Abstract
Purpose
Hyperglycemia, a hallmark of diabetes mellitus, is associated with retinal inflammation and impairment of endothelium-dependent nitric oxide (NO)–mediated dilation of retinal arterioles. However, molecular mechanisms involved in this diminished endothelial vasodilator function remain unclear. We examined whether inflammatory stress-activated kinases, c-Jun N-terminal kinase (JNK) and p38, contribute to retinal arteriolar dysfunction during exposure to acute and chronic hyperglycemia.
Methods
Retinal arterioles were isolated from streptozocin-induced diabetic pigs (2 weeks; chronic hyperglycemia, 471 ± 23 mg/dL) or age-matched control pigs (euglycemia, 79 ± 5 mg/dL), and then cannulated and pressurized for vasoreactivity study. For acute hyperglycemia study, vessels from nondiabetic pigs were exposed intraluminally to high glucose (25 mM ≈ 450 mg/dL) for 2 hours, and normal glucose (5 mM ≈ 90 mg/dL) served as the control.
Results
Endothelium-dependent vasodilation to bradykinin was reduced in a similar manner after exposure to acute or chronic hyperglycemia. Administration of NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) nearly abolished vasodilations either in control (euglycemia and normal glucose) or hyperglycemic (acute and chronic) vessels. Treatment of either acute or chronic hyperglycemic vessels with JNK inhibitor SP600125 or JNK-interacting protein-1 (JIP1) inhibitor BI-78D3, but not p38 inhibitor SB203580, preserved bradykinin-induced dilation in an L-NAME–sensitive manner. By contrast, endothelium-independent vasodilation to sodium nitroprusside was unaffected by acute or chronic hyperglycemia.
Conclusions
Activation of JIP1/JNK signaling in retinal arterioles during exposure to acute or chronic hyperglycemia leads to selective impairment of endothelium-dependent NO-mediated dilation. Therapeutic targeting of the vascular JNK pathway may improve retinal endothelial vasodilator function during early diabetes.
Keywords: hyperglycemia, retinal arterioles, stress-activated kinases, vasodilation, nitric oxide
Diabetes mellitus with chronic hyperglycemia can lead to microvascular complications in the retina and is a major cause of adult-onset blindness worldwide.1 Early nonproliferative stages of diabetic retinopathy are characterized by reduced retinal vasodilator function and retinal blood flow,2–7 as well as increased vascular permeability,8 with little effect on visual acuity. However, without proper management, the condition will progress to vision loss in the late stage of proliferative/ischemic retinopathy.9 Although laser photocoagulation and vitrectomy are available to manage proliferative retinopathy, there are treatment complications and a high rate of recurrence of neovascularization. Furthermore, there are no treatments for the early stage injury other than management of blood glucose level or intravitreal injection of glucocorticoids or VEGF neutralizing agents to mitigate hyperpermeability and macular edema.10,11 New approaches for treating the early stages of diabetes before proliferative retinopathy are needed, especially in the ability to preserve retinal blood flow and avoid ischemic insult. Unfortunately, this strategy is hampered due to the poor understanding of how the vasomotor dysfunction of resistance arterioles, the major site of flow regulation, is developed during the disease state. Although it is known that the endothelium has a key role in regulating vasomotor function and its dysfunction associated with hyperglycemia is regarded as an early event of diabetes,12 hitherto a limited number of animal studies have investigated the phenomenon of flow dysregulation during diabetes with endothelial impairment in the retinal microcirculation.13–19
One potent vasodilator produced by the retinal arteriolar endothelium is nitric oxide (NO).20,21 Clinical evidence indicates that NO produced from NO synthase (NOS) can influence retinal vascular tone and regulate retinal blood flow in humans.22–25 Metabolic activation of the neural retina in healthy human subjects with diffuse flickering light has been shown to increase retinal arteriolar diameter25,26 and retinal blood flow,26 which can be reduced by NOS blockade.25 In diabetic patients, vasodilations induced by flicker light were reduced,4,5,27–32 and this vascular dysregulation might be attributable to diminished NO bioavailability. In support of this notion, we recently provided the first direct evidence of impaired endothelium-dependent NO-mediated dilation of retinal arterioles in vitro following 2 to 12 weeks of chronic hyperglycemia/diabetes in the pig,33 a large animal model of type 1 diabetes relevant to the human retinal microcirculation.20 However, detrimental signaling molecules and mechanistic pathways within retinal arterioles that are responsible for vasomotor dysregulation related to endothelial NO deficiency remain unclear.
An intriguing idea from biochemical and molecular findings from animal models of diabetes suggests that local inflammation in the retina, primary or secondary to hyperglycemia, may contribute to damage of retinal endothelial cells during early diabetes.34 It remains to be addressed whether inflammatory signaling cascades within the endothelium of retinal arterioles contribute to diabetes-induced vasomotor dysfunction. In cultured endothelial cells, activation of inflammatory stress-activated kinases, c-Jun N-terminal kinase (JNK)35 and p38 kinase,36,37 is increased following short-term exposure to high glucose. However, the contribution of these stress-activated kinases to retinal arteriolar dysfunction during diabetes has not been evaluated. Therefore, we used an isolated vessel approach in vitro, which excludes neural-glial and humoral influences, to examine whether activation of stress-activated kinases contributes directly to the impairment of endothelium-dependent NO-mediated dilation of retinal arterioles following acute and chronic hyperglycemia.
Methods
Porcine Diabetes Model
All animal procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Baylor Scott & White Institutional Animal Care and Use Committee. Domestic male pigs (8–12 weeks old, 8–11 kg) were purchased from Real Farms (San Antonio, TX, USA). Diabetes (i.e., chronic hyperglycemia) was induced by selective ablation of pancreatic β-cells with intravenous injection of streptozocin (STZ; Zanosar, 150 mg/kg in saline) via an ear vein (22 pigs) as described in detail in our previous study.33 The control group was injected intravenously with saline instead of STZ (14 pigs). An additional group of nondiabetic pigs without saline injection was used for the acute hyperglycemia study in vitro as described below (24 pigs). The pigs were maintained for a period of 2 weeks. Following STZ or saline (control) injection, the animals were allowed free access to water and fed with syrup mixed with hog chow to prevent temporary hypoglycemia for 24 hours after STZ injection. The animals were allowed free access to water and commercial diet thereafter. The general condition, body weight, and level of blood glucose were closely monitored in all pigs, and only those that had sustained hyperglycemia with a fasting blood glucose level between 250 and 540 mg/dL were included in the study. Fasting blood glucose levels were obtained each day in the morning using a Bayer Contour glucometer (Bayer Corporation, Pittsburgh, PA, USA). Following the 2-week time period, pigs were sedated with Telazol (4–8 mg/kg, intramuscularly), anesthetized with 2% to 4% isoflurane, and intubated. The procedure used for harvesting eyes has been described previously.38
Isolation and Cannulation of Microvessels
The techniques used for identification, isolation, cannulation, pressurization, and visualization of the retinal vasculature have been described previously.38 In brief, the isolated retinal arterioles (∼40–60 μm in situ diameter) were cannulated with a pair of glass micropipettes containing a physiologic salt solution (PSS; in mM: NaCl 145.0, KCl 4.7, CaCl2 2.0, MgSO4 1.17, NaH2PO4 1.2, pyruvate 2.0, EDTA 0.02, and MOPS 3.0) with normal 5 mM D-glucose and 1% albumin. For some vessels, the D-glucose was increased to 25 mM in the PSS. The increased osmolarity in this solution was balanced to 290 mOsm by reducing the NaCl concentration. The vessels then were pressurized to 55 cm H2O intraluminal pressure without flow by two independent pressure reservoir systems. Vasomotor activity of isolated vessels was recorded using videomicroscopic techniques throughout the experiments.38
Study of Vasomotor Function
Cannulated, pressurized arterioles were bathed in PSS-albumin at 36° to 37°C to allow the development of basal tone. To evaluate the effect of chronic hyperglycemia on vasomotor function, endothelium-dependent vasodilation to bradykinin (1 pM to 1 nM) and endothelium-independent vasodilation to sodium nitroprusside (SNP; 1 nM to 10 μM) were established in vessels isolated from the 2-week diabetic and saline-control pigs. Vessels were exposed to each concentration of agonist for 4 to 5 minutes until a stable diameter was maintained. To assess the involvement of stress-activated kinases in the chronic hyperglycemia-induced effect, the vasodilator responses were examined following intraluminal treatment of vessels with JNK inhibitor SP600125 (5 μM),39,40 JNK-interacting protein-1 (JIP1) inhibitor BI-78D3 (7 μM; Bio-Techne/Tocris, Minneapolis, MN, USA),41,42 or p38 kinase inhibitor SB203580 (0.1 μM; EMD Millipore, Billerica, MA, USA)43,44 for 2 hours. For some vessels, the contribution of NO in the vasodilation to bradykinin was evaluated in the presence of NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME; 10 μM, 40-minute incubation) with or without stress-activated kinase inhibitor treatment.
The acute effect of high glucose on vasodilations to bradykinin and SNP was evaluated in vitro after intraluminal incubation of vessels from nondiabetic pigs with 25 mM D-glucose or 5 mM D-glucose for 2 hours. The impact of stress-activated kinases on the NO-mediated vasodilator responses was evaluated following co-incubation of 25 mM glucose with SP600125, BI-78D3, or SB203580 for 2 hours. In some vessels, the contribution of NO was examined in the presence of a stress-activated kinase inhibitor by adding L-NAME (10 μM, 40-minute incubation).
Chemicals
All drugs were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA) except as specifically stated. Bradykinin, SNP, and L-NAME were dissolved in PSS, whereas SP600125, BI-78D3, and SB203580 were dissolved in dimethyl sulfoxide (DMSO). Subsequent concentrations of drugs in DMSO were diluted in PSS. The final concentrations of DMSO in the vessel lumen did not exceed 0.07% by volume. The 0.07% DMSO had no significant effect on vessel viability, vasodilator responses, or maintenance of tone (data not shown).
Data Analysis
At the end of each experiment, the maximum diameter of the vessels was obtained at 0.1 mM SNP in the presence of calcium-free PSS with EDTA (1 mM).38 Diameter changes in response to vasodilator agonists were normalized to this maximum vasodilation and expressed as percentage maximum dilation. Data are reported as mean ± SEM and n value represents the number of vessels (1 per pig per treatment group) studied. Student's t-test or ANOVA followed by Bonferroni multiple-range test was used to determine the significance of experimental interventions, as appropriate. A value of P < 0.05 was considered significant.
Results
Animal Model of Diabetes Mellitus/Chronic Hyperglycemia
Two weeks following STZ injection, blood glucose levels in pigs elevated from 85 ± 6 (4.7 ± 0.3 mM) to 471 ± 23 (26.2 ± 1.3 mM) mg/dL, and the body weight increased from 11.1 ± 0.4 to 14.7 ± 0.6 kg. Pigs injected with saline (control) had unaltered blood glucose levels after 2 weeks, 77 ± 6 (4.3 ± 0.4 mM) vs. 79 ± 5 (4.4 ± 0.3 mM) mg/dL, and the body weight increased from 10.3 ± 0.6 to 17.1 ± 1.4 kg. The weight gain was significantly greater for control than diabetic pigs (Control, 6.8 ± 1.0 kg versus Diabetes, 3.7 ± 0.5 kg).
Effect of Chronic Hyperglycemia on NOS-Mediated Vasodilation
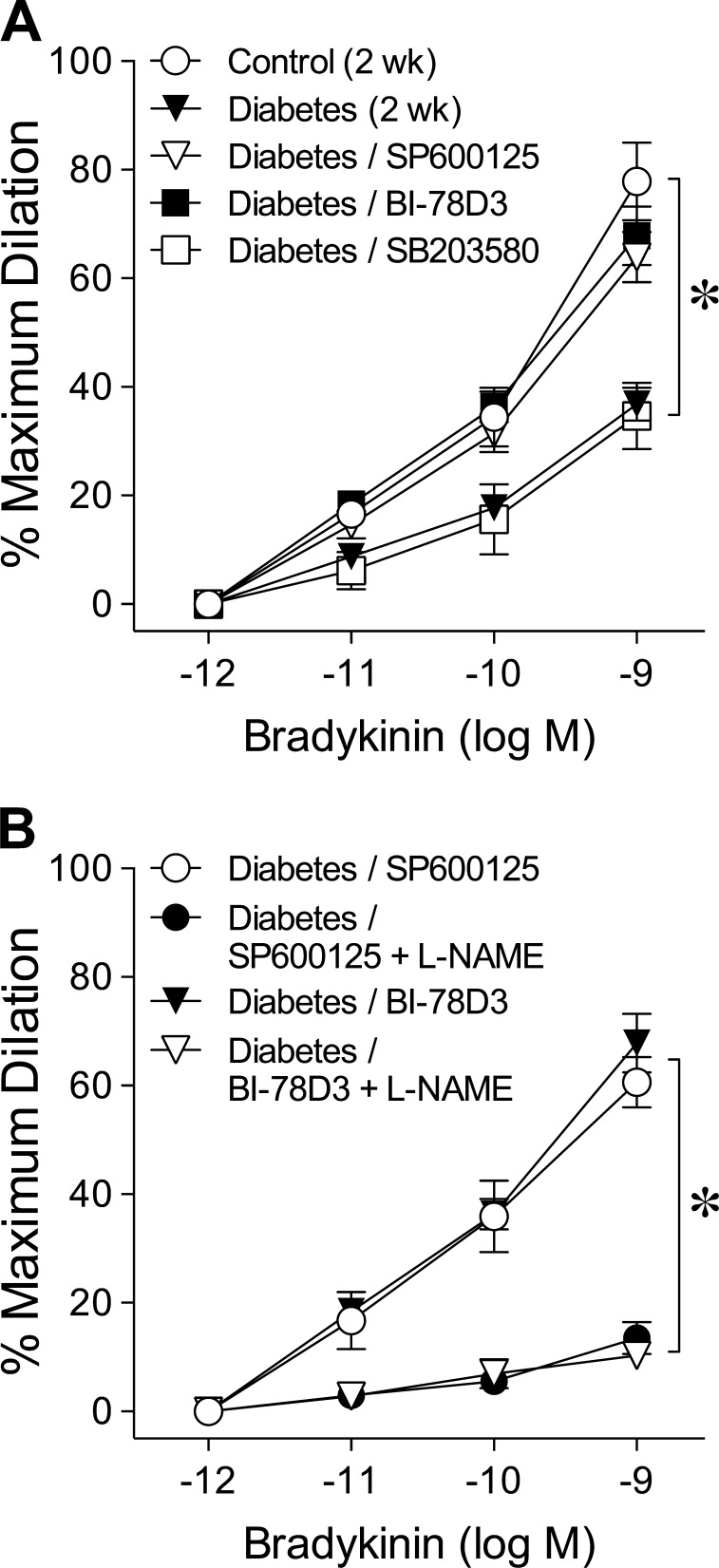
Retinal arterioles from control and diabetic pigs developed a comparable level of basal tone (Control, 49 ± 2% of maximum diameter, 97 ± 3 μm versus Diabetes, 48 ± 1% of maximum diameter, 97 ± 2 μm; P = 0.55), but the concentration-dependent dilation to bradykinin was significantly less in arterioles from diabetic pigs (Fig. 1). The maximum dilation to bradykinin at 1 nM was 37 ± 4% in diabetic vessels and 71 ± 5% in control vessels. In the presence of NOS inhibitor L-NAME, these vasodilations were nearly abolished (Fig. 1). The L-NAME treatment did not alter basal tone (data not shown).
Figure 1.
Chronic hyperglycemia impairs NOS-mediated dilation of retinal arterioles. Concentration-dependent dilation of isolated retinal arterioles to bradykinin was examined in the absence or presence of NOS inhibitor L-NAME after 2 weeks of euglycemia (Control; n = 6) or hyperglycemia (Diabetes; n = 9) in pigs. *P < 0.05 versus Control; #P < 0.05 versus Control or Diabetes.
Roles of JNK, JIP1, and p38 in Chronic Hyperglycemia-Induced Vasodilator Dysfunction
Incubation of diabetic vessels with JNK inhibitor SP600125 or with JIP1 inhibitor BI-78D3 restored the vasodilator response to bradykinin (Fig. 2A) without altering basal tone (data not shown). By contrast, p38 kinase inhibitor SB203580 did not affect the vasodilations to bradykinin (Fig. 2A). The restored bradykinin-induced vasodilations by SP600125 or BI-78D3 were significantly reduced by L-NAME (Fig. 2B). For control vessels, the SP600125, BI-78D3, and SB203580 treatments did not alter the bradykinin-induced vasodilations (data not shown).
Figure 2.
Blockade of JNK activation reverses chronic hyperglycemia-induced reduction of retinal arteriolar dilation to bradykinin. (A) Dilation of retinal arterioles to bradykinin was examined after 2 weeks of euglycemia (Control; n = 10) or hyperglycemia (Diabetes; n = 18) in pigs in the absence or presence of JNK inhibitor SP600125 (n = 9), JIP1 inhibitor BI-78D3 (n = 6), or p38 inhibitor SB203580 (n = 6). *P < 0.05 versus Control. (B) Dilation of retinal arterioles to bradykinin from diabetic pigs was examined in the presence of SP600125 (n = 4) or BI-78D3 (n = 6) before and after treatment with L-NAME. *P < 0.05 versus Diabetes/SP600125 or Diabetes/BI-78D3.
Effect of Acute Hyperglycemia on NOS-Mediated Vasodilation
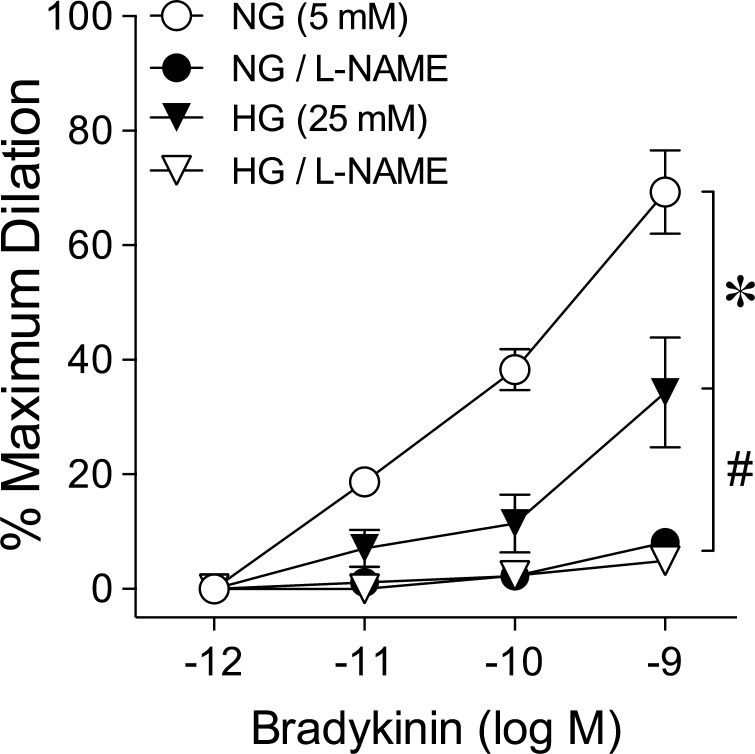
The retinal arterioles isolated from the nondiabetic pigs developed a comparable level of basal tone after intraluminal exposure to normal glucose (NG; 5 mM) or high glucose (HG; 25 mM) for 2 hours (NG, 50 ± 2% of maximum diameter versus HG, 49 ± 3% of maximum diameter, P = 0.85). Concentration-dependent dilation to bradykinin was significantly less in arterioles with intraluminal exposure to HG (Fig. 3). The maximum dilation to bradykinin at 1 nM was 34 ± 10% in HG-treated vessels and 69 ± 7% in NG-treated vessels. These vasodilator responses in HG- and NG-treated vessels were almost completely eliminated in the presence of L-NAME (Fig. 3).
Figure 3.
Acute hyperglycemia impairs NOS-mediated dilation of retinal arterioles. Dilation of retinal arterioles to bradykinin was examined in the absence or presence of L-NAME after 2-hour intraluminal exposure in vitro to NG (5 mM; n = 6) or HG (25 mM; n = 6). *P < 0.05 versus NG (5 mM); #P < 0.05 versus NG (5 mM) or HG (25 mM).
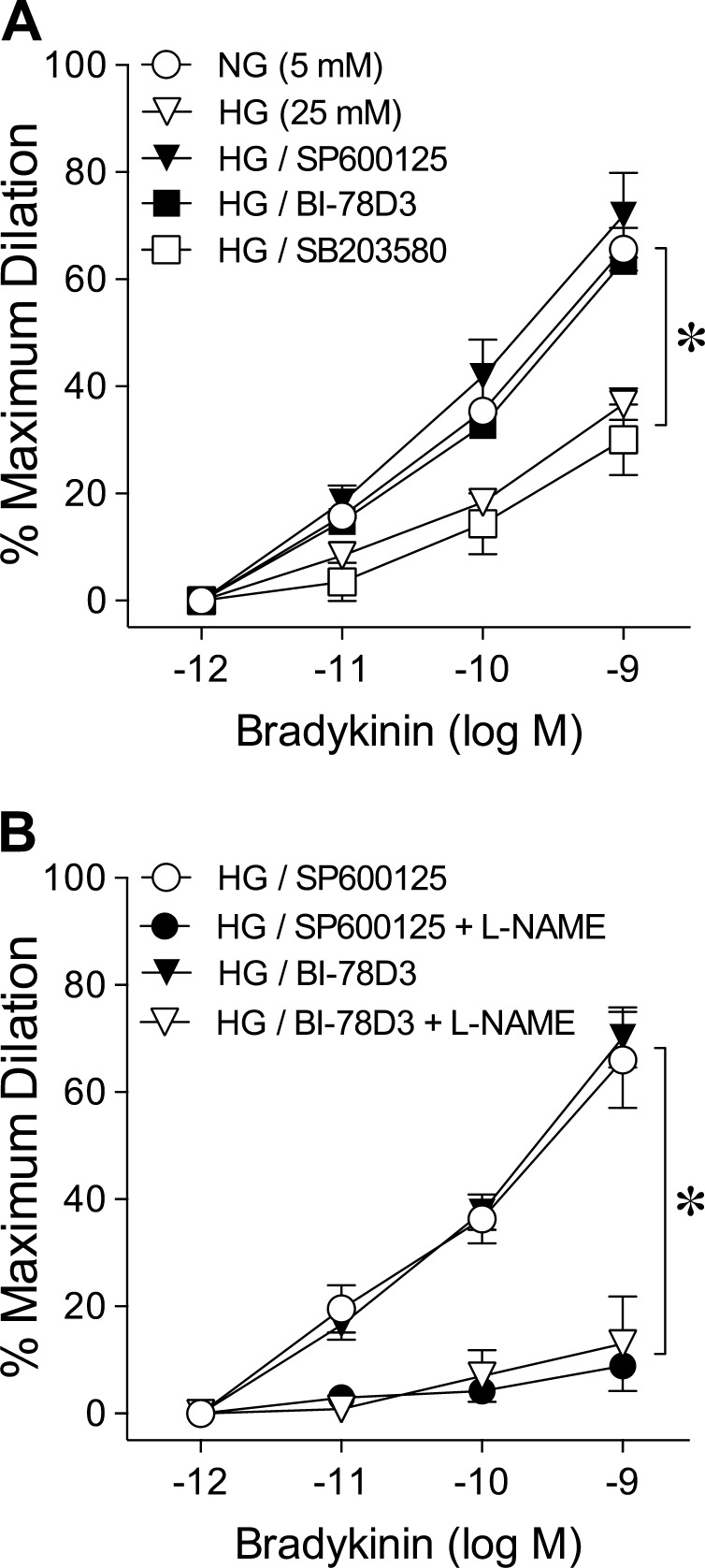
Roles of JNK, JIP1, and p38 in Acute Hyperglycemia-Induced Vasodilator Dysfunction
Incubation of HG-treated vessels with SP600125 or BI-78D3 but not SB203580 preserved the vasodilator response to bradykinin (Fig. 4A) without altering basal tone (data not shown). These preserved vasodilations were attenuated in a similar manner by L-NAME (Fig. 4B). For vessels exposed to normal glucose, the SP600125 and BI-78D3 treatments did not alter the bradykinin-induced vasodilations (data not shown).
Figure 4.
Blockade of JNK activation prevents acute hyperglycemia-induced reduction of retinal arteriolar dilation to bradykinin. (A) Dilation of retinal arterioles to bradykinin was examined after 2-hour intraluminal exposure in vitro to NG (5 mM; n = 19) or HG (25 mM; n = 22) in the absence or presence of SP600125 (n = 8), BI-78D3 (n = 11), or SB203580 (n = 5). *P < 0.05 versus NG (5 mM). (B) Dilation of retinal arterioles to bradykinin was examined following exposure to HG in the presence of SP600125 (n = 5) or BI-78D3 (n = 5) before and after treatment with L-NAME. *P < 0.05 versus HG/SP600125 or HG/BI-78D3.
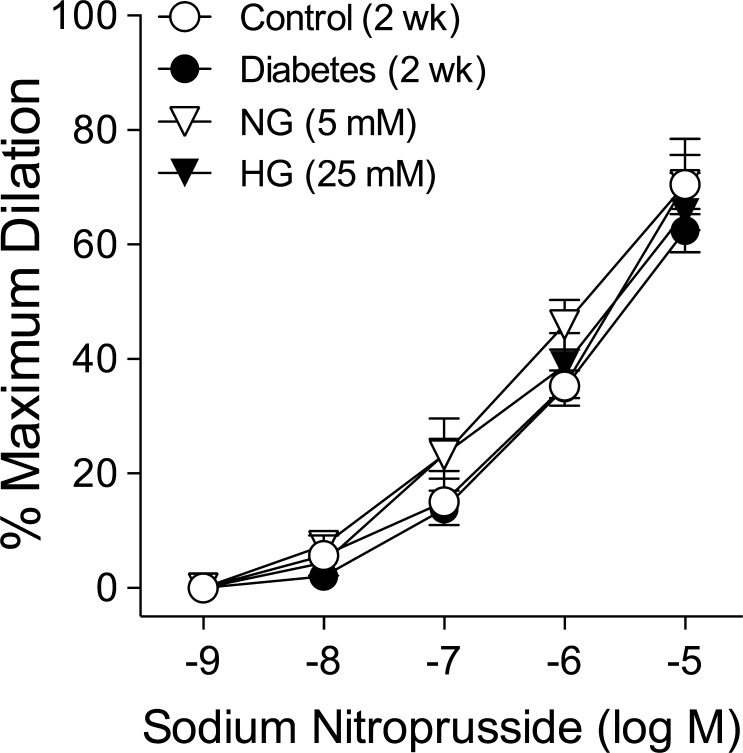
Vasodilation to SNP Following Chronic and Acute Hyperglycemia
Retinal arterioles from nondiabetic (control) and diabetic pigs dilated in a comparable manner to endothelium-independent NO donor SNP with maximum dilation of approximately 70% at 10 μM (Fig. 5). This vasodilator response also was similar between NG- and HG-treated vessels (Fig. 5).
Figure 5.
Chronic or acute hyperglycemia does not alter SNP-induced dilation of retinal arterioles. Concentration-dependent vasodilation to SNP was examined after 2 weeks of euglycemia (Control; n = 5) or hyperglycemia (Diabetes; n = 5) in pigs or after 2-hour intraluminal exposure in vitro to NG (5 mM; n = 5) or HG (25 mM; n = 5).
Discussion
This study showed that exposure of porcine retinal arterioles to chronic hyperglycemia in vivo or acute hyperglycemia in vitro selectively diminished endothelium-dependent NO-mediated dilation to bradykinin. Pharmacologic inhibition of stress-activated kinase JNK and its upstream interaction with JIP1 preserved retinal vasodilator function related to endothelial NO. It appears that JIP1/JNK signaling contributes directly to the impairment of endothelium-dependent NO-mediated dilation of retinal arterioles following acute and chronic hyperglycemia.
The current findings corroborated our previous report on selective impairment of endothelium-dependent dilation of retinal arterioles to bradykinin within 2 weeks of diabetes in pigs.33 This relatively rapid onset of endothelial vasodilator dysfunction in the pig model is consistent with previous evidence showing diminished increase in retinal blood flow following intravitreal administration of endothelial agonist acetylcholine in 2-week diabetic rats.13 However, these earlier studies did not investigate whether the endothelial impairment was a result of diminished NOS activation. We tested this possibility by treating the isolated retinal arterioles with nonselective NOS inhibitor L-NAME after 2 weeks of diabetes, and this series of experiments nearly abolished the remaining vasodilation to bradykinin. Because L-NAME also completely blocked the dilation of retinal arterioles from 2-week control pigs, it is likely that chronic hyperglycemia in vivo attenuates the ability of the endothelium in these vessels to produce and/or release NO via NOS.
The sources of NO production within the retina include neuronal NOS (nNOS)45 and inducible NOS (iNOS)46 in the perivascular tissue, and endothelial NOS (eNOS) in blood vessels.47 All three isoforms of NOS have been shown to contribute to regulation of retinal arteriolar tone under physiologic conditions. The activation of iNOS and nNOS in the perivascular retina during exposure to acute hypoxia (5 minutes) in pigs46 and flickering light stimulation in cats,45 respectively, promotes dilation of retinal arterioles. Interestingly, these vasodilator responses have been shown to be diminished in diabetic patients with27,29,32 and without28,31 retinopathy. Because bradykinin elicits endothelium-dependent dilation of porcine retinal arterioles48 and it predominantly activates eNOS in cultured endothelial cells,49 we believe that hyperglycemia can impair eNOS activation in the endothelium of arterioles. Although our studies used an isolated vessel preparation devoid of perivascular retinal tissue, the results do not exclude the possible contribution of iNOS and nNOS to the vascular impact of diabetes/hyperglycemia in vivo.
To demonstrate whether the endothelium of retinal arterioles is sensitive to hyperglycemia per se, we exposed these vessels to high glucose intraluminally in vitro. Within 2 hours of exposure to high glucose, the bradykinin-induced dilation of retinal arterioles was reduced to a similar level observed following 2 weeks of hyperglycemia in vivo. Notably, the high glucose concentration of 25 mM was comparable to the average glucose level in the diabetic pigs. In addition, osmolarity was maintained at a normal level in the high glucose-treated vessels to obviate the potential impact of hyperosmolarity on endothelial vasodilator function50 and basal tone,14,51 as well as stress-activated kinases.52 The complete blockade of the dilation of retinal arterioles to bradykinin by L-NAME in the presence of high glucose indicates that short-term hyperglycemia can directly impair endothelial NO function without triggering the activation of compensatory vasodilator mechanisms under these conditions. Because endothelium-independent vasodilation to NO donor SNP was unaltered following the 2-hour high glucose exposure, the ability of the smooth muscle to relax in response to NO appears to have remained intact. Therefore, the detrimental effect of acute hyperglycemia was selective for the impairment of NO synthesis/release from the endothelium. Taken together, our findings directly support the notion that acute or chronic hyperglycemia for up to 2 weeks reduces the endothelium-dependent NO-mediated dilation of retinal arterioles.
Accumulating evidence supports an association of inflammation and endothelial dysfunction within the retina during diabetes.34 Interestingly, the inflammatory stress-activated kinases, JNK and p38, have been shown to be activated in the neural retina53–55 and cultured retinal endothelium56,57 following diabetes or acute high glucose exposure. The potential impact of JNK and p38 activation on retinal arteriolar vasomotor function during hyperglycemia had not been explored. These kinases appeared to be reasonable targets because we have previously shown that inflammatory molecules C-reactive protein and TNF-α directly activate p38 and JNK, respectively, in isolated arterioles. In the present study, we found that simultaneous treatment of retinal arterioles with high glucose and JNK inhibitor SP600125 but not p38 inhibitor SB203580 prevented the reduction in dilation to bradykinin. These findings suggested a role for JNK in the initiation of endothelial vasodilator dysfunction during acute hyperglycemia. To assess the potential clinical significance of JNK blockade to restore vasodilator function after prolonged exposure to hyperglycemia in vivo, isolated retinal arterioles were treated with SP600125 after 2 weeks of diabetes. The intraluminal treatment of diabetic vessels with SP600125 preserved the dilation to bradykinin. Because these preserved vasodilator responses were sensitive to L-NAME, it is likely that SP600125 is able to effectively restore the ability of retinal arterioles to produce NO via NOS. On the other hand, SB203580 did not improve vasodilation to bradykinin following chronic hyperglycemia, suggesting that p38 kinase does not contribute to retinal endothelial dysfunction under these conditions. The concentration of SB203580 (0.1 μM) used in this study was sufficient because we have shown previously its efficacy in preventing endothelial vasodilator dysfunction in retinal arterioles induced by C-reactive protein.43 Collectively, our current findings indicate that activation of JNK within retinal arterioles contributes to diabetes-induced endothelial vasodilator dysfunction.
The JNKs belong to the mitogen-activated protein kinase (MAPK) family. Cellular activation of MAPKs, including JNK, involves a distinct protein kinase cascade, which is organized in signaling modules by scaffold proteins for precise regulation in response to various stress stimuli.58 A common feature of MAPK cascades is the organization of 3 kinases in which a MAPK kinase kinase (MAP3K) activates a MAP2K, which subsequently activates a MAPK.59 In the mammalian JNK module, the cytosolic JIP group of scaffold proteins selectively enhance JNK signaling by interacting with and linking the upstream kinases to JNK activation.60,61 Specifically, the JIP1 isoform serves as a docking site for the binding of JNK, MAP3Ks, and MAP2K7,60 to facilitate sequential kinase activation of the JNK signaling pathway.62 The physiologic role for JIP1 in JNK activation was supported by evidence that JIP1 knockout mice lack the ability to elicit anoxic and excitotoxic stress-induced activation of JNK in hippocampal neurons.61 At the vascular level, in vitro studies showed that cultured rat retinal endothelial cells containing overexpression of glucose transporter-1 and elevated intracellular glucose concentrations exhibited an increased JIP1 protein expression and JNK phosphorylation.63 Our current study extended these earlier findings63 to determine whether JIP1 influences retinal arteriolar vasodilator function during acute and chronic hyperglycemia. In 2-week diabetic pigs, we observed reversal of impaired retinal arteriolar dilation to bradykinin with the JIP1 inhibitor BI-78D3,41,42 which is a small molecule mimic of JIP1 that competitively blocks the JIP1 binding domain of JNK from interacting with its cognate substrates and endogenous JIP1. This JNK signaling inhibitor does not influence the ATP-binding region of JNK but instead blocks protein–protein interaction at the JNK-binding site of JIP1. Similarly, bradykinin-induced dilation of retinal arterioles during exposure to acute hyperglycemia was preserved in the presence of JIP1 blockade. Taken together, our findings with 2 structurally distinct JNK pathway inhibitors, SP600125 and BI-78D3, suggest that the interaction of JIP1 and JNK is important in initiating endothelial damage during hyperglycemia, and support the idea that this molecular event contributes to development of endothelial vasodilator dysfunction in retinal arterioles during the early stage of diabetes.
At the mechanistic level it is reasonable to speculate that JNK activation may be linked with increased oxidative stress during diabetes in retinal arterioles with concomitant reduction in NO bioavailability. This notion is supported by earlier work showing that pharmacologic blockade of superoxide production augmented the bradykinin-induced increase in retinal blood flow following 3 hours of hyperglycemia in cats in vivo.14 Our previous study showed that activation of JNK by TNF-α in porcine coronary arterioles leads to superoxide generation and subsequent reduction of NO release via xanthine oxidase activation.39 On the other hand, evidence also has been provided for superoxide-dependent activation of JNK in endothelial cells.64 Future molecular studies are warranted to investigate whether oxidative stress in retinal arterioles during hyperglycemia is requisite for impairing NOS-mediated vasodilation.
In summary, we demonstrated that acute and chronic hyperglycemia promote activation of JIP1/JNK signaling in retinal arterioles leading to impairment of endothelium-dependent NO-mediated dilation. The ability of pharmacologic blockade of JIP1 or JNK to fully restore arteriolar vasodilator function following 2 weeks of hyperglycemia suggests that these specific proteins may provide useful clinical targets to consider for retinal vascular treatment to ameliorate retinal blood flow during early diabetes.65,66
Acknowledgments
The authors thank Christina Du, Angie Hitt, and the animal facility staff for their technical assistance with animal care.
Supported by National Institutes of Health (Bethesda, MD, USA) Grants R01EY023335 and R01EY024624 (TWH), Retina Research Foundation (TWH, LK), the Scott & White Research Foundation Ophthalmic Vascular Research Program (LK), and the Kruse Chair Endowment Fund (LK).
Disclosure: T.W. Hein, None; W. Xu, None; X. Xu, None; L. Kuo, None
References
- 1. Sivaprasad S,, Gupta B,, Crosby-Nwaobi R,, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012. ; 57: 347–370. [DOI] [PubMed] [Google Scholar]
- 2. Konno S,, Feke GT,, Yoshida A,, Fujio N,, Goger DG,, Buzney SM. Retinal blood flow changes in type I diabetes. A long-term follow-up study. Invest Ophthalmol Vis Sci. 1996. ; 37: 1140–1148. [PubMed] [Google Scholar]
- 3. Feke GT,, Buzney SM,, Ogasawara H,, et al. Retinal circulatory abnormalities in type 1 diabetes. Invest Ophthalmol Vis Sci. 1994. ; 35: 2968–2975. [PubMed] [Google Scholar]
- 4. Mandecka A,, Dawczynski J,, Blum M,, et al. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care. 2007. ; 30: 3048–3052. [DOI] [PubMed] [Google Scholar]
- 5. Garhofer G,, Zawinka C,, Resch H,, Kothy P,, Schmetterer L,, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004. ; 88: 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bursell SE,, Clermont AC,, Kinsley BT,, Simonson DC,, Aiello LM,, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996. ; 37: 886–897. [PubMed] [Google Scholar]
- 7. Tayyari F,, Khuu LA,, Flanagan JG,, Singer S,, Brent MH,, Hudson C. Retinal blood flow and retinal blood oxygen saturation in mild to moderate diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015. ; 56: 6796–6800. [DOI] [PubMed] [Google Scholar]
- 8. Antonetti DA,, Barber AJ,, Khin S,, Lieth E,, Tarbell JM,, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998. ; 47: 1953–1959. [DOI] [PubMed] [Google Scholar]
- 9. Aiello LP,, Cahill MT,, Wong JS. Systemic considerations in the management of diabetic retinopathy. Am J Ophthalmol. 2001. ; 132: 760–776. [DOI] [PubMed] [Google Scholar]
- 10. Antonetti DA,, Klein R,, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012. ; 366: 1227–1239. [DOI] [PubMed] [Google Scholar]
- 11. Stitt AW,, Curtis TM,, Chen M,, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016. ; 51: 156–186. [DOI] [PubMed] [Google Scholar]
- 12. Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008. ; 45: 1–16. [DOI] [PubMed] [Google Scholar]
- 13. Horio N,, Clermont AC,, Abiko A,, et al. Angiotensin AT1 receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia. 2004. ; 47: 113–123. [DOI] [PubMed] [Google Scholar]
- 14. Sogawa K,, Nagaoka T,, Izumi N,, Nakabayashi S,, Yoshida A. Acute hyperglycemia-induced endothelial dysfunction in retinal arterioles in cats. Invest Ophthalmol Vis Sci. 2010. ; 51: 2648–2655. [DOI] [PubMed] [Google Scholar]
- 15. Nakazawa T,, Mori A,, Saito M,, Sakamoto K,, Nakahara T,, Ishii K. Vasodilator effects of adenosine on retinal arterioles in streptozotocin-induced diabetic rats. Naunyn-Schmiedebergs Arch Pharmacol. 2008. ; 376: 423–430. [DOI] [PubMed] [Google Scholar]
- 16. Mori A,, Saigo O,, Hanada M,, Nakahara T,, Ishii K. Hyperglycemia accelerates impairment of vasodilator responses to acetylcholine of retinal blood vessels in rats. J Pharmacol Sci. 2009. ; 110: 160–168. [DOI] [PubMed] [Google Scholar]
- 17. Mori A,, Saigo O,, Sakamoto K,, Nakahara T,, Ishii K. Hyperglycemia impairs acetylcholine-induced vasodilation of retinal arterioles through polyol pathway-independent mechanisms in rats. J Pharmacol Sci. 2010. ; 112: 336–342. [DOI] [PubMed] [Google Scholar]
- 18. Nakazawa T,, Kaneko Y,, Mori A,, et al. Attenuation of nitric oxide- and prostaglandin-independent vasodilation of retinal arterioles induced by acetylcholine in streptozotocin-treated rats. Vasc Pharmacol. 2007. ; 46: 153–159. [DOI] [PubMed] [Google Scholar]
- 19. Elms SC,, Toque HA,, Rojas M,, Xu Z,, Caldwell RW,, Caldwell RB. The role of arginase I in diabetes-induced retinal vascular dysfunction in mouse and rat models of diabetes. Diabetologia. 2013. ; 56: 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hein TW,, Rosa RH, Jr,, Yuan Z,, Roberts E,, Kuo L. Divergent roles of nitric oxide and Rho kinase in vasomotor regulation of human retinal arterioles. Invest Ophthalmol Vis Sci. 2010; 51: 1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Potts LB,, Bradley PD,, Xu W,, Kuo L,, Hein TW. Role of endothelium in vasomotor responses to endothelin system and protein kinase C activation in porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2013. ; 54: 7587–7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michelson G,, Warntges S,, Harazny J,, Oehmer S,, Delles C,, Schmieder RE. Effect of NOS inhibition on retinal arterial and capillary circulation in early arterial hypertension. Retina. 2006. ; 26: 437–444. [DOI] [PubMed] [Google Scholar]
- 23. Delles C,, Michelson G,, Harazny J,, Oehmer S,, Hilgers KF,, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke. 2004. ; 35: 1289–1293. [DOI] [PubMed] [Google Scholar]
- 24. Polak K,, Dorner G,, Kiss B,, et al. Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol. 2000. ; 84: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dorner GT,, Garhofer G,, Kiss B,, et al. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003. ; 285: H631–H636. [DOI] [PubMed] [Google Scholar]
- 26. Garhofer G,, Zawinka C,, Resch H,, Huemer KH,, Dorner GT,, Schmetterer L. Diffuse luminance flicker increases blood flow in major retinal arteries and veins. Vision Res. 2004. ; 44: 833–838. [DOI] [PubMed] [Google Scholar]
- 27. Hammer M,, Heller T,, Jentsch S,, et al. Retinal vessel oxygen saturation under flicker light stimulation in patients with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012. ; 53: 4063–4068. [DOI] [PubMed] [Google Scholar]
- 28. Lecleire-Collet A,, Audo I,, Aout M,, et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011. ; 52: 2861–2867. [DOI] [PubMed] [Google Scholar]
- 29. Lim LS,, Ling LH,, Ong PG,, et al. Dynamic responses in retinal vessel caliber with flicker light stimulation in eyes with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014. ; 55: 5207–5213. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen TT,, Kawasaki R,, Wang JJ,, et al. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care. 2009. ; 32: 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petersen L,, Bek T. Preserved pressure autoregulation but disturbed cyclo-oxygenase and nitric oxide effects on retinal arterioles during acute hypoxia in diabetic patients without retinopathy. Ophthalmologica. 2016. ; 235: 114–120. [DOI] [PubMed] [Google Scholar]
- 32. Petersen L,, Bek T. The diameter response of retinal arterioles in diabetic maculopathy is reduced during hypoxia and is unaffected by the inhibition of cyclo-oxygenase and nitric oxide synthesis [published online ahead of print June 7, 2016]. Graefes Arch Clin Exp Ophthalmol. doi:http://dx.doi.org/10.1007/s00417-016-3399-6. [DOI] [PubMed]
- 33. Hein TW,, Potts LB,, Xu W,, Yuen JZ,, Kuo L. Temporal development of retinal arteriolar endothelial dysfunction in porcine type 1 diabetes. Invest Ophthalmol Vis Sci. 2012. ; 53: 7943–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang J,, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011. ; 30: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho FM,, Liu SH,, Liau CS,, Huang PJ,, Lin-Shiau SY. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation. 2000. ; 101: 2618–2624. [DOI] [PubMed] [Google Scholar]
- 36. Nakagami H,, Morishita R,, Yamamoto K,, et al. Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high D-glucose in human endothelial cells. Diabetes. 2001. ; 50: 1472–1481. [DOI] [PubMed] [Google Scholar]
- 37. el-Remessy AB,, Bartoli M,, Platt DH,, Fulton D,, Caldwell RB. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci. 2005. ; 118: 243–252. [DOI] [PubMed] [Google Scholar]
- 38. Hein TW,, Yuan Z,, Rosa RH, Jr,, Kuo L. Requisite roles of A2A receptors, nitric oxide, and KATP channels in retinal arteriolar dilation in response to adenosine. Invest Ophthalmol Vis Sci. 2005; 46: 2113–2119. [DOI] [PubMed] [Google Scholar]
- 39. Zhang C,, Hein TW,, Wang W,, Ren Y,, Shipley RD,, Kuo L. Activation of JNK and xanthine oxidase by TNF-α impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol. 2006. ; 40: 247–257. [DOI] [PubMed] [Google Scholar]
- 40. Bennett BL,, Sasaki DT,, Murray BW,, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001. ; 98: 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stebbins JL,, De SK,, Machleidt T,, et al. Identification of a new JNK inhibitor targeting the JNK-JIP interaction site. Proc Natl Acad Sci U S A. 2008. ; 105: 16809–16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Posthumadeboer J,, van Egmond PW,, Helder MN,, et al. Targeting JNK-interacting-protein-1 (JIP1) sensitises osteosarcoma to doxorubicin. Oncotarget. 2012. ; 3: 1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagaoka T,, Kuo L,, Ren Y,, Yoshida A,, Hein TW. C-reactive protein inhibits endothelium-dependent nitric oxide-mediated dilation of retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci. 2008. ; 49: 2053–2060. [DOI] [PubMed] [Google Scholar]
- 44. Qamirani E,, Ren Y,, Kuo L,, Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005. ; 25: 995–1001. [DOI] [PubMed] [Google Scholar]
- 45. Yoshioka T,, Nagaoka T,, Song Y,, Yokota H,, Tani T,, Yoshida A. Role of neuronal nitric oxide synthase in regulating retinal blood flow during flicker-induced hyperemia in cats. Invest Ophthalmol Vis Sci. 2015. ; 56: 3113–3120. [DOI] [PubMed] [Google Scholar]
- 46. Overso Hansen P, Kringelholt S, Simonsen U, Bek T. Hypoxia-induced relaxation of porcine retinal arterioles in vitro depends on inducible NO synthase and EP4 receptor stimulation in the perivascular retina. Acta Ophthalmol. 2015; 93: 457–463. [DOI] [PubMed] [Google Scholar]
- 47. Gericke A,, Goloborodko E,, Sniatecki JJ,, Steege A,, Wojnowski L,, Pfeiffer N. Contribution of nitric oxide synthase isoforms to cholinergic vasodilation in murine retinal arterioles. Exp Eye Res. 2013. ; 109: 60–66. [DOI] [PubMed] [Google Scholar]
- 48. Hein TW,, Rosa RH, Jr,, Ren Y,, Xu W,, Kuo L. VEGF receptor-2-linked PI3K/calpain/SIRT1 activation mediates retinal arteriolar dilations to VEGF and shear stress. Invest Ophthalmol Vis Sci. 2015; 56: 5381–5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Venema RC. Post-translational mechanisms of endothelial nitric oxide synthase regulation by bradykinin. Int Immunopharmacol. 2002. ; 2: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 50. El-Remessy A,, Abou-Mohamed G,, Caldwell R,, Caldwell R. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003. ; 44: 3135–3143. [DOI] [PubMed] [Google Scholar]
- 51. Ishizaka H,, Kuo L. Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity. Am J Physiol. 1997. ; 273: H104–H112. [DOI] [PubMed] [Google Scholar]
- 52. Galcheva-Gargova Z,, Derijard B,, Wu IH,, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science. 1994. ; 265: 806–808. [DOI] [PubMed] [Google Scholar]
- 53. Jiang H,, Fang J,, Wu B,, et al. Overexpression of serine racemase in retina and overproduction of D-serine in eyes of streptozotocin-induced diabetic retinopathy. J Neuroinflammation. 2011. ; 8: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oshitari T,, Bikbova G,, Yamamoto S. Increased expression of phosphorylated c-Jun and phosphorylated c-Jun N-terminal kinase associated with neuronal cell death in diabetic and high glucose exposed rat retinas. Brain Res Bull. 2014. ; 101: 18–25. [DOI] [PubMed] [Google Scholar]
- 55. Du Y,, Tang J,, Li G,, et al. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci. 2010. ; 51: 2158–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li J,, Wang P,, Yu S,, Zheng Z,, Xu X. Calcium entry mediates hyperglycemia-induced apoptosis through Ca2+/calmodulin-dependent kinase II in retinal capillary endothelial cells. Mol Vis. 2012. ; 18: 2371–2379. [PMC free article] [PubMed] [Google Scholar]
- 57. Kowluru V,, Kowluru RA. Increased oxidative stress in diabetes regulates activation of a small molecular weight G-protein, H-Ras, in the retina. Mol Vis. 2007. ; 13: 602–610. [PMC free article] [PubMed] [Google Scholar]
- 58. Morrison DK,, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003. ; 19: 91–118. [DOI] [PubMed] [Google Scholar]
- 59. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000. ; 103: 239–252. [DOI] [PubMed] [Google Scholar]
- 60. Whitmarsh AJ,, Cavanagh J,, Tournier C,, Yasuda J,, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998. ; 281: 1671–1674. [DOI] [PubMed] [Google Scholar]
- 61. Whitmarsh AJ,, Kuan CY,, Kennedy NJ,, et al. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 2001. ; 15: 2421–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tournier C,, Dong C,, Turner TK,, Jones SN,, Flavell RA,, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001. ; 15: 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhou J,, Deo BK,, Hosoya K,, et al. Increased JNK phosphorylation and oxidative stress in response to increased glucose flux through increased GLUT1 expression in rat retinal endothelial cells. Invest Ophthalmol Vis Sci. 2005. ; 46: 3403–3410. [DOI] [PubMed] [Google Scholar]
- 64. Valente AJ,, Irimpen AM,, Siebenlist U,, Chandrasekar B. OxLDL induces endothelial dysfunction and death via TRAF3IP2: inhibition by HDL3 and AMPK activators. Free Radic Biol Med. 2014. ; 70: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gardiner TA,, Archer DB,, Curtis TM,, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation. 2007. ; 14: 25–38. [DOI] [PubMed] [Google Scholar]
- 66. Pournaras CJ,, Rungger-Brandle E,, Riva CE,, Hardarson SH,, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008. ; 27: 284–330. [DOI] [PubMed] [Google Scholar]