Abstract
Purpose
The neuromodulator dopamine (DA) has been implicated in the prevention of excessive ocular elongation and myopia in various animal models. This study used retina-specific DA knockout mice to investigate the role of retinal DA in refractive development and susceptibility to experimental myopia.
Methods
Measurements of refractive error, corneal curvature, and ocular biometrics were obtained as a function of age for both untreated and form-deprived (FD) groups of retina-specific tyrosine hydroxylase knockout (rTHKO) and control (Ctrl) mice. Retinas from each group were analyzed by HPLC for levels of DA and its primary metabolite (DOPAC).
Results
Under normal visual conditions, rTHKO mice showed significantly myopic refractions (F(1,188) = 7.602, P < 0.001) and steeper corneas (main effect of genotype F(1,180) = 5.1, P < 0.01) at 4 and 6 weeks of age compared with Ctrl mice. Retina-specific THKO mice also had thinner corneas (main effect of genotype F(1,181) = 37.17, P < 0.001), thinner retinas (F(6,181) = 6.07, P < 0.001), and shorter axial lengths (F(6,181) = 3.78, P < 0.01) than Ctrl mice. Retina-specific THKO retinas contained less than 15% of DA and DOPAC compared with Ctrl retinas, and the remaining DA had a significantly higher turnover, as indicated by DOPAC/DA ratios (Student's t-test, P < 0.05). Retina-specific THKO mice showed similar, yet more variable, responses to 6 weeks of FD compared with Ctrl mice.
Conclusions
Diminished retinal DA induced spontaneous myopia in mice raised under laboratory conditions without form deprivation. The relative myopic shift in rTHKO mice may be explained by steeper corneas, an unexpected finding. The chronic loss of DA did not significantly alter the FD myopia response in rTHKO mice.
Keywords: dopamine, myopia, refractive error
During normal ocular refractive development, the mammalian eye grows until the incoming light is focused by the cornea and lens onto the retina to produce an image that is in-focus, a process called emmetropization. In a large percentage of the human population (41.6% of US residents from 1999–20041 and 96.5% of 19-year-old males in South Korea in 20122) this process occurs abnormally, leading to near-sightedness, or myopia. Human myopia is characterized by excessive axial eye growth such that incoming light is focused in front of the photoreceptors, resulting in a blurred image of distant objects. Negative corrective lenses focus light back on the retina and provide improved vision. Even with corrective lenses, myopia is associated with long-term risk for ocular pathologies such as glaucoma, cataract, and retinal detachment.3
Over the past few decades, increasing evidence has indicated that retinal dopamine (DA) is an important modulator of refractive errors and eye growth. Dopamine concentration has been shown to decrease with myopia development4 and therefore DA has been suggested as a “stop” signal for eye growth (see review in Ref. 5). Traditionally, researchers have studied this pathway in primate and chick models, using pharmacological agents to affect DA receptors. For example, spiperone, a D2-like receptor antagonist, prevented the ameliorative effects of brief periods of unrestricted vision in chicks undergoing form deprivation (FD).6 This suggests that DA plays a key role in inhibiting excess eye growth during emmetropization. Another study showed that apomorphine, a DA agonist, inhibits axial growth and myopia development in primates during visual deprivation, again suggesting that DA prevents myopic growth.7 Overall, current findings support the idea that retinal DA is an important protective factor against myopia, yet these findings have been mostly supported by pharmacological experiments.
This study used a mouse model in which DA was selectively removed from the retina by genetically targeting the DA synthesis pathway through tyrosine hydroxylase (TH). This conditional knockout is specific to the retina, as a complete knockout would be lethal.8 Tyrosine hydroxylase catalyzes the formation of L-3,4-dihydroxyphenylalanine (L-DOPA) from the amino acid L-tyrosine. L-DOPA is then converted to DA by DOPA decarboxylase. Tyrosine hydroxylase is the rate-limiting enzyme in this process. To achieve retinal specificity, Cre-lox technology was used to target TH excision in retinal tissue using a Chx10 promoter. Retina-specific TH knockout (rTHKO) mice have approximately 90% reduction in retinal DA and DOPAC levels compared with wild-type (WT) controls, showing that a low level of retinal DA still remains.9
Previous studies in which visual input was altered, followed by measurements of DA levels, refractive error, and eye size, suggest that changes in dopaminergic amacrine cell activation may represent a “blur detector,” such that disrupted visual input decreases retinal DA release, leading to myopic refractive errors.5 Using rTHKO mice, we tested the effect of chronic removal of retinal DA on refractive error development under normal and FD conditions. Because DA is considered a “stop signal” for myopia, we hypothesized that the absence of DA during the critical period of refractive development would result in myopia without FD, mimicking the effect of altered visual input.
Materials and Methods
Retinal DA Knockout Model
In this study, mice were used according to the approved Institutional Animal Care and Use Committee protocol and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Retina-specific THKO mice were described previously and showed significantly reduced contrast sensitivity and light-adapted retinal functions.9 Briefly, Th(loxP/loxP) mice, in which exon 1 of the Th gene was flanked with two loxP sites, were bred with mice expressing Cre-recombinase driven by the Chx-10 promoter, which is expressed in retinal progenitor cells.10 The Th(loxP/loxP) mice were used as the WT control (“Ctrl”) for each experimental paradigm. Mice were genotyped by Transnetyx, Inc. (Cordova, TN, USA).
Experimental Overview
In order to better understand refractive development under normal and FD visual conditions, two experimental paradigms were followed. First, mice underwent testing to measure refractive error, corneal curvature, and ocular biometrics every 2 weeks starting at postnatal day 28 (P28) until P112 (n = 12 Ctrl; n = 17 rTHKO mice) while being raised in standard mouse cages with unrestricted visual input on a 12:12 light:dark cycle (∼70 [range, 20–200] lux; 4100K, 32W; Sylvania Octron@800 Ecologic fluorescent bulb; Sylvania, Wilmington, MA, USA). This lighting emits three major spectral peaks at 430, 545, and 610 nm with 70% of the spectral power greater than 530 nm.11 Retinas were collected from each mouse for DA analysis 2 days following the final measurement session to allow time for residual effects of anesthesia to be eliminated. This group is referred to as normal refractive development (NRD). In the second experimental paradigm (FD), the mice underwent a surgical procedure at P28 in which a pedestal was fitted to the top of the skull in order to hold a diffuser goggle over the right eye.12 Goggled (n = 11 Ctrl; n = 6 rTHKO mice) and untreated naïve littermates (n = 20 Ctrl; n = 19 rTHKO mice) subsequently underwent weekly ocular measurements, as described below, until P77. Two days following the final testing, retinas were collected for DA analysis, as described below.
Ocular Measurements
In order to quantify refractive development and ocular growth, we performed ocular biometry and measurements of refractive error and corneal curvature. Eyes were first dilated with 1% tropicamide. Refractive error of each eye was measured with an automated photorefractor.13 Refractive errors were first obtained with the mouse awake and allowed to move freely to get a baseline recording with a natural head position. After the mouse was anesthetized with ketamine and xylazine (ketamine 80 mg/kg; xylazine 16 mg/kg), a second set of refractive measurements was taken.12 Mice that showed a difference of greater than 2.0 diopter (D) in refractive error between the two eyes at P28 were excluded from the study. If a mouse exhibited significant tear film aberrations after anesthetization, the refractive values from the awake measurements were used instead. Next, a photokeratometer was used to measure the radius of curvature of the cornea using a ring of infrared light-emitting diodes (LED).13,14
Finally, biometric measurements of the mouse eye were taken with a 1310 nm spectral domain optical coherence tomography (SD-OCT) system (intrasubject variability: 10 ± 10 μm15; Bioptigen, Durham, NC, USA) calibrated with a refractive index of 1.43316 to obtain the following biometric lengths: corneal thickness (CT), anterior chamber depth (ACD), lens thickness (LT), vitreous chamber depth (VCD), and retinal thickness (RT). With these values, axial length (AL), defined as the distance from the anterior surface of the cornea to the anterior surface of the RPE, was calculated. For these measurements, we assumed that the refractive index of each structure was constant, and thus applied a single refractive index to allow for comparisons between groups (see Refs. 17, 18 for a discussion of refractive index measurements in mice). Following testing, the effects of xylazine were reversed using yohimbine (2.1 mg/kg) in order to reduce the possibility of corneal lesions.19 The mice were kept warm on a heating pad during recovery from anesthesia, and care was taken to ensure that their eyes remained moist at all times with saline drops.
During these experiments, the OCT system was upgraded to an Envisu R4300 SD-OCT (Bioptigen). Because the Envisu OCT produces significantly enhanced spatial resolution (intrasubject variability 4.1 ± 2.3 μm), especially in the retina, we were able to more accurately determine which structure in the OCT image corresponds with the RPE border. To correct for the difference between the instruments, a careful comparison of the images produced with the two devices was made. Based on this analysis, all RT and AL values acquired by the 1310 nm OCT were reduced by 0.0411 mm.
Head Pedestal Surgery
Under the FD experimental paradigm, P28 mice had ocular measurements taken and were subsequently outfitted with a head-mounted pedestal and a monocular diffuser goggle, as described previously.12 Briefly, the scalp and periosteum of the anesthetized mouse were removed, and three stainless steel screws were placed in the skull. A mix of cyanoacrylate glue (Krazy Glue, Westerville, OH, USA) and dental cement was used to create a pedestal that held in place a diffuser goggle over the right eye. Mice were checked daily to ensure proper goggle compliance. Goggles were repositioned when needed. Temporary loss of goggles (<4–6 hours) did not appear to alter the myopia shift, and no mice were removed from the study for lack of goggle compliance.
DA Analysis
In order to determine the levels of retinal DA and DOPAC (the primary metabolite of DA20) retinal samples were analyzed by HPLC. Mice were killed by cervical dislocation between 4 and 6 hours after light onset to control for circadian rhythms in retinal DA. Each eye was quickly enucleated under controlled lighting conditions (fluorescent lighting, 600 lux), and retinal tissue was collected, immediately frozen on dry ice, and stored at −80°C. Retinal samples were subsequently processed for DA analysis as described previously.21 The retinas were homogenized in 0.1 N HClO4 solution (0.01% sodium metabisulfite and 50 ng/mL internal standard 3,4-dihydroxybenzylamine hydrobromide) and centrifuged. Supernatant fractions were separated with HPLC using a 0.1 M sodium phosphate, 0.1 mM EDTA, 0.35 mM sodium octyl-sulfate, and 6% acetonitrile (pH 2.7) mobile phase to quantify the DA and DOPAC levels with coulometric detection. The DA and DOPAC levels were calculated using a standard curve generated with 0.1 to 1 ng DA and DOPAC and normalized to aggregate protein concentration (ng/mg). Dopamine and DOPAC levels were compared between groups, as well as the ratio of DOPAC/DA as an indicator of DA turnover in the eye.
Statistics
Two-way repeated-measures ANOVA with Holm Sidak post hoc comparisons (SigmaStat, San Jose, CA, USA) was performed to examine the differences between the two genotypes across age. Results are reported as an interaction effect unless otherwise stated. Normal distributions and equal variances were verified for each test. The differences between genotypes for DA levels were analyzed using an unpaired two-tailed Student's t-test. Because no significant differences were found between refractive errors of untreated NRD mice and naïve FD mice, the refractive error data were combined. To determine the effect of FD treatment, the difference in refractive error between the right (OD) and left (OS) eyes was calculated as a “shift.” We have previously shown that the naive left eye does not respond to FD treatment.22
Results
Loss of Retinal DA Leads to Myopia During Normal Refractive Development
Under normal visual conditions, rTHKO mice had significant myopic refractions compared with Ctrl mice from 6 to 14 weeks (average difference in refractive error, 3.28 ± 0.27 D, F(1,188) = 7.602, P < 0.001; Fig. 1). Refractive errors of rTHKO and Ctrl mice were similar at 4 weeks of age, but both genotypes became more hyperopic by 6 weeks, with Ctrl mice reaching 6.06 ± 0.72 D and rTHKO only 3.16 ± 0.58 D (Holm-Sidak post hoc comparison, P < 0.001). Within each genotype the refractive errors were not statistically different from 6 to 12 weeks of age, with refractions becoming less hyperopic at 14 and 16 weeks.
Figure 1.
Relative refractive error is shown across age for the two genotypes, rTHKO and Ctrl. The eyes of rTHKO mice had significantly less hyperopic refractive errors than Ctrl mice, corresponding with relative myopia (two-way repeated ANOVA interaction effect: F(1,188) = 7.602, P < 0.001; post hoc analysis: *P < 0.05; **P < 0.01; ***P < 0.001). Symbols represent average ± SEM.
In addition, rTHKO mice had significantly steeper corneas (smaller corneal radius of curvature) by 0.023 ± 0.003 mm from 4 to 16 weeks of age compared with Ctrl mice (Fig. 2; main effect of genotype F(1,180) = 5.1, P < 0.05). Unlike refractive errors that were similar at 4 weeks of age between the genotypes, the corneas of rTHKO mice were steeper at 4 and 6 weeks of age.
Figure 2.
Corneal radius of curvature is shown across age for the two genotypes, rTHKO and Ctrl. Retina-specific THKO mice had significantly smaller corneal radii of curvature, corresponding with steeper corneas and therefore, presumably shorter focal lengths (two-way repeated ANOVA main effect of genotype F(1,180) = 5.1, P < 0.05). Symbols represent average ± SEM.
Analysis of ocular parameters showed differences in ocular growth between the two genotypes. First, rTHKO mice had significantly smaller CTs at all age, with an average difference of 0.010 ± 0.001 mm (Fig. 3A; main effect of genotype F(1,181) = 37.17, P < 0.001). Additionally, as shown in Figure 3B, rTHKO mice had significantly thinner retinas compared with Ctrl mice (F(6,181) = 6.07, P < 0.001). Control and rTHKO RTs began at 0.170 ± 0.003 and 0.169 ± 0.002 mm, respectively at P28, but Ctrl mice showed a thickening trend, reaching 0.186 ± 0.003 mm at 12 weeks, while rTHKO mice showed a slight thinning trend, reaching 0.163 ± 0.003 mm at 12 weeks.
Figure 3.
Ocular parameters of both rTHKO and Ctrl mice measured at different ages in the refractive development experiment. (A) Retina-specific THKO mice had significantly thinner corneas compared with Ctrl mice (two-way repeated ANOVA main effect of genotype F(1,181) = 37.17, P < 0.001). (B) Retina-specific THKO mice had significantly thinner retinas compared to Ctrl mice (two-way repeated ANOVA interaction effect: F(6,181) = 6.07, P < 0.001). (C) Retina-specific THKO mice had significantly shorter ALs across time compared with Ctrl mice (two-way repeated ANOVA interaction effect: F(6,181) = 3.78, P < 0.01). Post hoc analysis: *P < 0.05; **P < 0.01; ***P < 0.001. All symbols represent average ± SEM. Note that some errors bars are obscured by the symbols.
Finally, eyes of rTHKO mice had significantly shorter ALs compared with Ctrl mice as a function of age (Fig. 3C; F(6,181) = 3.78, P < 0.01). Axial length was shorter by an average of 0.040 ± 0.005 mm in rTHKO compared with Ctrl across all ages. Measurements of ACD, LT, and VCD did not show any significant differences between the genotypes (Supplementary Table S1).
Retinal DA and DOPAC Significantly Reduced in rTHKO Mice
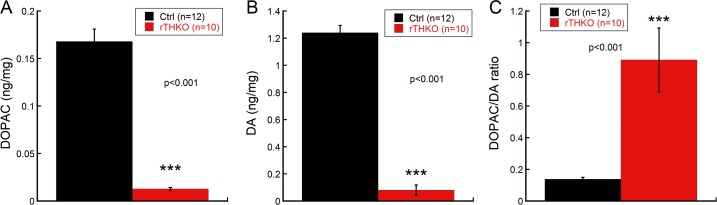
Figure 4 shows that retinal DA was reduced by 93.5 ± 3.1% and retinal DOPAC was reduced by 93.4 ± 0.8% in rTHKO mice compared with Ctrl mice in the NRD group (Fig. 4A, 4B; Student's t-test, ***P < 0.001). Retina-specific THKO mice exhibited higher DOPAC/DA turnover ratios compared with Ctrl mice (Fig. 4C; Student's t-test, ***P < 0.001).
Figure 4.
Retinal DA levels in rTHKO and Ctrl mice at P70 when housed under normal laboratory conditions. In rTHKO mice DA (A) and DOPAC (B) concentrations were significantly reduced compared with those in Ctrl mice (Student's t-test, P < 0.001). (C) Retina-specific THKO mice exhibited a significantly higher DOPAC/DA ratio than Ctrl mice (Student's t-test, P < 0.001). Bars represent average ± SEM.
Loss of Retinal DA Did Not Alter the Response to FD
Control mice underwent a significant myopic shift (OD-OS) of 3.54 ± 0.51 D after 2 weeks of treatment. This myopic shift showed a statistically significant difference from untreated Ctrl mice, and the post hoc analysis was significant for all time points after 4 weeks (Fig. 5A; F(3,90) = 5.54, P < 0.01). Retina-specific THKO mice showed a significant myopic shift of 4.07 ± 1.5 D after 6 weeks of FD, but there was markedly more variation in the degree of response to FD (Fig. 5B; main effect of treatment F F(1,93) = 11.1, P < 0.01). There was no statistical difference in the response to FD between the two genotypes (F(1,52) = 0.239, P = 0.63).
Figure 5.
The myopic shift (OD minus OS) induced by FD treatment is shown for the two genotypes, Ctrl (A) and rTHKO (B). The dashed lines show data for the FD treated mice, while the solid lines represent data from the naïve, untreated mice. (A) Control mice undergoing the FD treatment showed a significant myopic shift after 2 weeks of treatment (two-way repeated measures ANOVA interaction effect: F(3,90) = 5.54, P < 0.01; post hoc analysis: *P < 0.05; **P < 0.01; ***P < 0.001). All goggled time-points have greater than three Ctrl mice except at 10 weeks. (B) Retina-specific THKO mice undergoing the FD treatment showed a myopic shift after 2 weeks of treatment (two-way repeated measures ANOVA main effect of treatment: F(1,93) = 11.1, P < 0.01). Symbols represent average ± SEM.
Corneal curvature did not change as a result of the FD treatment for either genotype (Supplementary Table S1). Analysis of ocular parameters from the FD experiments yielded no statistically significant differences for either genotype when comparing goggled mice with untreated control mice or between genotypes (Supplementary Table S1). Dopamine and DOPAC analysis by HPLC also showed no statistically significant changes in either DA, DOPAC, or DOPAC/DA ratio as a result of the FD treatment.
Discussion
Our findings show that rTHKO mice raised under normal, unaltered visual conditions have relative myopia compared with Ctrl mice. In this mouse model, the shift toward myopia appeared to be due to increased corneal steepening, and not increased AL, indicating potential interactions between dopaminergic signaling in the retina and development of the cornea. The significant reduction of DA had no effect on the response to FD in rTHKO mice. Potential explanations for the normal response to FD include that residual retinal DA turnover preserved the signaling for FD myopia, or that DA signaling is not involved in the response to FD in mice.
Effectiveness of rTHKO in Eliminating Retinal DA
Retina-specific THKO mice have substantially reduced retinal DA and DOPAC levels. As previously reported, retinal DA and DOPAC levels were below 10% of Ctrl while concentrations of DA, DOPAC, and other catecholamines in the brain were completely unaltered.9 Thus, the results gathered from this model can be attributed to changes in retinal DA pathways, rather than higher level neural pathways or other systemic effects. The residual levels of DA and DOPAC may be attributed to either incomplete action of the Chx-10 promoter during development or alternative synthesis pathways of DA. The Chx-10 promoter serves as a good tool for studying the retina because it has been shown to be actively transcribed in all neuroblasts in the developing optic cup10; however, it has been shown to be only variably active in adult retinal tissue,23 leaving the possibility that some retinal neurons remain unaffected and evade Th excision by Cre recombinase.24 Consistent with this interpretation, Jackson et al.9 found that some TH-immunoreactive amacrine cells persist in the retinas of rTHKO mice, accounting for approximately 10% of the number of cells in control retinas. Alternatively, other DA synthesis pathways may become upregulated to compensate for the absence of TH. A previous study found 2% to 22% DA concentration in the brains of TH-null mice compared with WT controls, but found undetectable amounts of DA in TH-null mice that also had the enzyme tyrosinase knocked out.25 Thus, tyrosinase may be synthesizing DA in the absence of TH, which could account for the trace DA levels seen in this model.
Ocular Parameter Changes in rTHKO Mice Raised Under Unaltered Visual Conditions
Retina-specific THKO mice had significantly less hyperopic refractive errors and steeper corneas compared with Ctrl mice. The large difference in refractive index at the air and corneal interface as well as the asphericity of the anterior corneal surface makes the cornea the most important refracting surface of the eye. We hypothesize that the relative myopia seen in the rTHKO mice is due to steeper corneal curvature, instead of the typical axial elongation observed with myopia. A previous study found corneal curvature to be significantly correlated with refractive error in mice.26
It is possible that the corneal steepening in rTHKO mice is producing such a large myopic defocus that axial growth is slowed. Previous studies in several animal models, including tree shrews,27 guinea pigs,28 chicks,29 marmosets,30 and rhesus macaques31 have shown that positive lens defocus, which brings the focal point of incident light in front of the photoreceptors, slows eye growth and axial lengthening. Based on these observations, we predict that the short ALs in rTHKO mice may be due to slowed axial lengthening during development in response to myopic defocus produced by the decreased corneal radius of curvature.
The corneal curvature changes in the rTHKO mice may indicate that DA directly acts on the cornea, as some dopaminergic receptor activity is located in the corneas of rabbits32 and bovines.33 Alternatively, corneal changes may be due to DA-regulated growth factors released from the retina that act on the cornea, or due to DA-influenced retinal functions that alter parasympathetic output to the anterior segment. Future experiments on optical models of the mouse eye and the potential role of DA in corneal development may help elucidate the mechanisms driving corneal curvature and AL in mice.
The reduction in retinal DA may induce developmental changes that result in decreased RT in the rTHKO mice compared with Ctrl mice (Fig. 3B). The RT of the rTHKO mice was relatively stable from 4 to 16 weeks of age (0.169 ± 0.001 to 0.165 ± 0.002, respectively), indicating the absence of a progressive retinal degeneration phenotype. Because DA is an essential neuromodulator in the retina, the loss of DA likely influences retinal signaling and may lead to reduced survival of specific neurons. Future studies are needed to more fully characterize the retinal morphology of the rTHKO mice.
Absence of Retinal DA Does Not Significantly Alter Response to FD Myopia in rTHKO Mice
Retina-specific THKO mice showed no significant differences in mean magnitude of response to FD treatment compared with Ctrl mice. Previous studies have shown that retinal DA levels decrease after FD or lens defocus in animal models of experimental myopia (see review in ref. 5). However, in the mouse model of myopia, reductions in retinal DA levels with FD have not been reported.34–38 Furthermore, the consequences of chronically reduced DA levels on the response to FD have been variable. Several chicken studies have shown that using either nonselective DA antagonists39 or models in which retinal DA stores are reduced40–43 or abolished44 has either no effect on FD or a slight reduction in response to FD. In mice with retinal gene mutations that results in chronic reductions in DA signaling, the response to FD has had opposite effects: enhancing myopic shifts in models with ON pathway defects22 or photoreceptor degeneration,38 or producing no response to FD in a model with nonfunctional rod photoreceptors.37 The results of this study suggest that low levels of retinal DA do not substantially alter the response to FD in mice. Perhaps due to the residual levels of retinal DA in rTHKO mice, DA turnover was present and in fact, significantly greater, when expressed as the DOPAC/DA ratio, than in Ctrl mice, and may have provided sufficient signaling for a normal response to FD. Compensatory increases in DA turnover following partial DA depletion may be a common property of DA neurons. For example, compensatory increases in DA synthesis and turnover have been observed in brain DA neurons following partial lesions with 6-hydroxydopamine.45
It should be noted that the rTHKO mice responded to FD with a trend for smaller myopic shifts with greater SDs (−2.67 ± 3.80 D) compared with Ctrl mice (−4.3 ± 1.71 D), suggesting some abnormalities in signaling for myopic eye growth. Alternatively, it is possible that DA signaling is not critical to the development of FD myopia in mice, as previously reported.34,37 Future studies in which all retinal DA is removed are needed to determine the role of retinal DA in susceptibility to environmental myopia.
A puzzling aspect of the FD data is the absence of changes in ocular parameters to explain the measured refractive shift. One possible explanation is that the sensitivity of our instruments is not great enough to detect the changes in mouse eyes. Due to the small size of the mouse eye, small changes in AL have large effects on refractive power, such that an approximately 5 μm change in AL has been calculated to produce a 1 D myopic shift.18,46 The resolution of the newest SD-OCT used here is near this limit. A second possibility is that the mouse does not respond consistently with axial myopia as observed in other animal models.18 The absence of axial elongation in mice after FD-induced myopic shifts, as found in this study and others,16,35,36,38,47 is contrasted with studies reporting a correlation between AL and refractive error with FD48–53 or lens defocus.48,53 Finally, because we did not measure all possible ocular parameters, it is possible that there are changes in one or more of these parameters that could explain the myopic refractive errors in the rTHKO mice. For instance, LT changes have been reported for other experimental myopia models54–58 and during emmetropization in humans.59–62 We have previously reported that the crystalline lens refractive index increases with FD in a mouse model with an ON pathway defect, suggesting another potential factor influencing ocular parameter measurements.17 The development of new and improved instruments to image the eye and perform ocular biometry will improve our ability to determine which changes in ocular parameters produce the refractive change in the mouse eye.
Relevance of rTHKO Mice to Human Myopia
Axial length is the primary ocular component associated with myopia in human eyes,63 and changes in AL have been previously associated with DA level changes (see review in ref. 5). Thus, whether the results from rTHKO mice that show relative myopia and shorter ALs are relevant to human myopia has yet to be determined.
Retinopathy of prematurity (ROP) in human children and oxygen-induced retinopathy (OIR) in rats results in myopic eyes with shorter than normal ALs.35,64–67 The myopic refractive errors in ROP and OIR are due primarily to changes in optical power of the cornea.64,65 Oxygen-induced retinopathy in rats and mice is associated with decreased retinal DA67 and loss of TH-positive amacrine cells and processes.68 The present results suggest that the alterations of optical power of the anterior chamber in ROP and OIR are causally related to decreased retinal DA.
Animal models, even with their various advantages and disadvantages69 and possible inadequacies to model clinical myopia,70 have greatly increased our knowledge about visually driven eye growth and myopia.69 The power of using mice for experimental myopia is to explore the effects of gene mutations on refractive development under unaltered visual conditions22,35,36,38,71–74 or in response to form deprivation or lens defocus.18,75 Additionally, transgenic mice can be used to confirm that specific genes identified in humans are involved in refractive development.76,77 However, caution in interpreting these results is warranted because mutations that are present during pre- and postnatal development may cause secondary changes in retinal circuitry or signaling that also affect visually-driven eye growth. Finally, specific mutations may amplify signaling to certain ocular structures (for instance the change in corneal curvature in the rTHKO mice). While this may not produce the same phenotype as seen in most cases of human myopia, it may reveal new information about the importance or influence of particular pathways on refractive development in isolation.
Conclusions
Retina-specific THKO mice with low retinal DA developed spontaneous myopia and retained a myopic response to FD, albeit with greater variability. The spontaneous myopia in rTHKO mice was associated with steeper corneas rather than increased ALs. Additional studies are needed to further explore the role of DA in myopia development in mice, including using inducible knock-outs to maintain normal gene expression during early development and using pharmacological agents in combination with genetic mutations to further elucidate mechanisms. This knowledge from mouse models, combined with that from other animal models of experimental myopia, is important for elucidating the role of DA in human myopia in the future.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health Grants R01EY016435, R01EY004864, P30EY006360 (Bethesda, MD, USA), Research Career Scientist Award (MTP) from the Department of Veterans Affairs Rehab R&D Service (Washington DC, USA), and an unrestricted departmental grant from Research to Prevent Blindness (New York, NY, USA).
Disclosure: M.A. Bergen, None; H.N. Park, None; R. Chakraborty, None; E.G. Landis, None; C. Sidhu, None; L. He, None; P.M. Iuvone, None; M.T. Pardue, None
References
- 1. Vitale S,, Sperduto RD,, Ferris FL., III. Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009; 127: 1632–1639. [DOI] [PubMed] [Google Scholar]
- 2. Jung SK,, Lee JH,, Kakizaki H,, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci. 2012; 53: 5579–5583. [DOI] [PubMed] [Google Scholar]
- 3. Saw S-M,, Gazzard G,, Shih-Yen EC,, Chua W-H. Myopia and associated pathological complications. Ophthal Physiol Optics. 2005; 25: 381–391. [DOI] [PubMed] [Google Scholar]
- 4. Stone RA,, Lin T,, Laties AM,, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989; 86: 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feldkaemper M,, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 6. Nickla DL,, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011; 93: 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iuvone PM,, Tigges M,, Stone RA,, Lambert S,, Laties AM. Effects of apomorphine a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991; 32: 1674–1677. [PubMed] [Google Scholar]
- 8. Sotak BN,, Hnasko TS,, Robinson S,, Kremer EJ,, Palmiter RD. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005; 1061: 88–96. [DOI] [PubMed] [Google Scholar]
- 9. Jackson CR,, Ruan GX,, Aseem F,, et al. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012; 32: 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu IS,, Chen JD,, Ploder L,, et al. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994; 13: 377–393. [DOI] [PubMed] [Google Scholar]
- 11. Sylvania Spectral Power Distributions. Technical Information Bulletin: Spectral Power Distributions of SYLVANIA Fluorescent Lamps. 2000. Available at: http://assets.sylvania.com/assets/documents/faq0041-0800.83f1d8de-3fe1-4d24-a209-d95f6cac74b9.pdf.
- 12. Faulkner AE,, Kim MK,, Iuvone PM,, Pardue MT. Head-mounted goggles for murine form deprivation myopia. J Neurosci Methods. 2007; 161: 96–100. [DOI] [PubMed] [Google Scholar]
- 13. Schaeffel F. Test systems for measuring ocular parameters and visual function in mice. Front Biosci. 2008; 13: 4904–4911. [DOI] [PubMed] [Google Scholar]
- 14. Schaeffel F,, Howland H. Corneal accommodation in chick and pigeon. J Comp Physiol A. 1987; 160: 375–384. [DOI] [PubMed] [Google Scholar]
- 15. Park H,, Qazi Y,, Tan C,, et al. Assessment of axial length measurements in mouse eyes. Optom Vis Sci. 2012; 89: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmucker C,, Schaeffel F. In vivo biometry in the mouse eye with low coherence interferometry. Vision Res. 2004; 44: 2445–2456. [DOI] [PubMed] [Google Scholar]
- 17. Chakraborty R,, Lacy KD,, Tan CC,, Park HN,, Pardue MT. Refractive index measurement of the mouse crystalline lens using optical coherence tomography. Exp Eye Res. 2014; 125: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pardue MT,, Stone RA,, Iuvone PM. Investigating mechanisms of myopia in mice. Exp Eye Res. 2013; 114: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner PV,, Albassam MA. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med. 2005; 55: 175–182. [PubMed] [Google Scholar]
- 20. Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004; 108: 17–40. [DOI] [PubMed] [Google Scholar]
- 21. Nir I,, Haque R,, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000; 870: 118–125. [DOI] [PubMed] [Google Scholar]
- 22. Pardue MT,, Faulkner AE,, Fernandes A,, et al. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008; 49: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lefebvre JL,, Zhang Y,, Meister M,, Wang X,, Sanes JR. Gamma protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008; 135: 4141–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu Q,, Ivanova E,, Ganjawala TH,, Pan ZH. Cre-mediated recombination efficiency and transgene expression patterns of three retinal bipolar cell-expressing Cre transgenic mouse lines. Mol Vis. 2013; 19: 1310–1320. [PMC free article] [PubMed] [Google Scholar]
- 25. Rios M,, Habecker B,, Sasaoka T,, et al. Catecholamine synthesis is mediated by tyrosinase in the absence of tyrosine hydroxylase. J Neurosci. 1999; 19: 3519–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou X,, Shen M,, Xie J,, et al. The development of the refractive status and ocular growth in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2008; 49: 5208–5214. [DOI] [PubMed] [Google Scholar]
- 27. Metlapally S,, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008; 8 (3): 1. [DOI] [PubMed] [Google Scholar]
- 28. Howlett MHC,, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009; 49: 219–227. [DOI] [PubMed] [Google Scholar]
- 29. Irving EL,, Sivak JG,, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992; 12: 448–456. [PubMed] [Google Scholar]
- 30. Graham B,, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus). Vision Res. 1999; 39: 189–206. [DOI] [PubMed] [Google Scholar]
- 31. Zhu X,, McBrien NA,, Smith EL, III,, Troilo D,, Wallman J. Eyes in various species can shorten to compensate for myopic defocus. Invest Ophthalmol Vis Sci. 2013; 54: 2634–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cavallotti C,, Pescosolido N,, Artico M,, Feher J. Localization of dopamine receptors in the rabbit cornea. Cornea. 1999; 18: 721–728. [DOI] [PubMed] [Google Scholar]
- 33. Grub M,, Mielke J,, Rohrbach M,, Schlote T. Dopamine receptors of the corneal epithelium and endothelium [in German]. Klin Monbl Augenheilkd. 2012; 229: 822–825. [DOI] [PubMed] [Google Scholar]
- 34. Wu XH,, Li YY,, Zhang PP,, et al. Unaltered retinal dopamine levels in a C57BL/6 mouse model of form-deprivation myopia. Invest Ophthalmol Vis Sci. 2015; 56: 967–977. [DOI] [PubMed] [Google Scholar]
- 35. Chakraborty R,, Park H,, Aung MH,, et al. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol Vis. 2014; 20: 1318–1327. [PMC free article] [PubMed] [Google Scholar]
- 36. Chakraborty R,, Park HN,, Hanif AM,, Sidhu CS,, Iuvone PM,, Pardue MT. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res. 2015; 137: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park H,, Jabbar SB,, Tan CC,, et al. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014; 55: 6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park H,, Tan CC,, Faulkner A,, et al. Retinal degeneration increases susceptibility to myopia in mice. Mol Vis. 2013; 19: 2068–2079. [PMC free article] [PubMed] [Google Scholar]
- 39. McCarthy CS,, Megaw P,, Devadas M,, Morgan IG. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res. 2007; 84: 100–107. [DOI] [PubMed] [Google Scholar]
- 40. Schaeffel F,, Bartmann M,, Hagel G,, Zrenner E. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995; 35: 1247–1264. [DOI] [PubMed] [Google Scholar]
- 41. Ohngemach S,, Hagel G,, Schaeffel F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience deprivation, and after reserpine application. Vis Neurosci. 1997; 14: 493–505. [DOI] [PubMed] [Google Scholar]
- 42. Diether S,, Schaeffel F. Long-term changes in retinal contrast sensitivity in chicks from frosted occluders and drugs: relations to myopia? Vision Res. 1999; 39: 2499–2510. [DOI] [PubMed] [Google Scholar]
- 43. Kroger RH,, Hirt B,, Wagner HJ. Effects of retinal dopamine depletion on the growth of the fish eye. J Comp Physiol A. 1999; 184: 403–412. [DOI] [PubMed] [Google Scholar]
- 44. Li XX,, Schaeffel F,, Kohler K,, Zrenner E. Dose-dependent effects of 6-hydroxy dopamine on deprivation myopia, electroretinograms, and dopaminergic amacrine cells in chickens. Vis Neurosci. 1992; 9: 483–492. [DOI] [PubMed] [Google Scholar]
- 45. Zigmond MJ,, Berger TW,, Grace AA,, Stricker EM. Compensatory responses to nigrostriatal bundle injury. Studies with 6-hydroxydopamine in an animal model of Parkinsonism. Mol Chem Neuropathol. 1989; 10: 185–200. [DOI] [PubMed] [Google Scholar]
- 46. Schmucker C,, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004; 44: 1857–1867. [DOI] [PubMed] [Google Scholar]
- 47. Schaeffel F,, Burkhardt E,, Howland HC,, Williams RW. Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci. 2004; 81: 99–110. [DOI] [PubMed] [Google Scholar]
- 48. Barathi VA,, Boopathi VG,, Yap EP,, Beuerman RW. Two models of experimental myopia in the mouse. Vision Res. 2008; 48: 904–916. [DOI] [PubMed] [Google Scholar]
- 49. Qian YS,, Chu RY,, Hu M,, Hoffman MR. Sonic hedgehog expression and its role in form-deprivation myopia in mice. Curr Eye Res. 2009; 34: 623–635. [DOI] [PubMed] [Google Scholar]
- 50. Yu Y,, Chen H,, Tuo J,, Zhu Y. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Res. 2011; 46: 80–87. [DOI] [PubMed] [Google Scholar]
- 51. Tejedor J,, de la Villa P. Refractive changes induced by form deprivation in the mouse eye. Invest Ophthalmol Vis Sci. 2003; 44: 32–36. [DOI] [PubMed] [Google Scholar]
- 52. Zhou X,, Ji F,, An J,, et al. Experimental murine myopia induces collagen type Ialpha1 (COL1A1) DNA methylation and altered COL1A1 messenger RNA expression in sclera. Mol Vis. 2012; 18: 1312–1324. [PMC free article] [PubMed] [Google Scholar]
- 53. Tkatchenko TV,, Shen Y,, Tkatchenko AV. Mouse experimental myopia has features of primate myopia. Invest Ophthalmol Vis Sci. 2010; 51: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McBrien NA,, Moghaddam HO,, Cottriall CL,, Leech EM,, Cornell LM. The effects of blockade of retinal cell action potentials on ocular growth emmetropization and form deprivation myopia in young chicks. Vision Res. 1995; 35: 1141–1152. [DOI] [PubMed] [Google Scholar]
- 55. Lin T,, Zhu X,, Capehart C,, Stone RA. The ciliary ganglion and vitreous cavity shape. Curr Eye Res. 1996; 15: 453–460. [DOI] [PubMed] [Google Scholar]
- 56. Zhu X,, Wallman J. Opposite effects of glucagon and insulin on compensation for spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Siegwart JT, Jr,, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998; 38: 3505–3515. [DOI] [PubMed] [Google Scholar]
- 58. Ritchey ER,, Zelinka CP,, Tang J,, Liu J,, Fischer AJ. The combination of IGF1 and FGF2 and the induction of excessive ocular growth and extreme myopia. Exp Eye Res. 2012; 99: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mutti DO,, Mitchell GL,, Jones LA,, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005; 46: 3074–3080. [DOI] [PubMed] [Google Scholar]
- 60. Shih YF,, Chiang TH,, Lin LL. Lens thickness changes among schoolchildren in Taiwan. Invest Ophthalmol Vis Sci. 2009; 50: 2637–2644. [DOI] [PubMed] [Google Scholar]
- 61. Richdale K,, Bullimore MA,, Sinnott LT,, Zadnik K. The effect of age, accommodation, and refractive error on the adult human eye. Optom Vis Sci. 2016; 93: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iribarren R. Crystalline lens and refractive development. Prog Retin Eye Res. 2015; 47: 86–106. [DOI] [PubMed] [Google Scholar]
- 63. Meng W,, Butterworth J,, Malecaze F,, Calvas P. Axial length of myopia: a review of current research. Ophthalmologica. 2011; 225: 127–134. [DOI] [PubMed] [Google Scholar]
- 64. Chui TY,, Bissig D,, Berkowitz BA,, Akula JD. Refractive development in the “ROP rat.” J Ophthalmol. 2012; 2012: 956705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cook A,, White S,, Batterbury M,, Clark D. Ocular growth and refractive error development in premature infants with or without retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2008; 49: 5199–5207. [DOI] [PubMed] [Google Scholar]
- 66. Wang J,, Ren X,, Shen L,, Yanni SE,, Leffler JN,, Birch EE. Development of refractive error in individual children with regressed retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2013; 54: 6018–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang N,, Favazza TL,, Baglieri AM,, et al. The rat with oxygen-induced retinopathy is myopic with low retinal dopamine. Invest Ophthalmol Vis Sci. 2013; 54: 8275–8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Spix NJ,, Liu LL,, Zhang Z,, et al. Vulnerability of dopaminergic amacrine cells to chronic ischemia in a mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2016; 57: 3047–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schaeffel F,, Feldkaemper M. Animal models in myopia research. Clin Exp Optom. 2015; 98: 507–517. [DOI] [PubMed] [Google Scholar]
- 70. Fledelius HC,, Goldschmidt E,, Haargaard B,, Jensen H. Human parallels to experimental myopia? A literature review on visual deprivation. Acta Ophthalmol. 2014; 92: 724–729. [DOI] [PubMed] [Google Scholar]
- 71. Schippert R,, Burkhardt E,, Feldkaemper M,, Schaeffel F. Relative axial myopia in Egr-1 (ZENK) knockout mice. Invest Ophthalmol Vis Sci. 2007; 48: 11–17. [DOI] [PubMed] [Google Scholar]
- 72. Zhou G,, Strom RC,, Giguere V,, Williams RW. Modulation of retinal cell populations and eye size in retinoic acid receptor knockout mice. Mol Vis. 2001; 7: 253–260. [PubMed] [Google Scholar]
- 73. Zhou X,, Huang Q,, An J,, et al. Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice. Invest Ophthalmol Vis Sci. 2010; 51: 4362–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wisard J,, Faulkner A,, Chrenek MA,, et al. Exaggerated eye growth in IRBP-deficient mice in early development. Invest Ophthalmol Vis Sci. 2011; 52: 5804–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chakraborty R,, Pardue MT. Molecular and biochemical aspects of the retina on refraction. Prog Mol Biol Transl Sci. 2015; 134: 249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tkatchenko AV,, Tkatchenko TV,, Guggenheim JA,, et al. APLP2 regulates refractive error and myopia development in mice and humans. PLoS Genet. 2015; 11: e1005432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Miyake M,, Yamashiro K. Identification of myopia-associated WNT7B polymorphisms provides insights into the mechanism underlying the development of myopia. 2015; 6: 6689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.