Abstract
Purpose
Researchers have hypothesized that treatment with cyclosporine A (CyA), interleukin-1 receptor antagonists (IL-1RA; e.g., anakinra), P2Y2 receptor agonists (e.g., uridine triphosphate; UTP), and rebamipide may alleviate human meibomian gland dysfunction (MGD) and/or dry eye disease. Investigators have also proposed that prostaglandin analogues (e.g., bimatoprost) may induce MGD. Our goal was to determine whether these compounds directly influence human meibomian gland epithelial cell (HMGEC) function.
Methods
Multiple concentrations of each compound were tested for effects on immortalized (I) HMGEC morphology and survival. Nontoxic dosages were used for our studies. Immortalized HMGEC were cultured in the presence of vehicle, CyA, IL-1RA, UTP, rebamipide, or bimatoprost for up to 6 days in various media. Experiments included positive controls for proliferation (epidermal growth factor and bovine pituitary extract), differentiation (azithromycin), and signaling pathway activation (insulin-like growth factor 1). Cells were analyzed for neutral lipid staining, lysosome accumulation, lipid composition, and phosphatidylinositol-3-kinase/Akt (AKT), phosphorylation.
Results
Our findings demonstrate that CyA, IL-1RA, UTP, rebamipide, and bimatoprost had no effect on the proliferation; neutral lipid content; lysosome number; or levels of free cholesterol, triglycerides, or phospholipids in IHMGECs. Cylosporine A, IL-1RA, rebamipide, and bimatoprost significantly reduced the phosphorylation of AKT, as compared to control. Of interest, tested doses of CyA above 8 nM killed the IHMGECs.
Conclusions
Our results show that CyA, IL-1RA, UTP, rebamipide, and bimatoprost do not influence the proliferation or differentiation of IHMGEC. However, with the exception of UTP, these compounds do decrease the activity of the AKT signaling pathway, which is known to promote cell survival.
Keywords: dry eye disease, epithelial cells, human meibomian gland
Dry eye disease (DED) is one of the most frequent reasons for patient visits to eye care practitioners throughout the world, and afflicts over 40 million people in the United States alone.1–3 Dry eye disease is characterized by a vicious cycle of tear film hyperosmolarity and instability, and leads to increased ocular surface friction, stress, inflammation, and damage, as well as visual impairment.2,3 The primary cause of DED is obstructive meibomian gland dysfunction (MGD).4,5 Meibomian gland dysfunction, in turn, is due to hyperkeratinization of the external duct epithelium and a reduced meibum quality (i.e., elevated viscosity), resulting in lipid insufficiency and a heightened evaporation of the tear film.4,6
Three drugs have been approved by regulatory agencies in several countries for the treatment of DED. These include cyclosporine A (CyA), an immunosuppressant compound7; diquafosol (diuridine-5′-tetraphosphate), a P2Y2 receptor agonist8; and rebamipide, a quinolinone derivative.9 These agents have been reported to alleviate the symptoms and/or signs of DED.7–26
In contrast, there is no drug approved for the treatment of MGD. Recently, investigators have proposed that topical therapy with CyA,27–29 diquafosol,14,30 or an interleukin-1 receptor antagonist31 (IL-1RA, anakinra) may be helpful in ameliorating obstructive MGD and its associated evaporative DED. Our goal was to determine whether these compounds directly influence human meibomian gland epithelial cell (HMGEC) function.
Toward that end we examined whether CyA, anakinra, and the P2Y2 receptor agonist uridine-5′-triphosphate (UTP, a diqufosol analogue) regulate the proliferation, differentiation, lipid composition, and signaling in immortalized (I) HMGECs. The secretagogue UTP is analogous to diquafosol and elicits similar actions on the ocular surface.12,19,22 For comparative purposes, we also tested the effects of rebamipide and bimatoprost on HMGECs. Bimatoprost, a prostaglandin F2α analogue, is an antiglaucoma (i.e., Lumigan) and eyelash lengthening (i.e., Latisse) drug that has been reported to induce MGD and DED.32–37
Materials and Methods
Cell Culture
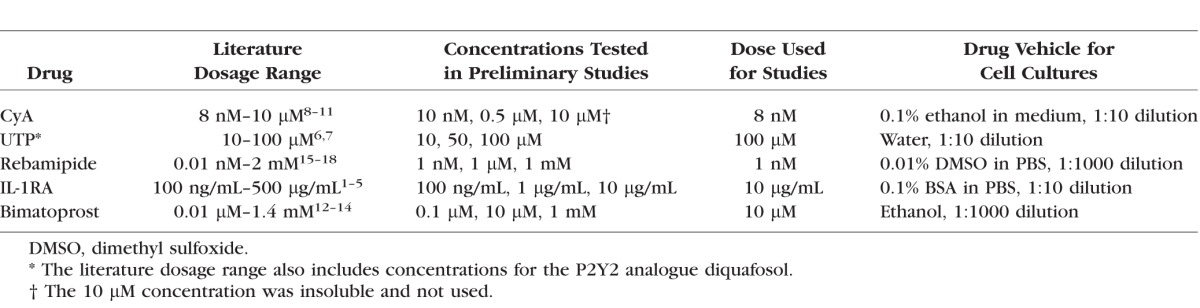
Immortalized HMGECs were grown in keratinocyte serum-free medium (Life Technologies, Grand Island, NY, USA), as previously described.38–40 Cells were treated with three doses of CyA, UTP, rebamipide, bimatoprost (all purchased from Santa Cruz Biotechnology, Dallas, TX, USA), or recombinant human IL-1RA (PeproTech, Rocky Hill, NJ, USA). Dosages tested were based upon literature reports evaluating the effects of CyA,41–44 P2Y2 agonists,45,46 rebamipide,47–50 IL-1RA,51–55 and bimatoprost56–58 on various primary and immortalized cells (Table). During these preliminary experiments IHMGECs were observed for morphologic changes and cell survival for up to 7 days. Based upon the results of these studies, we then selected the highest concentration for each drug that did not have a dramatic effect on cell survival or morphology. Because all cells were killed by the CyA doses tested, we used the lowest dose found in the literature for subsequent experiments. The following nontoxic doses were selected for all further studies: CyA, 8 nM; UTP, 100 μM; rebamipide, 1 nM; IL-1RA, 10 μg/mL; and bimatoprost, 10 μM (Table). To determine effects of each agent on proliferation, cells were cultured for 6 days with drug or vehicle and counted using a hemocytometer. As a positive control, 5 ng/mL epidermal growth factor (EGF) and 50 μg/mL bovine pituitary extract (BPE; Life Technologies) were added to the culture medium.39
Table.
Drugs and Vehicles Used in the IHMGEC Experiments
Lipid Analyses
Differentiation effects were observed in IHMGECs cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 (DMEM/F12; Mediatech, Inc., Manassas, VA, USA), supplemented with 10% fetal bovine serum (FBS; Life Technologies) and 10 ng/mL EGF for 5 days. Cells were exposed to LysoTracker Red DND-99 (Life Technologies) for 30 minutes, fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA), stained with LipidTOX green neutral lipid stain, and mounted using ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (both from Life Technologies). Slides were viewed using an Eclipse E800 fluorescent microscope and images captured with NIS-Elements Basic Research software, version 4.2 (Nikon Instruments, Melville, NY, USA). Azithromycin (AZM; 10 μg/mL; Santa Cruz Biotechnology) was used as a positive control for the accumulation of neutral lipids and lysosomes in IHMGECs.59–63
To identify alterations in specific lipid species, the lipid fraction was isolated from samples containing equivalent cell numbers, as previously described.61,62 In brief, lipid extracts were developed on a silica gel high-performance thin-layer chromatography (HPTLC) plate (EMD Millipore, Billerica, MA, USA) and compared to cholesterol oleate (Nu-Chek Prep, Elysian, MN, USA), free cholesterol (FC), triolein (both from Sigma-Aldrich Corp., St. Louis, MO, USA), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylcholine (PC) (all from Avanti Polar Lipids, Alabaster, AL, USA) standards. Nonpolar lipid separation was achieved with benzene:hexane (65:35, vol/vol), while polar lipids were separated using chloroform:methanol:water (65:25:4) followed by benzene:hexane (65:35). Bands were visualized using published techniques.64 Plates were heated, submerged in acetic acid:sulfuric acid:water (5:0.5:95) with 0.5% CuSO4, then charred. Densitometry calculations on scanned images were performed using ImageJ software (http;//imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). Azithromycin (10 μg/mL) was utilized as a positive control for the accumulation of free cholesterol and phospholipids, and a reduction in the content of triglycerides, in IHMGECs.59,61–63
SDS-PAGE and Immunoblots
Immortalized HMGECs were cultured in DMEM/F12 medium containing 10% FBS for 6 days, then starved in 1% FBS overnight before treatment with drugs or 10 nM recombinant human insulin-like growth factor-1 (IGF-1; National Hormone and Peptide Program, Torrance, CA, USA) for 15 minutes. Subsequently, cells were lysed in Laemmli buffer (Bio-Rad Laboratories, Hercules, CA, USA) supplemented with 1% protease inhibitor cocktail, 200 μM sodium orthovanadate, and 5% β-mercaptoethanol (all from Sigma-Aldrich Corp.). Lysates were denatured at 95°C for 10 minutes, separated by SDS-PAGE on 10% Tris/glycine precast gels (Life Technologies), and transferred to polyvinylidene difluoride (PVDF) membrane. Membranes were incubated with primary antibodies specific for phospho-phosphoinositide 3-kinase-protein kinase B (p-AKT) (1:4000, rabbit) or β-actin (1:10,000, mouse; both from Cell Signaling Technology, Danvers, MA, USA), followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG or Fc-specific goat anti-mouse IgG secondary antibodies, diluted 1:5000 (Sigma-Aldrich Corp.). Blocking and antibody incubation were performed in Tris-buffered saline containing 5% bovine serum albumin and 0.1% Tween 20. Proteins were visualized with Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Rockford, IL, USA) using a G-Box gel documentation station (Syngene, Frederick, MD, USA). Image analysis and densitometry were performed using GeneSys software (Syngene). In these studies, IGF-1 served as a positive control.59
Results
Influence of CyA, UTP, Rebamipide, IL-1RA, and Bimatoprost on the Proliferation of IHMGECs
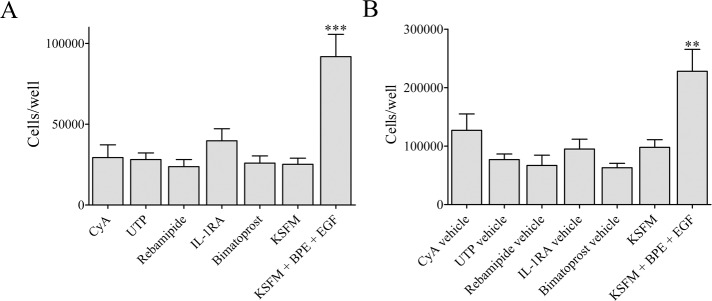
To determine whether CyA, UTP, rebamipide, IL-1RA, and bimatoprost modulate the proliferation of IHMGECs, we cultured cells with these drugs or their vehicles in serum-free media for 5 days (n = 3 wells/drug or vehicle/experiment; n = 3 experiments/drug). We also compared their effect, if any, to that of EGF plus BPE, a combination known to stimulate IHMGEC proliferation.39 As shown in Figure 1, and in contrast to EGF plus BPE exposure, neither these drug treatments nor their vehicles had any significant influence on the proliferation of IHMGECs.
Figure 1.
Drugs or vehicles do not alter IHMGEC survival or proliferation. Cells were exposed to treatments or vehicles for 5 days in keratinocyte serum-free medium (KSFM) and counted using a hemocytometer. Cells were exposed to drugs or vehicles at different times under the same conditions. (A) Cell counts from three experiments (mean ± standard error) are shown. (B) One representative of three control experiments is shown. The combination of epidermal growth factor (EGF, 5 ng/mL) and bovine pituitary extract (BPE, 50 μg/mL) is known to induce IHMGEC proliferation. **P < 0.01, ***P < 0.001, respectively, compared to all other conditions.
Effect of CyA, UTP, Rebamipide, IL-1RA, and Bimatoprost on Lipid and Lysosome Accumulation in IHMGECs
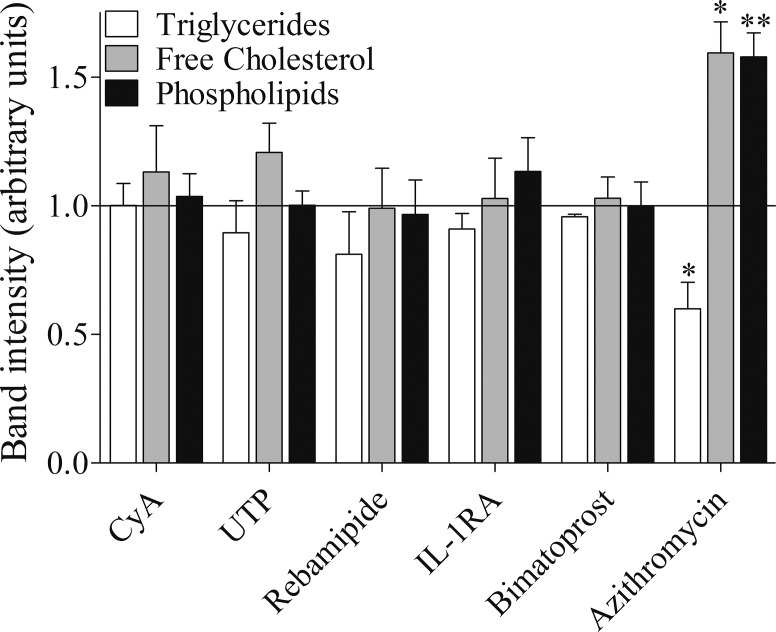
To examine whether CyA, UTP, rebamipide, IL-1RA, and bimatoprost influence lipid and lysosome accumulation in IHMGECs, we treated cells with these drugs, their vehicles, or AZM for 5 days and then processed samples for histologic and biochemical procedures (n = 3 wells/treatment/experiment; n = 3 experiments/drug). As demonstrated in Figure 2, none of these drugs or their vehicles had any effect on the accumulation of neutral lipids (i.e., LipidTOX staining) or lysosomes (i.e., LysoTracker staining) in IHMGECs. Similarly, these drug and vehicle treatments did not influence the expression of free cholesterol, triglycerides, or phospholipids (Fig. 3). For comparison, AZM increased the appearance of intracellular neutral lipids and lysosomes, elevated the levels of free cholesterol and phospholipids, and reduced the content of triglycerides (Figs. 2, 3).
Figure 2.
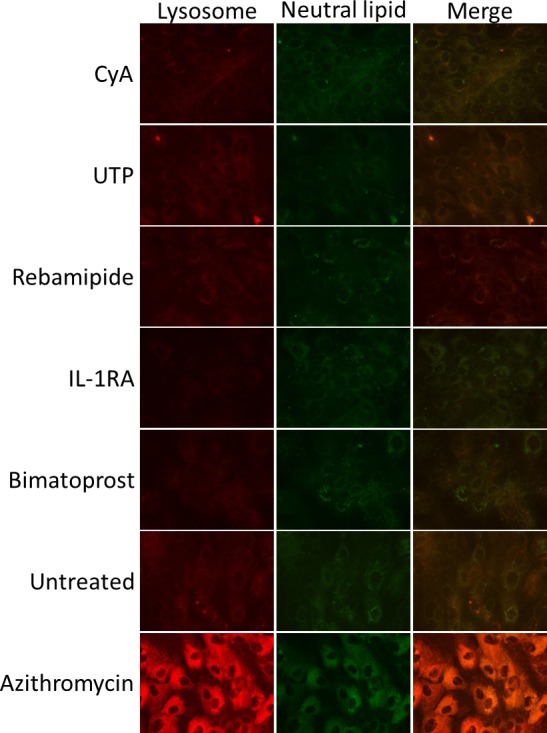
Drugs do not alter lipid accumulation or lysosomal expression in IHMGEC. Cells were treated for 5 days in DMEM supplemented with 10% FBS and 10 ng/mL EGF, then stained for lysosomes (LysoTracker Red) and neutral lipid (LipidTOX, green). All images ×400 magnification.
Figure 3.
Drugs do not alter the expression of triglycerides, free cholesterol, or phospholipids in IHMGEC. Cells were treated for 5 days in DMEM/F12 supplemented with 10% FBS and 10 ng/mL EGF. The lipid fraction was isolated from samples containing equivalent cell numbers and developed on a silica gel high-performance thin-layer chromatography (HPTLC) plate, compared to lipid standards. Fold change compared to vehicle-treated control cells is shown. Azithromycin (10 μg/mL) is a positive control. *P < 0.05, **P < 0.01, respectively, compared to control.
Impact of CyA, UTP, Rebamipide, IL-1RA, and Bimatoprost on AKT Signaling in IHMGECs
To assess whether CyA, UTP, rebamipide, IL-1RA, and bimatoprost alter the activity of a cell survival mediator, we evaluated whether these drugs influenced AKT signaling. Such a signal, as indicated by AKT phosphorylation, promotes cell growth, proliferation, and survival.65
As illustrated in Figure 4, we discovered that CyA, rebamipide, IL-1RA, and bimatoprost significantly reduced the phosphorylation of AKT as compared to control. Uridine triphosphate and the drug-specific vehicles had no effect, whereas IGF-1 significantly increased p-AKT levels (Fig. 4).
Figure 4.
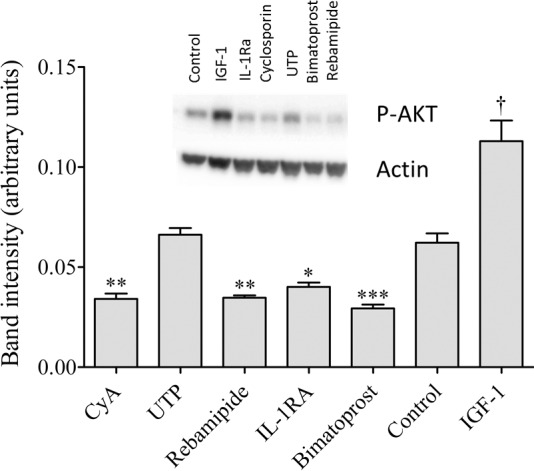
Drugs alter IHMGEC signaling. Cells were cultured for 6 days in DMEM/F12 supplemented with 10% FBS and 10 ng/mL EGF, serum starved overnight (1% FBS), and treated with drugs or vehicles for 15 minutes. Cell lysates were transferred to PVDF and incubated with antibodies specific for phospho-AKT or β-actin. Insulin-like growth factor (IGF-1, 10 nM) is a positive control. Band intensity was normalized to actin and analyzed using ImageJ. By analysis of variance, significant differences exist between groups: P < 0.0001. Post hoc analysis using Dunnett's multiple comparisons test indicates that individual treatments significantly decreased (*P < 0.05; **P < 0.01; ***P < 0.001) or significantly increased (†P < 0.001) AKT phosphorylation compared to control.
Discussion
Our results demonstrate that CyA, IL-1RA, UTP, rebamipide, and bimatoprost have no effect on the proliferation; neutral lipid content; lysosome number; or levels of free cholesterol, triglycerides, or phospholipids in IHMGECs. Further, our data show that CyA, IL-1RA, rebamipide, and bimatoprost significantly decrease the phosphorylation of AKT, a mediator of cell survival, and that tested CyA concentrations above 8 nM kill the IHMGECs. Our findings do not provide any evidence for a positive impact of CyA, IL-1RA, UTP, rebamipide, or bimatoprost on HMGEC function.
Researchers have reported that CyA ameliorates certain signs and/or symptoms of DED.7,11,24,26 These include improvements in Ocular Surface Disease Index scores, tear film breakup times (TBUT), and Schirmer tests and/or goblet cell densities, as well as a reduction in corneal fluorescein staining.24,26,66,67 The proposed mechanism(s) of CyA action have ranged from anti-inflammatory effects on the ocular surface,7 to neuroendocrine influence in the lacrimal gland,68 to a decrease in action potential generation by corneal cold nerve terminals (Kovacs I, et al. IOVS 2012;53:ARVO E-Abstract 1795). Researchers have also hypothesized that CyA treatment may be effective for the therapy of MGD,27–29 and have found that CyA may reduce the number of orifice inclusions in patients with symptomatic MGD.28 Given that CyA did not promote the function of HMGECs in our study, it may be that this compound indirectly targets the glandular external duct plugging by suppressing the release of conjunctival proinflammatory mediators that may influence the keratinization process. It is unlikely that CyA does this directly, because this compound is known to induce hyperkeratinization.69,70
Investigators have also reported that P2Y2 agonists suppress specific signs and/or symptoms of DED.8,12,14,19–22 These actions, whether by UTP or diquafosol, are mediated through guanosine triphosphate binding protein-coupled receptors that stimulate goblet cell mucin release and conjunctival chloride transport.8,12,19,71–77 This latter effect promotes an increase in fluid flow across the conjunctiva and thereby increases tear volume.8,22,78 The treatment result is a partial or consistent improvement in DED biomarkers, such as ocular surface staining, TBUT, Schirmer test scores, and symptoms.8,14,21 Because researchers identified P2Y2 receptors in ductal epithelial cells of rat, rabbit, and primate meibomian glands,79,80 a recent study was performed to evaluate topical diquafosol for the treatment of MGD.30 However, this 4- to 16-month-long study included no placebo-treated controls, which make it impossible to determine whether the P2Y2 agonist elicited an effect in humans. Based upon our findings, if diquafosol does influence the human meibomian gland, the effect might involve ductal, but not acinar, epithelial cells.
In contrast, a recent study with Cu,Zn–superoxide dismutase-1 knockout mice reported that 2 weeks of topical diquafosol treatment increased the number of lipid droplets, the acinar unit density, and keratins 4 and 13 staining in meibomian glands (Ikeda K, et al. IOVS 2016;57:ARVO E-Abstract 2869). However, given that this study also contained no placebo-treated controls, it is not clear whether these results are due to diquafosol or the vehicle.
Topical rebamipide has been reported to reduce various signs and/or symptoms of DED.9,10,13,15–18,23,25 These responses include an increase in mucin expression and optical quality and a reduction in corneal fluorescein staining, ocular surface inflammation, and foreign body sensation.10,13,16,18,23,81–88 Given that MGD is the most common cause of DED,4,5 we hypothesized that part of rebamipide's effect could involve promoting meibomian gland function. Our results, though, do not show a stimulatory effect of this quinolinone derivative on HMGECs.
Investigators have hypothesized that topical IL-1RA may have therapeutic benefit as a treatment for desiccating,89 aqueous-deficient,90 and MGD-associated evaporative31 DED. Mouse experiments have shown that IL-1RA may improve ocular surface integrity, increase tear secretion, and suppress corneal inflammation,89,90 and a human trial discovered that IL-1RA (i.e., anakinra) reduced corneal epitheliopathy in patients with MGD-related DED.31 However, this latter study did not observe any IL-1RA–associated change in meibomian gland secretion quality or the number of expressible glands as compared to baseline or placebo. This lack of a stimulatory effect of IL-1RA on the meibomian gland was also found in our present study. The rationale for administering IL-1RA is unclear, given that the levels of IL-1RA protein are increased in the tear film of MGD patients with evaporative DED91 and in the conjunctiva of patients with aqueous-deficient DED.92
Lastly, given that prostaglandins have been reported to induce MGD and DED,32–37 we hypothesized that bimatoprost, a prostaglandin F2α analogue, may exert a direct action on HMGECs. But like CyA, rebamipide, and IL-1RA, bimatoprost had no effect on the IHMGEC function other than decreasing the phosphorylation of AKT. In contrast, our control compounds, including EGF plus BPE, AZM, and IGF-1, all induced the anticipated alterations in the proliferation; neutral lipid content; lysosome number; levels of free cholesterol, triglycerides and phospholipids; and signaling pathway activity in IHMGECs. Overall, our findings suggest that treatment with CyA, IL-1RA, P2Y2 agonists, or rebamipide may not be effective for stimulating the function of HMGECs.
Acknowledgments
Supported by National Institutes of Health Grants EY05612 and 1K99EY023536-01A1, the Margaret S. Sinon Scholar in Ocular Surface Research Fund, and the Guoxing Yao Research Fund.
Disclosure: W.R. Kam, None; Y. Liu, None; J. Ding, None; D.A. Sullivan, None
References
- 1. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 93–107. [DOI] [PubMed] [Google Scholar]
- 2. Chao W,, Belmonte C. BenitezDel Castillo JM,, et al. Report of the Inaugural Meeting of the TFOS i(2) = initiating innovation Series: Targeting the Unmet Need for Dry Eye Treatment. Ocul Surf. 2016; 14: 264–316. [DOI] [PubMed]
- 3. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 179–193. [DOI] [PubMed] [Google Scholar]
- 4. Knop E,, Knop N,, Millar T,, Obata H,, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011; 52: 1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lemp MA,, Crews LA,, Bron AJ,, Foulks GN,, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012; 31: 472–478. [DOI] [PubMed] [Google Scholar]
- 6. Green-Church KB,, Butovich I,, Willcox M,, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011; 52: 1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Utine CA,, Stern M,, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010; 18: 352–361. [DOI] [PubMed] [Google Scholar]
- 8. Lau OC,, Samarawickrama C,, Skalicky SE. P2Y2 receptor agonists for the treatment of dry eye disease: a review. Clin Ophthalmol. 2014; 8: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kashima T,, Itakura H,, Akiyama H,, Kishi S. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: a critical appraisal. Clin Ophthalmol. 2014; 8: 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arimoto A,, Kitagawa K,, Mita N,, Takahashi Y,, Shibuya E,, Sasaki H. Effect of rebamipide ophthalmic suspension on signs and symptoms of keratoconjunctivitis sicca in Sjogren syndrome patients with or without punctal occlusions. Cornea. 2014; 33: 806–811. [DOI] [PubMed] [Google Scholar]
- 11. Brito-Zeron P,, Siso-Almirall A,, Bove A,, Kostov BA,, Ramos-Casals M. Primary Sjogren syndrome: an update on current pharmacotherapy options and future directions. Expert Opin Pharmacother. 2013; 14: 279–289. [DOI] [PubMed] [Google Scholar]
- 12. Fujihara T,, Murakami T,, Nagano T,, Nakamura M,, Nakata K. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002; 18: 363–370. [DOI] [PubMed] [Google Scholar]
- 13. Igarashi T,, Fujita M,, Yamada Y,, et al. Improvements in signs and symptoms of dry eye after instillation of 2% rebamipide. J Nippon Med Sch. 2015; 82: 229–236. [DOI] [PubMed] [Google Scholar]
- 14. Keating GM. Diquafosol ophthalmic solution 3 %: a review of its use in dry eye. Drugs. 2015; 75: 911–922. [DOI] [PubMed] [Google Scholar]
- 15. Kinoshita S,, Awamura S,, Nakamichi N,, Suzuki H,, Oshiden K,, Yokoi NA. Multicenter, open-label, 52-week study of 2% rebamipide (OPC-12759) ophthalmic suspension in patients with dry eye. Am J Ophthalmol. 2014; 157: 576–583. 583, e571. [DOI] [PubMed] [Google Scholar]
- 16. Kinoshita S,, Oshiden K,, Awamura S,, et al. A randomized, multicenter phase 3 study comparing 2% rebamipide (OPC-12759) with 0.1% sodium hyaluronate in the treatment of dry eye. Ophthalmology. 2013; 120: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 17. Kinoshita S,, Awamura S,, Oshiden K,, et al. Rebamipide (OPC-12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology. 2012; 119: 2471–2478. [DOI] [PubMed] [Google Scholar]
- 18. Koh S,, Inoue Y,, Sugmimoto T,, Maeda N,, Nishida K. Effect of rebamipide ophthalmic suspension on optical quality in the short break-up time type of dry eye. Cornea. 2013; 32: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 19. Murakami T,, Fujihara T,, Nakamura M,, Nakata K. P2Y(2) receptor stimulation increases tear fluid secretion in rabbits. Curr Eye Res. 2000; 21: 782–787. [DOI] [PubMed] [Google Scholar]
- 20. Shimazaki-Den S,, Iseda H,, Dogru M,, Shimazaki J. Effects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eye. Cornea. 2013; 32: 1120–1125. [DOI] [PubMed] [Google Scholar]
- 21. Takamura E,, Tsubota K,, Watanabe H,, Ohashi Y; Diquafosol Ophthalmic Solution Phase 3 Study Group. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012; 96: 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thelin WR,, Johnson MR,, Hirsh AJ,, Kublin CL,, Zoukhri D. Effect of topically applied epithelial sodium channel inhibitors on tear production in normal mice and in mice with induced aqueous tear deficiency. J Ocul Pharmacol Ther. 2012; 28: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ueda K,, Matsumiya W,, Otsuka K,, Maeda Y,, Nagai T,, Nakamura M. Effectiveness and relevant factors of 2% rebamipide ophthalmic suspension treatment in dry eye. BMC Ophthalmol. 2015; 15: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wan KH,, Chen LJ,, Young AL. Efficacy and safety of topical 0.05% cyclosporine eye drops in the treatment of dry eye syndrome: a systematic review and meta-analysis. Ocul Surf. 2015; 13: 213–225. [DOI] [PubMed] [Google Scholar]
- 25. Yamane M,, Ogawa Y,, Fukui M,, et al. Long-term rebamipide and diquafosol in two cases of immune-mediated dry eye. Optom Vis Sci. 2015; 92 (4 suppl 1): S25–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou XQ,, Wei RL. Topical cyclosporine A in the treatment of dry eye: a systematic review and meta-analysis. Cornea. 2014; 33: 760–767. [DOI] [PubMed] [Google Scholar]
- 27. Qiao J,, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013; 7: 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perry HD,, Doshi-Carnevale S,, Donnenfeld ED,, Solomon R,, Biser SA,, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006; 25: 171–175. [DOI] [PubMed] [Google Scholar]
- 29. Prabhasawat P,, Tesavibul N,, Mahawong W. A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea. 2012; 31: 1386–1393. [DOI] [PubMed] [Google Scholar]
- 30. Arita R,, Suehiro J,, Haraguchi T,, et al. Topical diquafosol for patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2013; 97: 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amparo F,, Dastjerdi MH,, Okanobo A,, et al. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmol. 2013; 131: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aydin Kurna S, Acikgoz S, Altun A, Ozbay N, Sengor T, Olcaysu OO. The effects of topical antiglaucoma drugs as monotherapy on the ocular surface: a prospective study. J Ophthalmol. 2014; 2014: 460483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wirta D,, Vandenburgh AM,, Weng E,, Whitcup SM,, Kurstjens S,, Beddingfield FC., III. Long-term safety evaluation of bimatoprost ophthalmic solution 0.03%: a pooled analysis of six double-masked, randomized, active-controlled clinical trials. Clin Ophthalmol. 2011; 5: 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Servat JJ,, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011; 28: 267–282. [DOI] [PubMed] [Google Scholar]
- 35. Chen HY,, Lin CL,, Tsai YY,, Kao CH. Association between glaucoma medication usage and dry eye in Taiwan. Optom Vis Sci. 2015; 92: e227–e232. [DOI] [PubMed] [Google Scholar]
- 36. Arita R,, Itoh K,, Maeda S,, et al. Comparison of the long-term effects of various topical antiglaucoma medications on meibomian glands. Cornea. 2012; 31: 1229–1234. [DOI] [PubMed] [Google Scholar]
- 37. Arita R,, Itoh K,, Maeda S,, et al. Effects of long-term topical anti-glaucoma medications on meibomian glands. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 38. Liu S,, Hatton MP,, Khandelwal P,, Sullivan DA. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2010; 51: 3993–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu S,, Kam WR,, Ding J,, Hatton MP,, Sullivan DA. Effect of growth factors on the proliferation and gene expression of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2013; 54: 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sullivan DA,, Liu Y,, Kam WR,, et al. Serum-induced differentiation of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2014; 55: 3866–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dykes PJ,, Brunt J,, Marks R. The effect of cyclosporin on human epidermal keratinocytes in vitro. Br J Dermatol. 1990; 122: 173–180. [DOI] [PubMed] [Google Scholar]
- 42. Teofoli P,, De Pita O,, Lotti TM. Cyclosporin G inhibits proliferation of A431 cells in a dose- and time-dependent manner comparable to cyclosporin A. Skin Pharmacol. 1997; 10: 79–84. [DOI] [PubMed] [Google Scholar]
- 43. Birraux J,, Kirby JA,, Thomason JM,, Taylor JJ. The effect of cyclosporin on cell division and apoptosis in human oral keratinocytes. J Periodont Res. 2006; 41: 297–302. [DOI] [PubMed] [Google Scholar]
- 44. Fenyvesi F,, Kiss T,, Fenyvesi E,, et al. Randomly methylated beta-cyclodextrin derivatives enhance taxol permeability through human intestinal epithelial Caco-2 cell monolayer. J Pharm Sci. 2011; 100: 4734–4744. [DOI] [PubMed] [Google Scholar]
- 45. Marcet B,, Horckmans M,, Libert F,, Hassid S,, Boeynaems JM,, Communi D. Extracellular nucleotides regulate CCL20 release from human primary airway epithelial cells monocytes and monocyte-derived dendritic cells. J Cell Physiol. 2007; 211: 716–727. [DOI] [PubMed] [Google Scholar]
- 46. Soto D,, Pintor J,, Peral A,, Gual A,, Gasull X. Effects of dinucleoside polyphosphates on trabecular meshwork cells and aqueous humor outflow facility. J Pharmacol Exp Ther. 2005; 314: 1042–1051. [DOI] [PubMed] [Google Scholar]
- 47. Suzuki M,, Miura S,, Mori M,, et al. Rebamipide, a novel antiulcer agent, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994; 35: 1375–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takagi T,, Naito Y,, Uchiyama K,, et al. Rebamipide promotes healing of colonic ulceration through enhanced epithelial restitution. World J Gastroenterol. 2011; 17: 3802–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nagano Y,, Matsui H,, Muramatsu M,, et al. Rebamipide significantly inhibits indomethacin-induced mitochondrial damage, lipid peroxidation, and apoptosis in gastric epithelial RGM-1 cells. Dig Dis Sci. 2005; 50 (suppl 1): S76–S83. [DOI] [PubMed] [Google Scholar]
- 50. Banan A,, Fitzpatrick L,, Zhang Y,, Keshavarzian A. OPC-compounds prevent oxidant-induced carbonylation and depolymerization of the F-actin cytoskeleton and intestinal barrier hyperpermeability. Free Radic Biol Med. 2001; 30: 287–298. [DOI] [PubMed] [Google Scholar]
- 51. Sahraoui A,, Kloster-Jensen K,, Ueland T,, Korsgren O,, Foss A,, Scholz H. Anakinra and tocilizumab enhance survival and function of human islets during culture: implications for clinical islet transplantation. Cell Transplant. 2014; 23: 1199–1211. [DOI] [PubMed] [Google Scholar]
- 52. Fernandez-Vojvodich P,, Palmblad K,, Karimian E,, Andersson U,, Savendahl L. Pro-inflammatory cytokines produced by growth plate chondrocytes may act locally to modulate longitudinal bone growth. Horm Res Paediatr. 2012; 77: 180–187. [DOI] [PubMed] [Google Scholar]
- 53. Muhr P,, Renne J,, Schaefer V,, Werfel T,, Wittmann M. Primary human keratinocytes efficiently induce IL-1-dependent IL-17 in CCR6+ T cells. Exp Dermatol. 2010; 19: 1105–1107. [DOI] [PubMed] [Google Scholar]
- 54. Schwarznau A,, Hanson MS,, Sperger JM,, et al. IL-1beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol. 2009; 220: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hillegass JM,, Miller JM,, MacPherson MB,, et al. Asbestos and erionite prime and activate the NLRP3 inflammasome that stimulates autocrine cytokine release in human mesothelial cells. Part Fibre Toxicol. 2013; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Draman MS,, Grennan-Jones F,, Zhang L,, et al. Effects of prostaglandin F2α on adipocyte biology relevant to Graves' orbitopathy. Thyroid. 2013; 23: 1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takano N,, Tsuruma K,, Ohno Y,, Shimazawa M,, Hara H. Bimatoprost protects retinal neuronal damage via Akt pathway. Eur J Pharmacol. 2013; 702: 56–61. [DOI] [PubMed] [Google Scholar]
- 58. Seibold LK,, Ammar DA,, Kahook MY. Acute effects of glaucoma medications and benzalkonium chloride on pre-adipocyte proliferation and adipocyte cytotoxicity in vitro. Curr Eye Res. 2013; 38: 70–74. [DOI] [PubMed] [Google Scholar]
- 59. Liu Y,, Ding J. The combined effect of azithromycin and insulin-like growth factor-1 on cultured human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2014; 55: 5596–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu Y,, Kam WR,, Ding J,, Sullivan DA. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA Ophthalmol. 2014; 132: 226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu Y,, Kam WR,, Ding J,, Sullivan DA. One man's poison is another man's meat: using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology. 2014; 320: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu Y,, Kam WR,, Ding J,, Sullivan DA. Can tetracycline antibiotics duplicate the ability of azithromycin to stimulate human meibomian gland epithelial cell differentiation? Cornea. 2015; 34: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Y,, Kam WR,, Sullivan DA. Influence of omega 3 and 6 fatty acids on human meibomian gland epithelial cells. Cornea. 2016; 35: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ponec M,, Weerheim A,, Kempenaar J,, Mommaas AM,, Nugteren DH. Lipid composition of cultured human keratinocytes in relation to their differentiation. J Lipid Res. 1988; 29: 949–961. [PubMed] [Google Scholar]
- 65. Song G,, Ouyang G,, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005; 9: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaswan R. Characteristics of a canine model of KCS: effective treatment with topical cyclosporine. Adv Exp Med Biol. 1994; 350: 583–594. [DOI] [PubMed] [Google Scholar]
- 67. Sacchetti M,, Mantelli F,, Lambiase A,, Mastropasqua A,, Merlo D,, Bonini S. Systematic review of randomised clinical trials on topical ciclosporin A for the treatment of dry eye disease. Br J Ophthalmol. 2014; 98: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 68. Kaswan RL,, Salisbury MA. A new perspective on canine keratoconjunctivitis sicca. Treatment with ophthalmic cyclosporine. Vet Clin North Am Small Anim Pract. 1990; 20: 583–613. [DOI] [PubMed] [Google Scholar]
- 69. Ayanoglou CM,, Lesty C. Cyclosporin A-induced gingival overgrowth in the rat: a histological, ultrastructural and histomorphometric evaluation. J Periodont Res. 1999; 34: 7–15. [DOI] [PubMed] [Google Scholar]
- 70. Seibel W,, Sundberg JP,, Lesko LJ,, Sauk JJ,, McCleary LB,, Hassell TM. Cutaneous papillomatous hyperplasia in cyclosporine-A treated beagles. J Invest Dermatol. 1989; 93: 224–230. [DOI] [PubMed] [Google Scholar]
- 71. Terakado K,, Yogo T,, Kohara Y,, et al. Conjunctival expression of the P2Y2 receptor and the effects of 3% diquafosol ophthalmic solution in dogs. Vet J. 2014; 202: 48–52. [DOI] [PubMed] [Google Scholar]
- 72. Brunschweiger A,, Müller CE. P2 receptors activated by uracil nucleotides--an update. Curr Med Chem. 2006; 13: 289–312. [DOI] [PubMed] [Google Scholar]
- 73. von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006; 110: 415–432. [DOI] [PubMed] [Google Scholar]
- 74. Diquafosol: DE 089 diquafosol tetrasodium, INS 365, INS 365 Ophthalmic, INS 365 Respiratory, KPY 998. Drugs R D. 2003; 4: 359–362. [DOI] [PubMed] [Google Scholar]
- 75. Jacobson KA,, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010; 15: 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Levin MH,, Verkman AS. CFTR-regulated chloride transport at the ocular surface in living mice measured by potential differences. Invest Ophthalmol Vis Sci. 2005; 46: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 77. Jumblatt JE,, Jumblatt MM. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp Eye Res. 1998; 67: 341–346. [DOI] [PubMed] [Google Scholar]
- 78. Hosoya K,, Ueda H,, Kim KJ,, Lee VH. Nucleotide stimulation of Cl(-) secretion in the pigmented rabbit conjunctiva. J Pharmacol Exp Ther. 1999; 291: 53–59. [PubMed] [Google Scholar]
- 79. Tanioka H,, Kuriki Y,, Sakamoto A,, Katsuta O,, Kawazu K,, Nakamura M. Expression of the P2Y(2) receptor on the rat ocular surface during a 1-year rearing period. Jpn J Ophthalmol. 2014; 58: 515–521. [DOI] [PubMed] [Google Scholar]
- 80. Cowlen MS,, Zhang VZ,, Warnock L,, Moyer CF,, Peterson WM,, Yerxa BR. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res. 2003; 77: 77–84. [DOI] [PubMed] [Google Scholar]
- 81. Fukuda K,, Ishida W,, Tanaka H,, Harada Y,, Fukushima A. Inhibition by rebamipide of cytokine-induced or lipopolysaccharide-induced chemokine synthesis in human corneal fibroblasts. Br J Ophthalmol. 2014; 98: 1751–1755. [DOI] [PubMed] [Google Scholar]
- 82. Arakaki R,, Eguchi H,, Yamada A,, et al. Anti-inflammatory effects of rebamipide eyedrop administration on ocular lesions in a murine model of primary Sjogren's syndrome. PLoS One. 2014; 9: e98390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Itoh S,, Itoh K,, Shinohara H. Regulation of human corneal epithelial mucins by rebamipide. Curr Eye Res. 2014; 39: 133–141. [DOI] [PubMed] [Google Scholar]
- 84. Ueta M,, Sotozono C,, Yokoi N,, Kinoshita S. Rebamipide suppresses PolyI:C-stimulated cytokine production in human conjunctival epithelial cells. J Ocul Pharmacol Ther. 2013; 29: 688–693. [DOI] [PubMed] [Google Scholar]
- 85. Tanaka H,, Fukuda K,, Ishida W,, Harada Y,, Sumi T,, Fukushima A. Rebamipide increases barrier function and attenuates TNFalpha-induced barrier disruption and cytokine expression in human corneal epithelial cells. Br J Ophthalmol. 2013; 97: 912–916. [DOI] [PubMed] [Google Scholar]
- 86. Kimura K,, Morita Y,, Orita T,, Haruta J,, Takeji Y,, Sonoda KH. Protection of human corneal epithelial cells from TNF-alpha-induced disruption of barrier function by rebamipide. Invest Ophthalmol Vis Sci. 2013; 54: 2572–2760. [DOI] [PubMed] [Google Scholar]
- 87. Urashima H,, Takeji Y,, Okamoto T,, Fujisawa S,, Shinohara H. Rebamipide increases mucin-like substance contents and periodic acid Schiff reagent-positive cells density in normal rabbits. J Ocul Pharmacol Ther. 2012; 28: 264–270. [DOI] [PubMed] [Google Scholar]
- 88. Ohguchi T,, Kojima T,, Ibrahim OM,, et al. The effects of 2% rebamipide ophthalmic solution on the tear functions and ocular surface of the superoxide dismutase-1 (sod1) knockout mice. Invest Ophthalmol Vis Sci. 2013; 54: 7793–7802. [DOI] [PubMed] [Google Scholar]
- 89. Okanobo A,, Chauhan SK,, Dastjerdi MH,, Kodati S,, Dana R. Efficacy of topical blockade of interleukin-1 in experimental dry eye disease. Am J Ophthalmol. 2012; 154: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vijmasi T,, Chen FY,, Chen YT,, Gallup M,, McNamara N. Topical administration of interleukin-1 receptor antagonist as a therapy for aqueous-deficient dry eye in autoimmune disease. Mol Vis. 2013; 19: 1957–1965. [PMC free article] [PubMed] [Google Scholar]
- 91. Enriquez-de-Salamanca A,, Castellanos E,, Stern ME,, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010; 16: 862–873. [PMC free article] [PubMed] [Google Scholar]
- 92. Solomon A,, Dursun D,, Liu Z,, Xie Y,, Macri A,, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001; 42: 2283–2292. [PubMed] [Google Scholar]