Overexpression of malic enzyme in the larval stage extends Drosophila lifespan. Biochem. Biophys. Res. Commun. 456: 676–682. [DOI] [PubMed] [Google Scholar]
- Kirchner J., Gross S., Bennett D., Alphey L., 2007. The nonmuscle myosin phosphatase PP1beta (flapwing) negatively regulates Jun N-terminal kinase in wing imaginal discs of Drosophila. Genetics 175: 1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockel L., Homsy J. G., Bohmann D., 2001. Drosophila AP-1: lessons from an invertebrate. Oncogene 20: 2347–2364. [DOI] [PubMed] [Google Scholar]
- Kraus W. L., Lis J. T., 2003. PARP goes transcription. Cell 113: 677–683. [DOI] [PubMed] [Google Scholar]
- Kunttas-Tatli E., Bose A., Kahali B., Bishop C. P., and A. P. Bidwai, 2009. Functional dissection of Timekeeper (Tik) implicates opposite roles for CK2 and PP2A during Drosophila neurogenesis. Genesis 47: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. H., Fedina T. Y., Hansen I., Dreisewerd K., Dierick H. A., et al. , 2012. Insulin signaling mediates sexual attractiveness in Drosophila. PLoS Genet. 8: e1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis G. N., Salomon M. P., Keroles D., Brookes N., Sekimura T., et al. , 2015. 2015 The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila. Aging (Albany, N.Y.) 7: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrovsky Y., Chatterjee B., Clark R. A., Roy A. K., 2000. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 35: 521–532. [DOI] [PubMed] [Google Scholar]
- Lee S. B., Cho K. S., Kim E., Chung J., 2003. blistery encodes Drosophila tensin protein and interacts with integrin and the JNK signaling pathway during wing development. Development 130: 4001–4010. [DOI] [PubMed] [Google Scholar]
- Llorens J. V., Metzendorf C., Missirlis F., Lind M. I., 2015. Mitochondrial iron supply is required for the developmental pulse of ecdysone biosynthesis that initiates metamorphosis in Drosophila melanogaster. J. Biol. Inorg. Chem. 20: 1229–1238. [DOI] [PubMed] [Google Scholar]
- Montagner A., Korecka A., Polizzi A., Y. Lippi, Y. Blum et al, 2016. Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Sci. Rep. 6: 20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T., Yoon K. S., White B. H., Keshishian H., 2001. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA 98: 12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik D. Y. G., Jang Y. E., Lee Y. N., Lee R., Yamamoto R., et al. , 2012. Misexpression screen delineates novel genes controlling Drosophila lifespan. Mech. Ageing Dev. 133: 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palandri A., L’hôte D., Cohen-Tannoudji J., Tricoire H., Monnier V., 2015. Frataxin inactivation leads to steroid deficiency in flies and human ovarian cells. Hum. Mol. Genet. 24: 2615–2626. [DOI] [PubMed] [Google Scholar]
- Pile L. A., Lee F. W., Wassarman D. A., 2001. The histone deacetylase inhibitor trichostatin A influences the development of Drosophila melanogaster. Cell. Mol. Life Sci. 58: 1715–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Hughes K. A., 2014. Vitellogenin family gene expression does not increase Drosophila lifespan or fecundity. F1000 Res. 3: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford, L. M., 1993 Hormones and Drosophila development, pp. 899–939 in The Development of Drosophila melanogaster, volume 2, edited by M. Bates and A. Martinez-Arias. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- Roman G., Endo K., Zong L., Davis R. L., 2001. P{Switch}, a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98: 12602–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Kauppinen A., Kaarniranta K., 2016. AMPK/Snf1 signaling regulates histone acetylation: Impact on gene expression and epigenetic functions. Cell Signal 28: 887–895. [DOI] [PubMed] [Google Scholar]
- Sanz A., Fernández-Ayala D. J., Stefanatos R. K., Jacobs H. T., 2010a Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging 2: 200–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A., Soikkeli M., Portero-Otín M., Wilson A., Kemppainen E., et al. , 2010b Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc. Natl. Acad. Sci. USA 107: 9105–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiewer M. J., Knudsen K. E., 2014. Transcriptional roles of PARP1 in cancer. Mol. Cancer Res. 12: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serviddio G., Blonda M., Bellanti F., Villani R., Iuliano L., et al. , 2013. Oxysterols and redox signaling in the pathogenesis of non-alcoholic fatty liver disease. Free Radic. Res. 47: 881–893. [DOI] [PubMed] [Google Scholar]
- Sfregola M., 2014. Centralspindlin is required for thorax development during Drosophila metamorphosis. Genesis 52: 387–398. [DOI] [PubMed] [Google Scholar]
- Shen J., Curtis C., Tavare S., Tower J., 2009. A screen of apoptosis and senescence regulatory genes for life span effects when over-expressed in Drosophila. Aging (Albany, N.Y.) 1: 191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Miyakura R., Otsuka Y., 2015. Nuclear receptor subfamily 4, group A, member 1 inhibits extrinsic apoptosis and reduces caspase-8 activity in H2O2-induced human HUC-F2 fibroblasts. Redox Rep. 20: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Dong Q., 2015. Regulation of a serine protease homolog by the JNK pathway during thoracic development of Drosophila melanogaster. FEBS Open Bio 5: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. A., Mohtashami M., Zhou L., Trimble W. S., Boulianne G. L., 2001. SNARE-dependent signaling at the Drosophila wing margin. Dev. Biol. 234: 13–23. [DOI] [PubMed] [Google Scholar]
- Sun X., Wheeler C. T., Yolitz J., Laslo M., Alberico T., et al. , 2014. A mitochondrial ATP synthase subunit interacts with TOR signaling to modulate protein homeostasis and lifespan in Drosophila. Cell Reports 8: 1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G. P., Bohmann D., 2008. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Makino Y., Okamoto K., Iida T., Yan K., et al. , 1999. Redox regulation of the glucocorticoid receptor. Antioxid. Redox Signal. 1: 403–423. [DOI] [PubMed] [Google Scholar]
- Thomas C., Rousset R., Noselli S., 2009. JNK signalling influences intracellular trafficking during Drosophila morphogenesis through regulation of the novel target gene Rab30. Dev. Biol. 331: 250–260. [DOI] [PubMed] [Google Scholar]
- Toivonen J. M., O’Dell K. M., Petit N., Irvine S. C., Knight G. K., et al. , 2001. Technical knockout, a Drosophila model of mitochondrial deafness. Genetics 159: 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire H., Battisti V., Trannoy S., Lasbleiz C., Pret A. M., et al. , 2009. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech. Ageing Dev. 130: 547–552. [DOI] [PubMed] [Google Scholar]
- Tripura C., Nulu-Prafulla C., Susmitha V. N., Noselli S., Shashidhara L. S., 2011. Regulation and activity of JNK signaling in the wing disc peripodial membrane during adult morphogenesis in Drosophila. Int. J. Dev. Biol. 55: 583–590. [DOI] [PubMed] [Google Scholar]
- Wan L., Dockendorff T. C., Jongens T. A., Dreyfuss G., 2000. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol. Cell. Biol. 20: 8536–8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Leung H. T., Mazalouskas M. D., Watkins G. R., R. J. Gomezet al. , 2012. Essential roles of the Tap42-regulated protein phosphatase 2A (PP2A) family in wing imaginal disc development of Drosophila melanogaster. PLoS One 7: e38569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskar M. G. N., Landis J., Shen C., Curtis C. K., Tozer K., et al. , 2009. Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging 1: 903–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T., Woods H. A., Hargand S., Elser J. J., Markow T. A., 2006. Biological stoichiometry of growth in Drosophila melanogaster. J. Insect Physiol. 52: 187–193. [DOI] [PubMed] [Google Scholar]
- Welshons W. J., 1958. The analysis of a pseudoallelic recessive lethal system at the notch locus of Drosophila melanogaster. Cold Spring Harb. Symp. Quant. Biol. 23: 171–176. [DOI] [PubMed] [Google Scholar]
- Wigby S., Slack C., Grönke S., Martinez P., Calboli F. C., et al. , 2011. Insulin signalling regulates remating in female Drosophila. Proc. Biol. Sci. 278: 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger J., Bohmann D., 1999. Thorax closure in Drosophila: involvement of Fos and the JNK pathway. Development 126: 3947–3956. [DOI] [PubMed] [Google Scholar]
- Zhu X. J., Liu Y., Yuan X., Wang M., Zhao W., et al. , 2016. Ectodermal Wnt controls nasal pit morphogenesis through modulation of the BMP/FGF/JNK signaling axis. Dev. Dyn. 245: 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.