Abstract
Purpose
We developed polycaprolactone (PCL) implants that achieve zero-order release of a proprietary ocular hypotensive agent (DE-117) over 6 months.
Methods
The release rates of DE-117–loaded PCL devices were tuned based on an established predictive model and confirmed by in vitro release studies. Devices containing DE-117 and empty devices were implanted intracamerally in normotensive rabbits for up to 8 weeks' duration. Devices were retrieved after rabbits were euthanized and evaluated for tissue adherence. The drug remaining in each device was analyzed by high performance liquid chromatography. Drug distribution in ocular tissues was measured by liquid chromatography coupled with a tandem mass spectrometry (LC/MS/MS).
Results
In vitro release of DE-117 showed zero-order release with a release rate of 0.5 μg/day over 6 months. Implantation in rabbit eyes demonstrated that the devices were well tolerated in the intracameral space. Quantification of DE-117 and hDE-117 (the hydrolyzed active form of DE-117) in ocular tissues (cornea, iris-ciliary body, aqueous humor, and vitreous humor) indicated sustained release of DE-117 and its conversion to hDE-117 when released from the device. Analysis of drug remaining in the device found that concentration of hDE-117 was below the limit of detection, indicating the encapsulated drug was protected from hydrolysis in the device.
Conclusions
Proof-of-concept PCL drug delivery devices containing DE-117 show promise as a long-term glaucoma treatment based on their zero-order drug release profile in vitro, biocompatibility in vivo, and effective distribution of released drug in relevant ocular tissues.
Keywords: drug delivery, glaucoma, glaucoma anterior segment
Glaucoma is one of the leading causes of blindness worldwide, with an estimated number of patients affected at over 60 million.1,2 For glaucoma management, topical therapy with ocular hypotensive medications is a mainstay. However, topical hypotensive agents typically require daily administration 1 to 3 times a day, and reports have repeatedly indicated that poor patient compliance with eye drops is a major obstacle.3,4 Particularly for the elderly population, who are more prone to glaucoma, 23% of glaucoma patients aged older than 65 years were nonadherent to glaucoma therapy, with mean number of days without therapy at 112 days in a year.4 Studies also estimate that less than half of elderly patients successfully administer topical drugs using eye droppers.3 Therefore, a long-term implant that ensures proper drug administration for an extended period of time has the potential to improve patient outcomes, by ensuring continuous therapy and obviating patient compliance issues.
Prostaglandin analogs are advantageous candidates for a drug delivery implant due to their high potency, which allows a small mass of loaded drug to last a long period of time. For example, the proprietary prostaglandin analog DE-117 will require a maximum desired release rate of 0.5 μg/day in the anterior chamber based on previous studies (Ihekoromadu N, et al. IOVS 2015;56:ARVO E-Abstract 5708) (Kirihara T, et al. IOVS 2015;56:ARVO E-Abstract 5709). The low desired release rate enables further miniaturization of the device, reducing bulk that may come from loading large amounts of less potent drugs. Furthermore, DE-117 is a promising candidate since it has been shown to reduce intraocular pressure (IOP) at a lower dose (0.002% [wt/vol]) (Ihekoromadu N, et al. IOVS 2015;56:ARVO E-Abstract 5708) than other prostaglandin analogs, such as latanoprost (0.005% [wt/vol]) or bimatoprost (0.01% [wt/vol]). DE-117, which is converted to its active form (hDE-117) by hydrolysis, is a selective prostaglandin EP2 agonist. It has been shown to significantly decrease IOP in animal models upon topical administration (Kirihara T, et al. IOVS 2015;56:ARVO E-Abstract 5709) and also in a phase 2a clinical study with a daily dose of 0.002% (wt/vol) eye drops (Ihekoromadu N, et al. IOVS 2015;56:ARVO E-Abstract 5708).
We developed an implantable DE-117 delivery device for treatment of glaucoma up to 6 months. Our proof-of-concept drug delivery device encapsulates DE-117 between two diffusion-limiting polycaprolactone (PCL) films. The biodegradable polyester PCL has been extensively used in drug delivery applications and has previously established ocular biocompatibility.5,6 Furthermore, a previous study supports the use of PCL films as a diffusion-limiting barrier for delivery of a highly hydrophobic drug, rapamycin, to the eye with zero-order release kinetics.6 To assess the potential of a PCL device as a long-term intracameral implant, we investigated drug release in vitro, device biocompatibility in rabbit anterior chambers, distribution of DE-117 and its active form in rabbit ocular tissues, and analysis of remaining device payload after in vivo release.
Methods
Materials
All chemicals were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA) unless noted otherwise. DE-117, its active form (hDE-117), and deuterium-labeled hDE-117 were prepared by Ube Industries, Ltd. (Ube, Japan). We obtained 10X stock PBS from Millipore (Billerica, MA, USA) and Tween 80 was obtained from Spectrum Chemical (New Brunswick, NJ, USA).
Device Fabrication
We made PCL thin films by spin-casting. First, PCL (Mn = 80 kDa) was dissolved in 2,2,2-trifluoroethanol at a concentration of 150 mg/mL. Then, the solution was casted on a silicon wafer using a spin-coater (Specialty Coating Systems, Indianapolis, IN, USA) at 1000 rpm for 10 seconds. The casting process was repeated twice. Films were annealed at 110°C to remove residual solvent and were left to cool at ambient temperature. Resulting films were thoroughly washed with Milli-Q deionized water and dried in air. Film thickness was measured using a film micrometer (iGaging, San Clemente, CA, USA). Four layers of PCL films were then stacked to create the desired thickness (224 μm). Drug delivery devices were assembled by placing DE-117 powder between two stacked films and heat-sealing the edges.6 Heat-sealing was performed with nichrome wire embedded in PDMS; PCL films were placed on the PDMS support above the wire and 1A current was applied to resistively heat the wire (Fig. 1). The resulting devices were approximately 3 × 3-mm in dimension.
Figure 1.
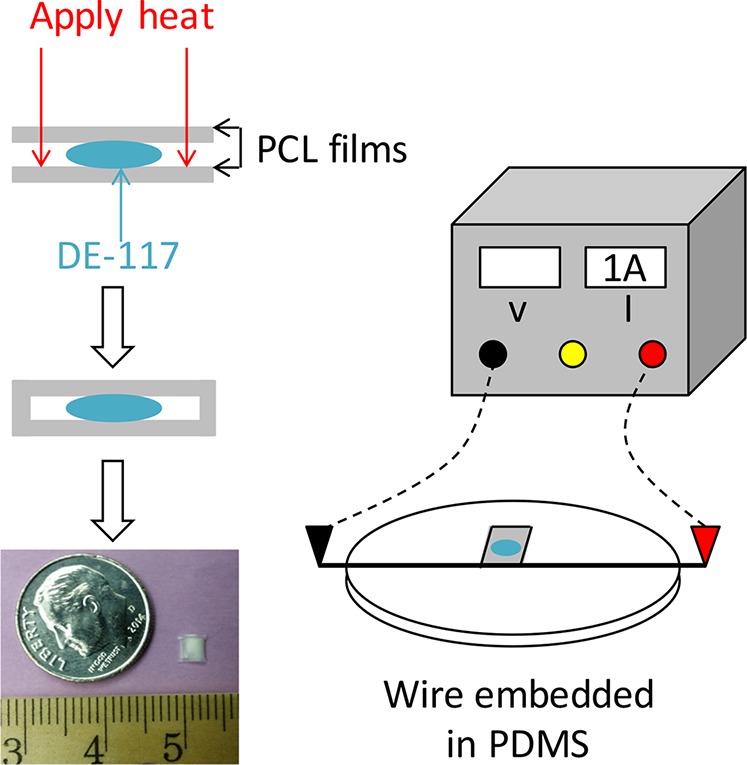
Schematic diagram of device fabrication and photo of a PCL device containing DE-117, with dime and metric ruler (millimeter tick marks) for scale. We encapsulated DE-117 between two PCL films and the edges of PCL films were heat-sealed by applying current to a wire embedded in PDMS.
In Vitro Release Studies and Quantification Analysis
All in vitro release studies used an elution buffer of 1× PBS with 0.1% Tween 80 (pH 7.4). Elution buffer was chosen based on the reported use of PBST (PBS + Tween 80) in the analysis of front-of-the-eye drug delivery devices7 and the aqueous humor composition (98.69% water and less than 0.1% wt/vol protein content).8 Devices (n = 4) were each submerged in 4 mL elution buffer. Volume of elution buffer was chosen to provide an absolute sink condition based on the solubility of DE-117 in buffer (25 μg/mL). The devices were placed on an orbital shaker at 120 rpm in a 37°C incubator to mimic physiological conditions. For each sampling time point, the elution buffer was collected and replaced with fresh buffer. Concentration of DE-117 in the collected elution buffer was measured using high performance liquid chromatography (HPLC; 1260 Infinity Quaternary LC System, Agilent Technologies, Santa Clara, CA, USA). A C18 reverse-phase column (Eclipse Plus C18, 4.6 × 100 mm, 3.5 μm; Agilent Technologies) was used with a gradient of mobile phase A:B (65:35–45:55 in 12 minutes). Mobile phase A contained 0.03% trifluoroacetic acid in deionized water and mobile phase B was HPLC-grade acetonitrile (Thermo Fisher Scientific, Waltham, MA, USA). Detection was done at a wavelength of 260 nm. The release rate at each time point was calculated by dividing the amount of drug collected by the duration between two time points. The average release rate was calculated by applying a linear regression to the cumulative released DE-117 versus time. Data is presented as mean ± standard deviation.
In Vivo Device Implantation
To evaluate the release rate of each device prior to implantation, in vitro release was studied for 10 days before implantation. Then, devices were collected, dried in a vacuum overnight, transferred to a sterile hood, washed in 70% ethanol, and subsequently dried before implantation.
Implantation of PCL devices in the rabbit eye was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. New Zealand white rabbits were premedicated with 0.03 mg/kg intramuscular buprenorphine and then anesthetized by inhalation of isoflurane (2%–4%). Proparacaine hydrochloride (0.5%) ophthalmic drops were given as topical anesthetic followed by Betadine drops (5% povidone iodine solution). A clear corneal incision was then made using a 2.8-mm slit knife (Alcon Laboratories, Ft. Worth, TX, USA) and widened to 4 mm, through which the PCL device was inserted into the anterior chamber. The incision was closed with 7-0 polyglactin 910 suture (Vicryl; Ethicon, Somerville, NJ, USA). Surgical procedures were performed on one eye and the contralateral eye was untreated. Clinical ophthalmologic exams by visual inspection of nonanesthetized animals were performed at day 1, week 1, and 7 with anterior segment photography performed at all time points using a digital single-lens reflex (DSLR) camera (Canon EOS Rebel T4i; Canon USA, San Jose, CA, USA). Exams utilizing the operating microscope were also performed immediately after implantation as well as prior to euthanization and photos were taken with the Canon camera body and a SLR camera-microscope adapter (Carl Zeiss Meditec, Inc., Dublin, CA, USA).
Analysis of Drug Distribution in Rabbit Ocular Tissues
Rabbits were anesthetized as described above and euthanized at various time points postimplantation (1, 2, 4, and 8 weeks) by intravenous injection of 2 mmol/kg potassium chloride into the marginal ear vein. The ocular globe was enucleated immediately after euthanasia. Aqueous humor was withdrawn by limbal paracentesis using a 30-gauge needle on a 1-mL syringe. The globe was dissected to collect cornea, iris-ciliary body, retina-choroid, and vitreous humor, and preserved by freezing at −80°C. We extracted DE-117 and hDE-117 from aqueous humor and homogenates of vitreous humor, cornea, iris-ciliary body, and retina-choroid by addition of organic solvents.
We determined DE-117 and hDE-117 concentrations in ocular tissues by a liquid chromatography coupled with a tandem mass spectrometry (LC/MS/MS) and calculated by the analytical concentration, tissue wet weight, and dilution factor. Deuterium-labeled hDE-117 was used for the internal standard of DE-117 and hDE-117 analyses. High performance liquid chromatographic system consists of system controller CBM-20A, solvent delivery unit LC30AD, auto-sampler SIL-30AC, and column oven CTO-30AC and degasser DGV-20A (Shimadzu Corp., Kyoto, Japan). An analytical column (Kinetex XB-C18, 2.1 × 50 mm inner diameter, 2.6 μm; Phenomenex, Torrance, CA, USA) was used with a gradient of mobile phase A:B (68:32–10:90). Mobile phase A contained 0.1% formic acid in deionized water and mobile phase B contained 0.1% formic acid in acetonitrile. The system (AB SCIEX QTRAP 5500; AB Sciex, Foster City, CA, USA) interfaced by turbo ion spray with positive ion source in multiple reaction monitoring mode was applied for detection. We used LC/MS grade of acetonitrile, methanol, and formic acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan) throughout sample preparation and LC/MS/MS analysis.
Postimplantation Device Analysis
Devices were collected at time of euthanization in the dissection of the rabbit eye and were photographed to macroscopically observe the degree of in vivo iris tissue adherence. Then, devices were rinsed with filtered water (Milli-Q; EMD Millipore Corp.) and dried in a vacuum chamber overnight. The dried devices were cut open and remaining drug was extracted from the device by serial extraction in diluent (50% acetonitrile and 50% water) over five iterations. Remaining drug in devices was characterized by HPLC as described above for in vitro release studies. Peak retention time of remaining drug was compared with those of DE-117 and hDE-117 in 1.5 μg/mL working standards. In addition, opened PCL devices were analyzed using gel permeation chromatography (GPC). Opened PCL devices were dissolved in tetrahydrofuran (THF; VWR International, Radnor, PA, USA) for GPC analysis. A series of GPC columns (Styragel HR 5, 2, and 0.5; Waters Corp., Milford, MA, USA) was used with THF flowing at 1 mL/minute for 40 minutes. A refractive index detector (RID) was used to detect the polymer. Reference standards of PCL (Mn = 80 kDa, 45 kDa, and 10 kDa) were freshly prepared in THF. The signal was normalized to baseline signal of fresh THF solution.
Results
To design our PCL ocular implant, design parameters were based on a predictive model of drug release.9 Previous work showed that by changing the thickness of diffusion-limiting PCL film and device dimensions, one can design devices to achieve a desired drug release rate.9 Based on the physiochemical properties of the drug, the following equation can be used to predict drug release from a PCL reservoir device (drug encapsulated between two PCL films):
 |
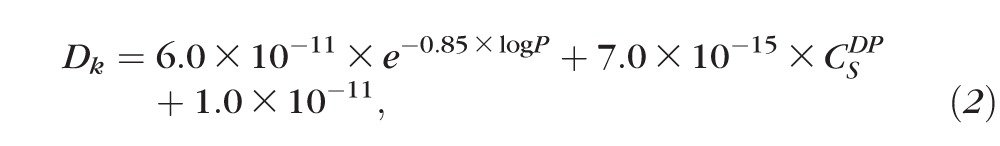 |
where J is mass flux of drug through PCL film, A is device surface area, Dk is a combined diffusion coefficient and partition coefficient, 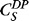 is the solubility of drug product at 25°C, and L is the thickness of PCL film.9 Based on the properties of DE-117 (log P = 3.15,
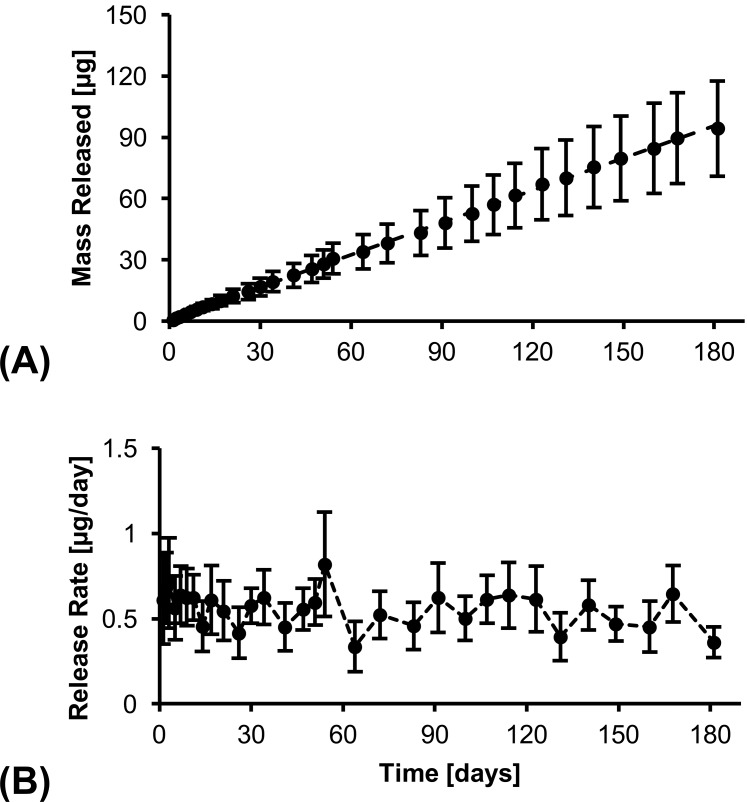
is the solubility of drug product at 25°C, and L is the thickness of PCL film.9 Based on the properties of DE-117 (log P = 3.15, 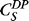 = 25 mg/L), the predicted Dk value of DE-117 is 1.43 × 10−11 m2/h. Considering the limited space of the anterior chamber, we chose to minimize device dimension as much as possible using current fabrication methods. Guided by this model, the final device was made with PCL film that was 224-μm thick and approximately 3 × 3 mm in dimension (Fig. 1). These DE-117–loaded PCL devices achieved a zero-order release rate of 0.53 μg/day in vitro (R2 = 0.9995 to linear fit) over 6 months (Fig. 2).
= 25 mg/L), the predicted Dk value of DE-117 is 1.43 × 10−11 m2/h. Considering the limited space of the anterior chamber, we chose to minimize device dimension as much as possible using current fabrication methods. Guided by this model, the final device was made with PCL film that was 224-μm thick and approximately 3 × 3 mm in dimension (Fig. 1). These DE-117–loaded PCL devices achieved a zero-order release rate of 0.53 μg/day in vitro (R2 = 0.9995 to linear fit) over 6 months (Fig. 2).
Figure 2.
(A) Cumulative mass released and (B) release rate of DE-117 over 6 months in vitro. Linear fit of cumulative mass released show zero-order release.
To obtain pharmacokinetic characteristics and confirm biocompatibility of these devices in the intracameral space, devices were surgically implanted into the anterior chamber of normotensive rabbits (Fig. 3). Preimplantation in vitro release studies of these devices yielded a linear release rate of 0.435 ± 0.075 μg/day (R2 = 0.989 to linear fit). The difference in release rate is due to the variability in device surface area that results from fabricating the devices by hand. Empty PCL devices were also implanted as controls (Fig. 3).
Figure 3.
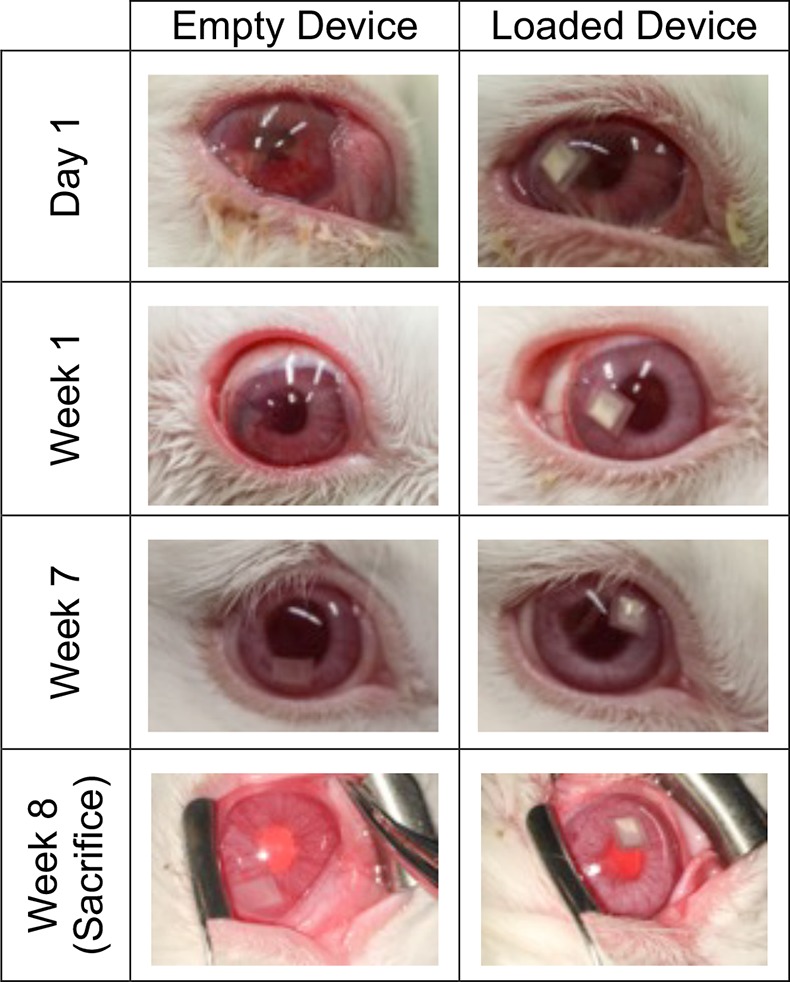
Representative photos of rabbit eye after implantation of empty or DE-117–loaded drug delivery devices. Devices were well tolerated in the anterior chamber of rabbit eyes.
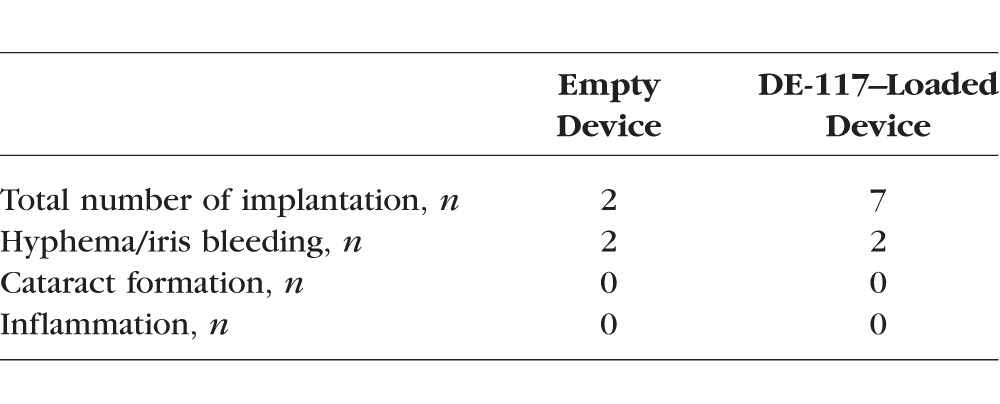
Devices with and without DE-117 were well tolerated in the anterior chamber (Table), with no incidence of infectious complications, uveitis, or cataract over 8 weeks. The most frequent ocular complications occurred during the implantation process related to surgical trauma. The scale of the rabbit eye being several times smaller than the human eye, these devices fit tightly in the shallow anterior chamber, positioning in direct contact with both the posterior cornea and the iris. Iris bleeding and/or hyphema occurred in four out of nine procedures overall. However, as the surgical technique was tuned, the rate of surgical trauma was reduced from two out of three surgeries (first day of surgery) to two out of six surgeries (second day) during the course of this study.
Table.
Summary of Biocompatibility Analysis
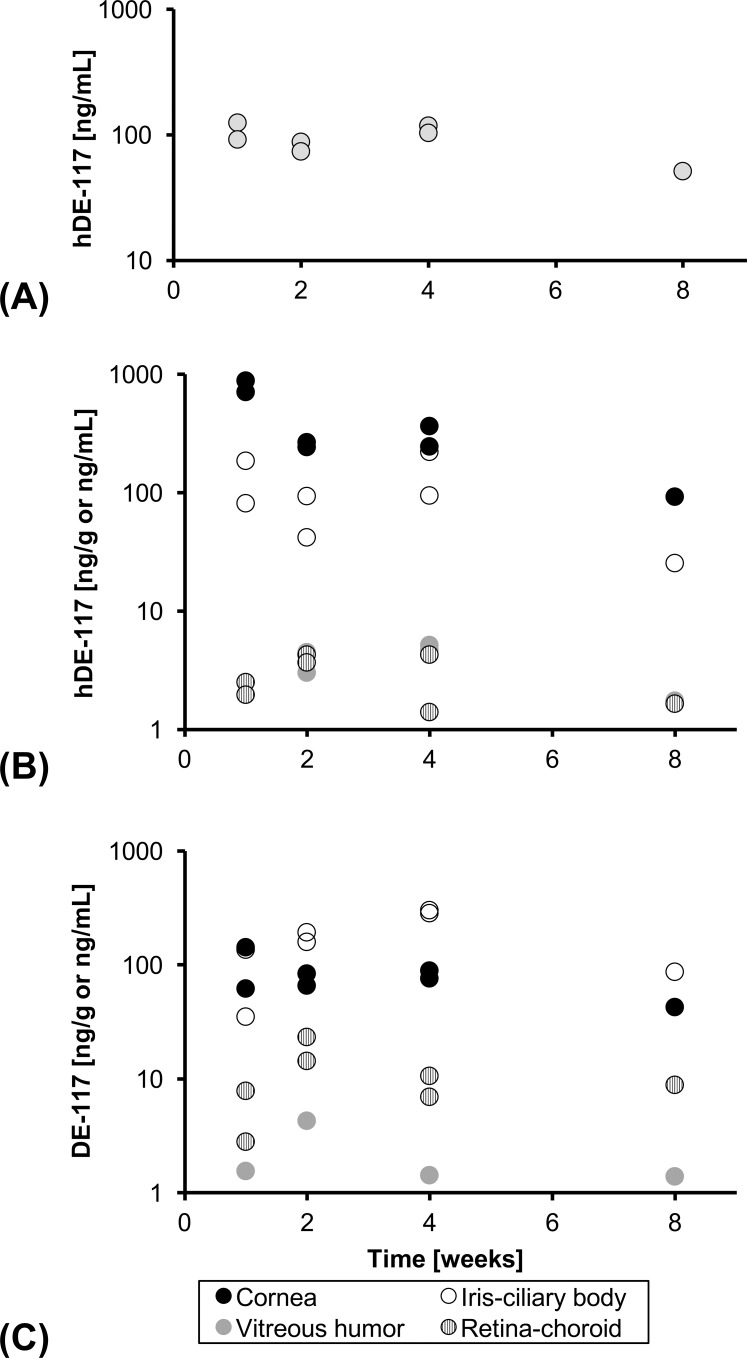
LC-MS analysis of DE-117 and hDE-117 showed that devices released DE-117 in the eye as expected from the in vitro release studies; once released, DE-117 was readily converted to its active form (Fig. 4). Furthermore, concentration of hDE-117 in the aqueous humor was maintained at a relatively steady level (93 ± 25 ng/mL) over the time course studied (Fig. 4), indicating sustained release of DE-117 and conversion to hDE-117 in vivo. Concentration of hDE-117 in the aqueous humor was also near the Cmax achieved by topical administration of 0.1% DE-117 solution (108 ± 23 ng/mL) (Kirihara T, et al. IOVS 2015;56:ARVO E-Abstract 5709). Concentration of DE-117 and hDE-117 in the blood was below the limit of detection.
Figure 4.
(A) Concentration of hDE-117 in the aqueous humor, distribution of (B) hDE-117 and (C) DE-117 in ocular tissues through 8 weeks after device implantation. Units are ng/mL for aqueous humor and vitreous humor and ng/g for cornea, iris-ciliary body, and retina-choroid. Concentration of DE-117 and hDE-117 in ocular tissues shows sustained release of DE-117 in the anterior chamber and its conversion to hDE-117 upon release.
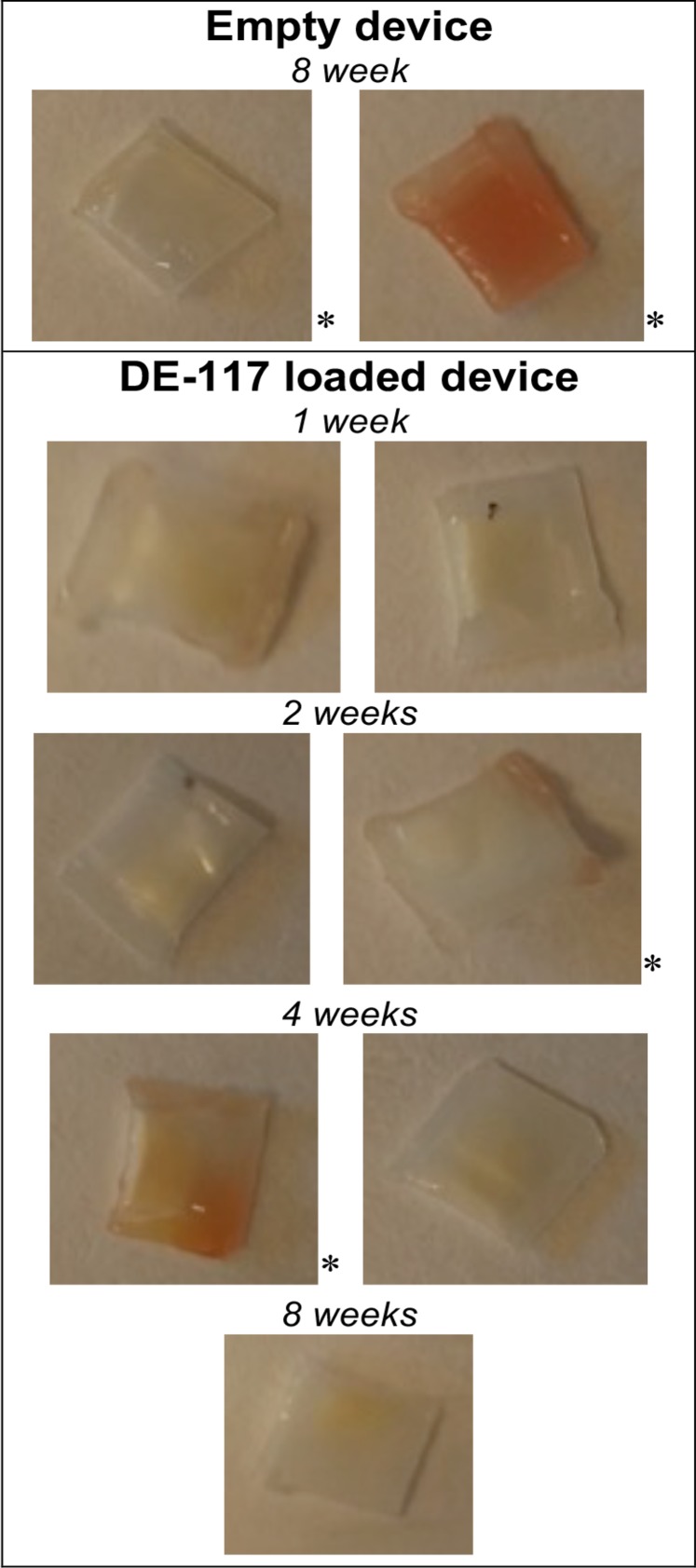
Devices showed some macroscopically observable iris stroma tissue adherence upon removal from the rabbit eye (Fig. 5). However, we noted that three out of four devices that experienced hemorrhage events during implantation (indicated with an asterisk) showed tissue adherence. All devices that underwent implantation without surgical complications lacked noticeable tissue adherence. This suggests that device tissue adherence is due to the surgical trauma, not due to the material properties of the device. Furthermore, LC-MS analysis of DE-117 and hDE-117 concentration in ocular tissues (Fig. 4) confirmed that the tissue adherence did not have an observable effect on the release of DE-117 from the device.
Figure 5.
Macroscopic photos of PCL devices collected from the rabbit eye through 8 weeks, showing tissue adherence after residence in the intracameral space. A correlation between tissue adherence and hemorrhage events during implantation (noted by an asterisk) has been observed.
Once devices were retrieved from the rabbit eye, the remaining drug was extracted and analyzed for uniformity of its contents. Analysis of HPLC showed that all remaining drug in the device was DE-117 (Supplementary Fig. S1). This confirmed that the drug payload was protected from esterases present in the anterior segment of the eye and was only converted to hDE-117 upon release. Lastly, PCL degradation in the intracameral space was analyzed by GPC (Supplementary Fig. S2). Based on the peak retention time of 80 kDa PCL standard, devices retrieved from the eye at various time points showed no change in average and distribution of retention time, indicating negligible change in the molecular weight distribution of PCL in the rabbit eye up to 4 weeks. While PCL is expected to degrade in vivo,10,11 the change in PCL molecular weight over 4 weeks in vivo was likely too small to be detectable considering the inherent variability of GPC analysis.
Discussion
Poor patient compliance in topical glaucoma treatment has been repeatedly reported.3,4,12 To overcome this challenge, several drug delivery implants for glaucoma are in development. These include an implant device for delivery of latanoprost (Durasert; Pfizer, New York, NY, USA) and a sustained release formulation of bimatoprost currently in phase 3 clinical trials (NCT02250651; Allergan, Irvine, CA, USA).13 We explored biodegradable polymers and formulations that can achieve a release of DE-117 of 6 months or more in the anterior chamber and chose PCL as our device material. Compared with other polymers that have been utilized in glaucoma drug delivery devices such as poly(lactic-co-glycolic acid),14 we noted that a slow degradation rate of PCL is important to achieve zero-order release of the therapeutic over several months. Since the device acts as a diffusion-limiting barrier in our design, a hypothetical polymer that degrades completely in 6 months would not be able to deliver the therapeutic at a constant rate for 6 months. While PCL has been utilized in intravitreous6,15,16 and subretinal implants,17 PCL has not been widely utilized as intracameral implants for treatment of diseases, such as glaucoma. Biocompatibility of PCL and its degradation profile in the ocular space have been well characterized and our study supports previous reports that indicate that PCL is well tolerated in the eye5,6 and degrades slowly15 with negligible change in Mn over 4 weeks.
Based on the concentration of DE-117 detected in the aqueous humor, in vivo release rates can be estimated. Previous studies have noted two main mechanisms of drug clearance from the anterior chamber: by convective flow due to aqueous humor turnover and by uveal blood flow.18 In our calculations, we assumed that the main mode of DE-117 clearance from the anterior chamber is due to aqueous humor turnover considering the rapid turnover rate (2.31 μL/min in rabbits,19 indicating complete turnover of aqueous humor in approximately 90 minutes). Based on this number, rate of drug release could be calculated by the equation below.
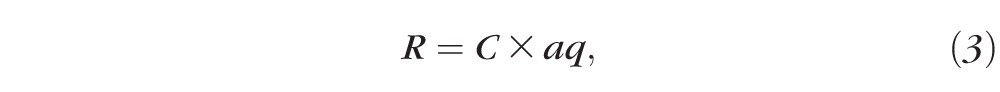 |
where R is the in vivo release rate of drug, C is the concentration of DE-117 found in the aqueous humor, and aq is the rate of aqueous humor turnover. Consequently, the estimated in vivo release rate of DE-117 is 0.42 ± 0.24 μg/day, which is comparable to the preimplantation in vitro release rate (0.44 ± 0.08 μg/day). We expect the actual in vivo release rate to be higher due to uveal blood flow or additional clearance mechanisms that increase the rate of DE-117 clearance. An additional term that accounts for uveal blood flow was not utilized in this calculation, as we noted that an accurate prediction of in vivo release rate is not possible due to the natural variability of the aq value19 used in the calculation. Due to the simplifying assumptions, the equation is intended to provide an estimate of in vivo release rate in the intracameral space instead of an accurate prediction. With the constraints of the equation in mind, this calculation suggests that the experimental conditions used in the in vitro release study resembled physiological conditions in the anterior chamber of the eye and in vitro release results can be used to estimate release in vivo.
The previously mentioned predictive model anticipates that the release rate of drug from PCL devices depend on the drug's solubility and log P.9 Therefore, hydrolysis of DE-117 in the device would impact the release over time: an undesirable property of a sustained release device. For example, complete hydrolysis of DE-117 to hDE-117 (Cs = 1000 mg/L and log P = −1.19) would change the Dk value of Equation 2 to 1.82 × 10−10 m2/h, increasing the predictive release rate 500-fold. Fortunately, DE-117 is protected from hydrolysis when encapsulated in the device and is only converted to its active form when released to the anterior humor. In addition, the uniformity of remaining drug suggests that the PCL devices were not physically compromised during the implantation or retrieval procedures.
However, there are a few limitations of this study that should be addressed. Current fabrication processes involve manual positioning of PCL films on the heat-sealing apparatus to achieve desired device dimensions, which can result in unintended variability. Limitations of our study also include small number of animals tested with device implantation. Since the study was focused on evaluating the biocompatibility of the device, the study was not designed to test the IOP reducing effect of released DE-117. Furthermore, considering the location of implantation, damage to the corneal endothelium is possible. However, as pharmacokinetic and GPC analysis required dissection of the rabbit eyes, histology could not be performed after euthanization to check for damages. Also, based on previous report studying degradation of PCL,10,11 80-kDa PCL can take up to 2 years to completely degrade in vivo. To ensure the device is not retained in the eye after full drug depletion, the degradation rate of PCL should be tuned to match the desired lifetime of a glaucoma implant.
Due to the potency of DE-117, the desired maximum release rate of DE-117 in the intracameral space is 0.5 μg/day, which only requires loading 90 μg of the drug for a 6-month release. In this study, we tested the smallest devices that could be reliably made via manual fabrication. However, there is potential to further miniaturize device size and reduce release rate to match the dose that has been successfully tested in clinical trials (0.002% wt/vol daily eye drops) (Ihekoromadu N, et al. IOVS 2015;56:ARVO E-Abstract 5708). Upon device miniaturization, the position of device implantation can also be optimized so that the devices are away from the angle of the eye. Future studies will be powered to evaluate the IOP-reducing effect of intracameral implants containing DE-117 over 6 months. Also, histologic analysis on the effect of intracameral implantation will be performed to check corneal endothelial damage. Furthermore, study of these devices in larger animals, such as dogs or monkeys, will provide better representations of the IOP-reducing effect in humans. In addition, blends of PCL with other biodegradable polymers, such as poly(D,L-lactide) (PLA), can be explored to tune degradation rates. Previous studies have shown that the degradation rate of physical blends of PLA and PCL is between those of pure PLA and PCL.20
In summary, we demonstrate the potential of DE-117 PCL devices based on in vitro release kinetics of DE-117, biocompatibility of device in the intracameral space, and distribution of DE-117 and hDE-117 in ocular tissues upon implantation. Future studies will focus on optimizing the devices and evaluating the major points described above for further development and clinical translation.
Supplementary Material
Acknowledgments
Supported by R01EY021574 from the National Institutes of Health, a research grant from Santen Pharmaceutical Co., Ltd., and an unrestricted grant from Research to Prevent Blindness.
Disclosure: J. Kim, None; M. Kudisch, None; S. Mudumba, Santen, Inc. (E); H. Asada, Santen Pharmaceutical Co, Ltd. (E); E. Aya-Shibuya, Santen Pharmaceutical Co., Ltd. (E); R.B. Bhisitkul, Santen Pharmaceutical Co., Ltd. (F), Santen, Inc. (C), P; T.A. Desai, Santen Pharmaceutical Co., Ltd. (F), Santen, Inc. (C), P
References
- 1. World Health Organization. Glaucoma is second leading cause of blindness globally. Available at: http://www.who.int/bulletin/volumes/82/11/feature1104/en/. Accessed December 15, 2015. [PMC free article] [PubMed]
- 2. Glaucoma Research Foundation. Glaucoma facts and stats. Available at: http://www.glaucoma.org/glaucoma/glaucoma-facts-and-stats.php. Accessed December 15, 2015.
- 3. Burns E,, Mulley GP. Practical problems with eye-drops among elderly ophthalmology outpatients. Age Ageing. 1992; 21: 168–170. [DOI] [PubMed] [Google Scholar]
- 4. Gurwitz JH,, Glynn RJ,, Monane M,, et al. Treatment for glaucoma: Adherence by the elderly. Am J Public Health. 1993; 83: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernards DA,, Bhisitkul RB,, Wynn P,, et al. Ocular biocompatibility and structural integrity of micro- and nanostructured poly(caprolactone) films. J Ocul Pharmacol Ther. 2013; 29: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lance KD,, Good SD,, Mendes TS,, et al. In vitro and in vivo sustained zero-order delivery of rapamycin (sirolimus) from a biodegradable intraocular device. Invest Opthalmol Vis Sci. 2015; 56: 7331–7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiang B,, Kim YC,, Doty AC,, et al. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release. 2016; 228: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agarwal S,, Agarwal A,, Apple DJ. Textbook of Ophthalmology. New Delhi India: Jaypee Brothers; 2002. [Google Scholar]
- 9. Schlesinger E,, Ciaccio N,, Desai TA. Polycaprolactone thin-film drug delivery systems: empirical and predictive models for device design. Mater Sci Eng C Mater Biol Appl. 2015; 57: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun H,, Mei L,, Song C,, Cui X,, Wang P. The in vivo degradation absorption and excretion of PCL-based implant. Biomaterials. 2006; 27: 1735–1740. [DOI] [PubMed] [Google Scholar]
- 11. Pitt CG,, Chaslow FI,, Hibionada YM,, Klimas DM,, Schindler A. Alphatic polyesters. I. The degradation of poly(ε-caprolactone) in vivo. J Appl Polym Sci. 1981; 26: 3779–3787. [Google Scholar]
- 12. Robin A,, Grover DS. Compliance and adherence in glaucoma management. Indian J Ophthalmol. 2011; 59: S93–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Safety and efficacy of bimatoprost sustained-release (SR) in patients with open-angle glaucoma or ocular hypertension. ClinicalTrials.gov. available at: https://www.clinicaltrials.gov/ct2/show/NCT02250651?term=bimatoprost+sr&rank=2. Accessed December 15, 2015.
- 14. Safety study of latanoprost slow release insert (latanoprost SR). ClinicaTrials.gov. Available at: https://www.clinicaltrials.gov/ct2/show/NCT01180062?term=latanoprost+SR&rank=1. Accessed December 15, 2015.
- 15. Fialho S,, Behar-Cohen F,, Silva-Cunha A. Dexamethasone-loaded poly(ε-caprolactone) intravitreal implants: a pilot study. Eur J Pharm Biopharm. 2008; 68: 637–646. [DOI] [PubMed] [Google Scholar]
- 16. Silva-Cunha A,, Fialho SL,, Naud MC,, Behar-Cohen F. Poly-ε-caprolactone intravitreous devices: an in vivo study. Invest Opthalmol Vis Sci. 2009; 50: 2312. [DOI] [PubMed] [Google Scholar]
- 17. Beeley NRF,, Rossi JV,, Mello-Filho P,, et al. Fabrication, implantation, elution, and retrieval of a steroid-loaded polycaprolactone subretinal implant. J Biomed Mater Res A. 2005; 73: 437–444. [DOI] [PubMed] [Google Scholar]
- 18. Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006; 58: 1131–1135. [DOI] [PubMed] [Google Scholar]
- 19. Alvarez LJ,, Zamudio AC,, Candia OA. Sildenafil stimulates aqueous humor turnover in rabbits. Exp Eye Res. 2013. ; 111: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang L,, Ma W,, Gross RA,, McCarthy SP. Reactive compatibilization of biodegradable blends of poly(lactic acid) and poly(ε-caprolactone). Polym Degrad Stab. 1998; 59: 161–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.